Abstract
Background
The relationships between coffee, tea, and sugar-sweetened carbonated soft drink consumption and colon cancer risk remain unresolved.
Methods
We investigated prospectively the association between coffee, tea, and sugar-sweetened carbonated soft drink consumption and colon cancer risk in a pooled analysis of primary data from 13 cohort studies. Among 731 441 participants followed for up to 6–20 years, 5604 incident colon cancer case patients were identified. Study-specific relative risks (RRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models and then pooled using a random-effects model. All statistical tests were two-sided.
Results
Compared with nonconsumers, the pooled multivariable relative risks were 1.07 (95% CI = 0.89 to 1.30, Ptrend = .68) for coffee consumption greater than 1400 g/d (about six 8-oz cups) and 1.28 (95% CI = 1.02 to 1.61, Ptrend = .01) for tea consumption greater than 900 g/d (about four 8-oz cups). For sugar-sweetened carbonated soft drink consumption, the pooled multivariable relative risk comparing consumption greater than 550 g/d (about 18 oz) to nonconsumers was 0.94 (95% CI = 0.66 to 1.32, Ptrend = .91). No statistically significant between-studies heterogeneity was observed for the highest category of each beverage consumed (P > .20). The observed associations did not differ by sex, smoking status, alcohol consumption, body mass index, physical activity, or tumor site (P > .05).
Conclusions
Drinking coffee or sugar-sweetened carbonated soft drinks was not associated with colon cancer risk. However, a modest positive association with higher tea consumption is possible and requires further study.
CONTEXTS AND CAVEATS
Prior knowledge
Coffee and tea are the most commonly consumed beverages worldwide, and consumption of sweetened soft drinks is increasing. Although polyphenols in coffee and tea have been thought to protect against colorectal cancer, all three beverages have been studied as potential risk factors.
Study design
The association between the consumption of coffee, tea, and sweetened soft drinks and the risk of developing colorectal cancer was examined in a pooled analysis of 13 prospective cohort studies conducted in North America and Europe.
Contribution
No statistically significant association was found between the risk of developing colorectal cancer and consumption of coffee or sweetened soft drinks, although a small positive association was found for higher tea consumption. The results were similar regardless of sex, smoking status, alcohol consumption, body mass index, physical activity, and tumor site.
Implications
Drinking more than six 8-oz cups of coffee or 18 oz of sweetened soft drinks is not associated with a risk of colorectal cancer. The modest association with consumption of more than four 8-oz cups of non-herbal tea requires further study.
Limitations
The association between colon cancer risk and coffee consumption was studied only in a European population, and thus, the lack of such an association cannot be generalized to other populations. Only 2% of the population consumed more than four 8-oz cups of tea or 18 oz of soft drinks, and thus, the study may be underpowered to draw conclusions for high consumption levels. Addition of milk and sugar to coffee and tea and consumption of different types of tea or diet soft drinks were not directly measured in the studies and may result in unmeasured confounding.
From the Editors
Colorectal cancer is the third most common cancer worldwide (1). Incidence rates vary more than 25-fold across countries (1), suggesting that environmental and lifestyle factors such as diet may play a role in the development of colorectal cancer (2). Approximately 70%–80% of colorectal cancer in developed countries is colon cancer (3). We focus on colon cancer in this report because colon and rectal cancers may have distinct etiologies (3,4).
Coffee and tea are among the most commonly consumed beverages worldwide, and they have been hypothesized to decrease the risk of colon cancer (5,6). Polyphenols present in coffee and tea protect against colon tumor formation in animal studies, possibly through their antioxidant properties (5,6). In addition, coffee consumption may reduce the synthesis and secretion of bile acids, potential promoters of colon carcinogenesis (7) and increase colonic motility (8), thus decreasing exposure of epithelial cells to potential carcinogens in the colon (6). On the other hand, coffee and tea also contain compounds with mutagenic and genotoxic properties (6,9,10), which could increase the risk of colon cancer. In 2007, a report by the World Cancer Research Fund and the American Institute for Cancer Research determined that no firm conclusions could be reached on the associations between consumption of coffee and tea and risk of colon cancer because of inconsistent epidemiological evidence (2).
Worldwide, consumption of caloric sweeteners (such as glucose, fructose, sucrose, honey, and high-fructose corn syrup) increased approximately 30% from 1962 to 2000, resulting in a 74 kcal/d average increase in energy intake (11). In addition, consumption of sugar-sweetened carbonated soft drinks (ie, those containing caloric sweeteners) has been positively associated with weight gain, obesity, insulin resistance, and type 2 diabetes (11), all of which are potential risk factors for colon cancer (2). However, the relationship between soft drink intake and colon cancer risk has been examined rarely, and no conclusion could be made regarding this association in a recent review by the World Cancer Research Fund (2).
Given the inconsistent associations between consumption of coffee, tea, and sugar-sweetened carbonated soft drinks and risk of colon cancer, and the potential implications for colon cancer prevention, we conducted a pooled analysis examining the association between consumption of these beverages and colon cancer risk in 13 prospective cohort studies conducted in North America and Europe. We also examined whether these associations were modified by colon cancer risk factors and tumor site.
Methods
Study Population
This study was conducted within the Pooling Project of Prospective Studies of Diet and Cancer (Pooling Project), an international consortium of cohort studies (12). For the analyses on colorectal cancer, 13 prospective cohort studies (13–24) were identified that met the following prespecified criteria: identification of at least 50 incident case patients of colorectal cancer, at least one publication of a diet and cancer association, comprehensive assessment of long-term dietary intake, and evaluation of the dietary assessment method or a closely related instrument in a validation study. The New York University Women’s Health Study, which has been included in other colon cancer analyses in the Pooling Project, was excluded from the current analyses because this study did not assess coffee, tea, and sugar-sweetened carbonated soft drink intakes. All studies included here were reviewed and approved by the institutional review board of the institution at which the study was conducted.
Identification of Colon Cancer Case Patients
Incident colon cancer case patients were identified in each study by follow-up questionnaires with subsequent review of medical records (21,24), linkage to cancer registries (16,18–20,22,23), or both (13–15,17). Some studies also used mortality registries (13,15,17,18,21) as an additional source for identifying colon cancer case patients. The follow-up rate has generally been estimated to be greater than 90% in most of the studies (12). Colon cancer was further classified into proximal colon cancers (neoplasms from the cecum to the splenic flexure) and distal colon cancers (neoplasms in the descending and sigmoid colon) (25).
Dietary Assessment
Each study assessed diet at baseline using a self-administered food-frequency questionnaire (12). Information on coffee consumption was obtained in 11 studies (Table 1). Four studies (Health Professionals Follow-up Study, Iowa Women's Health Study, Nurses’ Health Study (a) and (b), and Women's Health Study) obtained information on both regular and decaffeinated coffee consumption, two studies (Adventist Health Study and Canadian National Breast Screening Study) measured consumption of regular (caffeinated) coffee only, and five studies (Alpha-Tocopherol Beta-Carotene Cancer Prevention Study; Netherlands Cohort Study; New York State Cohort; Swedish Mammography Cohort; and Prospective Study on Hormones, Diet, and Breast Cancer) did not distinguish the type of coffee consumed. Ten percent of the coffee consumed was estimated to be decaffeinated coffee based on a validation study on a subsample of the Prospective Study on Hormones, Diet, and Breast Cancer Study; all the coffee consumed was assumed to be caffeinated based on consumption patterns in the remaining four studies. Information on tea consumption was obtained from 10 cohort studies (Table 1). The consumption of “tea (not herbal tea)” was directly measured in the food-frequency questionnaires specifically in four US-based cohorts (Adventist Health Study, Health Professionals Follow-up Study, Iowa Women's Health Study, and Women's Health Study). Based on personal communications from the principal investigators in each study, herbal tea consumption was low in the remaining studies. Information on sugar-sweetened carbonated soft drinks was measured in 10 studies (Table 1), although how this variable was measured differed across studies.
Table 1.
