Abstract
Serous tubal intraepithelial carcinoma (STIC) has been proposed as a precursor for many pelvic high-grade serous carcinomas. Our previous analysis of the ovarian cancer genome identified several genes with oncogenic potential that are amplified and/or overexpressed in the majority of high-grade serous carcinomas. Determining whether these genes are upregulated in STICs is important in further elucidating the relationship of STICs to high-grade serous carcinomas and is fundamental in understanding the molecular pathogenesis of high-grade serous carcinomas. In this study, 37 morphologically defined STICs were obtained from 23 patients with stage IIIC/IV high-grade serous carcinomas. Both STICs and the high-grade serous carcinomas were analyzed for expression of Rsf-1, cyclin E, fatty acid synthase (FASN), and mucin-4. In addition, they were examined for expression of established markers including p53, Ki-67 and p16. We found that diffuse nuclear p53 and p16 immunoreactivity was observed in 27 (75%) of 36 and 18 (55%) of 33 STICs, respectively, while an elevated Ki-67 labeling index (≥10%) was detected in 29 (78%) of 37 STICs. Cyclin E nuclear staining was seen in 24 (77%) of 35 STICs while normal tubal epithelial cells were all negative. Increased Rsf-1 and FASN immunoreactivity occurred in 63%, and 62% of STICs, respectively, compared to adjacent normal-appearing tubal epithelium. Interestingly, only one STIC demonstrated increased mucin-4 immunoreactivity. Carcinomas, as compared to STICs, overexpressed p16, Rsf-1, cyclin E and FASN in a higher proportion of cases. In conclusion, STICs express several markers including Rsf-1, cyclin E and FASN in high-grade serous carcinomas. In contrast, mucin-4 immunoreactivity either did not change or was reduced in most STICs. These results suggest that overexpression of Rsf-1, cyclin E and FASN occurs early in tumor progression.
Keywords: ovarian cancer, tubal intraepithelial carcinoma, HBXAP (Rsf-1)
Introduction
Characterization of putative precursor lesions is fundamental in elucidating the molecular pathogenesis of cancer and has profound implications for early detection, prevention and treatment. In contrast to other major types of carcinomas including colorectal, prostate, pancreatic and breast cancer, the cell origin of ovarian cancer has not yet been identified despite numerous studies that have carefully examined the ovaries for precursor lesions. Recent histopathological studies, however, have provided provocative new evidence that the fallopian tube, specifically the fimbria, rather than the ovarian surface epithelium may be the source of most ovarian high-grade serous carcinomas. 1 The basis for this hypothesis comes from morphologic, immunohistochemical and molecular genetic studies of a lesion designated “serous tubal intraepithelial carcinoma” (STIC) which closely resembles high-grade ovarian serous carcinoma and co-exists with small, early invasive tubal carcinomas in women with a genetic predisposition to ovarian cancer. 2, 3 In addition, STICs are also detected in prophylactic specimens of fallopian tubes from women who do not have an associated carcinoma. 4 More recently, Kindelberger et al. have reported that over 70% of sporadic (nonhereditary) ovarian and peritoneal high-grade serous carcinomas displayed mucosal tubal involvement by carcinoma including STICs suggesting that the latter are potential precursors for sporadic as well as hereditary high-grade serous carcinomas.5 Further support for the tubal origin of high-grade ovarian serous carcinoma comes from the finding of strong, diffuse p53 immunoreactivity in the majority of STICs 4 as well as the detection of identical TP53 mutations in the STICs and concurrent ovarian serous carcinomas, indicating a clonal relationship between them.5
Moreover, STICs with TP53 mutations have also been detected in the absence of a concomitant ovarian serous carcinoma, suggesting that STICs precede the development of high-grade serous carcinoma rather than representing a metastasis from a primary ovarian carcinoma. Finally, a gene profiling study showing that serous carcinomas from the fallopian tube and ovary are indistinguishable 6 and that the expression profile of high-grade serous carcinoma is more closely related to the fallopian tube than to ovarian surface epithelium.7
In order to further characterize the molecular changes in STICs, we analyzed them for gene expression using several well-established ovarian cancer-associated genes including Rsf-1 8, cyclin E 9, fatty acid synthase (FASN) 10 and mucin-4 11 that are frequently amplified and/or upregulated in high-grade serous carcinoma. Because STICs are almost always incidental microscopic findings detected during histopathology review it is very difficult to harvest sufficient fresh tissue from STICs to perform mRNA-based gene expression analysis. We therefore applied immunohistochemistry on paraffin sections using well characterized antibodies in order to study gene expression in STICs. We found that all the selected genes except mucin-4 were upregulated in most STICs as compared to adjacent normal-appearing tubal epithelium. The frequency of overexpression is similar to that found in a large series of high-grade serous carcinoma that has been previously reported. 9, 12, 13 Our results provide additional evidence that STICs are the likely precursors of high-grade serous carcinomas and suggest that overexpression of Rsf-1 (HBXAP), cyclin E and FASN occurs at an early stage in tumor development.
Materials and Methods
Case selection
The criteria for case selection were based on the lesions exhibiting three or more of following histologic features including 1) abnormal chromatin pattern, 2) nuclear enlargement, 3) marked pleomorphism, 4) epithelial stratification and/or loss of polarity, and 5) nuclear molding. Thirty seven morphologically defined STICs were obtained from 23 patients with stage IIIC/IV high-grade serous carcinoma collected from the Johns Hopkins Hospital. Many of the specimens were sent as consultation cases from the Legacy Health Systems, Portland, Oregon. Tissue collection conformed to the guidelines of the Institutional Research Board of The Johns Hopkins Hospital.
