Abstract
Objective
Although the term ‘reflex sympathetic dystrophy’ has been replaced by ‘complex regional pain syndrome’ (CRPS) type I, there remains a widespread presumption that the sympathetic nervous system is actively involved in mediating chronic neuropathic pain [“sympathetically maintained pain” (SMP)], even in the absence of detectable neuropathophysiology.
Methods
We have used microneurography to evaluate possible electrophysiological interactions in 24 patients diagnosed with CRPS I (n=13), or CRPS II (n=11) by simultaneously recording from single identified sympathetic efferent fibers and C nociceptors, while provoking sympathetic neural discharges in cutaneous nerves.
Results
We assessed potential effects of sympathetic activity upon 35 polymodal nociceptors and 19 mechano-insensitive nociceptors, recorded in CRPS I (26 nociceptors) and CRPS II patients (28 nociceptors). No evidence of activation of nociceptors related to sympathetic discharge was found, although nociceptors in 6 CRPS II patients exhibited unrelated spontaneous pathological nerve impulse activity.
Conclusion
We conclude that activation of nociceptors by sympathetic efferent discharges is not a cardinal pathogenic event in either CRPS I or CRPS II patients.
Significance
This study shows that sympathetic-nociceptor interactions, if they exist in patients communicating chronic neuropathic pain, must be the exception.
Keywords: CRPS, sympathetically maintained pain, nociceptor, sympathetic efferent, sympathetic-sensory interaction
1. Introduction
Historically it has been assumed that the sympathetic nervous system plays a pathogenic role in some chronic pain conditions, variously labeled RSD (Reflex Sympathetic Dystrophy), SMP (sympathetically maintained pain) or CRPS (complex regional pain syndrome) (Ochoa and Verdugo, 1993), largely on the grounds of physical signs commonly regarded as autonomic, and of subjective symptom relief following sympatholysis (Loh and Nathan, 1978). The presumptive site of interaction between the sympathetic efferent fibers and the afferent system has shifted over time, from the nerve trunk (Nathan, 1947), to the nociceptor terminal (Hannington-Kiff, 1974; Loh and Nathan, 1978; Sato and Perl, 1991; Gibbs et al., 2008), to low threshold mechanoreceptors (Roberts, 1986), and to the dorsal root ganglion (Michaelis et al., 1996). An abnormal state of excitability in the dorsal horn, triggered or maintained by sympathetic activity, has also been postulated (Evans, 1946).
In animals, several studies have investigated possible sympathetic/nociceptor interactions following experimental nerve damage. Roberts and Elardo (1985) reported that electrical stimulation of the sympathetic chain in cats might activate Aδ nociceptor afferents after experimental inflammation. Also, following nerve injury in animals, nociceptor terminals may develop sensitivity to catecholamines, and become excitable during sympathetic stimulation (Häbler et al., 1987; Sato and Perl, 1991; Jänig et al., 1996; O’Halloran and Perl, 1997; Birder and Perl, 1999). According to Ren et al. (2005) sensitization of cutaneous Aδ and C nociceptors by capsaicin was prevented by sympathectomy and rekindled by adrenergic agonists. In a previous report, however, sympathectomy did not prevent discharge of chemically sensitized afferents (Koltzenburg et al., 1992).
In behavioral studies in human volunteers, the capsaicin-evoked pain and mechanical hyperalgesia remained unchanged during full body warming or cooling, maneuvers known to decrease and increase maximally the sympathetic vasoconstrictor tone (Baron et al., 1999). Moreover, in volunteers, mechano-insensitive C-nociceptors, sensitized by capsaicin and tested through microneurography, were not responsive to sympathetic reflex maneuvers (Serra et al., 2004). Furthermore, the putative abnormal interaction between sympathetic efferent activity and afferent activity in human polymodal nociceptors sensitized with mustard oil has also been addressed through microneurography with negative results (Elam et al., 1999).
In chronic pain patients with or without demonstrable nerve damage, psychophysical studies led to the assumption that sympathetic efferent activity is a significant determinant of the pains. Indeed, diagnostic and therapeutic sympathetic blocks are often followed by transient symptom relief, although this effect has been attributed directly to placebo (Verdugo and Ochoa, 1994; Verdugo et al., 1994; Jadad et al., 1995; Ramamurthy and Hoffman, 1995). Conversely, there are examples where exogenous norepinephrine might trigger a pain complaint. An injection of norepinephrine near a stump neuroma in amputees reportedly evoked significant pain (Chabal et al., 1992). Further, noradrenaline injected in previously symptomatic skin of patients diagnosed with SMP, neuralgia or causalgia, was reported to rekindle the symptom (Wallin et al., 1976; Torebjörk et al., 1995). Ali et al. (2000) reported that norepinephrine injections produced pain in SMP patients at doses that were just at the threshold for producing vasoconstriction. Mailis-Gagnon and Bennett (2004) describe enhanced pain responses to intradermal norepinephrine in asymptomatic skin in patients with SMP, and suggest a central rather than a peripheral mechanism for this phenomenon. All these claims are based upon subjective self-reports.
In fact, no pathologically increased sympathetic neuro-secretion to symptomatic areas has been documented in patients labeled with RSD (Goldstein et al., 2000) and sympathetic efferent neural activity is not abnormally increased in patients assessed with SMP (Elam, 1998). However, a recent case report (Jørum et al., 2007) described a patient with a chronic pain condition in whom spontaneous activity recorded by microneurography from skin nociceptor units was enhanced by maneuvers that normally increase sympathetic outflow. Further direct neurophysiological studies in patients therefore seemed warranted.
Here, we present a series of 24 patients with complex regional pain syndromes type I and II (CRPS I and II, without and with nerve injury, respectively) in whom we searched, through the use of microneurography, for potential interactions between natural sympathetic activity documented for individual efferent fibers and afferent activity in subtype-identified nociceptors innervating symptomatic areas. No evidence of interaction was found for either polymodal or mechanically-insensitive C fiber nociceptors in these patients.
2. Methods
2.1. Subjects
All subjects (24 patients and 1 healthy volunteer) gave informed written consent to be studied; the consent form was approved by the Legacy System and Universidad del Desarrollo local ethics committees.
