Abstract
Quercetin, a member of the flavonoid family, is one of the most prominent dietary antioxidants. This study investigates the mechanisms for the effects of quercetin on cultured human RPE cells and in Ccl2/Cx3cr1 double knock-out (DKO) mice, which spontaneously develop progressive retinal lesions mimicking age-related macular degeneration (AMD). In the in vitro experiment, cultured ARPE-19 cells were exposed to 1mM H2O2 with or without 50μM quercetin for 2 hours. Cellular viability, mitochondrial function, and apoptosis were assessed using crystal violet staining, MTT assay, and comet assay, respectively. Apoptotic molecular transcripts of BCL-2, BAX, FADD, CASPASE-3 and CASPASE-9 were measured by RQ-PCR. COX activity and nitric oxide (NO) level were determined in the supernatant of the culture medium. Quercetin treatment protected ARPE-19 cells from H2O2-induced oxidative injury, enhanced BCL-2 transcript levels, increased the BCL-2/BAX ratio, suppressed the transcription of pro-apoptotic factors such as BAX, FADD, CASPASE-3 and CASPASE-9, inhibited the transcription of inflammatory factors such as TNF-α, COX-2 and INOS, and decreased the levels of COX and NO in the culture medium. In the in vivo experiment, DKO and C57/B6 mice were treated with 25mg/kg/day quercetin by intraperitoneal injection daily for two months. Funduscopy was performed monthly. After two months, serum was collected to measure NADP+/NADPH, COX, PGE-2, and NO levels. The eyes were harvested for histology and A2E measurement. Ocular transcripts of Bcl-2, Bax, Cox-2, Inos, Tnf-α, Fas, FasL and Caspase-3 were detected by RQ-PCR. Quercetin treatment did not reverse the progression of retinal lesions in DKO mice funduscopically or histologically. Although quercetin treatment could recover systemic anti-oxidative capacity, suppress the systemic expression of NO, COX and PGE-2, and decrease ocular A2E levels, it could not effectively suppress the transcripts of the ocular inflammatory factors Tnf-α, Cox-2 and Inos, or the pro-apoptotic factors Fas, FasL and Caspase-3 in DKO mice. Our data demonstrate that quercetin can protect human RPE cells from oxidative stress in vitro via inhibition of pro-inflammatory molecules and direct inhibition of the intrinsic apoptosis pathway. However, quercetin (25mg/kg/day) does not improve the retinal AMD-like lesions in the Ccl2−/−/Cx3cr1−/− mice, likely due to its insufficient suppression of the inflammatory and apoptosis pathways in the eye.
Keywords: Quercetin, RPE, age-related macular degeneration, oxidative stress, inflammation, apoptosis, AMD mouse model
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible central vision loss in elderly people worldwide (Friedman et al., 2004). AMD accounts for more than 54% of all vision loss in Caucasians age 55 years and older in the United States (Congdon et al., 2004). Owing to the rapidly growing elderly population, the number of persons with AMD is expected to increase by 50% to 2.95 million in the US in 2020 (Friedman et al., 2004).
Clinically and histologically, the hallmarks of AMD are drusen and degeneration of photoreceptor and retinal pigment epithelial (RPE) cells (Coleman et al., 2008; Ding et al., 2009a). The current pathophysiologic concept of AMD assigns a primary role to age-related, cumulative oxidative damage in the RPE due to an imbalance between the generation and the elimination of reactive oxygen species (ROS) (Winkler et al., 1999; Beatty et al., 2000; Dunaief et al., 2002). This is a major cause of RPE damage in AMD (Dunaief et al., 2002; King et al., 2004). RPE cells are also susceptible to damage by ROS (Cai et al., 2000). Accordingly, oxidative stress may promote AMD pathogenesis by interfering with RPE function, decreasing RPE junctional integrity, enhancing RPE expression of pro-inflammatory and pro-angiogenic cytokines, and/or promoting RPE apoptosis (Higgins et al., 2003; Bailey et al., 2004; Binder et al., 2007).
Apoptosis occurs through two broad pathways: the intrinsic pathway (also known as the mitochondrial pathway) and the extrinsic pathway (also known as the death receptor pathway) (Lorenzo & Susin, 2007). Caspases are key players in the apoptotic pathway, especially the effector caspases, caspase-3 and caspase-9 (Oliver & Vallette, 2005; Iannolo et al., 2008). One of the important elements in the mitochondrial apoptosis pathway is the Bcl-2 (B-cell leukemia/lymphoma 2) family (Antonsson & Martinou, 2000; Guo et al., 2001). The Bcl-2 family controls mitochondrial outer membrane permeability and can either enhance or inhibit apoptosis. The levels of anti-apoptotic proteins such as Bcl-2 and the expression of pro-apoptotic factors such as Bax are associated with the survival of cells under stress (Eberle et al., 2007; Zhang et al., 2007; Wu et al., 2008; Kernt et al., 2009). The extrinsic pathway is initiated by the binding of transmembrane death receptors (Fas, TNF receptor, and TRAIL receptor) to their respective ligands (FasL, TNF, and TRAIL), with the aid of the adaptor molecule FADD, to activate membrane-proximal caspases (Mollinedo & Gajate, 2006).
Quercetin is the most studied member of the flavonoid family and is also thought to be one of the most prominent dietary antioxidants. Previous studies have shown that quercetin can decrease the risk of asthma, lung and colorectal cancer, and pulmonary and cardiovascular diseases, and that it can also protect against aging. These beneficial effects are thought to be due to the ability of quercetin to scavenge highly reactive species (Boots et al., 2008). Quercetin has been shown to be an excellent in vitro antioxidant. Within the flavonoid family, quercetin is the most potent scavenger of ROS, including O2•−, and reactive nitrogen species (RNS), such as NO• and ONOO− (Hanasaki et al., 1994; van Acker et al., 1995; Heijnen et al., 2001; Cushnie & Lamb, 2005). Quercetin is also known to exhibit strong anti-inflammatory capacities (Read, 1995; Orsolic et al., 2004). Several in vitro studies using different cell lines have shown that the flavonoid is capable of inhibiting LPS-induced cytokine production. For instance, quercetin inhibits LPS-induced TNF-α production in macrophages (Manjeet & Ghosh, 1999) and LPS-induced IL-8 production in lung cells (Geraets et al., 2007). In glial cells, it was shown that quercetin could inhibit LPS-induced transcription of TNF-α and IL-1α (Bureau et al., 2008). In a microglial-neuronal co-culture, the effects of this flavonoid resulted in diminished apoptotic neuronal cell death induced by microglial activation (Bureau et al., 2008). Previous studies have also shown that quercetin can protect RPE cells from oxidative damage in vitro (Hanneken et al., 2006; Kook et al., 2008). The major purpose of the present study is to investigate the anti-inflammatory effects of quercetin on cultured RPE monolayer cells subjected to oxidative stress and in the retinas of Ccl2−/−/Cx3cr1−/− DKO mice, which show oxidative stress- and inflammation-related retinal degeneration (Tuo et al., 2007; Ross et al., 2008).
2. Materials and Methods
The study was conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animal experiments were performed under the protocols approved by the National Eye Institute Institutional Animal Care and Use Committee.
2.1. Cell cultures
Human RPE cells (ARPE-19) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured as described previously (Ding et al., 2009b). This cell line is not transformed and has structural and functional properties characteristic of RPE cells in vivo.