Baseline characteristics of the cohort studies included in the pooled analyses of coffee, tea, and sugar-sweetened carbonated soft drink consumption and colon cancer risk
| Study | Baseline cohort size* | Follow-up years | No. of colon cancer case patients† | Baseline age range, years | Coffee‡ |
Tea‡ |
Sugar-sweetened carbonated soft drinks‡ |
|||
| Nondrinkers (%) | Median (10th, 90th percentile) intake among drinkers, g/d | Nondrinkers (%) | Median (10th, 90th percentile) intake among drinkers, g/d | Nondrinkers (%) | Median (10th, 90th percentile) intake among drinkers, g/d | |||||
| Men | ||||||||||
| Adventist Health Study | 12 368 | 1976–1982 | 46 | 25–90 | 57 | 237 (17, 1066) | 78 | 17 (17, 237) | § | § |
| Alpha-Tocopherol Beta-Carotene Cancer Prevention Study | 26 987 | 1984–1999 | 187 | 50–69 | 2 | 600 (220, 1100) | 64 | 157 (31, 440) | 58 | 47 (11, 236) |
| Cancer Prevention Study II Nutrition Cohort | 66 068 | 1992–1999 | 467 | 50–74 | § | § | § | § | 45 | 77 (18, 270) |
| Health Professionals Follow-up Study | 45 128 | 1986–2000 | 430 | 40–75 | 30 | 237 (33, 1066) | 42 | 102 (19, 592) | 44 | 88 (30, 372) |
| Netherlands Cohort Study | 58 279 | 1986–1993 | 393 | 55–69 | 3 | 500 (250, 875) | 15 | 375 (125, 625) | 47 | 54 (6, 175) |
| New York State Cohort | 30 363 | 1980–1987 | 335 | 50–93 | 12 | 710 (237, 1421) | 64 | 237 (237, 710) | § | § |
| Women | ||||||||||
| Adventist Health Study | 17 397 | 1976–1982 | 60 | 25–90 | 67 | 237 (17, 592) | 74 | 17 (17, 237) | § | § |
| Breast Cancer Detection Demonstration Project Follow-up Study | 41 987 | 1987–1999 | 349 | 40–93 | § | § | § | § | 61 | 47 (6, 247) |
| Canadian National Breast Screening Study | 49 613 | 1980–2000 | 431 | 40–59 | 15 | 448 (96, 1120) | 23 | 336 (32, 896) | 63 | 40 (7, 224) |
| Cancer Prevention Study II Nutrition Cohort | 74 046 | 1992–1999 | 349 | 50–74 | § | § | § | § | 64 | 29 (10, 202) |
| Iowa Women's Health Study | 33 844 | 1986–2001 | 784 | 55–69 | 10 | 597 (191, 1421) | 42 | 102 (19, 592) | 57 | 58 (30, 298) |
| Netherlands Cohort Study | 62 573 | 1986–1993 | 353 | 55–69 | 4 | 500 (250, 750) | 11 | 375 (125, 750) | 54 | 29 (6, 162) |
| New York State Cohort | 22 550 | 1980–1987 | 223 | 50–93 | 15 | 474 (237, 1184) | 49 | 474 (237, 947) | § | § |
| Nurses’ Health Study (a) | 86 769 | 1980–1986 | 155 | 34–59 | 23 | 592 (189, 1421) | 30 | 237 (17, 592) | 45 | 78 (26, 422) |
| Nurses’ Health Study (b) | 64 449‖ | 1986–2000 | 401 | 40–65 | 26 | 592 (102, 1066) | 37 | 102 (19, 592) | 62 | 52 (30, 370) |
| Prospective Study on Hormones, Diet and Breast Cancer¶ | 9079 | 1987–2001 | 45 | 34–70 | 29 | 320 (92, 596) | § | § | § | § |
| Swedish Mammography Cohort | 56 741 | 1987–2003 | 437 | 40–74 | 4 | 443 (177, 768) | 29 | 181 (16, 555) | 60 | 23 (12, 75) |
| Women's Health Study | 37 649 | 1993–2002 | 159 | 45–89 | 14 | 592 (189, 1189) | 33 | 102 (19, 592) | 64 | 82 (30, 400) |
Total cohort size = 731 441. Baseline cohort size is after applying study-specific exclusion criteria and further excluding participants with energy intakes beyond 3 SDs of their loge-transformed study-specific mean energy intake, history of cancer diagnosis at baseline (except for nonmelanoma skin cancer), and missing values for coffee, tea, and sugar-sweetened carbonated soft drink intake (if the beverage was measured in the study); the Canadian National Breast Screening Study and the Netherlands Cohort Study were analyzed as case-cohort studies, and the above exclusions were not applied to their baseline cohort size.
Total number of incident colon cancer case patients was 5604.
For coffee and tea, 8 oz weighs approximately 237 g; for sugar-sweetened carbonated soft drinks, 12 oz weighs approximately 355 g.
Intake of the beverage was not assessed.
Nurses’ Health Study (b) was not included in the total cohort size because these participants were included in Nurses’ Health Study (a).
In the Prospective Study on Hormones, Diet and Breast Cancer, the consumption of espresso coffee, not regular coffee, was measured. To approximate the amount of regular coffee consumed, we multiplied the espresso coffee intake by 4.
The quantity of each beverage or food consumed was provided by each study as the amount (in grams) or frequency. We converted the frequency data to grams consumed per day based on the study-specific serving size for each food item. We calculated the consumption of coffee, tea, and sugar-sweetened carbonated soft drinks by summing up the related individual beverages listed in each study. Each study also provided intake data for caffeine and energy. Caffeine intake was calculated by multiplying the frequency of consumption of each food item, the portion size of the food, and the caffeine content of the portion.
The food-frequency questionnaire used in each study or a closely related instrument was evaluated in a validation study (16,23,26–32) (V.K., personal communication, 2005). However, we were unable to conduct measurement error correction analyses because only a few studies assessed the validity of specific foods or food groups (23,27,29). Among these studies, the correlation coefficient comparing intakes assessed by the food-frequency questionnaire with intakes assessed by multiple dietary records or 24-hour recalls generally exceeded 0.5 for coffee, tea, and sugar-sweetened carbonated soft drinks.
Statistical Analyses
Studies that enrolled both women and men were analyzed separately as sex specific cohorts. To take advantage of the more detailed dietary assessment available in 1986, we analyzed the Nurses’ Health Study as two different cohorts (1980–1986, Nurses’ Health Study[a]; 1986–2000, Nurses’ Health Study[b]) because blocks of person-time, derived from the same participants, are asymptotically uncorrelated according to the underlying theory of survival analysis (33). We analyzed the Canadian National Breast Screening Study and Netherlands Cohort Study as case-cohort studies (34) because these two studies had each processed dietary questionnaires for only a sample of their noncase participants. We applied the exclusion criteria used by each study and further excluded participants with a history of cancer at baseline (except for nonmelanoma skin cancer) and with energy intakes beyond 3 SDs from their study-specific loge-transformed mean energy intake. We additionally excluded participants with missing data on coffee, tea, or sugar-sweetened carbonated soft drinks.
We conducted all analyses using the Statistical Analysis System (SAS) software (SAS Institute, Inc, Version 9; Cary, NC). We modeled intake of each beverage as a categorical variable across studies; the cut points used to categorize intakes captured approximate multiples of 8 oz (1 fl oz = 30 mL) servings of coffee and tea and 12-oz servings of sugar-sweetened carbonated soft drinks (35). If there were no case patients in the highest category in a study, the relative risk of that category could not be estimated in that study, and the person-time and noncase participants in the highest category were then included in the second highest category. To calculate P for the test for trend, we assigned the median value for each intake category and modeled that variable as a continuous term. Given that coffee and tea are good sources of caffeine (5,6), we analyzed caffeine intake using study-specific quintiles.
We used a two-stage analysis strategy to estimate pooled relative risks (RRs) and 95% confidence intervals (CIs) (12). First, a Cox proportional hazards model (36) using the SAS proportional hazards regression procedure (PROC PHREG) (37) was used to estimate study- and sex-specific relative risks and 95% confidence intervals. Person-years of follow-up were calculated from the date of questionnaire return to the date of diagnosis of incident colon cancer, death, loss to follow-up (if applicable), or end of the follow-up, whichever came first. The person-time for participants diagnosed with rectal cancer was censored at their date of diagnosis. Age at baseline (in years) and year of questionnaire return were entered as stratification variables. Thus, we created a time metric simultaneously accounting for age, calendar time, and time since entry into the study (12). We also conducted multivariable analyses to adjust for other established and potential colon cancer risk factors, including family history of colorectal cancer, education, physical activity, body mass index, height, smoking habits, alcohol intake, red meat intake, dietary folate intake, total energy intake, total milk consumption, use of nonsteroidal anti-inflammatory drugs and multivitamins, and, in women, use of oral contraceptives and postmenopausal hormones (see Table 2 for categories). We conducted additional analyses in which we mutually adjusted for tea and coffee drinking. Furthermore, we conducted analyses by excluding body mass index from the multivariable model to evaluate whether body mass index is in the causal pathway of beverage intakes and colon cancer risk. Because the proportion of participants with missing data for the covariates was generally low in these studies, we created an indicator variable for missing data where applicable (12).