Thirteen consecutive patients fulfilling diagnostic criteria for CRPS I (Merskey and Bogduk, 1994) (see Discussion) were included, as well as 11 patients with CRPS II, of which 10 had painful neuropathy and 1 a traumatic nerve injury. Patients reporting chronic pain associated with motor and sensory symptoms, usually restricted to one extremity, without clinical or standard neurophysiological evidence of nerve pathology, were classified as CRPS I. Typically, these patients reported minor injuries (such as sprains, soft tissue contusions, etc.) as a triggering event.
All patients underwent a complete clinical neurological evaluation, with special attention to sensation. The sensory examination included testing for light touch, pinprick, position and vibration senses, two-point discrimination thresholds, bedside cold and warm assessment, and mapping of the areas of tactile or pinprick hypoesthesia, and static or dynamic mechanical hyperalgesia. The territories of abnormal sensory modalities were color-coded with ink and then photographed.
2.2 Neurophysiological studies
All patients underwent routine electrodiagnostic studies, including nerve conduction studies and needle EMG (Teca TD50 and Synergy Oxford Instruments). All tests were performed using standardized methods. Patients also underwent routine quantitative somatosensory testing to measure cold, warm, and thermal (cold and heat) pain sensory thresholds, in symptomatic areas (QST Thermotest Somedic, Sweden, and TSA II, Medoc, Israel).
2.3 Thermography
In most patients, the thermal emission profile of the symptomatic areas was recorded through infrared telethermography (Flexitherm Mark IV) to determine sympathetic vasomotor function and presence of neurogenic vasodilatation.
2.4 Microneurography
Single action potentials of C fibers were recorded from cutaneous nerve fascicles of the superficial peroneal nerve at the ankle, the superficial radial nerve at the wrist, or the median nerve at the elbow. The subjects sat relaxed with either an arm or a leg firmly supported on a padded platform. The general technique of microneurography has been described in detail elsewhere (Vallbo and Hagbarth, 1968). Identification of different functional types of C fiber was performed as described by Serra et al. (1999; 2004). Briefly, a lacquer-insulated tungsten microelectrode, with a shaft diameter of 200 μm (nominal 100 kΩ impedance; FHC, Bowdoinham, ME, USA) was inserted into the nerve and guided into a sensory fascicle. The intraneural signal, referred to a second tungsten needle inserted into the skin close by, was amplified (FHC Inc., 3+ Cell Isolated Microamplifier), noise was eliminated (Hum Bug, Quest Scientific, North Vancouver, Canada), and then digitized at 20 kHz using a PC with QTRAC software (Institute of Neurology, University College London, London, UK). Electrical stimuli were delivered through twin needles resting on the surface of the skin in the area (dorsum of the foot, or hand) of the cutaneous receptive field. Stimuli (0.2 ms duration) were delivered every 4 s and multiple C-unit responses were recognized in the neurogram by the individual horizontal tracks on a raster plot of latencies to action potential peaks versus elapsed time. To identify functional classes of the C units, the baseline stimulation rate of 0.25 Hz was interrupted by a 3-minute period with one stimulus every 30 s (“pause”), followed by a 3-minute period of stimulation at 2 Hz. Units with conduction velocities that slowed progressively, by at least 10% during the 2 Hz train, were identified as nociceptors (Type 1; Serra et al., 1999). These were further separated into polymodal (CMH) and mechano-insensitive nociceptors (Types 1A and 1B, respectively; Serra et al., 2004) on the basis of their behavior immediately after the 3-minute pause in stimulation, since only the mechano-insensitive afferents (MIAs) slow by >2% at 0.25 Hz after the pause. Skin temperature was measured with a thermocouple taped to the skin, close to the receptive field(s) of the unit(s) under study. It was controlled simply by moving a shielded infra-red lamp closer to, or further away from, the patient, or by switching it off. This procedure was preferred to an automatic lamp controller using temperature feedback because it avoided electrical interference due to electronic switching of the lamp current and it did not generate small oscillations in temperature, and therefore in C-fiber latency, which would interfere with interpretation of changes in impulse latency.
Once a single unit identified as a nociceptor was characterized on the basis of mechanical and thermal receptor responses (measured using von Frey monofilaments and a feedback-controlled Peltier device), the patient was challenged with maneuvers known to increase sympathetic nerve discharge: loud sudden noise (startle), arithmetic exercises (mental stress) and changes in intrathoracic pressure (Valsalva) (Hallin and Torebjörk, 1974). These maneuvers were performed in all the experiments, either when only nociceptors were recorded, or when both nociceptors and sympathetic efferents were simultaneously recorded from the same intraneural site. This was our key strategy intended to discern possible latency changes, or ectopic discharge, in nociceptors during natural activation of sympathetic efferents.
3. Results
3.1 Clinical Aspects
Twenty-four patients (Table 1) that fulfilled the diagnosis of CRPS (13 CRPS I and 11 CRPS II) underwent successfully one or more microneurography recordings. Mean age of CRPS I patients (8 females, 5 males) was 40.2 years, slightly younger than the CRPS II patients (4 females, 7 males) (mean age 44.5 years). All patients communicated spontaneous pain and various degrees of static or dynamic mechanical hyperalgesias. Their main clinical features are summarized in Table 1. The sensory symptoms of most patients were in stocking distribution, in which case the superficial peroneal nerve at the ankle was studied. In four patients with CRPS I who expressed symptoms in the hand, the recording was obtained from the superficial radial nerve (three patients) or the median nerve at the elbow (one patient).
Table 1.