2.2. Animals
The development of Ccl2−/−/Cx3cr1−/− DKO mice has been described previously (Tuo et al., 2007; Chan et al., 2008). These DKO mice are characterized by a broad spectrum of AMD pathology with early onset and high penetrance. Funduscopy findings include drusen-like retinal lesions, histology findings include photoreceptor degeneration, and biochemistry reveals increased A2E lipofuscin accumulation and altered mRNA and protein levels for endoplasmic reticulum proteins (Tuo et al., 2007; Chan et al., 2008).
2.3. Drugs and chemicals
To induce oxidative stress in this study, 1mM hydrogen peroxide (H2O2) conditioned media was freshly made by dissolving 30% H2O2 (Fisher Scientific, Fair lawn, NJ) in growth media just before use. Quercetin is hydrophobic, and DMSO is the most commonly used hydrotrope to facilitate the dissolution of quercetin in water. It is not easy or barely possible to use other solvents, for example ethanol. Moreover, DMSO can trap free radical hydroxide and is often used as a free radical scavenger (Beilke et al., 1987). Thus, DMSO has been used as a common control or vehicle in these types of in vivo and in vitro studies. A 100 mM stock solution of quercetin (Sigma, St Louis, MO, USA) was prepared in DMSO (Mediatech, Inc, Herndon, VA, USA), and it was used in growth media at a final concentration of 50μM. The control group was treated with DMSO in growth media at the same final concentration (0.05% v/v). Before stimulation with H2O2/quercetin, the cells were serum starved for 24 hours. All analyses were carried out 4 hours after H2O2 exposure, except for the measurement of COX enzymatic activity, which was carried out 24 hours after H2O2 exposure.
DKO and WT mice were randomly divided into quercetin-treated and vehicle control groups. As the oral bioavailability of quercetin is very low in mice, at approximately 0.98% (Meyers et al., 2008), intraperitoneal injection was used. Starting at an age of 3 to 5 weeks, the quercetin-treated group was injected intraperitoneally with quercetin in 40% v/v DMSO/PBS (25mg/kg/day) for two months. Equivalent volumes of 40% v/v DMSO/PBS were delivered intraperitoneally to the vehicle control group. Funduscopy was performed monthly. After 2 months of treatment, the mice were sacrificed. Serum from each mouse was collected. The eyes were harvested for histology, RNA isolation, and A2E measurement.
2.4. Cell viability by crystal violet assay
The assessment of mitochondrial damage was performed using crystal violet staining to determine the proportion of living ARPE-19 cells in the control, quercetin treated, H2O2 treated, and H2O2 + quercetin treated groups. After 2 hours of treatment, 24-well cultures were washed three times with phosphate buffered saline (PBS) and stained with crystal violet solution (0.4% crystal violet and 20% ethanol in water) for 20 minutes at room temperature. They were then washed three times with distilled water and air dried before photos were taken under light microscopy. The cultures were then incubated with extraction buffer (10% methanol and 10% acetic acid in water) for 15 minutes at room temperature. A 100 μl volume of the resultant dye mixture was transferred to a 96-well plate. The proportion of viable cells was determined by measuring the optical density (OD) of each sample at 590nm using a plate reader (Synergy 2, Biotek Instruments, Winooski, VT). Three wells were exposed to each treatment, and the mean optical density for each group of cultures was compared.
2.5. Measurement of mitochondrial damage by MTT assay
The assessment of mitochondrial damage was performed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, which has been described previously (Cao et al., 2007), to determine mitochondrial function in control, quercetin treated, H2O2 treated, and H2O2 + quercetin treated ARPE-19 cells.
2.6. Cellular apoptosis by comet assay
Apoptotic cells were detected using the Comet Assay Kit (Trevigen, Gaithersburg, MD), performed under alkaline conditions according to the manufacturer’s instructions.
2.7. Fundus Photography
Funduscopic examinations were performed every 4 weeks for two months using a Kowa fundus camera (Kowa Optimed, Torrance, CA) and Volk 90D lens (Volk Optical, Mentor, OH), as previously described(Tuo et al., 2009).
2.8. Histopathology
After 2 months of treatment, the mice were euthanized, and the eyes were harvested for histopathology, as previously described (Tuo et al., 2009). An ophthalmic pathologist (CCC) reviewed the pupillary-optic nerve sections in a mass fashion.
2.9. Quantitative RT-PCR (RQ-PCR)
Total RNA was extracted from ARPE-19 cells, retinal tissue or eyes from Ccl2−/−/Cx3cr1−/− or wild type (WT) mice (RNeasy Mini Kit; Qiagen, Hilden, Germany) and was treated with DNase I (Qiagen, Hilden, Germany). Five μg RNA from each sample was used for cDNA synthesis (Superscript II RNase H Reverse Transcriptase; Invitrogen, Grand Island, NY, USA) and RQ-PCR (Stratagene Mx3000 Real-Time PCR System and Brilliant SYBR Green QPCR Master Mix; Stratagene, La Jolla, CA, USA). The specific primers used were forward (F): 5′-CCATTTGGTGTTCGGAGTTTA-3′ and reverse (R):5′-TTCGCAGAAGTCCTGTGATGT-3′ for human BCL-2; F: 5′-CCAGGGTGGTTGGGTGAGACT-3′ and R: 5′-TGGGAGGTCAGCAGGGTAGAT-3′ for human BAX; F: 5′-GAACTGGACTGTGGCATTGAG-3′ and R: 5′-CAAAGCGACTGGATGAACCA-3′ for human CASPASE-3; F: 5′-CAGTAACCCCGAGCCAGAT-3′ and R: 5′-GAAACAGCATTAGCGACCCT-3′ for human CASPASE-9; and F: 5′-GGGAAGAAGACCTGTGTGC-3′ and R: 5′-CTGTTCTGGAGGTCACGC-3′ for human FADD. Primers for human COX-2, INOS, TNF-α and murine Bcl-2, Bax, Cox-2, Inos, Tnf-α, Fas, FasL, Caspase-3 and universal total RNA were purchased from SABiosciences Corporation (SABiosciences Corporation, Frederick, MD). Real-time quantitative PCR was performed using SYBR Green qPCR Master Mix according to the manufacturer’s protocol (SABiosciences Corporation, Frederick, MD) using a Stratagene Mx3000P QPCR System (SABioscience Corporation, Frederick, MD). Real-time PCR amplification of β-actin, with primers F: 5′-TCCCCCAACTTGAGATGTATGAAG-3′ and R: 5′-AACTGGTCTCAAGTCAGTGTACAGG-3′, served as an internal control.
2.10. Measurement of COX enzymatic activity and nitric oxide (NO) level in the supernatant of the culture medium of RPE cells, and serum COX enzymatic activity, PGE-2, nitric oxide and NADP+/NADPH concentration for DKO mice
COX enzymatic activity was measured using the COX Fluorescence Activity Assay Kit (Cayman Chemical Company, Ann Arbor, MI), according to the manufacturer’s instructions. PGE-2 concentration was measured using the Prostaglandin E2 EIA Kit (Cayman Chemical Company, Ann Arbor, MI), according to the manufacturer’s instructions. NO concentration was measured using the modified Griess colorimetric reaction, which has been previously described (Ding et al., 1988; Ding et al., 2009b). Serum NADP+/NADPH was measured using the NADP+/NADPH assay kit (ECNP-100) (BioAssay System, Hayward, CA), which is based on a glucose dehydrogenase cycling reaction (Zhao et al., 1987; Ding et al., 2009b).