Table 2.
Pooled relative risks (RRs) and 95% confidence intervals (CIs) of colon cancer for coffee, tea, and sugar-sweetened carbonated soft drink consumption
| Beverages | Categories of beverage intake | Ptrend | Pbetween-studies heterogeneity, highest category | Pbetween-studies heterogeneity due to sex, highest category | |||||
| Coffee* | |||||||||
| Intake category, g/d† | Nondrinkers | >0–150‡ | >150–400 | >400–900 | >900–1400§ | >1400§,‖ | |||
| Median intake, g/d | 0 | 59 | 237 | 592 | 1066 | 1421 | |||
| No. of case patients (women, men) | 416, 197 | 205, 111 | 591, 310 | 1413, 593 | 290, 131 | 133, 49 | |||
| Age-adjusted RR (95% CI) | |||||||||
| Total | 1 | 1.00 (0.87 to 1.16) | 1.06 (0.94 to 1.19) | 1.05 (0.93 to 1.17) | 1.00 (0.87 to 1.16) | 1.13 (0.94 to 1.36) | .64 | .93 | .22 |
| Women | 1 | 0.96 (0.81 to 1.15) | 1.03 (0.90 to 1.18) | 1.04 (0.90 to 1.21) | 0.99 (0.84 to 1.16) | 1.05 (0.84 to 1.31) | .64 | .81 | |
| Men | 1 | 1.09 (0.85 to 1.40) | 1.13 (0.92 to 1.39) | 1.06 (0.87 to 1.30) | 1.05 (0.81 to 1.37) | 1.36 (0.96 to 1.92) | .85 | .98 | |
| Multivariable RR (95% CI)¶ | |||||||||
| Total | 1 | 0.97 (0.84 to 1.12) | 1.02 (0.91 to 1.15) | 0.99 (0.86 to 1.15) | 0.95 (0.82 to 1.09) | 1.07 (0.89 to 1.30) | .68 | .97 | .35 |
| Women | 1 | 0.95 (0.80 to 1.14) | 1.01 (0.88 to 1.16) | 1.01 (0.85 to 1.21) | 0.95 (0.80 to 1.12) | 1.01 (0.81 to 1.27) | .77 | .84 | |
| Men | 1 | 1.01 (0.78 to 1.30) | 1.00 (0.75 to 1.34) | 0.92 (0.66 to 1.27) | 0.95 (0.72 to 1.25) | 1.24 (0.87 to 1.77) | .69 | .99 | |
| Tea* | |||||||||
| Intake category, g/d† | Nondrinkers | >0–150‡ | >150–400# | >400–900#,** | >900#,** | ||||
| Median intake, g/d | 0 | 33 | 237 | 592 | 1066 | ||||
| No. of case patients (women, men) | 1021, 578 | 772, 256 | 618, 309 | 483, 223 | 109, 25 | ||||
| Age-adjusted RR (95% CI) | |||||||||
| Total | 1 | 0.97 (0.89 to 1.06) | 0.99 (0.89 to 1.11) | 1.02 (0.91 to 1.15) | 1.26 (1.00 to 1.58) | .10 | .21 | .73 | |
| Women | 1 | 0.93 (0.84 to 1.03) | 0.98 (0.88 to 1.09) | 0.96 (0.83 to 1.12) | 1.28 (0.99 to 1.66) | .29 | .21 | ||
| Men | 1 | 1.11 (0.93 to 1.31) | 1.05 (0.80 to 1.37) | 1.16 (0.95 to 1.40) | 1.17 (0.67 to 2.06) | .23 | .21 | ||
| Multivariable RR (95% CI)¶ | |||||||||
| Total | 1 | 0.99 (0.91 to 1.08) | 1.02 (0.92 to 1.12) | 1.05 (0.94 to 1.18) | 1.28 (1.02 to 1.61) | .01 | .23 | .63 | |
| Women | 1 | 0.95 (0.86 to 1.05) | 1.01 (0.90 to 1.13) | 1.01 (0.88 to 1.15) | 1.32 (1.02 to 1.71) | .08 | .24 | ||
| Men | 1 | 1.12 (0.94 to 1.33) | 1.05 (0.81 to 1.35) | 1.17 (0.96 to 1.42) | 1.17 (0.66 to 2.06) | .24 | .22 | ||
| Sugar-sweetened carbonated soft drinks* | |||||||||
| Intake category, g/d† | Nondrinkers | >0–250 | >250–550 | >550†† | |||||
| Median intake, g/d | 0 | 50 | 349 | 925 | |||||
| No. of case patients (women, men) | 2119, 729 | 1191, 641 | 87, 91 | 21, 16 | |||||
| Age-adjusted RR (95% CI) | |||||||||
| Total | 1 | 0.96 (0.91 to 1.02) | 1.12 (0.90 to 1.40) | 0.98 (0.71 to 1.37) | .61 | .81 | .35 | ||
| Women | 1 | 0.96 (0.89 to 1.03) | 1.01 (0.76 to 1.34) | 1.13 (0.73 to 1.76) | .97 | .55 | |||
| Men | 1 | 0.97 (0.87 to 1.08) | 1.29 (0.90 to 1.86) | 0.82 (0.50 to 1.36) | .48 | .93 | |||
| Multivariable RR (95% CI)¶ | |||||||||
| Total | 1 | 0.96 (0.90 to 1.02) | 1.08 (0.87 to 1.34) | 0.94 (0.66 to 1.32) | .91 | .64 | .33 | ||
| Women | 1 | 0.96 (0.89 to 1.03) | 0.98 (0.76 to 1.26) | 1.08 (0.67 to 1.73) | .89 | .39 | |||
| Men | 1 | 0.96 (0.85 to 1.07) | 1.24 (0.86 to 1.79) | 0.77 (0.46 to 1.29) | .74 | .85 | |||
The Breast Cancer Detection Demonstration Project Follow-up Study and Cancer Prevention Study II Nutrition Cohort were not included in the analyses on coffee and tea consumption because coffee and tea consumption was not measured in these studies; the Prospective Study on Hormones, Diet and Breast Cancer was excluded from the analyses on tea and sugar-sweetened carbonated soft drinks because intake of these beverages was not measured in this study. The Adventist Health Study and New York State Cohort were not included in the analyses on sugar-sweetened carbonated soft drink because this variable was not measured.
For coffee and tea, 8 oz weighs approximately 237 g; for sugar-sweetened carbonated soft drinks, 12 oz weighs approximately 355 g.
The New York State Cohort was excluded from this category because there were no participants in this category based on how frequency of intake of this beverage was assessed on the food-frequency questionnaire.
The female cohort of the Adventist Health Study and the Swedish Mammography Cohort were excluded from the >900–1400 and >1400 g/d categories because there were no cases in these categories. The participants who were not cases and who would have been in these categories were included in the >400–900 g/d category.
The Prospective Study on Hormones, Diet and Breast Cancer was excluded from the >1400 g/d category because there were no cases in this category. The participants who were not cases and who would have been in this category were included in the >900–1400 g/d category.