Characteristics of the patients
| Patient number |
Age | Sex | Dg/ CRPS Type |
Spont pain |
Mech. Hyperalg / Allodynia |
Spatial distribution |
Weak- ness |
Spasm | TR | NCS/ EMG |
QST | Symp. blocks |
Ectopic Nociceptor Activity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | M | CRPS I | Y | Y/N | Arm/legs | Y | N | norm | norm | Cold hyper | np | N |
| 2 | 38 | F | CRPS I | Y | N/Y | Non-dermat | N | N | norm | norm | Normal | np | N |
| 3 | 34 | F | CRPS I | Y | Y/Y | Glove | Y | N | norm | norm | Cold hyper | np | N |
| 4 | 43 | M | CRPS I | Y | Y/Y | Stocking | Y | N | norm | norm | Cold hyper | Y | N |
| 5 | 49 | F | CRPS I | Y | Y/Y | Stocking | N | N | norm | norm | Cold hyper | np | N |
| 6 | 35 | F | CRPS I | Y | Y/Y | Stocking | N | N | norm | norm | Normal | np | N |
| 7 | 46 | M | CRPS I | Y | N/N | Stocking | N | N | norm | norm | Warm hypo |
np | N |
| 8 | 53 | M | CRPS I | Y | Y/Y | Stocking | N | N | norm | norm | np | np | N |
| 9 | 53 | F | CRPS I | Y | Y/Y | Non-dermat | Y | N | norm | norm | np | Y | N |
| 10 | 25 | M | CRPS I | Y | Y/Y | Stocking | N | N | norm | norm | Cold hyper | Y | N |
| 11 | 32 | F | CRPS I | Y | Y/Y | Non-dermat | Y | N | norm | norm | Normal | np | N |
| 12 | 38 | F | CRPS I | Y | Y/Y | Stocking | N | N | norm | norm | Cold hyper | Y | N |
| 13 | 39 | F | CRPS I | Y | Y/Y | Stocking | Y | Y | norm | norm | Cold hyper | Y | N |
| 14 | 49 | F | CRPS II | Y | Y/Y | Sp peroneal | N | N | norm | norm | Warm hypo |
np | N |
| 15 | 48 | F | CRPS II (PNP) |
Y | Y/N | Stocking | N | N | norm | norm | Heat hyper | np | Y |
| 16 | 47 | M | CRPS II (PNP) |
Y | Y/N | Stocking | N | N | Abs | Abn | Warm hypo |
np | Y |
| 17 | 78 | M | CRPS II (PNP) |
Y | Y/N | Stocking | N | N | Abs | Abn | Warm hypo |
np | N |
| 18 | 32 | F | CRPS II (PNP) |
Y | Y/N | Stocking | N | N | norm | norm | Warm hypo |
np | Y |
| 19 | 47 | M | CRPS II (PNP) |
Y | Y/N | Stocking | N | N | Redu | Abn | Warm hypo |
np | N |
| 20 | 42 | M | CRPS II (PNP) |
Y | Y/N | Stocking | Y | N | norm | norm | Warm hypo |
np | Y |
| 21 | 35 | M | CRPS II (PNP) |
N | N/N | Stocking | N | N | norm | norm | Warm hypo |
np | Y |
| 22 | 54 | F | CRPS II (PNP) |
N | Y/N | Stocking | N | N | norm | norm | Warm hypo |
np | N |
| 23 | 49 | M | CRPS II (PNP) |
Y | N/N | Stocking | N | N | Abs | Abn | Cold hyper | np | Y |
| 24 | 13 | F | CRPS II (PNP) |
Y | Y | Stocking | N | N | norm | norm | Heat hyper | np | N |
Dg = diagnosis; traumatic = nerve injury; PNP = polyneuropathy; Spont pain = spontaneous pain; Mech. hyperalg = hyperalgesia; Y = yes; N = no; Sp peroneal = superficial peroneal; TR = tendon reflexes; norm = normal; Abs = absent; Abn = abnormal; Redu = reduced; NCS/EMG = nerve conduction studies/electromyography; QST = quantitative sensory testing; Cold hyper = cold hyperalgesia; Heat hyper = heat hyperalgesia; Warm hypo = Warm hypoesthesia; Symp. blocks = sympathetic blocks; np = not performed.
3.2 Microneurography
Fifty-four single identified C nociceptor units that displayed an adequate signal-to-noise ratio to allow analysis were pooled from the raster plots in 35 recordings: 35 were 1A fibers (CMHs) and 19 were 1B (MIAs) (classified using the same criteria as in previous studies (Serra et al., 2004)). Table 2 shows the mean conduction velocities and degree of slowing for the two nociceptor subtypes. For both CRPS I and CRPS II patients these values were in agreement with previous report in normal volunteers (Serra et al., 2004). As in previous studies (Schmelz et al., 2000; Serra et al., 2004), conduction velocities of MIAs were significantly slower than those of CMHs (p<0.01), both in CPRS I and CRPS II patients.
Table 2. Characteristics of the C fiber nociceptors and sympathetic efferents.
| CRPS I (13 patients) |
CRPS II (11 patients) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C fiber subtype | N | CV (m.s−1) |
Slowing (%) | N | CV (m.s−1) |
Slowing (%) | |||
| after “pause” |
2 Hz | after “pause” | 2 Hz | ||||||
| CMH | 1a | 18 | 0.72±0.16 | 1.06±0.16 | 28.1±7.63 | 17 | 0.74±0.19 | 1.21±0.71 | 30.9±13.6 |
| MIA | 1b | 8 | 0.47±0.17 | 4.36±1.16 | 34.4±13.7 | 11 | 0.48±0.24 | 3.86±1.20 | 39.8±22.5 |
| Sympathetic | 4 | 18 | 0.63±0.19 | 1.67±1.54 | 4.74±1.93 | 4 | 0.51±0.35 | 1.20±0.54 | 4.80±0.18 |
CV = conduction velocity; CMH = mechano-sensitive C fiber nociceptor; MIA = mechano-insensitive C fiber nociceptor; Sympathetic = sympathetic efferent C fiber; Slowing = activity-dependent slowing of CV; “pause” refers to the 3-min period of 1/30 Hz stimulation, following which stimulation at 0.25 Hz resumed; 2 Hz = refers to the end of the 3-min period of stimulation at 2-Hz.
Type 1A units (CMHs) suitable for dependable analysis were only activated by adequate mechanical and heat stimuli. In CRPS I patients, mechanical thresholds were tested 18 CMHs (median 52.3 mN, range 7.9 to >108.5 mN). Heat thresholds were obtained for 6/8 1A units. Three had thresholds of 40, 42, and 48°C, respectively, 3 did not respond when tested with temperatures up to 48°C. In CRPS II patients, mechanical thresholds were obtained in 12 CMHs, with a median of 7.9 mN (range 6.1 to >108.5 mN). Heat threshold was obtained in 5 CMHs and ranged from 44 to >46°C (median 46°C). All type 1B units (MIAs) in both CRPS I and CRPS II patients responded only to electrical stimuli applied to the skin and were unresponsive to mechanical and heat stimuli.