2.11. 2-[2,6-dimethyl-8-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1E,3E,5E,7E-octatetra-enyl]_1-(2-hydroxyethyl)_4-[4-methyl-6(2,6,6-trimethyl-1-cyclohexen-1-yl) 1E,3E,5E,7Ehexatrienyl]-pyridinium (A2E) Extraction and Quantification
A2E is the major component of the lipofuscin fluorophores generated from the visual cycle flux of all-trans-retinal. The molecule is particularly relevant to aging and AMD pathogenesis (Ben-Shabat et al., 2001). The mice were kept in the dark for >12 hours, and the eyes were removed in a dark room under dim red light after being sacrificed. One eye from each mouse was homogenized for A2E extraction with chloroform/methanol as previously described (Karan et al., 2005). The amount of A2E was measured using HPLC (Agilent 1100 LC system, Agilent, Wilmington, DE) and UV absorption at 435 nm. A volume of 15 μl of resuspended A2E was injected into a reverse-phase C18 Nucleosil column (4.6 mm 25cm, 5 μM, Beckman USA) and eluted with a gradient of 50% to 80% methanol in 0.1% TPA at a flow-rate of 0.5 ml/min. Quantification of A2E was performed using a pure A2E standard curve.
2.12. Statistical analysis
Multiple comparisons within experimental groups were made using one-way analysis of variance (ANOVA), and comparisons between two groups were made using independent group t-tests with a significance level of p<0.05 using SPSS Version 17.0 (SPSS Inc., IL, U.S). The results are presented as mean±S.E.
3. Results
In vitro experiments
3.1. H2O2 caused ARPE-19 cell apoptosis, which was inhibited by quercetin
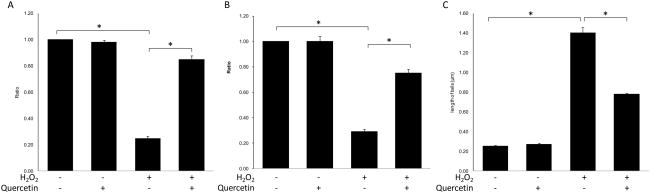
Compared to control cells, ARPE-19 cells treated with H2O2 exhibited not only significant decreases in cell viability (p=0.001) (Fig 1A) and mitochondrial function (p=0.001) (Fig 1B), but they also showed a significant increase in cellular apoptosis (p=0.001) (Fig 1C). Quercetin treatment rescued cell viability and mitochondrial function in ARPE-19 cells under oxidative stress induced by H2O2 (p=0.001 and p=0.001, respectively) (Fig 1A&B). Moreover, quercetin also significantly reduced cellular apoptosis (p=0.001) (Fig 1C).
Figure 1. Quercetin treatment could protect APRE-19 cells from the damage induced by H2O2.
RPE cells were treated with l.0 mM H2O2 with/without 50μM quercetin for 2 hrs and were then incubated with normal culture medium for 4hrs. (A) The cell viability was measured by crystal violet staining. (B) The mitochondrial function was measured by MTT assay. (C) The level of cellular apoptosis was measured by Comet assay. The severity of apoptosis was evaluated by the length of comet tails. Bars represent the mean ± S.E. of three independent experiments and indicate fold change relative to control cells. (*: p < 0.05)
3.2. H2O2 enhanced the intrinsic apoptosis pathway, but it had no significant effect on the extrinsic apoptosis pathway in APRE-19 cells, and quercetin inhibited both apoptosis pathways in APRE-19 cells under H2O2-induced oxidative stress
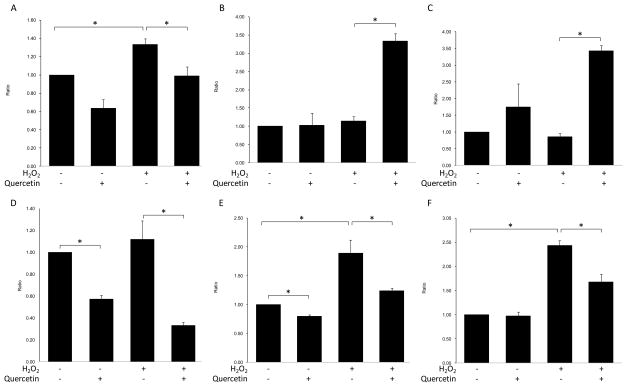
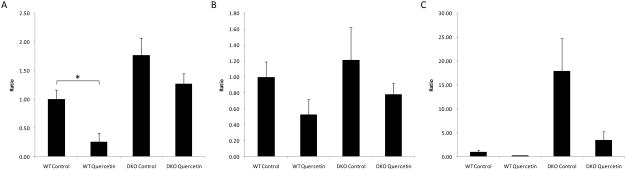
Compared to control cells, ARPE-19 cells treated with H2O2 exhibited enhanced transcription of BAX, a pro-apoptotic factor in the intrinsic apoptosis pathway (p=0.012) (Fig 2A), and an increase in the effecter caspases CASPASE-3 and CASPASE-9 (p=0.008 and p=0.005, respectively) (Fig 2E&F), but they did not exhibit significantly enhanced transcription of FADD, the key factor in the extrinsic apoptosis pathway (p=0.499) (Fig 2D). Quercetin treatment not only inhibited the increases in BAX, CASPASE-3 and CASPASE-9 (p=0.028, p=0.031 and p=0.016, respectively) (Fig 2A), but it also enhanced the transcription of BCL-2, an anti-apoptotic factor in the intrinsic apoptosis pathway (p=0.001) (Fig 2B). This resulted in an increase in the ratio of BCL-2/BAX transcription in APRE-19 cells under oxidative stress (p=0.001) (Fig 2C). Interestingly, quercetin treatment decreased the transcription of FADD in ARPE-19 cells with or without H2O2 induced oxidative stress (p=0.002 and p=0.001, respectively) (Fig 2D).
Figure 2. Quercetin treatment could inhibit the apoptotic pathway in ARPE-19 cells activated by H2O2.