Immunohistochemistry
Both STICs and the high-grade serous carcinomas were analyzed for expression of ovarian cancer-associated markers including Rsf-1 (HBXAP), FASN, cyclin E and mucin-4. These four proteins were selected because they are expressed in a high proportion of high-grade serous carcinomas. In addition, STICs and high-grade serous carcinoma were examined for expression of “conventional” markers including p53, Ki-67 and p16. The sources and dilution for each antibody were: Rsf-1 antibody (Millipore Upstate Cell Signaling, Billerica, MA; 1:2000 dilution), fatty acid synthase antibody (FASgen, Baltimore, MD; 1:50 dilution), cyclin E antibody (Zymed, Invitrogen; 1:250 dilution), mucin-4 antibody (Abcam, Cambridge, MA; 1:1500 dilution), p53 antibody (Ventana, Tucson, AZ; 1:1 dilution), Ki-67 antibody (Ventana, Tucson, AZ; 1:1 dilution) and p16 antibody (Cintec, Kirkland, WA; 1:1 dilution).
Antigen retrieval was performed by steaming the sections in citrate buffer (pH 6.0) for 20 min. After incubation with the primary antibodies at room temperature for 2 hrs, a positive reaction in tissue sections was detected by the EnVision+System (DAKO, Carpinteria, CA) and developed with 3,3′-diaminobenzidine. The percentage of intensely immunoreactive nuclei were determined for p53, Ki-67, p16 and cyclin E while a three-tier intensity score was used to compare the immunoreactivity of Rsf-1, FASN and mucin-4 in STICs to adjacent normal-appearing tubal epithelium. This grading system for Rsf-1, FASN and mucin-4 was employed because the normal fallopian tube epithelium expresses a low level of these markers. Accordingly, the final intensity score for these markers was derived by subtracting the intensity of the normal tubal epithelium from the initial intensity score of the STIC in the same specimen. In the process of cutting sections for immunohistochemistry, some of the STICs were exhausted and therefore not all of them could be evaluated for all of the markers.
Results
Thirty seven STICs from 23 patients were analyzed. All were flat lesions except for three that exhibited a papillary architecture (patient 7, patient 9 and STIC-1 in patient 23). Twenty two (59.4%) STICs were located in the fimbriated end of the tubes. Multiple STICs were detected in 8 patients. Twelve high-grade serous carcinomas co-existed with STICs in the same tissue sections. The morphologic features of the STICs and associated ovarian carcinomas were similar. The expression of all markers in STICs and high-grade serous carcinomas using immunohistochemistry is summarized in Table 1 and is discussed below.
Table 1.
Immunostaining results in all TICs and associated high-grade serous carcinomas.
| Patient | STIC | p53 (%) | Ki-67 (%) | p16 (%) | Rsf-1 | cyclin E (%) | FASN | Muc-4 |
|---|---|---|---|---|---|---|---|---|
| 1 | STIC-1 | 100 | 70 | 100 | 1 | 25 | 2 | na |
| 1 | STIC-2 | 100 | 70 | 100 | 1 | 85 | 3 | na |
| 1 | STIC-3 | 100 | 70 | 100 | 1 | 90 | 2 | na |
| 2 | STIC-1 | 90 | 10 | 50 | 2 | 90 | 0 | −2 |
| 2 | STIC-2 | 70 | 50 | 30 | 2 | 20 | 0 | −1 |
| 2 | STIC-3 | 100 | 50 | 90 | 2 | 90 | 1 | 0 |
| 2 | STIC-4 | 100 | 20 | 20 | 2 | 0 | 2 | na |
| 2 | STIC-5 | 90 | 3 | 0 | 2 | 0 | 2 | na |
| 2 | STIC-6 | 60 | 20 | 10 | 2 | 0 | 2 | na |
| 2 | CA | 100 | 60 | 10 | 2 | 10 | 2 | na |
| 3 | STIC-1 | 100* | 8 | 0 | na | 25 | 1 | 0 |
| 3 | STIC-2 | 100* | 5 | 10 | 1 | 40 | 2 | 0 |
| 3 | CA | 100* | 90 | 100 | 1 | 100 | 2 | na |
| 4 | STIC-1 | 100* | 5 | 50 | 0 | 30 | 1 | 0 |
| 4 | STIC-2 | 100* | 20 | 40 | 0 | 85 | 1 | 0 |
| 5 | STIC-1 | 70 | 8 | 0 | 0 | 0 | 0 | na |
| 5 | CA | 100 | 40 | 100 | 0 | 60 | 2 | na |