A total of 22 identified sympathetic units were recorded along with the nociceptors (Table 2). Sympathetic efferents were readily recognized by their characteristic profile of activity-dependent slowing: a fast initial slowing during the 2 Hz tetanus followed by a plateau and a partial recovery during the last phase (Campero et al., 2004). The other typical feature of many of sympathetic efferent fibers was their ongoing impulse activity.
3.3 Measures of sympathetic nerve fiber discharge: activity dependent slowing of sympathetics and cutaneous vasoconstriction
We used two methods to document sympathetic fiber discharge. First, direct detection of activity-dependent slowing of individual sympathetic efferents just following the sympathetic activating maneuver. The second strategy was to measure similar mild gradual increases in latency of C nociceptor fibers being recorded (CMHs and MIAs) at the time of sympathetic activation. These latency changes reflected a decrease in limb temperature induced by sympathetic mediated vasoconstriction, which directly followed a Valsalva maneuver or mental stress. Both activity-dependent slowing of C sympathetic units and passive slowing of C nociceptors by mild cooling due to vasoconstriction are illustrated in Figure 1 from a normal human subject.
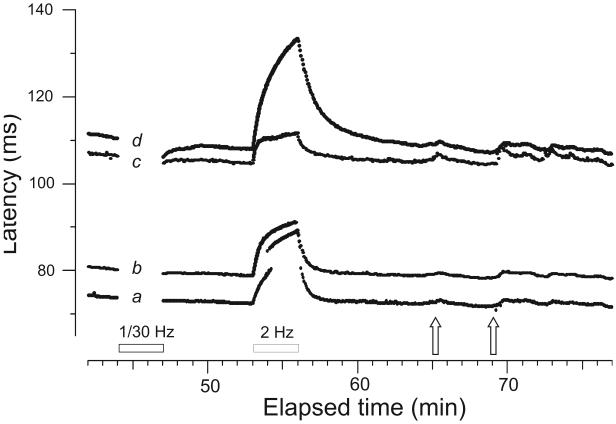
Figure 1.
Simultaneous recording of sensory and sympathetic units from the left superficial peroneal nerve in a healthy 28 year-old female. Each line is made of the confluence of dots that each represent one evoked nerve action potential plotted at the intersection of conduction latency and elapsed time. The electrical stimulation protocol consisted of a 0.25 Hz baseline, followed by 3 min of 1 pulse per 30 s (1/30 Hz) (“pause”), then a 6-min return to 0.25 Hz baseline, a 3-min 2-Hz tetanus, and finally a 20-min period resuming 0.25 Hz stimulation for recovery of excitability and natural stimulus testing. Four units are shown with different latencies, classified according to their activity-dependent slowing profile as follows: (a) CMH; (b) CMH; (c) sympathetic unit; (d) MIA. The 2 CMHs (conducting 0.87 and 0.81 m.s−1, respectively), display a small latency effect after the pause (0.4 and 0.7% slowing, respectively) in comparison to the significant activity-dependent slowing (3.4%) of the MIA (conducting at a nominal velocity of 0.58 m.s−1). The sympathetic efferent (c) had an intermediate conduction velocity (0.61 m.s−1) and slowed by 1.1% following the pause. During the 2-Hz tetanus the CMHs and MIA (a,b,d) slowed by 18.6, 16%, and 23.7%, respectively, whereas the sympathetic efferent (c) slowed by only 6.2%. (The faster conducting CMH showed jumps to longer latency due to branch switching, resulting from threshold increase during the rapid electrical stimulation.) Mental stress (naming country capitals) (left open arrow), while causing a sudden latency increase reflecting a burst of impulse activity in the sympathetic efferent (c), was associated with a rather gradual increase in latency of CMHs and MIA, reflecting skin cooling. A similar, but more pronounced phenomenon was induced by a sudden loud startling noise (right open arrow).
3.4 Sympathetic discharge and neural impulse activity in nociceptors
All C nociceptors recorded were tested for discharges temporally related to sympathetic activation. In 12 patients (including 3 of 5 CRPS I patients who responded to sympathetic block), identified single sympathetic efferents and nociceptors were recorded simultaneously, while in the 12 remaining patients, no single sympathetic efferents were recorded simultaneously, but the C nociceptors from patients (as well as from normal subjects) displayed the effect of vasoconstriction, as evidenced by a gradual latency increase during the maneuver (see Figure 1).
In CRPS I patients, CMH units never exhibited ongoing impulse activity and their latencies remained stable during sympathetic maneuvers, except for the subtle increase in latency associated with skin cooling. Eight mechano-insensitive afferents were tested similarly (sometimes along with the CMHs), and, the same as with mechano-sensitive nociceptors, there was no indication of a quantal latency shift due to single action potential or burst discharge during sympathetic maneuvers (examples shown in Figure 2).
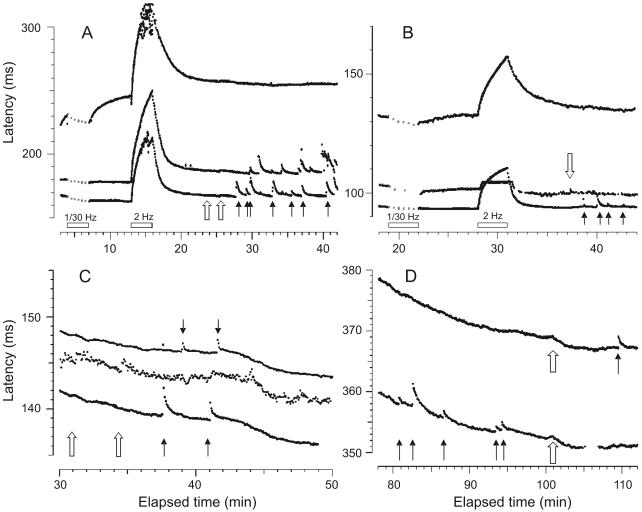
Figure 2.