RPE cells were treated with l.0 mM H2O2 with/without 50μM quercetin for 2 hrs and were then incubated with normal culture medium for 4hrs. Cells were then harvested, and total RNA was isolated and converted to cDNA. RQ-PCR analysis was then performed to determine the levels of targeted gene transcripts. (A) BAX; (B) BCL-2; (C) ratio of BCL-2/BAX; (D) FADD; (E) CASPASE-3 and (F) CASPASE-9. Bars represent the mean ± S.E. of three independent experiments and indicate fold change relative to control cells. (*: p < 0.05)
3.3. H2O2 induced the expression of inflammatory mediators in APRE-19 cells, and quercetin inhibited their expression in APRE-19 cells under H2O2-induced oxidative stress
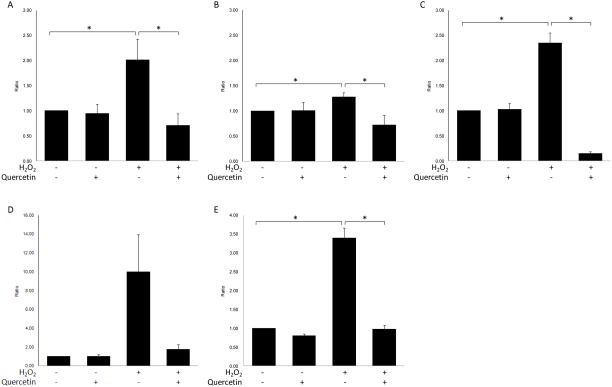
Compared to control cells, ARPE-19 cells treated with H2O2 showed slightly enhanced transcription of TNF-α (p=0.052) (Fig 3A), significantly enhanced transcription of COX-2 and INOS (p= 0.027 and p=0.002) (Fig 3B&C), slightly increased COX production (p=0.108) (Fig 3D) and significantly increased NO production (p=0.001) (Fig 3E) in the culture medium. Quercetin treatment inhibited the transcription of TNF-α, COX-2 and INOS (p= 0.018, p= 0.023 and p=0.001, respectively) (Fig 3A-C) and suppressed COX production slightly and NO production significantly in the culture medium of ARPE-19 cells under oxidative stress (p=0.129 and p=0.001, respectively) (Fig 3D&E).
Figure 3. Quercetin treatment could inhibit the increased expression of inflammatory factors in H2O2-treated ARPE-19 cells.
RPE cells were treated with l.0 mM H2O2 with/without 50μM quercetin for 2 hrs and were then incubated with normal culture medium for 24 hrs before measuring COX enzymatic activity or 4hrs before isolating RNA and measuring nitric oxide (NO) level. Cells were harvested and total RNA was isolated and converted to cDNA. RQ-PCR analysis was then performed to determine the levels of (A) TNF-α, (B) COX-2 and (C) INOS transcription. The enzymatic activity of COX (D) and the level of NO (E) in the supernatants of the culture medium were corrected for cell viability. Bars represent the mean ± S.E. of three independent experiments and indicate fold change relative to control cells. (*: p < 0.05)
In vivo experiments
3.4. Quercetin did not rescue the progression of retinal lesions in DKO mice clinically or histologically
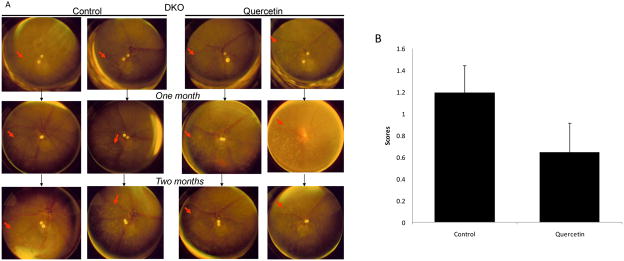
Funduscopically, there were no retinal changes after quercetin treatment in WT mice. The retinal lesions in quercetin treated DKO mice showed no significant differences compared with the control DKO group, in which retinal lesions continuously progressed (Fig 4A). No improvement was observed in the DKO mice that received quercetin daily for 2 months (p=0.146) (Fig 4B).
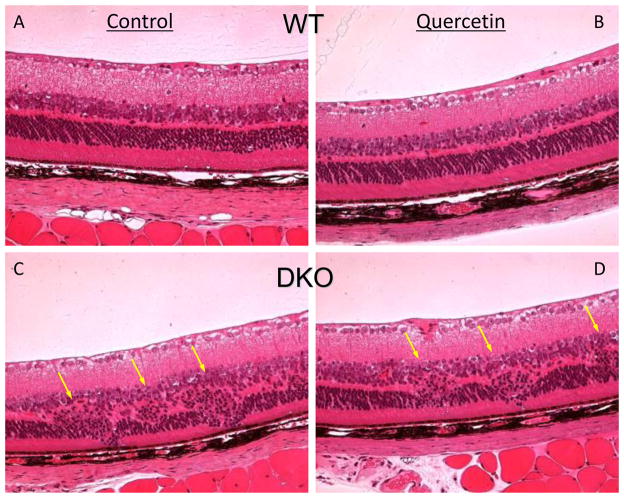
Figure 4. Quercetin treatment could not rescue the retinal lesions in DKO mice.
(A) Funduscopy for DKO mice. Quercetin in vehicle (40% v/v DMSO/PBS) was injected intraperitoneally (25mg/kg/day) for two months. Equivalent volumes of solvent were delivered intraperitoneally to the control vehicle groups. Funduscopy was performed monthly. The retinal lesions of the DKO mice in the control group progressed quickly. The quercetin treated DKO mice (quercetin group) showed no significant differences (arrows: retinal lesions). (B) The evaluation of retinal lesion progression in DKO mice. Progression was defined as a >10% increase in the number of deep retinal and subretinal spots (lesions), a >50% increase in spot diameter in at least 1/3 of the spots, >5 fused spots, or the appearance of >2 chorioretinal scars relative to the previous observation. Regression was defined as a >10% decrease in the number of deep retinal and subretinal spots (lesions) or a >50% decrease in spot diameter in at least 1/3 of the spots. Bars represent the mean ± S.E. (n ≥ 19).
Histologically, the retinas of WT mice that received quercetin were normal (Fig 5A&B). The DKO mice in the control group and the quercetin treated group showed similar progressive retinal lesions characterized by focal degeneration in the outer retinal layers and RPE of the posterior pole (Fig 5C and D). Quercetin did not ameliorate retinal lesions in the DKO mice.
Figure 5. Quercetin treatment could not improve the retinal lesions in DKO mice histologically.
Quercetin in 40% v/v DMSO/PBS was injected intraperitoneally (25mg/kg/day) for two months (B, Quercetin treated WT mice; D, Quercetin treated DKO mice). Equivalent volumes of 40% v/v DMSO/PBS were delivered intraperitoneally to the control vehicle groups (A, WT mice; C, DKO mice). Retinal lesions could be seen in the control and quercetin treated DKO mice (C&D, arrows). (n ≥ 19).