| 6 | STIC-1 | 0 | 50 | 0 | 1 | 30 | 0 | −1 |
| 6 | CA | 0 | 40 | 0 | 1 | 20 | 0 | −2 |
| 7 | STIC-1 | 100 | 30 | 0 | 2 | 100 | 2 | −2 |
| 8 | STIC-1 | 80 | 30 | 5 | 2 | 80 | 2 | 0 |
| 9 | STIC-1 | 0 | 10 | 50 | 1 | 100 | 2 | −1 |
| 10 | STIC-1 | 90 | 40 | 100 | 2 | 100 | 0 | −3 |
| 11 | STIC-1 | 100 | 70 | 90 | 2 | 100 | 2 | −2 |
| 11 | CA | 100 | 70 | 100 | 2 | 100 | 2 | −2 |
| 12 | STIC-1 | 0 | 10 | 20 | 1 | 0 | 0 | −2 |
| 12 | CA | 0 | 6 | 5 | 1 | 100 | 1 | −2 |
| 13 | STIC-1 | 100 | 80 | na | 0 | 70 | 1 | 0 |
| 13 | STIC-1 | 100 | 80 | na | 0 | 70 | 1 | 0 |
| 13 | CA | 100 | 50 | na | 0 | na | 2 | 0 |
| 14 | STIC-1 | 100 | 90 | 90 | 0 | 35 | 2 | na |
| 15 | STIC-1 | 90 | 2 | 0 | 0 | 0 | 0 | 0 |
| 16 | STIC-1 | 100 | 60 | 50 | 0 | 70 | 0 | na |
| 16 | STIC-2 | 90 | 40 | 70 | 1 | 0 | 1 | 0 |
| 16 | CA | 100 | 80 | 30 | 1 | 20 | 2 | 1 |
| 17 | STIC-1 | 0 | 80 | 10 | 0 | 0 | 1 | −1 |
| 17 | CA | 0 | 85 | 25 | 0 | na | na | −1 |
| 18 | STIC-1 | 100 | 7 | na | 0 | 90 | 1 | −1 |
| 18 | CA | 100 | 20 | na | 0 | 95 | 1 | 0 |
| 19 | STIC-1 | 100 | 30 | 100 | 0 | 0 | 0 | 0 |
| 19 | STIC-2 | 100 | 80 | 100 | 0 | 40 | 0 | 1 |
| 19 | STIC-3 | 0 | 10 | 20 | 0 | 0 | 0 | −1 |
| 20 | STIC-1 | 100 | 2 | 10 | 1 | 100 | 0 | 0 |
| 20 | CA | 100 | 80 | 100 | 3 | 80 | 2 | 0 |
| 21 | STIC-1 | 90 | 20 | 0 | na | 0 | 0 | 0 |
| 21 | CA | 0 | 50 | 0 | 3 | 80 | 2 | 0 |
| 22 | STIC-1 | na | 50 | na | 2 | 75 | 1 | 0 |
| 23 | STIC-1 | 100 | 45 | 100 | 1 | na | 0 | −1 |
| 23 | STIC-2 | 100 | 75 | 100 | 1 | na | 1 | 0 |
The percentage of intensely immunoreactive nuclei were determined for p53, Ki-67, p16 and cyclin E while a three-tier intensity score was used to compare the immunoreactivity of Rsf-1, FASN and mucin-4 in STICs to adjacent normal-appearing tubal epithelium. The final intensity score was the intensity score of the lesion minus the intensity score of normal-appearing tubal epithelium in the same specimens. na: not analyzed;
faint nuclear staining. Faint staining was interpreted as negative.
p53
Diffuse nuclear p53 (≥50% of nuclei) immunoreactivity was observed in 27 (75%) of 36 STICs (Fig 1) and defined as intense immunoreactivity. In contrast, the normal-appearing tubal epithelium adjacent to the STICs did not demonstrate detectable nuclear p53 immunoreactivity except in the immediate adjacent tubal epithelium, which showed patchy, less intense p53 nuclear staining (Fig. 1). Five STICs were completely negative for p53. Thus, a total of 32 (89%) of 36 STICs showed either intense p53 (n= 27) or negative p53 (n= 5) immunoreactivity. Both of these patterns are considered surrogate markers for TP53 mutations because misense mutations are almost always associated with intense nuclear stain whereas nonsense mutations encode truncated proteins that are not recognized by the p53 antibody. In cases with multiple STICs, the p53 immunostaining pattern was identical in all STICs except in one patient (patient 19) in whom one of the three STICs was completely negative for p53 whereas the other two were intensely positive, suggesting different TP53 mutations.
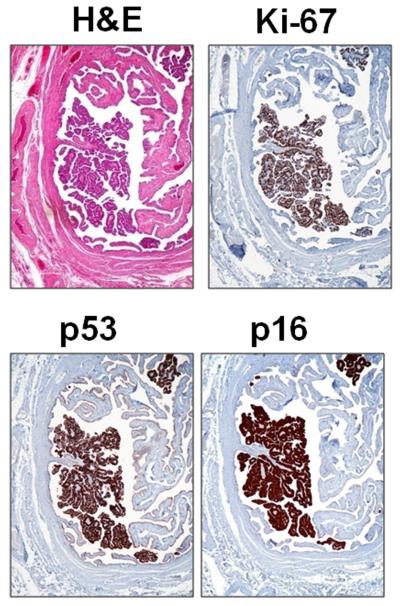
Fig. 1.
An example of a serous tubal intraepithelial carcinoma (STIC) (from patient 9). Hematoxylin and eosin (H&E) stain shows a discrete papillary growth of STIC without evidence of stromal invasion. The epithelial cells of the STIC are intensely positive for p53 and p16. The Ki-67 labeling index is high (>70%).
p16
p16 staining was variable in the STICs. Diffuse nuclear p16 immunoreactivity was observed in 18 (55%) of 33 STICs (Fig. 1 and Fig. 2). p16 nuclear immunoreactivity was not detected in normal-appearing tubal epithelium.