Adjusted for education (<high school graduate, high school graduate, >high school graduate); smoking habits (never, past [<20, 20 to <40, ≥40 years], current [<25 cigarettes per day and <40 years, ≥25 cigarettes per day and <40 years, <25 cigarettes per day and ≥40 years, ≥25 cigarettes per day and ≥40 years]); height (women: <1.60, 1.60 to <1.65, 1.65 to <1.70, 1.70 to <1.75, ≥1.75 m; men: <1.70, 1.70 to <1.75, 1.75 to <1.80, 1.80 to <1.85, ≥1.85 m); body mass index (<23, 23 to <25, 25 to <30, ≥30 kg/m2); physical activity (low, medium, high); family history of colorectal cancer (no, yes); use of nonsteroidal anti-inflammatory drugs (no, yes; only five out of 13 cohorts have this information); multivitamin use (no, yes <6 times/wk, yes ≥6 times/wk, yes missing dose for the Adventist Health Study, Breast Cancer Detection Demonstration Project Follow-up Study, Health Professionals Follow-up Study, Iowa Women's Health Study, Nurses’ Health Study [a and b], and Women's Health Study; no, yes, for the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, Cancer Prevention Study II Nutrition Cohort, Netherlands Cohort Study, and New York State Cohort); red meat intake (quintiles); total milk consumption (quartiles); alcohol consumption (0, >0 to <5, 5 to <15, 15 to <30, ≥30 g/d); dietary folate intake (quintiles); total energy intake (continuous); and in women, oral contraceptive use (never, ever); and postmenopausal hormone use (premenopausal, never, ever). Age in years and questionnaire return year were included as stratification variables.
The male cohort of the Adventist Health Study was excluded from the >150–400, >400–900, and >900 g/d categories because there were no cases in this category. The participants who were not cases and who would have been in these categories were included in the >0–150 g/d category.
The female cohort of the Adventist Health Study was excluded from the >400–900 and >900 g/d categories because there were no cases in these categories. The participants who were not cases and who would have been in these categories were included in the >150–400 g/d category.
The Breast Cancer Detection Demonstration Project Follow-up Study, the Canadian National Breast Screening Study, and the Swedish Mammography Cohort were excluded from the >550 g/d category because there were no cases in this category. The participants who were not cases and who would have been in this category were included in the >250–550 g/d category.
In the second stage of the analyses, we combined the study-specific relative risks using a random-effects model (38,39). The individual studies were weighted by the inverse of their variance. We tested for heterogeneity across studies using the Q statistic (39,40), which follows an approximate χ2 distribution. All statistical analyses were two-sided, and a P less than .05 was considered statistically significant. To verify the assumption of proportional hazards, we constructed models for each beverage that included an interaction term between age and intake of that beverage. We pooled the study-specific parameter estimates for these interaction terms using the random-effects model and used a Wald test to evaluate the statistical significance of the pooled interaction term. We observed no evidence of violation of the proportional hazards assumption.
To evaluate whether the associations between coffee, tea, and sugar-sweetened carbonated soft drink intakes and colon cancer risk were linear, we conducted nonparametric regression analyses using restricted cubic splines (41). For these analyses, studies were combined into one dataset. The participants were stratified by age, year of questionnaire return, and study, and the risk estimates were adjusted for other covariates. We excluded participants with extremely high intakes of each beverage (approximately the highest 1%) to reduce the influence of outliers in the nonparametric regression analyses. We used a likelihood ratio test to compare the model with linear and cubic spline terms selected by a stepwise regression procedure to the model with only the linear term for the beverage of interest. If linearity in the association between intake of a beverage and colon cancer risk was suggested, we further analyzed that beverage as a continuous variable.
To assess whether the association for each beverage varied by alcohol consumption, physical activity, body mass index, and postmenopausal hormonal use among postmenopausal women, we used a two-sided Wald test of the cross product term between consumption of the beverage of interest and the potential effect modifiers, each modeled as a continuous or binary variable. We examined potential heterogeneity of the effect of each beverage by sex, age at colon cancer diagnosis, and smoking status using a mixed-effects meta-regression model (42) because effect modification by sex and age of colon cancer diagnosis can be assessed only between studies, and smoking status is a polytomous nominal variable. We used a contrast test (43), which followed an approximate χ2 distribution, to test whether associations for proximal and distal colon cancer differed. We conducted sensitivity analyses based on different follow-up periods (<5 vs ≥5years) and used the mixed-effects meta-regression model to test whether the period-specific associations for each beverage differed (12).
Results
In the 13 studies of this pooled analysis, 5604 incident invasive colon cancer case patients (1858 men and 3746 women) were identified among 239 193 men and 492 248 women followed for up to 6–20 years (Table 1). Slightly more proximal colon cancer case patients (n = 2928, 52%) than distal colon cancer case patients (n = 2228, 40%) were identified. Coffee consumption varied substantially across studies. Except for the Adventist Health Study, at least 70% of the participants in each study drank coffee, with higher coffee consumption levels observed in the European studies compared with the North American studies. There was a threefold difference in median coffee consumption (grams per day) among consumers across studies (Table 1). Intake levels (grams per day) of tea and sugar-sweetened soft drinks were lower than that observed for coffee consumption.
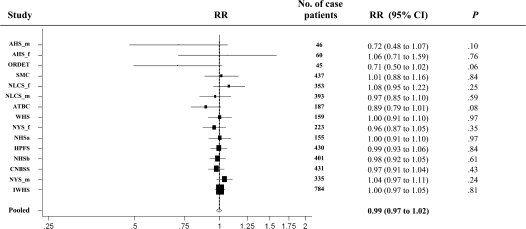
Coffee drinking was not associated with colon cancer risk (pooled multivariable RR = 1.07, 95% CI = 0.89 to 1.30) comparing >1400 g/d (about six 8-oz cups or 1.4 L) vs nondrinkers (Table 2). The test for heterogeneity between studies was not statistically significant. The nonparametric regression analysis did not detect nonlinearity in the association between coffee consumption and colon cancer risk (P, test for nonlinearity >.10). Thus, we conducted additional analyses in which we modeled coffee consumption as a continuous variable; no association was observed (for an increment of 250 g/d [237 g is about 8 oz or 240 mL], the pooled multivariable RR = 0.99, 95% CI = 0.97 to 1.02) (Figure 1 and Table 3).
Figure 1.
Forest plot of coffee drinking (increment, 250 g/d; 8-oz cup is about 237 g) and relative risk (RR) of colon cancer. The black squares and the horizontal lines represent the study-specific relative risk and corresponding 95% confidence intervals (CIs), respectively. The area of the black square reflects the weight of each study, measured by the inverse of the variance. The diamond represents the pooled multivariable relative risk with 95% confidence interval. The vertical dashed line provides a visual comparison of the pooled relative risk with the study-specific relative risk. AHS_f = Adventist Health Study (women); AHS_m = Adventist Health Study (men); ATBC = Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (men); CNBSS = Canadian National Breast Screening Study (women); HPFS = Health Professionals Follow-up Study (men); IWHS = Iowa Women's Health Study (women); NHSa = Nurses’ Health Study (a) (women); NHSb = Nurses’ Health Study (b) (women); NLCS_f = Netherlands Cohort Study (women); NLCS_m = Netherlands Cohort Study (men); NYS_f = New York State Cohort (women); NYS_m = New York State Cohort (men); ORDET = Prospective Study on Hormones, Diet and Breast Cancer (women); SMC = Swedish Mammography Cohort (women); WHS = Women's Health Study (women).
Table 3.