3.5. Quercetin partly inhibited the increased systemic oxidative stress and inflammatory responses in DKO mice
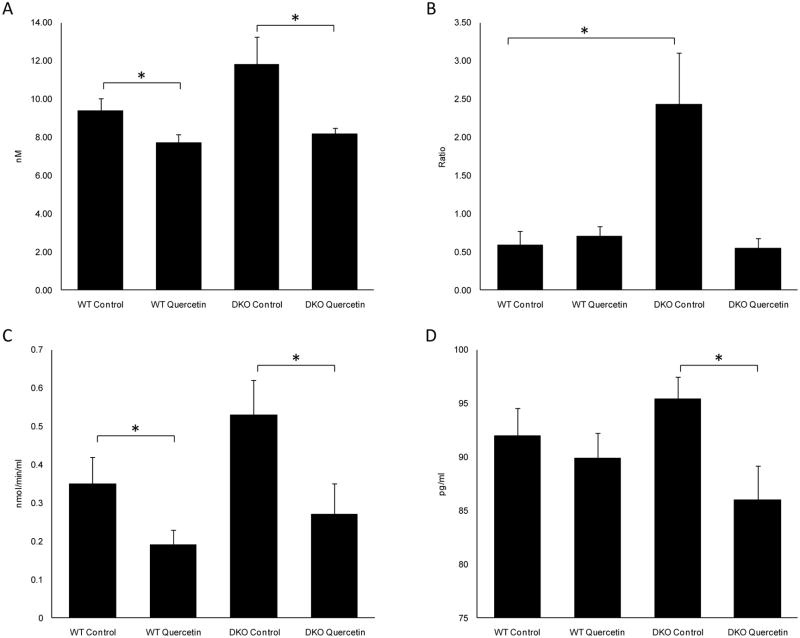
Serum NO levels were 11.81±1.46 nM in DKO mice and 9.36±0.65 nM in WT mice (p=0.118) (Fig 6A). The ratio of NADP+/NADPH in the serum was 2.43±0.68 in DKO mice and 0.58±0.19 in WT mice (p=0.03) (Fig 6B). NAD(P)H oxidase-derived oxidative stress is a critical biomarker for oxidative stress. These results indicated that DKO mice had increased levels of oxidative stress as reported previously (Ding et al., 2009b). Moreover, serum COX enzymatic activity for DKO mice was 0.53±0.09 nmol/min/ml, compared to 0.35±0.07 nmol/min/ml for WT mice (p=0.128) (Fig 6C). This may imply enhanced systemic inflammatory responses in DKO mice. The serum PGE-2 level was 95.41±2.02 pg/ml in DKO mice, which was not statistically different from 91.93±2.63 pg/ml measured in WT mice (p=0.336) (Fig 6D). The DKO mice treated with quercetin showed not only decreased serum NO levels (8.17±0.32nM, p=0.047) and slightly decreased NADP+/NADPH ratios (0.55±0.13, p=0.10), but also significantly decreased COX enzymatic activity (0.27±0.08 nmol/min/ml, p=0.043) and PGE-2 levels (85.98±3.19pg/ml, p=0.028) (Fig 6).
Figure 6. Quercetin treatment inhibited the increased systemic oxidative stress and inflammatory reaction in DKO mice.
Quercetin in the vehicle (40% v/v DMSO/PBS) was injected intraperitoneally (25mg/kg/day) for two months. Equivalent volumes of solvent were delivered intraperitoneally to the control vehicle groups. After two months of treatment, the mice were sacrificed, and the serum was taken to measure nitric oxide (NO) level (A), NADP+/NADPH ratio (B), COX enzymatic activity (C) and PGE-2 level (D). Bars represent the mean ± S.E. (n ≥ 7) (*: p < 0.05)
3.6. Quercetin did not effectively inhibit the increased expression of ocular pro-apoptotic molecules in DKO mice
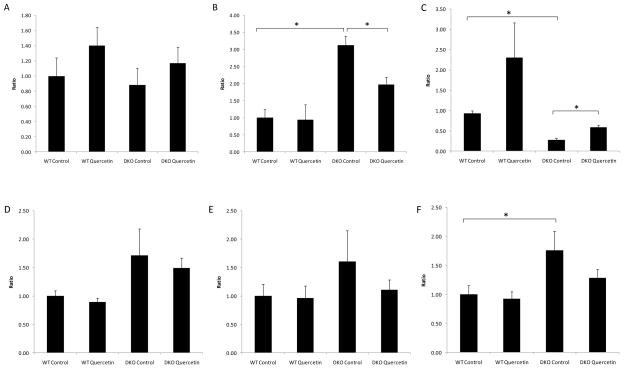
Compared with WT mice, DKO mice showed similar levels of ocular transcription of Bcl-2 (p=0.737) (Fig 7A), significantly higher levels of Bax (p=0.001) (Fig 7B) and Caspase-3 (p=0.045) (Fig 7F), lower ratios of Bcl-2/Bax (p=0.001) (Fig 7C), and slightly increased Fas and FasL levels (p=0.187 and p=0.335, respectively) (Fig 7D&E). Quercetin treatment had no effect on the ocular transcription levels of Bcl-2 (p=0.394) (Fig 7A), but it inhibited the increase in Bax transcription (p=0.017) (Fig 7B) and increased the ratio of Bcl-2/Bax transcription (p=0.009) (Fig 7C). Quercetin treatment did not inhibit the transcription of Fas, Fasl or Caspase-3 (p=0.669, p=0.414 and p=0.184, respectively) (Fig 7D–F).
Figure 7. Quercetin treatment could not effectively suppress the increased ocular transcription of pro-apoptotic factors in DKO mice.
Quercetin in the vehicle (40% v/v DMSO/PBS) was injected intraperitoneally (25mg/kg/day) for two months. Equivalent volumes of solvent were delivered intraperitoneally to the control vehicle groups. After two months of treatment, the mice were sacrificed, and the eyes were enucleated and homogenized. Total RNA was isolated and converted to cDNA. RQ-PCR analysis was then performed to determine the levels of Bcl-2 (A), Bax (B), Bcl-2/Bax ratio (C), Fas (D), FasL (E) and Caspase-3 (F). Bars represent the mean ± S.E. (n ≥ 3) (*: p < 0.05)
3.7. Quercetin did not inhibit the increased ocular transcription of pro-inflammatory mediators in DKO mice
Compared with WT mice, DKO mice showed slightly increased levels of ocular transcription of Cox-2, Inos and Tnf-α (p=0.057, p=0.653 and p=0.066, respectively) (Fig 8A–C). Quercetin treatment did not effectively inhibit the increases in the levels of transcription for these factors (p=0.175, p=0.363 and p=0.099, respectively) (Fig 8A–C).
Figure 8. Quercetin treatment could not inhibit the increased ocular transcription levels of pro-inflammatory factors in DKO mice.
Quercetin in 40% v/v DMSO/PBS was injected intraperitoneally (25mg/kg/day) for two months. Equivalent volumes of 40% v/v DMSO/PBS were delivered intraperitoneally to the control vehicle groups. After two months of treatment, the mice were sacrificed, and the eyes were enucleated and homogenized. Total RNA was isolated and converted to cDNA. RQ-PCR analysis was then performed to determine the levels of Cox-2 (A), Inos (B) and Tnf-α (C). Bars represent the mean ± S.E. (n ≥ 5) (*: p < 0.05)
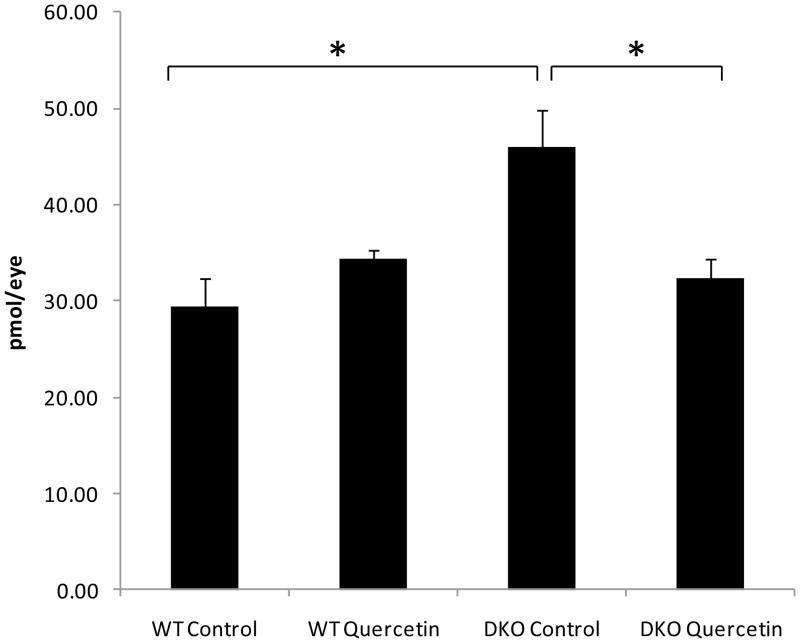
3.8. Quercetin lowered the ocular A2E levels of DKO mice
Compared with WT mice, DKO mice produced elevated ocular A2E levels (p=0.011). Quercetin treatment lowered these levels (p=0.016) (Fig 9).