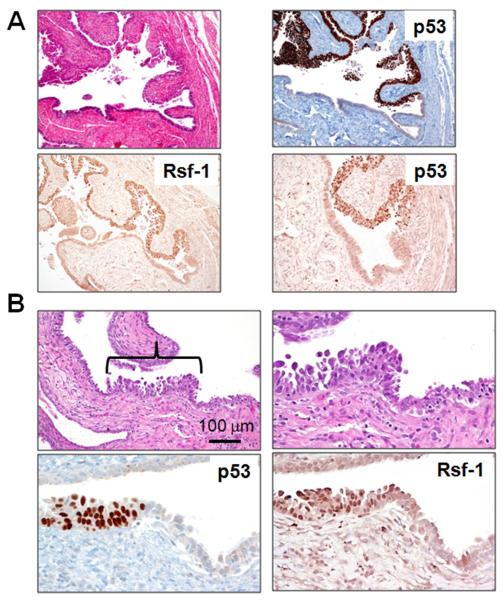
Fig. 2.
Rsf-1 upregulation in two representative STICs (Panels A and B). Panel A shows a relatively large STIC (patient 7) and the panel B shows a small STIC (patient 23, STIC-1). Rsf-1 immunoreactivity is more intense in the epithelial cells within both STICs than in the adjacent normal-appearing tubal epithelium and parallels p53 expression.
Ki-67
The percentage of Ki-67 positive cells was generally low and varied in different regions of normal-appearing tubal epithelium. In order to determine a cutoff value for the Ki-67 labeling index (percentage of Ki-67 positive cells) that corresponded to the level of proliferation in normal fallopian tubes, we stained 15 normal fallopian tubes which were removed for benign reasons. We found that the mean plus one standard deviation of the Ki-67 labeling index in normal fallopian tubes was 2.5% ± 0.8%. Therefore, we used an arbitrary cut off of 10% to define an elevated Ki-67 labeling index in this study. Using this cutoff, an elevated Ki-67 labeling index (≥10%) was observed in 29 (78%) of 37 STICs. All the markers were expressed in STICs with very low Ki-67 proliferation indices (<10%). In STICs with a higher Ki-67 index (≥ 10%) the number expressing either diffuse nuclear p53 or demonstrating complete absence of immunoreactivity for p53 was significantly increased (p= 0.028, Fisher's exact test) (Table 2). As previously noted, the total STICs that were available for immunostaining varied since some small STICs were completely depleted during sectioning and, therefore, were not available for immunohistochemical analysis. Of particular interest were the immunostaining patterns in 8 cases in which the Ki-67 proliferation index in the STICs was very low (<10%) (Table 2). The p53 staining pattern was consistent with a p53 mutation (≥ 50% positive cells or completely negative) in 63% of STICs and Rsf-1 was expressed in 43% and both cyclin E and FASN in 63%.
Table 2.
Increased Marker Expression in STICs Correlated with Ki-67 Proliferation Index.
| Ki-67 Index |
No. of Cases |
p53 | p16 | Rsf-1 | Cyclin E | FASN |
|---|---|---|---|---|---|---|
| <10% | 8 | 5/8 (63%) | 3/7(43%) | 3/7 (43%) | 5/8 (63%) | 5/8 (63%) |
| 10-49% | 14 | 13/14 (93%) | 6/14 (43%) | 10/13 (77%) | 6/13 (46%) | 7/14 (50%) |
| ≥ 50% | 15 | 14/14 (100%) | 9/12 (75%) | 9/15 (60%) | 13/14 (93%) | 11/15 (73%) |
| total | 37 | 32/36 (89%) | 18/33 (55%) | 22/35 (63%) | 24/35 (77%) | 23/37 (62%) |
For p53 a case was considered positive if the intensity score was 0 or ≥50% (intense nuclear) since both of these patterns are associated with TP53 mutations. Increased expression for p16 was defined as ≥ 50% of positive cells, for cyclin E ≥ 10% of positive cells, for Rsf-1 ≥ 1 (final score) and for FASN ≥ 1 (final score). The final score for Rsf-1 and FASN was determined by subtracting the intensity of the stain in normal adjacent tubal epithelium from the initial intensity score in the STIC.
Rsf-1
Normal tubal epithelium expresses Rsf-1 at a low level. Increased Rsf-1 immunoreactivity compared to that expressed in normal-appearing tubal epithelium occurred in 22 (63%) of 35 STICs, (Fig. 2). Fifteen (75%) of 20 STICs with elevated Rsf-1 expression were also positive for cyclin E. Similarly, 15 (68%) of 22 STICs with elevated Rsf-1 expression showed increased immunoreactivity of FASN.
FASN
Normal tubal epithelium (secretory cells) expresses FASN but FASN was increased in 23 (62%) of 37 STICs compared to adjacent normal tubal epithelium (Fig 3).