Pooled multivariable relative risks for consumption of coffee, tea, and sugar-sweetened carbonated soft drinks overall and by tumor site and risk factors for colon cancer
| Tumor site, sex, and risk factors | Coffee (increment, 250 g/d) |
Tea (increment, 250 g/d) |
Sugar-sweetened carbonated soft drinks (increment, 375 g/d) |
|||||||||
| No. of case patients | RR* (95% CI) | Pbetween-studies heterogeneity | Pinteraction | No. of case patients | RR* (95% CI) | Pbetween-studies heterogeneity | Pinteraction | No. of case patients | RR* (95% CI) | Pbetween-studies heterogeneity | Pinteraction | |
| Total colon | 4439 | 0.99 (0.97 to 1.02) | .45 | 4394 | 1.04 (1.00 to 1.07) | .50 | 4895 | 1.00 (0.91 to 1.10) | .57 | .37 | ||
| Women | 3048 | 0.99 (0.97 to 1.02) | .68 | .97 | 3003 | 1.03 (0.99 to 1.08) | .23 | .82 | 3418 | 0.96 (0.84 to 1.09) | .44 | |
| Men | 1391 | 0.98 (0.92 to 1.04) | .12 | 1391 | 1.03 (0.96 to 1.09) | .78 | 1477 | 1.05 (0.91 to 1.22) | .62 | |||
| Proximal colon | 2295 | 0.99 (0.96 to 1.02) | .98 | 2277 | 1.04 (1.00 to 1.09) | .64 | 2626 | 1.01 (0.88 to 1.15) | .80 | .13 | ||
| Women | 1625 | 0.99 (0.95 to 1.02) | .91 | .87 | 1605 | 1.04 (0.99 to 1.09) | .49 | .80 | 1846 | 0.91 (0.76 to 1.10) | .79 | |
| Men | 670 | 0.98 (0.92 to 1.04) | .82 | 670 | 1.05 (0.96 to 1.15) | .53 | 780 | 1.13 (0.93 to 1.36) | .87 | |||
| Distal colon | 1820 | 0.99 (0.96 to 1.03) | .71 | 1795 | 1.04 (0.99 to 1.10) | .34 | 1939 | 1.01 (0.88 to 1.17) | .81 | .68 | ||
| Women | 1195 | 1.00 (0.96 to 1.04) | .83 | .70 | 1169 | 1.05 (0.98 to 1.12) | .26 | .40 | 1283 | 1.04 (0.86 to 1.25) | .73 | |
| Men | 625 | 0.98 (0.90 to 1.06) | .24 | 626 | 1.01 (0.92 to 1.11) | .44 | 656 | 0.97 (0.76 to 1.24) | .53 | |||
| Smoking status† | ||||||||||||
| Never‡ | 1674 | 1.00 (0.96 to 1.03) | .75 | .54 | 1674 | 1.07 (1.00 to 1.14) | .06 | .51 | 1972 | 0.98 (0.80 to 1.18) | .17 | .97 |
| Past‡ | 1354 | 1.02 (0.97 to 1.06) | .34 | 1354 | 1.04 (0.98 to 1.10) | .88 | 1619 | 1.10 (0.93 to 1.30) | .68 | |||
| Current | 775 | 0.99 (0.92 to 1.06) | .08 | 775 | 1.01 (0.93 to 1.10) | .34 | 837 | 1.02 (0.81 to 1.29) | .31 | |||
| Alcohol consumption | ||||||||||||
| Nondrinkers | 1397 | 0.98 (0.94 to 1.02) | .33 | .33 | 1397 | 1.05 (0.98 to 1.11) | .23 | .59 | 1813 | 1.07 (0.93 to 1.22) | .43 | .87 |
| <15 g/d | 2117 | 1.01 (0.98 to 1.04) | .59 | 2117 | 1.04 (0.99 to 1.08) | .67 | 2227 | 0.94 (0.80 to 1.10) | .47 | |||
| ≥15 g/d | 761 | 0.99 (0.94 to 1.04) | .64 | 761 | 1.03 (0.93 to 1.13) | .23 | 845 | 1.14 (0.89 to 1.47) | .26 | |||
| Physical activity§ | ||||||||||||
| Low | 1499 | 1.00 (0.97 to 1.04) | .81 | .44 | 1499 | 1.03 (0.97 to 1.09) | .61 | .83 | 1926 | 0.94 (0.82 to 1.09) | .94 | .09 |
| Medium | 1127 | 0.99 (0.95 to 1.03) | .56 | 1127 | 1.07 (1.01 to 1.13) | .86 | 1482 | 0.94 (0.75 to 1.19) | .16 | |||
| High | 614 | 0.97 (0.88 to 1.06) | .03 | 614 | 1.04 (0.95 to 1.13) | .62 | 930 | 1.21 (0.99 to 1.48) | .99 | |||
| Body mass index | ||||||||||||
| <25 kg/m2 | 1985 | 0.99 (0.95 to 1.02) | .44 | .40 | 1985 | 1.02 (0.98 to 1.07) | .43 | .56 | 2254 | 0.94 (0.77 to 1.14) | .23 | .40 |
| ≥25 kg/m2 | 2210 | 1.01 (0.98 to 1.04) | .78 | 2210 | 1.04 (0.99 to 1.09) | .42 | 2519 | 1.03 (0.91 to 1.17) | .56 | |||
| Postmenopausal hormone use‖ | ||||||||||||
| Never | 1343 | 0.99 (0.95 to 1.03) | .40 | .24 | 1343 | 1.02 (0.96 to 1.08) | .73 | .60 | 1686 | 1.11 (0.94 to 1.31) | .64 | .13 |
| Ever | 677 | 1.00 (0.94 to 1.07) | .28 | 677 | 1.06 (0.96 to 1.17) | .18 | 1001 | 0.72 (0.48 to 1.08) | .11 | |||
The relative risks were adjusted for the covariates listed in Table 2. The Adventist Health Study and the Prospective Study on Hormones, Diet and Breast Cancer were excluded from all interaction analyses except for sex because of sparse stratum-specific case numbers.
The Swedish Mammography Cohort was not included in this analysis because smoking habits were not measured at baseline.
The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was excluded from the never and past smoker strata because all participants were current smokers.
The New York State Cohort and the Swedish Mammography Cohort were not included in this analysis because physical activity was not measured in these studies.
Among postmenopausal women. The New York State Cohort was not included in the analysis because this variable was not measured.
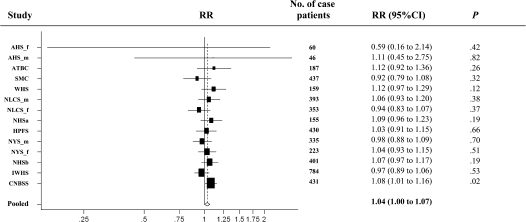
Tea consumption was associated with a modestly higher risk of colon cancer (pooled multivariable RR = 1.28, 95% CI = 1.02 to 1.61, comparing >900 g/d [about four 8-oz cups or 0.9 L] vs nondrinkers; Ptrend = .01). Many of the study-specific estimates for this comparison exceeded 1.0; however, only the result in the Canadian National Breast Screening Study was statistically significant. Because the test for nonlinearity was not statistically significant (P = .61), we conducted additional analyses in which tea consumption was modeled as a continuous variable; the pooled multivariable relative risk for an increment of 250 g/d (237 g is about 8 oz or 240 mL) was 1.04 (95% CI = 1.00 to 1.07) (Figure 2 and Table 3). Results for coffee and tea drinking did not change substantially when both were included in the model (data not shown). We could not examine associations with different types of tea (green, black, herbal, caffeinated, or decaffeinated) because none of the studies distinguished the type of tea consumed.
Figure 2.
Forest plot of tea drinking (increment, 250 g/d; 8-oz cup is about 237 g) and relative risk (RR) of colon cancer. The black squares and the horizontal lines represent the study-specific relative risk and the corresponding 95% confidence intervals (CIs), respectively. The area of the black square reflects the weight of each study, measured by the inverse of the variance. The diamond represents the pooled multivariable relative risk with 95% confidence interval. The vertical dashed line provides a visual comparison of the pooled relative risk with the study-specific relative risk. AHS_f = Adventist Health Study (women); AHS_m = Adventist Health Study (men); ATBC = Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (men); CNBSS = Canadian National Breast Screening Study (women); HPFS = Health Professionals Follow-up Study (men); IWHS = Iowa Women's Health Study (women); NHSa = Nurses’ Health Study (a) (women); NHSb = Nurses’ Health Study (b) (women); NLCS_f = Netherlands Cohort Study (women); NLCS_m = Netherlands Cohort Study (men); NYS_f = New York State Cohort (women); NYS_m = New York State Cohort (men); SMC = Swedish Mammography Cohort (women); WHS = Women's Health Study (women).
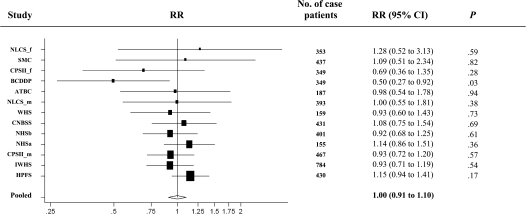
Sugar-sweetened carbonated soft drink consumption was not associated with colon cancer risk (pooled multivariable RR = 0.94, 95% CI = 0.66 to 1.32, comparing >550 g/d [about 18 oz or 0.54 L] vs nondrinkers, P, test for between-studies heterogeneity = .64) (Table 2). We further modeled sugar-sweetened carbonated soft drink consumption as a continuous variable because the nonparametric regression analyses suggested that the association was linear (P, test for nonlinearity = .31). The pooled multivariable relative risk for an increment of 375 g/d (355 g is about 12 oz or 0.36 L) was 1.00 (95% CI = 0.91 to 1.10) (Table 3 and Figure 3). When we examined diet carbonated soft drinks, the pooled multivariable relative risk for a 375 g/d increment was 1.01 (95% CI = 0.94 to 1.08; measured in four studies, n = 1928 case patients). We further divided sugar-sweetened carbonated soft drinks into cola and noncola beverages, and neither was associated with risk of colon cancer (data not shown). Moreover, the point estimates and the corresponding 95% confidence intervals for coffee, tea, and sugar-sweetened carbonated soft drink consumption did not change materially after exclusion of body mass index from the multivariable model.