Figure 9. Quercetin treatment could decrease the ocular A2E level in DKO mice.
Quercetin in 40% v/v DMSO/PBS was injected intraperitoneally (25mg/kg/day) for two months. Equivalent volumes of 40% v/v DMSO/PBS were delivered intraperitoneally to the control vehicle groups. After two months of treatment, the mice were sacrificed, and the eyes were enucleated and homogenized for A2E extraction with chloroform/methanol. Bars represent the mean ± S.E. (n ≥ 5) (*: p < 0.05)
4. Discussion
Severe oxidative stress is known to induce RPE cell apoptosis and is involved in the pathogenesis of AMD (Kasahara et al., 2005). The susceptibility of RPE cells to oxidative damage is well documented in the literature (Newsome et al., 1990; Esterbauer et al., 1991; Liles et al., 1991; Tate et al., 1993; Gaillard et al., 1995; Rozanowska et al., 1995; Snodderly, 1995; Srivastava et al., 1995; Tate et al., 1995; Akeo et al., 1996; Ueda & Armstrong, 1996; Kayatz et al., 1999; Wu & Rao, 1999; Beatty et al., 2001; Jin et al., 2001; Tyni et al., 2002). Being a major producer of ROS, mitochondria are subjected to direct attacks by ROS. Mitochondrial DNA is particularly prone to oxidative damage, more so than nuclear DNA (Salazar & Van Houten, 1997; Yakes & Van Houten, 1997; Ballinger et al., 1999; Ballinger et al., 2000; Mandavilli et al., 2000; Jin et al., 2001; Sawyer et al., 2001). As a result, H2O2 mainly damages the mitochondria and activates the intrinsic apoptosis pathway by increasing the transcription of BAX. Our results show that quercetin can effectively protect human RPE cells from oxidative stress by inhibiting cellular apoptosis that is induced by high doses of H2O2 in vitro. Several recent studies have shown that quercetin inhibits apoptosis induced by oxidative stress in neural cells, lens epithelial cells, and RPE cells in vitro (Cao et al., 2007; Chen et al., 2008; Kook et al., 2008; Ansari et al., 2009). Our in vitro experiments show that quercetin inhibits the intrinsic apoptotic pathway by increasing the ratio of BCL-2/BAX, thus preventing apoptosis and DNA damage in RPE cells under oxidative stress. Furthermore, quercetin also effectively protects mitochondrial function and recovers cell viability.
In addition to directly or indirectly damaging various bio-molecules, ROS are also involved in inflammation. We find that under oxidative stress, RPE cells express higher levels of TNF-α and COX-2 and produce higher levels of NO and COX. Quercetin is able to inhibit the release of these inflammatory mediators in human RPE cells. ROS can activate several transcription factors, including NF-κB and AP-1, which induce pro-inflammatory cytokine production (Schreck et al., 1991; MacNee, 2001; Rahman, 2002; Rahman et al., 2002). In vitro studies have shown that oxidative stress induces the release of inflammatory molecules such as IL-8 and NO in macrophages and lung cells (Antonicelli et al., 2002; Boots et al., 2008). Therefore, scavenging ROS can prevent damage from oxidative stress as well as alleviate inflammatory responses. Indeed, quercetin has been shown to inhibit LPS-induced TNF-α production in macrophages, IL-8 in lung cells, and TNF-α and IL-1α in glial cells. A possible mechanism is due to down-regulation of NF-κB activation by inhibiting the degradation of IκBα, the inhibitor of NF-κB (Peet & Li, 1999; Nair et al., 2006).
The Ccl2−/−/Cx3cr1−/− mouse, a murine model of AMD, produces elevated markers of systemic oxidative stress and inflammation, such as higher levels of serum NADP+/NADPH, COX enzymatic activity, PGE-2 and NO levels as compared to normal WT mice (Ding et al., 2009a; Tuo et al., 2009). These mice also express elevated levels of ocular inflammatory markers, such as Tnf-α and Inos (Tuo et al., 2009), as well as elevated ocular A2E lipofuscin (Tuo et al., 2007; Chan et al., 2008). Inflammation plays an important role in the pathogenesis of AMD, as evidenced by the identification of inflammatory cells in AMD lesions (Ding et al., 2009a) and inflammation-associated genetic variants that modulate AMD risk (Klein et al., 2008; Ding et al., 2009a). The natural environment of the eye is designed to downregulate inflammation, as suggested by the theory of the Downregulatory Immune Environment (DIE) (Nussenblatt & Ferris, 2007). Both oxidative stress and inflammatory processes that occur in the DKO eyes may break the DIE, resulting in increases in Bax and Caspase-3 and leading to apoptosis and retinal degeneration.
Quercetin decreased several serum oxidative and inflammatory molecules, including NADP+/NADPH, NO, COX and PGE-2 at least to a degree in the DKO mice, which is consistent with previous studies on the antioxidative effects of quercetin (Arts et al., 2004). However, quercetin does not repress the progression of retinal lesions in these mice, in spite of its ability to lower ocular A2E, the major autofluorescent component of lipofuscin, in the DKO mice. Quercetin could not effectively downregulate the increased transcripts of the ocular inflammatory mediators Tnf-α, Cox-2 and Inos. These findings imply that ocular inflammation may play an important role in the pathogenesis of AMD. Additionally, quercetin could not effectively suppress ocular transcripts of the pro-apoptotic factors Fas, FasL and Caspase-3. As a result, quercetin does not ameliorate or rescue retinal AMD-like lesions in the DKO mice.
Another explanation for the ineffectiveness of quercetin on the progression of retinal lesions in the DKO mice may be inadequate quercetin dosage and/or duration of administration. High doses of quercetin can cause toxicity (Cao et al., 2007; Kook et al., 2008) by inducing chromosomal damage or cytotoxicity in vitro (Yen et al., 2003; Saito et al., 2004; Cao et al., 2007). As the oral bioavailability of quercetin is very low in mice, at approximately 0.98% (Meyers et al., 2008), intraperitoneal injection was performed. Intraperitoneal injection of quercetin at a dose of 15mg/kg/day for 1 month has been shown to confer significant protection against streptozotocin-induced diabetes in rats (Kanter et al., 2007), 10–30mg/kg single intraperitoneal injection could inhibit pain-like behaviors in mice (Valerio et al., 2009), and 25mg/kg intraperitoneal injection twice a week for only 2 weeks showed significant antioxidant and anticancer effects in mice as well (Kamaraj et al., 2007). When compared with humans, for whom the daily intake of flavonols has been estimated at only 20–35 mg/day (Manach et al., 2005), of which quercetin represents 70% of the total (Hertog et al., 1993; de Vries et al., 1997), 25mg/kg/day is indeed a relatively high dosage for this experiment. Nevertheless, after two months of treatment, quercetin (25mg/kg/day) did not effectively modulate the retinal lesions of the DKO mice.