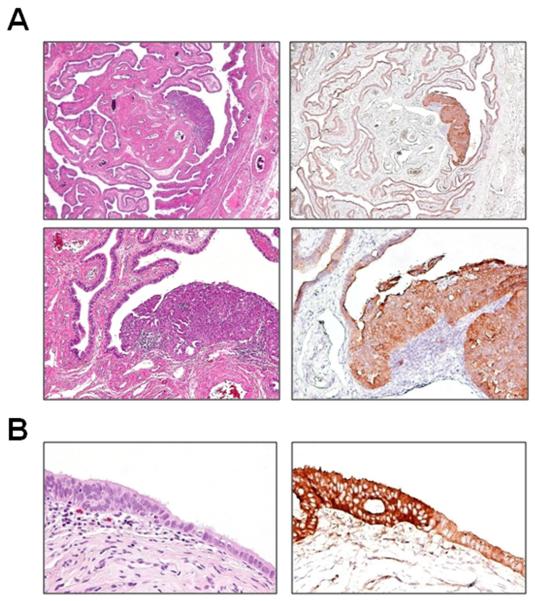
Fig. 3.
Overexpression of FASN in STICs. A: a large STIC (patient 9). B: a small STIC (patient 1, STIC-2). For both cases, FASN immunoreactivity in the STIC (3+) is significantly higher than that in the normal-appearing tubal epithelium (1+).
Cyclin E
Cyclin E nuclear staining was seen in 24 (77%) of 35 STICs while normal-appearing tubal epithelial cells were all negative.
Mucin-4
As compared to normal-appearing tubal epithelium, only one of 28 STICs demonstrated increased mucin-4 immunoreactivity while 12 (43%) and 15 (54%) of 28 STICs showed decreased or no change in staining, respectively. In order to further address the expression pattern of mucin-4 in fallopian tubes and ovarian surface epithelium from individuals without carcinoma, we performed mucin-4 immunohistochemistry on 8 pairs of normal fallopian tubes and ovaries. Mucin-4 immunoreactivity was focally positive in tubal epithelium but not in ovarian surface epithelium (data not shown).
Comparison of Immunohistochemistry of Multiple STICs with or without associated ovarian serous carcinomas
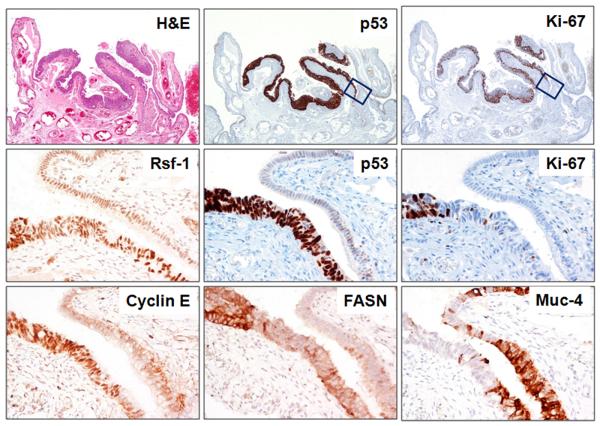
Of the 12 invasive carcinomas 4 did not express increased Rsf-1 immunoreactivity, 2 did not express p16 and one of these two also failed to express increased FASN. Aside from these, all informative invasive carcinomas expressed all of the markers. In the 12 cases in which there were concomitant invasive carcinomas with STICs the marker pattern was concordant in 5 and discordant in 7 (Table 3). Among these discordant cases one (patient 21) contained a STIC in which the p53 was positive in 90% of the tumor cells and an associated carcinoma in which the p53 was completely negative suggesting that the STIC and the carcinoma had different TP53 mutations. In addition, discordant patterns were observed in 2 cases (patients 19 and 23) with multiple STICs but in which there was not an associated carcinoma. In one of these cases (patient 19) with three separate STICs, p53 was expressed in 100% of cells in 2 STICs and in none of the cells in the third STIC suggesting that the last STIC had a different mutation from the other two. In another case of multiple STICs without an associated carcinoma (patient 23) one STIC expressed increased FASN expression and the other did not. Cyclin E and FASN were more frequently expressed in high-grade serous carcinomas than in STICs. One case (in patient 11) was of particular interest. A stretch of epithelium measuring approximately 1 mm located at the margin of a STIC showed cytologic atypia that was not as marked as in the STIC. It was diffusely p53 positive but the Ki-67 proliferation index was not elevated (Fig. 5). This lesion may represent a potential precursor of a STIC, where nuclear atypia and probably a TP53 mutation occurred before an aberration in cell cycle progression. We have tentatively designated this lesion as “serous tubal intraepithelial lesion (STIL)”. Rsf-1 and cyclin E proteins were overexpressed in this lesion whereas FASN upregulation was largely confined to the STIC. Mucin-4 immunoreactivity demonstrated a reverse pattern in which mucin-4 immunoreactivity was decreased in the STIC compared to the STIL and the normal-appearing tubal epithelium.
Table 3.
Discordant expression patterns in cases with multiple STICs and/or an associated carcinoma.
| Patient | lesions | p53 | p16 | Rsf-1 | cyclin E | FASN |
|---|---|---|---|---|---|---|
| 2 | STIC-1 | |||||
| STIC-2 | ||||||
| STIC-3 | ||||||
| STIC-4 | ||||||
| STIC-5 | ||||||
| STIC-6 | ||||||
| CA | ||||||
| 3 | STIC-1 | |||||
| STIC-2 | ||||||
| CA | ||||||
| 5 | STIC-1 | |||||
| CA | ||||||
| 12 | STIC-1 | |||||
| CA | ||||||
| 16 | STIC-1 | |||||
| STIC-2 | ||||||
| CA | ||||||
| 20 | STIC-1 | |||||
| CA | ||||||
| 21 | STIC-1 | |||||
| CA |
Filled box: p53 = 0 or ≥50% (intense nuclear); p16 ≥ 50%; cyclin E ≥ 10%; Rsf-1 ≥ 1 (net score); FASN ≥ 1 (net score).