Four panels showing examples of simultaneous recordings of two or more C nociceptors in patients during sympathetic maneuvers, one (A) without and three (B-D) with concurrent single-fiber sympathetic efferent recordings. (A) CRPS I patient (patient #11, Table 1): Recording of three units, the fastest two (bottom) are mechanosensitive nociceptors and the slow unit a mechano-insensitive afferent, as based upon slowing of conduction velocity after the stimulus pause and upon receptor responses to mechanical stimuli (solid arrows). During startle (open arrows) there was a subtle generalized slowing of the three fibers, due to skin cooling from reflex vasoconstriction, but no ectopic discharge. (B) Patient #10 (CRPS I): Three units, identified (going from short to long latency) as a mechano-sensitive nociceptor, a sympathetic efferent and a mechano-insensitive afferent. During reflex activation of the sympathetic efferent by startle (open arrow) no excitation of either type of nociceptor was recognized. The mechano-sensitive nociceptor readily responded with bursts to mechanical stimulation (solid arrows). (C) Patient #3 (CRPS I): At this intraneural site, two CMH nociceptors and an active sympathetic efferent (in between) were recorded simultaneously. Despite spontaneous activity of the sympathetic efferent fiber and its reflex activation (open arrows), no activation was seen for the nociceptors which otherwise readily discharged upon mechanical stimulation (solid arrows). (D) Patient #2 (CRPS I): A recording of two mechanosensitive C nociceptor afferents, without concomitant sympathetic efferent recording. Mechanical stimuli briskly activate the fibers (black arrows), while sympathetic maneuvers only induce a gradual cooling-related slowing (open arrows). The gradual shortening in latency seen for all the units in panels C and D was due to warming of the skin by an infrared lamp.
Figure 3 shows recordings from the superficial radial nerve of a CRPS I patient. Based on the activity-dependent slowing of the 4 fibers recorded simultaneously, 2 were mechano-sensitive nociceptors and 2 sympathetic efferents. The faster sympathetic efferent fiber exhibited more pronounced ongoing activity and responded vigorously to reflex provocation. In spite of the overt sympathetic reflex, both nociceptors remained unresponsive.
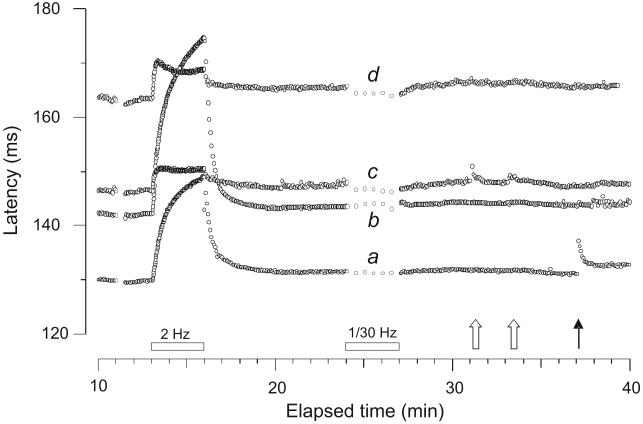
Figure 3.
Recording from the right superficial radial nerve of a CRPS I patient (#3, Table 1). Four units are recorded simultaneously following the routine electrical stimulation protocol. The 2 fastest units are nociceptors, a CMH (a) and a MIA (b), conducting at 0.76 m.s−1 and 0.69 m.s−1, respectively. Units (c) and (d) are sympathetic efferents. Unit (c) displays random latency changes due to its natural ongoing discharge and responds vigorously to startle (open arrows). Both nociceptors maintain a stable latency, indicating absence of extra impulse activity during the sympathetic activation maneuvers. Only unit (a) responded to a subsequent mechanical stimulus (51.9 mN) (solid arrow).
As in CRPS I patients, no evidence of firing induced by effective sympathetic maneuvers was registered in any of the CMH or MIA nociceptors recorded in CRPS II patients.
3.5 Abnormal nociceptor discharges in patients, unrelated to sympathetic activity
In none of the 13 CRPS I patients, but 6/11 CRPS II patients (a significantly different proportion, Fisher’s exact test, p=0.003), there was evidence of abnormal electrical behavior of afferent C nociceptor fibers (Table 1). In two CRPS II patients (#15 and #23) there were abnormal spontaneous discharges in MIAs. This discharge is illustrated for one of the patients (Figure 4). In 4 other CRPS II patients (#16, 18, 20, and 21) there was ectopic discharge in CMHs which is illustrated for one of the patients (Figure 5). This particular patient (#16) had severe ongoing pain and mechanical hyperalgesia associated with diabetic neuropathy.
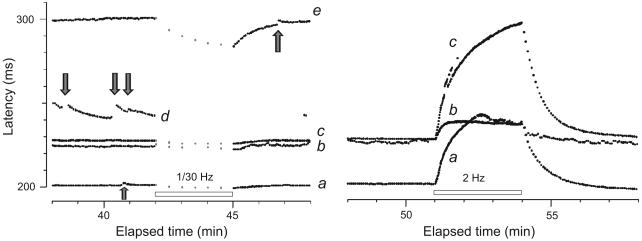
Figure 4.
Recording from the right superficial peroneal nerve of a CRPS II patient (#15, Table 1) with a painful neuropathy, ongoing pain and both heat and mechanical hyperalgesia. Left panel: Initial recording of 5 units, three (a,d,e) had abnormal bursting discharge (arrows); one fiber (b) was as a sympathetic efferent with natural on-going discharge. The fifth fiber (c) behaved as a normal CMH. The fastest conducting unit (0.9 m.s−1, (a)) with the abnormal discharge was a mechano-sensitive nociceptor. The slowest conducting unit (0.63 m.s−1, (e)), classified as a mechano-insensitive nociceptor (based on slowing after “pause”), also engaged in abnormal bursting discharge of two or more action potentials (arrow). The nociceptor with the longest latency (e) and an unidentified unit (d) with pronounced bursting (arrows) were lost. Right panel: Response to the classification protocol (2 Hz electrical stimulation) of the CMH (a), MIA (c) and sympathetic efferent (b).
Figure 5.