Figure 3.
Forest plot of sugar-sweetened carbonated soft drink consumption (increment, 375 g/d; 12-oz cup is about 355 g) and relative risk (RR) of colon cancer. The black squares and the horizontal lines represent the study-specific relative risk and the corresponding 95% confidence intervals (CIs), respectively. The area of the black square reflects the weight of each study, measured by the inverse of the variance. The diamond represents the pooled multivariable relative risk with 95% confidence interval. The vertical dashed line provides a visual comparison of the pooled relative risk with the study-specific relative risk. ATBC = Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (men); BCDDP = Breast Cancer Detection Demonstration Project Follow-up Study; CNBSS = Canadian National Breast Screening Study (women); CPSII_f = Cancer Prevention Study II Nutrition Cohort (women); CPSII_m = Cancer Prevention Study II Nutrition Cohort (men); HPFS = Health Professionals Follow-up Study (men); IWHS = Iowa Women's Health Study (women); NHSa = Nurses’ Health Study (a) (women); NHSb = Nurses’ Health Study (b) (women); NLCS_f = Netherlands Cohort Study (women); NLCS_m = Netherlands Cohort Study (men); SMC = Swedish Mammography Cohort (women); WHS = Women's Health Study (women).
Caffeine intake (measured in six studies, n = 2886 case patients) was not associated with colon cancer risk (pooled multivariable RR = 1.04, 95% CI = 0.91 to 1.19, comparing the highest to lowest quintile, P, test for between-studies heterogeneity = .63; data not shown).
The associations between coffee, tea and sugar-sweetened carbonated soft drink consumption and colon cancer risk did not vary by sex (Table 3) or by smoking status, alcohol consumption, body mass index, physical activity, and, in women, postmenopausal hormone use (Table 3), with all P for interaction > .05. The pooled multivariable relative risks for coffee, tea, and sugar-sweetened carbonated soft drink intakes also did not vary substantially according to age of colon cancer diagnosis (<65 vs ≥65 years; all P, test for difference > .25) and follow-up period (<5 vs ≥5 years; all P, test for difference > .25). Furthermore, results did not vary by tumor site (all P, test for difference between proximal and distal colon cancer > .5; data not shown). Finally, the results for these three beverages did not change after we excluded the case patients with diagnoses that occurred in the first 2 years.
Discussion
In this pooled analysis of 13 cohort studies, coffee drinking was not associated with risk of colon cancer. The relative risk was null even for comparison of consumption of more than 1400 g/d (about six 8-oz cups or 1.4 L) of coffee vs none. Drinking sugar-sweetened carbonated soft drinks was not associated with risk of colon cancer. In contrast, a modest positive association was observed for relatively high tea intake. The association for each beverage did not vary by tumor site, various colon cancer risk factors, or follow-up period.
As observed in our study, several case–control studies have also reported null associations between coffee consumption and colon cancer risk (44–49). However, other case–control studies (50–57) have reported statistically significant modest reductions in colon cancer risk (20%–40%), whereas one study (58) found a statistically significant 120% increased risk among men, but a non-statistically significant 10% decreased risk in women, comparing high vs low coffee consumption. Furthermore, most (59–64) of the cohort studies (59–66) not included in our analyses also found no association between coffee consumption and colon cancer risk. Six (60–64,66) of these cohort studies were excluded from our analyses because they did not assess long-term dietary intake or did not conduct a validation study of the dietary assessment method used in their study, failing to satisfy our inclusion criteria. Another two cohort studies (59,65) were excluded because they did not meet the inclusion criteria at the time the dataset for the colon cancer analyses was finalized but have since joined the Pooling Project. Only two cohort studies in Japan (65,66) have reported statistically significant inverse associations with high vs infrequent coffee consumption. However, these results were only observed in women and were based on relatively small numbers (<15 colon cancer case patients in the highest category of coffee consumption). Last, the null association between coffee consumption and colon cancer risk observed in our study is consistent with the finding from one recent meta-analysis synthesizing the publications on cohort studies up to June 2008 (67).
Although null associations for coffee consumption have been observed frequently, coffee drinking has been hypothesized to decrease the risk of colon cancer (6). Coffee consumption may increase colonic motility, thereby decreasing the exposure of epithelial cells to potential carcinogens in the colon (6). In addition, coffee consumption may reduce the synthesis and secretion of bile acids, potential promoters of colon carcinogenesis (7). Furthermore, coffee contains some phenolic compounds such as chlorogenic acid and caffeic acid, which have antioxidant properties (6). However, coffee also contains chemical compounds such as caffeine, which could increase the risk of colon cancer. Caffeine has genotoxic and mutagenic properties at high concentrations (6). In addition, caffeine has been shown to lower insulin sensitivity (68), which could increase colon cancer risk (69). Thus, the complex compounds in coffee with opposing effects may explain the null results observed.
Tea drinking has been hypothesized to decrease the risk of colon cancer. Antioxidants present in tea, such as polyphenols, can protect colonic epithelial cells against oxidative damage to DNA by free radicals (70). Tea also lowers the formation of nitrosamine compounds and heterocyclic aromatic amines, potential carcinogens for colon cancer (71). On the other hand, tea also contains compounds with mutagenic and genotoxic properties such as tannins and caffeine (9,10), which could increase the risk of colon cancer.
Animal studies have consistently demonstrated a protective effect of tea on the development of colon cancer (5); however, results from human observational studies have been inconsistent. Tea is the second most commonly consumed beverage worldwide following water (5). About 78% of the tea produced is black tea, 20% is green tea, and 2% is oolong tea (5). Most case–control studies have found no association between black tea consumption and colon cancer risk (47,53,55,57,72,73), although a few case–control studies have reported statistically significantly higher (48,50) risks of colon cancer of at least 40% when comparing the highest vs lowest level of intake. Among the three cohort studies (74–76) that did not meet our inclusion criteria (as described above), one reported a statistically significant (about 40%) reduced risk (75), whereas the other two studies found no association with black tea consumption (74,76). For green tea, a statistically significant 30%–40% reduced risk of colon cancer has been observed in two case–control studies (50,77) and in one cohort study (78); the remaining studies have reported null associations (48,65,74,79,80). The studies that have found the statistically significant inverse associations for green tea consumption were all conducted in Japanese and Chinese populations in which green tea was commonly consumed with a wide range of intake, unlike the other populations in which green tea consumption was low.
The observed increased risk with tea consumption in our analysis was unexpected. One possibility is that the positive association we observed was because of chance. However, a cohort study conducted in Singapore observed a stronger positive association between green tea intake and risk of advanced colon cancer compared with localized colon cancer, suggesting that tea may have a promoting effect on tumor progression and metastasis, but this difference by tumor stage was not observed for black tea consumption (74). As noted previously, although we did not have data on the type of tea consumed in most studies included in our study, black tea is the major type of tea consumed in Western populations. Furthermore, we were not able to examine whether the association that we observed differed by stage.
Consumption of soft drinks containing caloric sweeteners has been positively associated with colon cancer risk factors, including excess body weight (11); however, this relationship has rarely been examined (2). We found null associations with consumption of sugar-sweetened carbonated soft drinks, sugar-sweetened colas, sugar-sweetened noncolas, and diet carbonated soft drinks; all of the relative risks were close to 1.0 for an increment of 375 g/d (approximately 12 oz). However, we cannot exclude the possibility that a weak association was missed in our study because about 2% of the study population consumed more than 550 g/d of soft drinks.
