Abstract
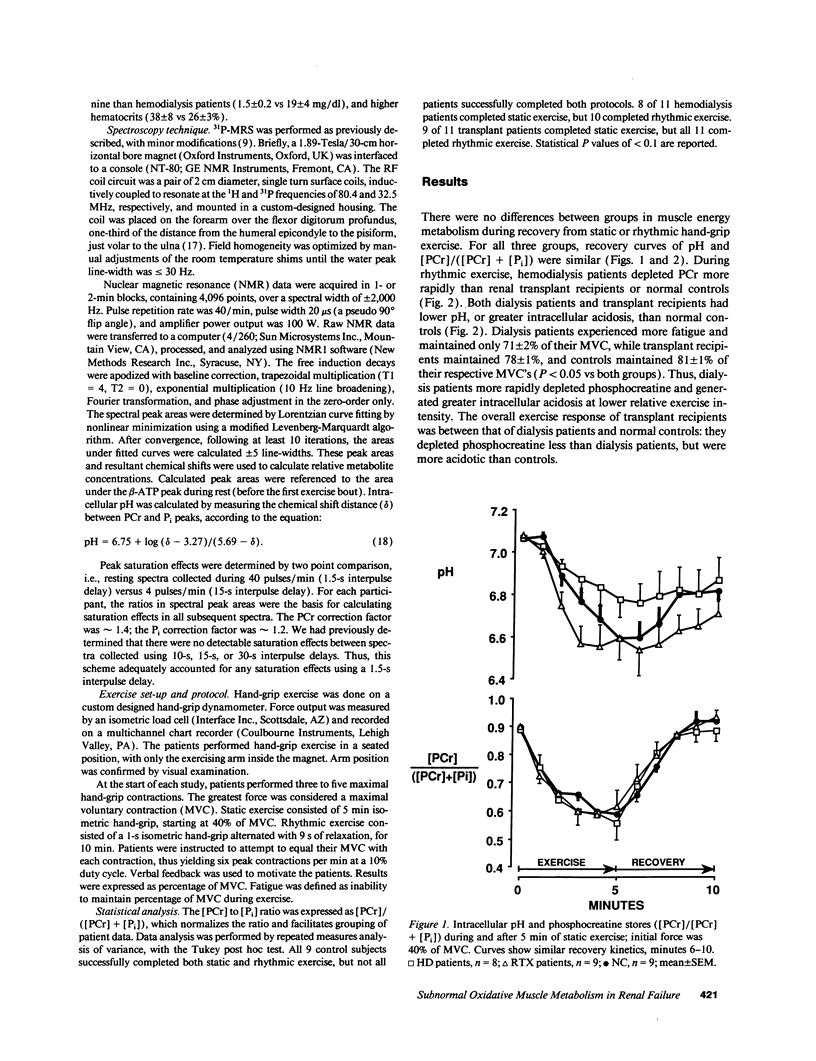
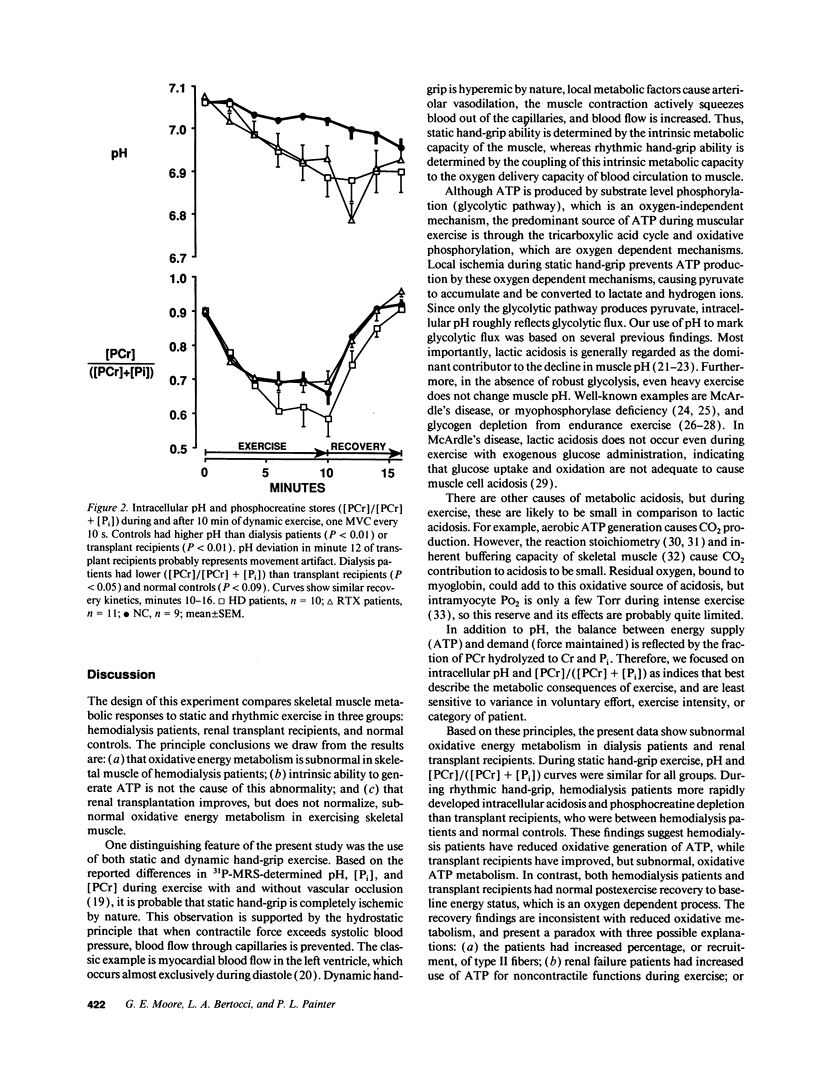
In hemodialysis patients, erythropoietin increases hemoglobin, but often the corresponding increase in peak oxygen uptake is low. The disproportionality may be caused by impaired energy metabolism. 31P-magnetic resonance spectroscopy was used to study muscle energy metabolism in 11 hemodialysis patients, 11 renal transplant recipients, and 9 controls. Measurements were obtained during rest, static hand-grip, and rhythmic hand-grip; recoveries were followed to baseline. During static hand-grip, there were no between-group differences in phosphocreatine (PCr), inorganic phosphate (Pi), or PCr/(PCr + Pi), although intracellular pH was higher in hemodialysis patients than transplant recipients. During rhythmic hand-grip, hemodialysis patients exhibited greater fatigue than transplant recipients or controls, and more reduction in PCr/(PCr + Pi) than transplant recipients. Intracellular pH was higher in controls than either hemodialysis patients or transplant recipients. Recoveries from both exercises were similar in all groups, indicating that subnormal oxidative metabolism was not caused by inability to make ATP. The rhythmic data suggest transplantation normalizes PCr/(PCr + Pi), but not pH. In hemodialysis patients, subnormal oxidative metabolism is apparently caused by limited exchange of metabolites between blood and muscle, rather than intrinsic oxidative defects in skeletal muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonen R. E. Light microscopic study of striated muscle in uremia. Acta Neuropathol. 1980;49(1):51–55. doi: 10.1007/BF00692219. [DOI] [PubMed] [Google Scholar]
- Ahonen R. E. Striated muscle ultrastructure in uremic patients and in renal transplant recipients. Acta Neuropathol. 1980;50(2):163–166. doi: 10.1007/BF00692869. [DOI] [PubMed] [Google Scholar]