Fig. 5.
Immunoreactivity of p53, Ki-67, Rsf-1, cyclin E, FASN and mucin-4 in a STIC located in the fimbria (patient 11). Upper panel shows the STIC at low magnification. The epithelial cells at the right margin of the STIC show intense p53 expression but lack Ki-67 immunoreactivity (boxes). In the Middle and Lower panels the increased Rsf-1 and cyclin E immunoreactivity is confined to the same region (intense p53 expression but no Ki-67 immunoreactivity). In contrast, FASN is overexpressed only in the region where there is strong p53 expression and an elevated Ki-67 proliferation index. Mucin-4 immunoreactivity (lower panel) is largely absent in the STIC but positive in the normal tubal epithelium.
Discussion
The findings in this study indicate that in addition to TP53, several genes including Rsf-1, cyclin E, p16, and FASN that are amplified or upregulated in high-grade ovarian serous carcinoma are also overexpressed in the majority of STICs. In contrast, mucin-4 expression was either down regulated or showed no change in most STICs. Approximately 90% of STICs displayed intense or virtually no p53 immunoreactivity. Both of these immunostaining patterns are highly associated with TP53 mutations. A complete absence of p53 immunoreactivity has been shown in these lesions and in high-grade serous carcinoma to be associated with nonsense TP53 mutations that result in a truncated protein which is not recognized by the p53 antibody.
Rsf-1, also known as HBXAP, was upregulated in 63% of STICs. It encodes a nuclear protein 14 which is a subunit in a chromatin assembly factor, called ISWI-containing factor RSF. 15 Rsf-1 has been shown to function as a histone chaperone while its binding partner, hSNF2H, possesses nucleosome-dependent ATPase activity. 16 The Rsf-1/hSNF2H complex (RSF complex) mediates ATP-dependent chromatin remodeling by mobilizing nucleosomes, a process that is essential for transcriptional activation or repression,17 DNA replication 18 and cell cycle progression. 19 In ovarian cancer, Rsf-1 amplification has been detected in approximately 15% and Rsf-1 overexpression in the majority of high-grade serous carcinomas. 8, 12, 20 It appears that in these serous carcinomas cellular proliferation and survival depend on the expression of Rsf-1, especially in the presence of paclitaxel. 8, 21, 22 In addition, Rsf-1 amplification or upregulation is associated with shorter overall survival in patients with high-grade serous carcinoma patients as compared to tumors in which Rsf-1 is not amplified or upregulated. 8
Cyclin E was expressed in 77% of STICs. 20 (83%) of 24 STICs that expressed cyclin E showed either intense or no p53 staining. Cyclin E which is encoded by CCNE1 is a well established cell cycle protein that complexes with Cdk2 to mediate the transition from G1 to S phase by inactivating the retinoblastoma protein. Cyclin E expression is upregulated in a variety of neoplasms through amplification of the CCNE1 or through its transcriptional activation. Among amplicons in high-grade serous carcinomas, the CCNE1 locus is the most common, occurring in approximately 36% of specimens. 20 Cyclin E has been demonstrated to play a role in the pathogenesis of ovarian carcinoma 9, 23-25 and therefore the high proportion of STICs expressing cyclin E underscores the important role of cyclin E in the early events of high-grade serous carcinogenesis.
Fatty acid synthase was expressed in 62% of STICs. 19 (86%) of 22 STICs that overexpressed fatty acid synthase showed either intense or no p53 staining. Fatty acid synthase (FASN) encodes a cytoplasmic enzyme that is responsible for biochemical processes involving de novo fatty acid synthesis. 26 Since normal adult tissues contain abundant dietary lipids, only a minimal amount of FASN is expressed. In contrast, in a variety of neoplastic diseases including ovarian serous carcinomas 13, 26, 27 elevated FASN levels are detected. In mouse xenograft ovarian tumor models that overexpress FASN, inhibition of the enzyme activity led to a reduction in tumor volume.10 Although the mechanism by which FASN participates in tumor progression remains unclear, it has been proposed that de novo synthesis of long chain fatty acid is important to promote tumor growth and survival. In addition, FASN may act in concert with other signaling pathways including AMP-activated kinase, AKT and ErbB2 28-31 to mediate tumor initiation and progression.