In summary, quercetin protects human RPE cells from oxidative stress in vitro via inhibition of pro-inflammatory molecules and direct inhibition of the intrinsic apoptosis pathway. However, quercetin (25mg/kg/day) cannot improve the retinal lesions in the Ccl2−/−/Cx3cr1−/− mice, most likely due to insufficient suppression of ocular inflammatory and apoptotic pathways.
Acknowledgments
The study was supported by the Intramural Research Program of the National Eye Institute, NIH.
Footnotes
Financial Disclosure(s)
The authors have no proprietary or commercial interest in any materials discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akeo K, Hiramitsu T, Kanda T, Yorifuji H, Okisaka S. Comparative effects of linoleic acid and linoleic acid hydroperoxide on growth and morphology of bovine retinal pigment epithelial cells in vitro. Curr Eye Res. 1996;15:467–476. doi: 10.3109/02713689609000758. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1–42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20:269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonicelli F, Parmentier M, Drost EM, Hirani N, Rahman I, Donaldson K, MacNee W. Nacystelyn inhibits oxidant-mediated interleukin-8 expression and NF-kappaB nuclear binding in alveolar epithelial cells. Free Radic Biol Med. 2002;32:492–502. doi: 10.1016/s0891-5849(01)00820-6. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- Arts MJTJ, Sebastiaan Dallinga J, Voss H-P, Haenen GRMM, Bast A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chemistry. 2004;88:567–570. [Google Scholar]
- Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765–772. doi: 10.1006/exer.1998.0661. [DOI] [PubMed] [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001;42:439–446. [PubMed] [Google Scholar]
- Beilke MA, Collins-Lech C, Sohnle PG. Effects of dimethyl sulfoxide on the oxidative function of human neutrophils. J Lab Clin Med. 1987;110:91–96. [PubMed] [Google Scholar]
- Ben-Shabat S, Parish CA, Hashimoto M, Liu J, Nakanishi K, Sparrow JR. Fluorescent pigments of the retinal pigment epithelium and age-related macular degeneration. Bioorg Med Chem Lett. 2001;11:1533–1540. doi: 10.1016/s0960-894x(01)00314-6. [DOI] [PubMed] [Google Scholar]
- Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Progress in retinal and eye research. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Bureau G, Longpre F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Progress in retinal and eye research. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Cao XG, Li XX, Bao YZ, Xing NZ, Chen Y. Responses of human lens epithelial cells to quercetin and DMSO. Invest Ophthalmol Vis Sci. 2007;48:3714–3718. doi: 10.1167/iovs.06-1304. [DOI] [PubMed] [Google Scholar]
- Chan CC, Ross RJ, Shen D, Ding X, Majumdar Z, Bojanowski CM, Zhou M, Salem N, Jr, Bonner R, Tuo J. Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic research. 2008;40:124–128. doi: 10.1159/000119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li XX, Xing NZ, Cao XG. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch Clin Exp Ophthalmol. 2008;246:373–378. doi: 10.1007/s00417-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries JH, Janssen PL, Hollman PC, van Staveren WA, Katan MB. Consumption of quercetin and kaempferol in free-living subjects eating a variety of diets. Cancer Lett. 1997;114:141–144. doi: 10.1016/s0304-3835(97)04645-4. [DOI] [PubMed] [Google Scholar]
- Ding A, Nathan C, Stuehr D. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;131:2407–2412. [PubMed] [Google Scholar]
- Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009a;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Patel M, Shen D, Herzlich AA, Cao X, Villasmil R, Klupsch K, Tuo J, Downward J, Chan CC. Enhanced HtrA2/Omi expression in oxidative injury to retinal pigment epithelial cells and murine models of neurodegeneration. Invest Ophthalmol Vis Sci. 2009b;50:4957–4966. doi: 10.1167/iovs.09-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- Eberle J, Kurbanov BM, Hossini AM, Trefzer U, Fecker LF. Overcoming apoptosis deficiency of melanoma-hope for new therapeutic approaches. Drug Resist Updat. 2007;10:218–234. doi: 10.1016/j.drup.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Gaillard ER, Atherton SJ, Eldred G, Dillon J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol. 1995;61:448–453. doi: 10.1111/j.1751-1097.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Geraets L, Moonen HJ, Brauers K, Wouters EF, Bast A, Hageman GJ. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr. 2007;137:2190–2195. doi: 10.1093/jn/137.10.2190. [DOI] [PubMed] [Google Scholar]
- Guo B, Godzik A, Reed JC. Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J Biol Chem. 2001;276:2780–2785. doi: 10.1074/jbc.M005889200. [DOI] [PubMed] [Google Scholar]
- Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci. 2006;47:3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- Heijnen CG, Haenen GR, van Acker FA, van der Vijgh WJ, Bast A. Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol In Vitro. 2001;15:3–6. doi: 10.1016/s0887-2333(00)00053-9. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- Higgins GT, Wang JH, Dockery P, Cleary PE, Redmond HP. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest Ophthalmol Vis Sci. 2003;44:1775–1782. doi: 10.1167/iovs.02-0742. [DOI] [PubMed] [Google Scholar]
- Iannolo G, Conticello C, Memeo L, De Maria R. Apoptosis in normal and cancer stem cells. Crit Rev Oncol Hematol. 2008;66:42–51. doi: 10.1016/j.critrevonc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Jin GF, Hurst JS, Godley BF. Rod outer segments mediate mitochondrial DNA damage and apoptosis in human retinal pigment epithelium. Curr Eye Res. 2001;23:11–19. doi: 10.1076/ceyr.23.1.11.5423. [DOI] [PubMed] [Google Scholar]
- Kamaraj S, Vinodhkumar R, Anandakumar P, Jagan S, Ramakrishnan G, Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo(a)pyrene. Biol Pharm Bull. 2007;30:2268–2273. doi: 10.1248/bpb.30.2268. [DOI] [PubMed] [Google Scholar]
- Kanter M, Altan MF, Donmez S, Ocakci A, Kartal ME. The effects of quercetin on bone minerals, biomechanical behavior, and structure in streptozotocin-induced diabetic rats. Cell Biochem Funct. 2007;25:747–752. doi: 10.1002/cbf.1397. [DOI] [PubMed] [Google Scholar]
- Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y, Thirumalaichary S, Li C, Birch DG, Vollmer-Snarr HR, Williams DS, Zhang K. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc Natl Acad Sci U S A. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara E, Lin LR, Ho YS, Reddy VN. SOD2 protects against oxidation-induced apoptosis in mouse retinal pigment epithelium: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46:3426–3434. doi: 10.1167/iovs.05-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayatz P, Heimann K, Schraermeyer U. Ultrastructural localization of light-induced lipid peroxides in the rat retina. Invest Ophthalmol Vis Sci. 1999;40:2314–2321. [PubMed] [Google Scholar]