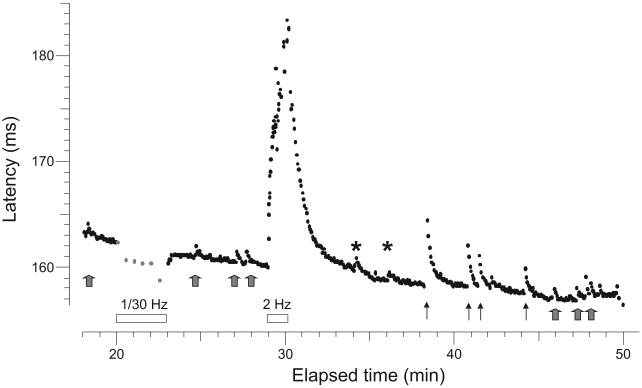
Record from the superficial peroneal nerve of a patient with painful neuropathy (#16, Table 1). This fiber with a conduction velocity of 0.59 m.s−1, had a latency increase of 0.5% after the 1/30 Hz pause, typical of a CMH. It displayed abnormal discharges (gray arrows) detected as activity-dependent conduction slowing equivalent to one or two action potential spikes. This was determined according to a calibration after the 2-Hz tetanus when 3 extra electrical stimuli were given during the ongoing 0.25 Hz stimulation to demonstrate the extent of activity-dependent slowing (asterisks). This unit was abnormally sensitive to mechanical stimulation (threshold 7.8 mN) (solid arrows).
4. Discussion
Our search for abnormal excitation of single identified CMH and mechano-insensitive nociceptor afferents, induced by documented sympathetic efferent activation in symptomatic painful limbs of 24 patients fitting the descriptive categories CRPS I and CRPS II (Merskey and Bogduk, 1994), has been negative. The present results differ from those in a single case study that also used microneurography (Jørum et al., 2007; Ochoa, 2007). In all 24 CRPS patients we have failed to detect any evidence of sympathetic-nociceptor interaction, including the 6 patients who were previously diagnosed by the treating physicians as having SMP on the basis of their responses to diagnostic sympathetic blocks (uncontrolled for placebo effect). It is most unlikely that this failure to observe any nociceptor activation by sympathetic activity is a false negative due to insufficient sensitivity, because the ‘marking’ method of monitoring latencies to regular electrical stimuli can definitely detect single impulses in C nociceptors, and otherwise did reveal random spontaneous pathological discharges in CRPS II patients. We did observe a gradual slowing of conduction velocity due to cooling of skin resulting from vasoconstriction during our sympathetic maneuvers, but no excitation of extra nociceptor impulses. Given the substantial number of fibers and the abundant opportunities for potential interactions studied, the negative results cannot simply be dismissed as due to inadequate sampling. The negative result in the present study for a large number of both CRPS I and CRPS II patients indicates that if there is any activation of C nociceptors by sympathetic discharges in these patients, it must be very rare, and therefore unlikely to make a significant contribution to their subjective pain reports.
4.1 Alternative interpretations
4.1.1 Timing and site of microneurography recordings
All but two patients expressed ongoing chronic pain at the time of recording. Thus, the possibility of having tested the patient population during spontaneous symptom remission is ruled out. The possibility that the recordings may not have covered symptomatic areas is also ruled out by the experimental design because the cutaneous innervations territories of the nerve fascicles sampled did cover those areas.
4.1.2 Catchment area of the testing method
As applied, microneurography sampling in mid-nerve trunk could not have missed distal or proximal sources of abnormal sympathetic/nociceptor interaction. Orthodromic sympathetic activation of C nociceptors afferents at peripheral nerve endings would have been readily detected in the mid-nerve trunk recordings. The same applies to potential interaction at dorsal root or ganglion level, since that would have generated sensory impulses propagated antidromically which would also have caused activity-dependent slowing at the recording site. Some of the antidromic impulses would have been blocked proximally by collision with the orthodromic, electrically activated impulses, but the baseline electrical stimulation rate was low enough (once every 4 s) so the majority of antidromic impulses would have escaped collision and would have been detected. A general example of pathological antidromic activity recorded microneurographically from peripheral sensory fascicles are the single-fiber ectopic discharges during provocation of cervical radicular paresthesias through the compressive maneuver of Spurling (Ochoa et al., 1987).
4.1.3. Small fiber neuropathy
In the present sample the paucity of sympathetic efferents recorded concurrently with nociceptors in patients with CRPS II (4 efferents) compared with CRPS I (18 efferents) could be explained by a sampling bias because sympathetic efferents amount to only one quarter of all unmyelinated fibers in human peroneal nerve(Namer et al., 2009). Besides, most of the CRPS II patients had various degrees of small fiber neuropathy which may have further depopulated sympathetic efferents among the C fibers in distal nerves.
4.1.4 Changes in nociceptor latency during sympathetic reflex activation
Conduction velocity slowing in C nociceptors was observed during sympathetic maneuvers. However, the slowing of conduction velocity in C nociceptors that followed sympathetic efferent activation (Figure 1) did not have the features of ectopic impulse generation in response to electrical or chemical actions (Figures 4 and 5). It was a sluggish conduction delay of the kind that is commonly induced both in sensory and sympathetic C fibers by passive cooling of the skin during experiments (Figure 2, c,d). The slowing in each case confirmed that sympathetic activity was increased by the maneuvers even in instances when this could not be confirmed directly because in some of the experiments sympathetic efferents were not seen by the microelectrode.
Spontaneous ectopic nociceptor discharge was found only in patients with the diagnosis of CRPS II. However, the discharge occurred without any temporal relationship to documented activation of individual sympathetic efferents. Therefore, sympathetic-nociceptor interactions, if they exist in patients communicating chronic neuropathic pain, must be the exception.
4.2 The complex nature of CRPS I
The mechanisms of chronic pains following injury to a limb without evidence of nerve pathology (CRPS I) are puzzling. Sensitization of second order neurons in the central nervous system has long been suspected, based upon the spreading of the symptoms pain and hyperalgesia, and on associated movement disorders none of which are explainable at the periphery. Animal models of nerve injury have shown that temporary central sensitization may occur after nociceptive input to the spinal cord, but hypothetical extrapolation to chronic pain syndromes in patients without nerve pathology is unjustified. An interaction between the sympathetic nerves and nociceptors has been postulated to provide a maintaining nociceptive input, by cross-talk at the site of nerve damage, or at the nerve terminal by adrenergic supersensitivity of C nociceptors. In either case we would have expected to find spontaneous, ongoing discharge in C nociceptors, related to sympathetic activity. However, in none of the 13 patients in the present study who were referred to us with the diagnosis of CRPS I did find any spontaneous, ongoing discharge in C nociceptors, and moreover there was complete lack of any sympathetic-nociceptor interaction, even in the 6 CRPS I patients who responded to placebo-uncontrolled sympathetic block. This leaves the clinical observation that sympathetic block may transiently relieve pain as the only argument that sympathetic nerves commonly maintain pain in CRPS I. However, as previously discussed, this evidence can be discounted since the response rate for sympathetic blocks is no higher than for placebo (Verdugo and Ochoa, 1994; Verdugo et al., 1994; Jadad et al., 1995; Ramamurthy and Hoffman, 1995).