In this study 89% of STICs demonstrated immunostaining patterns consistent with mutations of TP53 confirming that TP53 mutations are highly prevalent in STICs with a mutation frequency similar to high-grade serous carcinomas. Our data also demonstrate that these p53 staining patterns were substantially higher than the other markers examined in this study (Table 2). In other words, several STICs showing either intense or no p53 staining but did not show upregulation of p16, Rsf-1, cyclin E and FASN (Table 1). Moreover, analysis of eight STICs with very low proliferation indices (Ki-67 labeling <10%) showed that most of these lesions displayed p53 immunostaining patterns consistent with TP53 mutations, whereas 43% expressed increased Rsf-1 immunoreactivity. All of the associated carcinomas in this study expressed increased levels all of the markers including Rsf-1, cyclin E and FASN. These findings indicate that TP53 mutations probably represent a very early molecular genetic change in the development of high-grade serous carcinoma. It is likely that TP53 mutation, since it is a gatekeeper that regulates transformation, initiates a cascade of molecular changes that allow cells to survive in the presence of DNA damage and oncogenic activation without undergoing p53-dependent senescence or apoptosis.32
The newly proposed hypothesis of the tubal as opposed to the ovarian surface epithelial (OSE) origin of pelvic serous carcinomas challenges many of the previous reports demonstrating “overexpressed” ovarian cancer-associated genes in which the expression levels in the carcinomas were almost always compared to ovarian surface epithelium. Low-molecular-weight forms of cyclin E have been reported to be reliable markers for the distinction of ovarian carcinoma from benign and malignant mesothelial lesions.9 Accordingly, the high frequency of cyclin E expression in STICs and high-grade ovarian serous carcinoma casts further doubt on the OSE as the site of origin of these tumors. Furthermore, as the gene expression profiles in ovarian surface epithelium, which is of mesothelial origin, are different from the mullerian-derived fallopian tube epithelium it will be important to validate whether these previously reported overexpressed genes are indeed upregulated using fallopian tube epithelium rather than OSE as a control.33 For example, mucin-4 is regarded as a biomarker of ovarian cancer but in the present study we found that mucin-4 unlike Rsf-1, cyclin E and FASN, mucin-4 was not overexpressed in the majority of STICs as compared to the adjacent normal-appearing tubal epithelium. In fact, 50% of STICs either retained the same level of mucin-4 immunoreactivity as the adjacent normal-appearing tubal epithelium or exhibited decreased mucin-4 expression, suggesting that mucin-4 expression is tissue lineage-specific rather than tumor-specific. These findings argue against the role of mucin-4 in the pathogenesis of early high-grade serous carcinogenesis.
In conclusion, this report demonstrates that in addition to p53 several other oncogenic proteins associated with high-grade serous carcinomas are upregulated in STICs. In this study, 95% of STICs that overexpressed Rsf-1 showed either intense or no p53 staining, suggesting that TP53 mutations are required for Rsf-1 upregulation. The upregulation of Rsf-1 in STICs compared to the adjacent normal-appearing tubal epithelium suggests that excessive RSF (Rsf)-mediated chromatin remodeling occurs early in the development of high-grade serous carcinoma. Thus, it appears that overexpression of Rsf-1, cyclin E and FASN acts in concert with TP53 mutations to propel tumor development. We also found that STICs rarely demonstrated upregulation of mucin-4, which has been reported to be a tumor-associated protein in ovarian cancer, arguing against the role of mucin-4 in the pathogenesis of high-grade serous carcinoma. This finding underscores the importance of reassessing the validity of other previously reported overexpressed genes in ovarian high-grade cancer-associated proteins in which ovarian surface epithelium was used as a control. This has important clinical implications for the identification of candidate biomarkers for the development of screening tests and novel prevention strategies. Given the possibility that STICs that are detected in advanced cases of high-grade serous carcinoma may be different from those discovered incidentally, such as during risk reducing procedures in women with inherited BRCA1 or BRCA2 mutations, future studies should analyze the expression patterns of Rsf-1, cyclin E, FASN and mucin-4 in the STICs not associated with invasive carcinomas.
Fig. 4.
Expression of mucin-4 in STICs. Panel A shows that mucin-4 immunoreactivity is largely lost in a large STIC (patient 10). Panel B shows that the intensity of the immunostain in the small STIC (patient 13, STIC-1) is the same as in the adjacent tubal epithelium.
Acknowledgements
The authors appreciate the technical assistance of Mr. Joe Vasoontara for immunohistochemistry. This study was supported by NIH/NCI RO1CA129080 and DoDOC080469.