- Kernt M, Neubauer AS, Liegl R, Eibl KH, Alge CS, Lackerbauer CA, Ulbig MW, Kampik A, Wu PC, Tai MH, Hu DN, Lai CH, Chen YH, Wu YC, Tsai CL, Shin SJ, Kuo HK. Cytoprotective effects of a blue light-filtering intraocular lens on human retinal pigment epithelium by reducing phototoxic effects on vascular endothelial growth factor-alpha, Bax, and Bcl-2 expression. Cyclin-dependent kinase inhibitor roscovitine induces cell cycle arrest and apoptosis in rabbit retinal pigment epithelial cells. J Cataract Refract Surg. 2009;35:354–362. doi: 10.1016/j.jcrs.2008.10.052. [DOI] [PubMed] [Google Scholar]
- King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem Photobiol. 2004;79:470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Klein BE, Wong TY, Cotch MF, Liu K, Cheng CY, Burke GL, Saad MF, Jacobs DR, Jr, Sharrett AR. Inflammation, complement factor h, and age-related macular degeneration: the Multi-ethnic Study of Atherosclerosis. Ophthalmology. 2008;115:1742–1749. doi: 10.1016/j.ophtha.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, Welge-Lussen UC. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008;49:1712–1720. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- Liles MR, Newsome DA, Oliver PD. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch Ophthalmol. 1991;109:1285–1288. doi: 10.1001/archopht.1991.01080090111033. [DOI] [PubMed] [Google Scholar]
- Lorenzo HK, Susin SA. Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist Updat. 2007;10:235–255. doi: 10.1016/j.drup.2007.11.001. [DOI] [PubMed] [Google Scholar]
- MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Mandavilli BS, Ali SF, Van Houten B. DNA damage in brain mitochondria caused by aging and MPTP treatment. Brain Res. 2000;885:45–52. doi: 10.1016/s0006-8993(00)02926-7. [DOI] [PubMed] [Google Scholar]
- Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol. 1999;21:435–443. doi: 10.1016/s0192-0561(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Meyers KJ, Rudolf JL, Mitchell AE. Influence of dietary quercetin on glutathione redox status in mice. J Agric Food Chem. 2008;56:830–836. doi: 10.1021/jf072358l. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C. Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy. Drug Resist Updat. 2006;9:51–73. doi: 10.1016/j.drup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol. 2006;13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome DA, Dobard EP, Liles MR, Oliver PD. Human retinal pigment epithelium contains two distinct species of superoxide dismutase. Invest Ophthalmol Vis Sci. 1990;31:2508–2513. [PubMed] [Google Scholar]
- Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver L, Vallette FM. The role of caspases in cell death and differentiation. Drug Resist Updat. 2005;8:163–170. doi: 10.1016/j.drup.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Orsolic N, Knezevic AH, Sver L, Terzic S, Basic I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol. 2004;94:307–315. doi: 10.1016/j.jep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Peet GW, Li J. IkappaB kinases alpha and beta show a random sequential kinetic mechanism and are inhibited by staurosporine and quercetin. J Biol Chem. 1999;274:32655–32661. doi: 10.1074/jbc.274.46.32655. [DOI] [PubMed] [Google Scholar]
- Rahman I. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem Pharmacol. 2002;64:935–942. doi: 10.1016/s0006-2952(02)01153-x. [DOI] [PubMed] [Google Scholar]
- Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem. 2002;234–235:239–248. [PubMed] [Google Scholar]
- Read MA. Flavonoids: naturally occurring anti-inflammatory agents. Am J Pathol. 1995;147:235–237. [PMC free article] [PubMed] [Google Scholar]
- Ross RJ, Zhou M, Shen D, Fariss RN, Ding X, Bojanowski CM, Tuo J, Chan CC. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp Eye Res. 2008;86:675–683. doi: 10.1016/j.exer.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270:18825–18830. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- Saito A, Sugisawa A, Umegaki K, Sunagawa H. Protective effects of quercetin and its metabolites on H2O2-induced chromosomal damage to WIL2-NS cells. Biosci Biotechnol Biochem. 2004;68:271–276. doi: 10.1271/bbb.68.271. [DOI] [PubMed] [Google Scholar]
- Salazar JJ, Van Houten B. Preferential mitochondrial DNA injury caused by glucose oxidase as a steady generator of hydrogen peroxide in human fibroblasts. Mutat Res. 1997;385:139–149. doi: 10.1016/s0921-8777(97)00047-5. [DOI] [PubMed] [Google Scholar]
- Sawyer DE, Roman SD, Aitken RJ. Relative susceptibilities of mitochondrial and nuclear DNA to damage induced by hydrogen peroxide in two mouse germ cell lines. Redox Rep. 2001;6:182–184. doi: 10.1179/135100001101536157. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. Embo J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, Ansari NH. Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem Biophys Res Commun. 1995;217:741–746. doi: 10.1006/bbrc.1995.2835. [DOI] [PubMed] [Google Scholar]
- Tate DJ, Jr, Miceli MV, Newsome DA. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36:1271–1279. [PubMed] [Google Scholar]
- Tate DJ, Jr, Newsome DA, Oliver PD. Metallothionein shows an age-related decrease in human macular retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34:2348–2351. [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Ross RJ, Herzlich AA, Shen D, Ding X, Zhou M, Coon SL, Hussein N, Salem N, Jr, Chan CC. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyni T, Johnson M, Eaton S, Pourfarzam M, Andrews R, Turnbull DM. Mitochondrial fatty acid beta-oxidation in the retinal pigment epithelium. Pediatr Res. 2002;52:595–600. doi: 10.1203/00006450-200210000-00021. [DOI] [PubMed] [Google Scholar]
- Ueda T, Armstrong D. Preventive effect of natural and synthetic antioxidants on lipid peroxidation in the mammalian eye. Ophthalmic Res. 1996;28:184–192. doi: 10.1159/000267901. [DOI] [PubMed] [Google Scholar]
- Valerio DA, Georgetti SR, Magro DA, Casagrande R, Cunha TM, Vicentini FT, Vieira SM, Fonseca MJ, Ferreira SH, Cunha FQ, Verri WA., Jr Quercetin reduces inflammatory pain: inhibition of oxidative stress and cytokine production. J Nat Prod. 2009;72:1975–1979. doi: 10.1021/np900259y. [DOI] [PubMed] [Google Scholar]
- van Acker SA, Tromp MN, Haenen GR, van der Vijgh WJ, Bast A. Flavonoids as scavengers of nitric oxide radical. Biochem Biophys Res Commun. 1995;214:755–759. doi: 10.1006/bbrc.1995.2350. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- Wu GS, Rao NA. Activation of NADPH oxidase by docosahexaenoic acid hydroperoxide and its inhibition by a novel retinal pigment epithelial protein. Invest Ophthalmol Vis Sci. 1999;40:831–839. [PubMed] [Google Scholar]
- Wu PC, Tai MH, Hu DN, Lai CH, Chen YH, Wu YC, Tsai CL, Shin SJ, Kuo HK. Cyclin-dependent kinase inhibitor roscovitine induces cell cycle arrest and apoptosis in rabbit retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2008;24:25–33. doi: 10.1089/jop.2007.0044. [DOI] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen GC, Duh PD, Tsai HL, Huang SL. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci Biotechnol Biochem. 2003;67:1215–1222. doi: 10.1271/bbb.67.1215. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Hu X, Ross C. Comparison of Tissue Preparation Methods for Assay of Nicotinamide Coenzymes. Plant Physiol. 1987;84:987–988. doi: 10.1104/pp.84.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]