We can fairly conclude, therefore, that the pain in CRPS I is not in general maintained by a peripheral nociceptive input, whether involving sympathetic outflow or not, so the source must lie elsewhere. As Jänig (2001) stated:”.... CRPS-I can only be understood as a pain syndrome or disease that is actively generated by the brain.” But description of CRPS I as “a disease” is misleading. Clinical features assigned to CRPS I amount to self-reported pains plus seemingly neurological motor and sensory displays, in the absence of demonstrable neuropathophysiology. Moreover, as the American Medical Association (Rondinelli, 2008) writes: “CRPS is a challenging and controversial concept... Since a subjective complaint of pain is the hallmark...and since all of the associated physical signs and radiological findings can be the result of disuse, an extensive differential diagnostic process is necessary”, to include “unrecognized general medical problems, somatoform disorders and malingering... The diagnosis of CRPS has not been scientifically validated as representing a specific and discrete health condition... There is no gold standard diagnostic feature that distinguishes CRPS... Scientific findings indicate that whenever this diagnosis is made, it is probably incorrect.”
In practice, dedicated neurological differential diagnoses can reveal a variety of discrete organic and psychogenic entities that lie behind the descriptive label CRPS I (Ochoa, 2002). Because of this inhomogeneity of CRPS I, recurrent attempts to identify a unifying mechanism (such as SMP), that might lead to a single therapeutic approach, are almost certainly doomed to failure.
4.3 Conclusions
In 13 CRPS I and 11 CRPS II patients we could find no electrophysiological single nerve fiber evidence for activation of C nociceptors related to efferent activity in sympathetic efferent fibers. Therefore an a priori supposition of sympathetic involvement in pain generation at the C nociceptor level in either CRPS I or CRPS II is likely to be unfounded and may distract attention away from potentially treatable mechanisms.
Acknowledgments
This work was supported by NIH Grant Ro1-NS48932.
Footnotes
The authors do not have conflicts of financial interests, and there are no sponsors from the industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mario Campero, Departamento de Neurología, Clínica Alemana-Universidad del Desarrollo, Santiago, Chile. Av. Manquehue Norte 1410, piso 10, Vitacura, Santiago, Chile. mcampero@med.uchile.cl
Hugh Bostock, Institute of Neurology, Queen Square, London WC1N 3BG, U.K., mhbostock@ion.ucl.ac.uk
Thomas K. Baumann, Department of Neurological Surgery, CR-137, Oregon Health & Science University, Portland, OR, U.S.A., baumannt@ohsu.edu
José L. Ochoa, Legacy Good Samaritan Hospital & Medical Center and Oregon Health & Science University, Portland, OR, U.S.A., jochoa@nervesense.net
References
- Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;88:161–68. doi: 10.1016/S0304-3959(00)00327-4. [DOI] [PubMed] [Google Scholar]
- Baron R, Wasner G, Borgstedt R, Hastedt E, Schulte H, Binder A, Kopper F, Rowbotham M, Levine JD, Fields HL. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatation. Neurology. 1999;52:923–32. doi: 10.1212/wnl.52.5.923. [DOI] [PubMed] [Google Scholar]
- Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J. Physiol. 1999;515:533–42. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Partial reversal of conduction slowing during repetitive stimulation of single sympathetic efferents in human skin. Acta Physiol. Scand. 2004;182:305–11. doi: 10.1111/j.1365-201X.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- Chabal C, Jacobson L, Russell LC, Burchiel KJ. Pain response to perineuromal injection of normal saline, epinephrine, and lidocaine in humans. Pain. 1992;49:9–12. doi: 10.1016/0304-3959(92)90181-A. [DOI] [PubMed] [Google Scholar]
- Elam M. The relation between sympathetically maintained pain, regional vasomotor disturbances and sympathetic nerve activity: a remaining enigma. Schmerz. 1998;12:272–5. doi: 10.1007/s004829800030. [DOI] [PubMed] [Google Scholar]
- Elam M, Olausson B, Skarphedinsson JO, Wallin BG. Does sympathetic nerve discharge affect the firing of polymodal C-fibre afferents in humans? Brain. 1999;122:2237–44. doi: 10.1093/brain/122.12.2237. [DOI] [PubMed] [Google Scholar]
- Evans JA. Reflex sympathetic dystrophy. Sur. Gynec. & Obstet. 1946;82:36–44. [PubMed] [Google Scholar]
- Gibbs GF, Drummond PD, Finch PM, Phillips JK. Unravelling the pathophysiology of complex regional pain syndrome: focus on sympathetically maintained pain. Clin. Exp. Pharmacol. Physiol. 2008;35:717–24. doi: 10.1111/j.1440-1681.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Tack C, Li ST. Sympathetic innervation and function in reflex sympathetic dystrophy. Ann. Neurol. 2000;48:49–59. [PubMed] [Google Scholar]
- Häbler H-J, Jänig W, Koltzenburg M. Activation of unmyelinated afferents in chronically lesioned nerves by adrenaline and excitation of sympathetic efferents in the cat. Neurosci. Lett. 1987;82:35–40. doi: 10.1016/0304-3940(87)90167-4. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Torebjörk HE. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol. Scand. 1974;92:303–17. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Hannington-Kiff JG. Intravenous regional sympathetic block with guanethidine. Lancet. 1974;1:1019–20. doi: 10.1016/s0140-6736(74)90418-8. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Carroll D, Glynn CJ, McQuay HJ. Intravenous regional sympathetic blockade for pain relief in reflex sympathetic dystrophy: a systematic review and a randomized, double-blind crossover study. J. Pain. Symptom. Manage. 1995;10:13–20. doi: 10.1016/0885-3924(94)00064-R. [DOI] [PubMed] [Google Scholar]
- Jänig W. CRPS-I and CRPS-II: A strategic view. In: Harden RN, Baron R, Jänig W, editors. Complex regional pain syndrome. IASP Press; Seattle, WA: 2001. pp. 3–15. Progress in Pain Research and Management. [Google Scholar]