Our pooled analyses have several strengths. The large sample size of the study allowed us to conduct subgroup analyses by colon site and to examine whether the associations for each beverage varied by other colon cancer risk factors. The prospective design with high follow-up rate for each study minimized the potential for selection bias and recall bias.
There are also several limitations in this pooled analysis. The null association observed for coffee consumption on colon cancer risk may not be generalized to different ethnic groups because our study population is primarily of European origin. Some degree of measurement error inevitably exists for our estimates of the intake of each beverage, and we were unable to conduct measurement error correction analyses because few studies in our analyses evaluated the validity of coffee, tea, and carbonated soft drink intake. Although high correlations (ie, r > .5) between the intake estimates from the food-frequency questionnaires and from multiple dietary records or 24-hour recalls were observed for these beverages (23,27,29), we cannot rule out the possibility that the high correlation may result from the correlated errors in the food-frequency questionnaires and in the referent methods. Moreover, for tea and sugar-sweetened carbonated soft drink consumption, we were limited by relatively low consumption levels and a narrow intake range with only 2% of the study population consuming more than 550 g/d of soft drinks. Furthermore, none of the studies measured the type of tea consumed, and few studies measured the consumption of diet soft drinks. Last, personal history of colorectal screening and the addition of milk and sugar to coffee and tea was not directly assessed in several studies, which may have resulted in unmeasured confounding.
In summary, we did not observe an association between coffee drinking and colon cancer risk across a wide range of coffee consumption. In contrast, we observed a modest increase in risk with higher tea consumption, despite a limited range of tea consumption. To further evaluate this unexpected finding, future studies should be conducted in populations with a wider range of intake, and information should be collected on the types of tea consumed and preparation methods. Data on stage of disease should also be collected to confirm or refute previous data suggesting that green tea may promote tumor progression and metastasis. For sugar-sweetened carbonated soft drinks, although we observed no association with colon cancer risk, future studies with a wider intake range and with detailed information on sugar composition would be desirable.
Funding
National Institutes of Health (CA55075 to W.C.W.); the National Colorectal Cancer Research Alliance of the Entertainment Industry Foundation (to W.C.W.).
Footnotes
The authors thank Shiaw-Shyuan Yaun and Ruifeng Li for their assistance with data management. The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin D. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5 version 2.0. Lyon, France: IARC Press; 2004. [Google Scholar]
- 2.Word Cancer Research Fund. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. American Institute for Cancer Research Expert Panel. [DOI] [PubMed] [Google Scholar]
- 3.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potter JD. Nutrition and colorectal cancer. Cancer Causes Control. 1996;7(1):127–146. doi: 10.1007/BF00115644. [DOI] [PubMed] [Google Scholar]
- 5.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 6.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46(2):101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 7.Potter JD. Reconciling the epidemiology, physiology, and molecular biology of colon cancer. JAMA. 1992;268(12):1573–1577. [PubMed] [Google Scholar]
- 8.Brown SR, Cann PA, Read NW. Effect of coffee on distal colon function. Gut. 1990;31(4):450–453. doi: 10.1136/gut.31.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariza RR, Dorado G, Barbancho M, Pueyo C. Study of the causes of direct-acting mutagenicity in coffee and tea using the Ara test in Salmonella typhimurium. Mutat Res. 1988;201(1):89–96. doi: 10.1016/0027-5107(88)90114-5. [DOI] [PubMed] [Google Scholar]
- 10.Savolainen H. Tannin content of tea and coffee. J Appl Toxicol. 1992;12(3):191–192. doi: 10.1002/jat.2550120307. [DOI] [PubMed] [Google Scholar]
- 11.Popkin BM. Understanding global nutrition dynamics as a step towards controlling cancer incidence. Nat Rev Cancer. 2007;7(1):61–67. doi: 10.1038/nrc2029. [DOI] [PubMed] [Google Scholar]
- 12.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 13.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148(8):761–774. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 14.Hartman TJ, Tangrea JA, Pietinen P, et al. Tea and coffee consumption and risk of colon and rectal cancer in middle-aged Finnish men. Nutr Cancer. 1998;31(1):41–48. doi: 10.1080/01635589809514676. [DOI] [PubMed] [Google Scholar]
- 15.Calton BA, Lacey JV, Jr, Schatzkin A, et al. Physical activity and the risk of colon cancer among women: a prospective cohort study (United States) Int J Cancer. 2006;119(2):385–391. doi: 10.1002/ijc.21840. [DOI] [PubMed] [Google Scholar]
- 16.Terry P, Jain M, Miller AB, Howe GR, Rohan TE. Dietary intake of folic acid and colorectal cancer risk in a cohort of women. Int J Cancer. 2002;97(6):864–867. doi: 10.1002/ijc.10138. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Prevention Study II The American Cancer Society Prospective Study. Stat Bull Metrop Insur Co. 1992;73(4):21–29. [PubMed] [Google Scholar]
- 18.Lee DH, Anderson KE, Harnack LJ, Folsom AR, Jacobs DR., Jr Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women's Health Study. J Natl Cancer Inst. 2004;96(5):403–407. doi: 10.1093/jnci/djh047. [DOI] [PubMed] [Google Scholar]
- 19.Goldbohm RA, Hertog MG, Brants HA, van Poppel G, van den Brandt PA. Consumption of black tea and cancer risk: a prospective cohort study. J Natl Cancer Inst. 1996;88(2):93–100. doi: 10.1093/jnci/88.2.93. [DOI] [PubMed] [Google Scholar]
- 20.Graham S, Zielezny M, Marshall J, et al. Diet in the epidemiology of postmenopausal breast cancer in the New York State Cohort. Am J Epidemiol. 1992;136(11):1327–1337. doi: 10.1093/oxfordjournals.aje.a116445. [DOI] [PubMed] [Google Scholar]
- 21.Michels KB, Willett WC, Fuchs CS, Giovannucci E. Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J Natl Cancer Inst. 2005;97(4):282–292. doi: 10.1093/jnci/dji039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieri S, Krogh V, Pala V, et al. Dietary patterns and risk of breast cancer in the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2004;13(4):567–572. [PubMed] [Google Scholar]
- 23.Terry P, Bergkvist L, Holmberg L, Wolk A. Coffee consumption and risk of colorectal cancer in a population based prospective cohort of Swedish women. Gut. 2001;49(1):87–90. doi: 10.1136/gut.49.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Zhang SM, Cook NR, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States) Cancer Causes Control. 2005;16(3):225–233. doi: 10.1007/s10552-004-4025-1. [DOI] [PubMed] [Google Scholar]
- 25.Puckett CD. The Educational Annotation of ICD-9-CM; Diseases and Procedures Tabular Lists. Reno, NV: Channel Publishing Ltd; 1986. [Google Scholar]
- 26.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136(2):192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 27.Goldbohm RA, van den Brandt PA, Brants HA, et al. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr. 1994;48(4):253–265. [PubMed] [Google Scholar]
- 28.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 29.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–666. doi: 10.1093/oxfordjournals.aje.a115013. [DOI] [PubMed] [Google Scholar]
- 30.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11(4):462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Feskanich D, Marshall J, Rimm EB, Litin LB, Willett WC. Simulated validation of a brief food frequency questionnaire. Ann Epidemiol. 1994;4(3):181–187. doi: 10.1016/1047-2797(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 32.Fraser GE, Lindsted KD, Knutsen SF, Beeson WL, Bennett H, Shavlik DJ. Validity of dietary recall over 20 years among California Seventh-day Adventists. Am J Epidemiol. 1998;148(8):810–818. doi: 10.1093/oxfordjournals.aje.a009703. [DOI] [PubMed] [Google Scholar]
- 33.Rothman K. Modern Epidemiology. Boston, MA: Little Brown and Company; 1986. [Google Scholar]