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- 1.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 2.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 3.Paley PJ, Swisher EM, Garcia RL, Agoff SN, Greer BE, Peters KL, Goff BA. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176–180. doi: 10.1006/gyno.2000.6071. [DOI] [PubMed] [Google Scholar]
- 4.Shaw PA, Rouzbahman M, Pizer ES, Pintilie M, Begley H. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;22:1133–1138. doi: 10.1038/modpathol.2009.89. [DOI] [PubMed] [Google Scholar]
- 5.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 6.Tone AA, Begley H, Sharma M, Murphy J, Rosen B, Brown TJ, Shaw PA. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin Cancer Res. 2008;14:4067–4078. doi: 10.1158/1078-0432.CCR-07-4959. [DOI] [PubMed] [Google Scholar]
- 7.Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L, Fishman D, Berchuck A, Whitaker R, Gershenson DM, Mills GB, Bast RC, Jr., Lu KH. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–6126. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 8.Shih Ie M, Sheu JJ, Santillan A, Nakayama K, Yen MJ, Bristow RE, Vang R, Parmigiani G, Kurman RJ, Trope CG, Davidson B, Wang TL. Amplification of a chromatin remodeling gene, Rsf-1/HBXAP, in ovarian carcinoma. Proc Natl Acad Sci U S A. 2005;102:14004–14009. doi: 10.1073/pnas.0504195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson B, Skrede M, Silins I, Shih Ie M, Trope CG, Florenes VA. Low-molecular weight forms of cyclin E differentiate ovarian carcinoma from cells of mesothelial origin and are associated with poor survival in ovarian carcinoma. Cancer. 2007;110:1264–1271. doi: 10.1002/cncr.22918. [DOI] [PubMed] [Google Scholar]
- 10.Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–1193. [PubMed] [Google Scholar]
- 11.Davidson B, Baekelandt M, Shih Ie M. MUC4 is upregulated in ovarian carcinoma effusions and differentiates carcinoma cells from mesothelial cells. Diagn Cytopathol. 2007;35:756–760. doi: 10.1002/dc.20771. [DOI] [PubMed] [Google Scholar]
- 12.Mao TL, Hsu CY, Yen MJ, Gilks B, Sheu JJ, Gabrielson E, Vang R, Cope L, Kurman RJ, Wang TL, Shih Ie M. Expression of Rsf-1, a chromatin-remodeling gene, in ovarian and breast carcinoma. Hum Pathol. 2006;37:1169–1175. doi: 10.1016/j.humpath.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Gansler TS, Hardman W, 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28:686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 14.Shamay M, Barak O, Shaul Y. HBXAP, a novel PHD-finger protein, possesses transcription repression activity. Genomics. 2002;79:523–529. doi: 10.1006/geno.2002.6717. [DOI] [PubMed] [Google Scholar]
- 15.Loyola A, Huang J-Y, LeRoy G, Hu S, Wang Y-H, Donnelly RJ, Lane WS, Lee S-C, Reinberg D. Functional Analysis of the Subunits of the Chromatin Assembly Factor RSF. Mol. Cell. Biol. 2003;23:6759–6768. doi: 10.1128/MCB.23.19.6759-6768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aihara T, Miyoshi Y, Koyama K, Suzuki M, Takahashi E, Monden M, Nakamura Y. Cloning and mapping of SMARCA5 encoding hSNF2H, a novel human homologue of Drosophila ISWI. Cytogenet Cell Genet. 1998;81:191–193. doi: 10.1159/000015027. [DOI] [PubMed] [Google Scholar]
- 17.Vignali M, Hassan AH, Neely KE, Workman JL. ATP-dependent chromatin-remodeling complexes. Mol Cell Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan JF, Peterson CL. A role for the yeast SWI/SNF complex in DNA replication. Nucleic Acids Res. 1999;27:2022–2028. doi: 10.1093/nar/27.9.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosma MP, Tanaka T, Nasmyth K. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama K, Nakayama N, Jinawath N, Salani R, Kurman RJ, Shih Ie M, Wang TL. Amplicon profiles in ovarian serous carcinomas. Int J Cancer. 2007;120:2613–2617. doi: 10.1002/ijc.22609. [DOI] [PubMed] [Google Scholar]
- 21.Sheu JJ, Choi JH, Yildiz I, Tsai FJ, Shaul Y, Wang TL, Shih IM. The Roles of Human Sucrose Nonfermenting Protein 2 Homologue in the Tumor-Promoting Functions of Rsf-1. Cancer Res. 2008;68:4050–4057. doi: 10.1158/0008-5472.CAN-07-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JH, Sheu JJ, Guan B, Jinawath N, Markowski P, Wang TL, Shih Ie M. Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer Res. 2009;69:1407–1415. doi: 10.1158/0008-5472.CAN-08-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courjal F, Louason G, Speiser P, Katsaros D, Zeillinger R, Theillet C. Cyclin gene amplification and overexpression in breast and ovarian cancers: evidence for the selection of cyclin D1 in breast and cyclin E in ovarian tumors. Int J Cancer. 1996;69:247–253. doi: 10.1002/(SICI)1097-0215(19960822)69:4<247::AID-IJC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Farley J, Smith LM, Darcy KM, Sobel E, O'Connor D, Henderson B, Morrison LE, Birrer MJ. Cyclin E expression is a significant predictor of survival in advanced, suboptimally debulked ovarian epithelial cancers: a Gynecologic Oncology Group study. Cancer Res. 2003;63:1235–1241. [PubMed] [Google Scholar]
- 25.Marone M, Scambia G, Giannitelli C, Ferrandina G, Masciullo V, Bellacosa A, Benedetti-Panici P, Mancuso S. Analysis of cyclin E and CDK2 in ovarian cancer: gene amplification and RNA overexpression. Int J Cancer. 1998;75:34–39. doi: 10.1002/(sici)1097-0215(19980105)75:1<34::aid-ijc6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 27.Alo PL, Visca P, Framarino ML, Botti C, Monaco S, Sebastiani V, Serpieri DE, Di Tondo U. Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncol Rep. 2000;7:1383–1388. doi: 10.3892/or.7.6.1383. [DOI] [PubMed] [Google Scholar]
- 28.Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, Lupu R. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci U S A. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HQ, Altomare DA, Skele KL, Poulikakos PI, Kuhajda FP, Di Cristofano A, Testa JR. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24:3574–3582. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 31.Grunt TW, Wagner R, Grusch M, Berger W, Singer CF, Marian B, Zielinski CC, Lupu R. Interaction between fatty acid synthase- and ErbB-systems in ovarian cancer cells. Biochem Biophys Res Commun. 2009;385:454–459. doi: 10.1016/j.bbrc.2009.05.085. [DOI] [PubMed] [Google Scholar]
- 32.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–1197. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]