- Jänig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog. Brain Res. 1996;113:161–84. doi: 10.1016/s0079-6123(08)61087-0. [DOI] [PubMed] [Google Scholar]
- Jørum E, Ørstavik K, Schmidt R, Namer B, Carr RW, Kvarstein G, Hilliges M, Handwerker H, Torebjörk E, Schmelz M. Catecholamine-induced excitation of nociceptors in sympathetically maintained pain. Pain. 2007;127:296–301. doi: 10.1016/j.pain.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Kress M, Reeh PW. The nociceptor sensitization by bradykinin does not depend on sympathetic neurons. Neurosci. 1992;46:465–73. doi: 10.1016/0306-4522(92)90066-b. [DOI] [PubMed] [Google Scholar]
- Loh L, Nathan PW. Painful peripheral states and sympathetic blocks. J. Neurol. Neurosurg. Psych. 1978;41:664–71. doi: 10.1136/jnnp.41.7.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailis-Gagnon A, Bennett GJ. Abnormal contralateral pain responses from an intradermal injection of phenylephrine in a subset of patients with complex regional pain syndrome (CRPS) Pain. 2004;111:378–84. doi: 10.1016/j.pain.2004.07.019. [DOI] [PubMed] [Google Scholar]
- IASP Task Force on Taxonomy . In: Classification of chronic Pain: Descriptions of chronic pain syndromes and definitions of pain terms. 2nd edn. Merskey H, Bogduk N, editors. IASP Press; Seattle, WA: 1994. pp. 5–222. [Google Scholar]
- Michaelis M, Devor M, Jänig W. Sympathetic modulation of activity in rat dorsal root ganglion neurons changes over time following peripheral nerve injury. J. Neurophysiol. 1996;76:753–63. doi: 10.1152/jn.1996.76.2.753. [DOI] [PubMed] [Google Scholar]
- Namer B, Barta B, Ørstavik K, Schmidt R, Carr R, Schmelz M, Handwerker HO. Microneurographic assessment of C-fibre function in aged healthy subjects. J. Physiol. 2009;587:419–28. doi: 10.1113/jphysiol.2008.162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW. On the pathogenesis of causalgia in peripheral nerve injuries. Brain. 1947;70:145–170. doi: 10.1093/brain/70.2.145. [DOI] [PubMed] [Google Scholar]
- Ochoa JL. Pathophysiology of chronic “neuropathic pains”. In: Burchiel KJ, editor. Surgical Management of Pain. Thieme Medical and Scientific Publishers; New York: 2002. pp. 25–41. [Google Scholar]
- Ochoa JL. Letter to the Editor of Pain on Jørum et al: Catecholamine-induced excitation of nociceptors in sympathetically maintained pain. Pain. 2007;127:296–301. doi: 10.1016/j.pain.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Verdugo RJ. Reflex sympathetic dystrophy. Definitions and history of the ideas. A critical review of human studies. In: Low PA, editor. The evaluation and management of clinical autonomic disorders. Little, Brown and Co.; Boston: 1993. pp. 473–92. [Google Scholar]
- Ochoa JL, Cline MA, Dotson R, Marchettini P. Pain and paresthesias provoked mechanically in human cervical root entrapment (sign of Spuling). Single sensory unit antidromic recording of ectopic, bursting, propagated nerve impulse activity. In: Publos LM, Sessle BJ, editors. Effects of injury on trigeminal and spinal somatosensory systems. Alan R. Liss Publishers; New York: 1987. pp. 389–97. [Google Scholar]
- O’Halloran KD, Perl ER. Effects of partial nerve injury on the responses of C-fiber polymodal nociceptors to adrenergic agonists. Brain Res. 1997;759:233–40. doi: 10.1016/s0006-8993(97)00261-8. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Hoffman J, Guanethidine Study Group Intravenous regional guanethidine in the treatment of reflex sympathetic dystrophy/causalgia: a randomized, double-blind study. Anesth. Analg. 1995;81:718–23. doi: 10.1097/00000539-199510000-00011. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zou X, Fang L, Lin Q. Sympathetic modulation of activity in Adelta-and C-primary nociceptive afferents after intradermal injection of capsaicin in rats. J. Neurophysiol. 2005;93:365–77. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- Roberts WJ. A hypothesis on the physiological basis for causalgia and related pains. Pain. 1986;24:297–311. doi: 10.1016/0304-3959(86)90116-8. [DOI] [PubMed] [Google Scholar]
- Roberts WJ, Elardo SM. Sympathetic activation of A-delta nociceptors. Somatosens. Res. 1985;3:33–44. doi: 10.3109/07367228509144575. [DOI] [PubMed] [Google Scholar]
- Rondinelli RD. Complex regional pain syndrome impairment. In: Rondinelli RD, Andersson G, Dobie R, Frenkel E, Genovese E, Katz R, Mueller K, Parish B, Robinson J, Sataloff R, Smith S, Wetmore S, Zirwas M, editors. Guides to the evaluation of permanent impairment. 6th edn. American Medical Association; 2008. pp. 450–51. [Google Scholar]
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–10. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmid R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123:560–71. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J. Physiol. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Campero M, Bostock H, Ochoa J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J. Neurophysiol. 2004;91:2770–81. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]
- Torebjörk E, Wahren L, Wallin G, Hallin R, Koltzenburg M. Noradrenaline-evoked pain in neuralgia. Pain. 1995;63:11–20. doi: 10.1016/0304-3959(95)00140-N. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB, Hagbarth K-E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp. Neurol. 1968;21:270–89. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Verdugo RJ, Ochoa JL. ‘Sympathetically maintained pain.’ I. Phentolamine block questions the concept. Neurology. 1994;44:1003–10. doi: 10.1212/wnl.44.6.1003. [DOI] [PubMed] [Google Scholar]
- Verdugo RJ, Campero M, Ochoa JL. Phentolamine sympathetic block in painful polyneuropathies. II. Further questioning of the concept of ‘sympathetically maintained pain’. Neurology. 1994;44:1010–14. doi: 10.1212/wnl.44.6.1010. [DOI] [PubMed] [Google Scholar]
- Wallin G, Torebjörk E, Hallin R. Preliminary observations on the patho-physiology of hyperalgesia in the causalgic pain syndrome. In: Zotterman Y, editor. Sensory function of the skin. Pergamon Press; Oxford: 1976. pp. 489–502. [Google Scholar]