- 34.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 35.Pennington JAT. Bowes and Church's Food Values of Portions Commonly Used. 17th ed. New York, NY: Lippincott-Raven; 1998. [Google Scholar]
- 36.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 37.SAS Institute. SAS/STAT Software: The PHREG Procedure: Preliminary Documentation. Cary, NC: SAS Institute; 1991. [Google Scholar]
- 38.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 39.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 40.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 41.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 42.Stram DO. Meta-analysis of published data using a linear mixed-effects model. Biometrics. 1996;52(2):536–544. [PubMed] [Google Scholar]
- 43.Anderson TW. Introduction to Multivariate Statistics. 2nd ed. New York, NY: John Wiley & Sons; 1984. [Google Scholar]
- 44.Munoz SE, Navarro A, Lantieri MJ, et al. Alcohol, methylxanthine-containing beverages, and colorectal cancer in Cordoba, Argentina. Eur J Cancer Prev. 1998;7(3):207–213. doi: 10.1097/00008469-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE. Colorectal cancer and diet in an Asian population—a case-control study among Singapore Chinese. Int J Cancer. 1989;43(6):1007–1016. doi: 10.1002/ijc.2910430609. [DOI] [PubMed] [Google Scholar]
- 46.Bidoli E, Franceschi S, Talamini R, Barra S, La Vecchia C. Food consumption and cancer of the colon and rectum in North-Eastern Italy. Int J Cancer. 1992;50(2):223–229. doi: 10.1002/ijc.2910500211. [DOI] [PubMed] [Google Scholar]
- 47.Slattery ML, Caan BJ, Anderson KE, Potter JD. Intake of fluids and methylxanthine-containing beverages: association with colon cancer. Int J Cancer. 1999;81(2):199–204. doi: 10.1002/(sici)1097-0215(19990412)81:2<199::aid-ijc6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Inoue M, Tajima K, Hirose K, et al. Tea and coffee consumption and the risk of digestive tract cancers: data from a comparative case-referent study in Japan. Cancer Causes Control. 1998;9(2):209–216. doi: 10.1023/a:1008890529261. [DOI] [PubMed] [Google Scholar]
- 49.Shannon J, White E, Shattuck AL, Potter JD. Relationship of food groups and water intake to colon cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(7):495–502. [PubMed] [Google Scholar]
- 50.Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res. 1990;81(11):1101–1108. doi: 10.1111/j.1349-7006.1990.tb02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuyns AJ, Kaaks R, Haelterman M. Colorectal cancer and the consumption of foods: a case-control study in Belgium. Nutr Cancer. 1988;11(3):189–204. doi: 10.1080/01635588809513986. [DOI] [PubMed] [Google Scholar]
- 52.La Vecchia C, Ferraroni M, Negri E, et al. Coffee consumption and digestive tract cancers. Cancer Res. 1989;49(4):1049–1051. [PubMed] [Google Scholar]
- 53.Franceschi S, Favero A, La Vecchia C, et al. Food groups and risk of colorectal cancer in Italy. Int J Cancer. 1997;72(1):56–61. doi: 10.1002/(sici)1097-0215(19970703)72:1<56::aid-ijc8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S. Food groups and colorectal cancer risk. Br J Cancer. 1999;79(7–8):1283–1287. doi: 10.1038/sj.bjc.6690206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woolcott CG, King WD, Marrett LD. Coffee and tea consumption and cancers of the bladder, colon and rectum. Eur J Cancer Prev. 2002;11(2):137–145. doi: 10.1097/00008469-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg L, Werler MM, Palmer JR, et al. The risks of cancers of the colon and rectum in relation to coffee consumption. Am J Epidemiol. 1989;130(5):895–903. doi: 10.1093/oxfordjournals.aje.a115422. [DOI] [PubMed] [Google Scholar]
- 57.Baron JA, Gerhardsson de Verdier M, Ekbom A. Coffee, tea, tobacco, and cancer of the large bowel. Cancer Epidemiol Biomarkers Prev. 1994;3(7):565–570. [PubMed] [Google Scholar]
- 58.Slattery ML, West DW, Robison LM, et al. Tobacco, alcohol, coffee, and caffeine as risk factors for colon cancer in a low-risk population. Epidemiology. 1990;1(2):141–145. doi: 10.1097/00001648-199003000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Larsson SC, Bergkvist L, Giovannucci E, Wolk A. Coffee consumption and incidence of colorectal cancer in two prospective cohort studies of Swedish women and men. Am J Epidemiol. 2006;163(7):638–644. doi: 10.1093/aje/kwj067. [DOI] [PubMed] [Google Scholar]
- 60.Naganuma T, Kuriyama S, Akhter M, et al. Coffee consumption and the risk of colorectal cancer: a prospective cohort study in Japan. Int J Cancer. 2007;120(7):1542–1547. doi: 10.1002/ijc.22505. [DOI] [PubMed] [Google Scholar]
- 61.Stensvold I, Jacobsen BK. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control. 1994;5(5):401–408. doi: 10.1007/BF01694753. [DOI] [PubMed] [Google Scholar]
- 62.Jacobsen BK, Bjelke E, Kvale G, Heuch I. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst. 1986;76(5):823–831. [PubMed] [Google Scholar]
- 63.Klatsky AL, Armstrong MA, Friedman GD, Hiatt RA. The relations of alcoholic beverage use to colon and rectal cancer. Am J Epidemiol. 1988;128(5):1007–1015. doi: 10.1093/oxfordjournals.aje.a115045. [DOI] [PubMed] [Google Scholar]
- 64.Nomura A, Heilbrun LK, Stemmermann GN. Prospective study of coffee consumption and the risk of cancer. J Natl Cancer Inst. 1986;76(4):587–590. doi: 10.1093/jnci/76.4.587. [DOI] [PubMed] [Google Scholar]
- 65.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Coffee consumption and risk of colorectal cancer in a population-based prospective cohort of Japanese men and women. Int J Cancer. 2007;121(6):1312–1318. doi: 10.1002/ijc.22778. [DOI] [PubMed] [Google Scholar]
- 66.Oba S, Shimizu N, Nagata C, et al. The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Lett. 2006;244(2):260–267. doi: 10.1016/j.canlet.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 67.Je Y, Liu W, Giovannucci E. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer. 2008;124(7):1662–1668. doi: 10.1002/ijc.24124. [DOI] [PubMed] [Google Scholar]
- 68.Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25(2):364–369. doi: 10.2337/diacare.25.2.364. [DOI] [PubMed] [Google Scholar]
- 69.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(Suppl 11):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 70.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224(3):265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weisburger JH, Nagao M, Wakabayashi K, Oguri A. Prevention of heterocyclic amine formation by tea and tea polyphenols. Cancer Lett. 1994;83(1–2):143–147. doi: 10.1016/0304-3835(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 72.Cerhan JR, Putnam SD, Bianchi GD, Parker AS, Lynch CF, Cantor KP. Tea consumption and risk of cancer of the colon and rectum. Nutr Cancer. 2001;41(1–2):33–40. doi: 10.1080/01635581.2001.9680609. [DOI] [PubMed] [Google Scholar]
- 73.Il’yasova D, Martin C, Sandler RS. Tea intake and risk of colon cancer in African-Americans and whites: North Carolina colon cancer study. Cancer Causes Control. 2003;14(8):767–772. doi: 10.1023/a:1026371307954. [DOI] [PubMed] [Google Scholar]
- 74.Sun CL, Yuan JM, Koh WP, Lee HP, Yu MC. Green tea and black tea consumption in relation to colorectal cancer risk: the Singapore Chinese Health Study. Carcinogenesis. 2007;28(10):2143–2148. doi: 10.1093/carcin/bgm171. [DOI] [PubMed] [Google Scholar]
- 75.Su LJ, Arab L. Tea consumption and the reduced risk of colon cancer—results from a national prospective cohort study. Public Health Nutr. 2002;5(3):419–425. doi: 10.1079/phn2001314. [DOI] [PubMed] [Google Scholar]
- 76.Heilbrun LK, Nomura A, Stemmermann GN. Black tea consumption and cancer risk: a prospective study. Br J Cancer. 1986;54(4):677–683. doi: 10.1038/bjc.1986.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ji BT, Chow WH, Hsing AW, et al. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. 1997;70(3):255–258. doi: 10.1002/(sici)1097-0215(19970127)70:3<255::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 78.Yang G, Shu XO, Li H, et al. Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1219–1223. doi: 10.1158/1055-9965.EPI-07-0097. [DOI] [PubMed] [Google Scholar]
- 79.Nagano J, Kono S, Preston DL, Mabuchi K. A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan) Cancer Causes Control. 2001;12(6):501–508. doi: 10.1023/a:1011297326696. [DOI] [PubMed] [Google Scholar]
- 80.Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Jpn J Cancer Res. 1985;76(8):705–716. [PubMed] [Google Scholar]