- Argov Z., Bank W. J., Maris J., Leigh J. S., Jr, Chance B. Muscle energy metabolism in human phosphofructokinase deficiency as recorded by 31P nuclear magnetic resonance spectroscopy. Ann Neurol. 1987 Jul;22(1):46–51. doi: 10.1002/ana.410220112. [DOI] [PubMed] [Google Scholar]
- Arnold D. L., Taylor D. J., Radda G. K. Investigation of human mitochondrial myopathies by phosphorus magnetic resonance spectroscopy. Ann Neurol. 1985 Aug;18(2):189–196. doi: 10.1002/ana.410180205. [DOI] [PubMed] [Google Scholar]
- Bautista J., Gil-Necija E., Castilla J., Chinchon I., Rafel E. Dialysis myopathy. Report of 13 cases. Acta Neuropathol. 1983;61(1):71–75. doi: 10.1007/BF00688389. [DOI] [PubMed] [Google Scholar]
- Bertocci L. A., Haller R. G., Lewis S. F., Fleckenstein J. L., Nunnally R. L. Abnormal high-energy phosphate metabolism in human muscle phosphofructokinase deficiency. J Appl Physiol (1985) 1991 Mar;70(3):1201–1207. doi: 10.1152/jappl.1991.70.3.1201. [DOI] [PubMed] [Google Scholar]
- Davies C. T., Thompson M. W. Aerobic performance of female marathon and male ultramarathon athletes. Eur J Appl Physiol Occup Physiol. 1979 Aug;41(4):233–245. doi: 10.1007/BF00429740. [DOI] [PubMed] [Google Scholar]
- Diesel W., Noakes T. D., Swanepoel C., Lambert M. Isokinetic muscle strength predicts maximum exercise tolerance in renal patients on chronic hemodialysis. Am J Kidney Dis. 1990 Aug;16(2):109–114. doi: 10.1016/s0272-6386(12)80563-4. [DOI] [PubMed] [Google Scholar]
- Duboc D., Jehenson P., Tran Dinh S., Marsac C., Syrota A., Fardeau M. Phosphorus NMR spectroscopy study of muscular enzyme deficiencies involving glycogenolysis and glycolysis. Neurology. 1987 Apr;37(4):663–671. doi: 10.1212/wnl.37.4.663. [DOI] [PubMed] [Google Scholar]
- Fleckenstein J. L., Bertocci L. A., Nunnally R. L., Parkey R. W., Peshock R. M. 1989 ARRS Executive Council Award. Exercise-enhanced MR imaging of variations in forearm muscle anatomy and use: importance in MR spectroscopy. AJR Am J Roentgenol. 1989 Oct;153(4):693–698. doi: 10.2214/ajr.153.4.693. [DOI] [PubMed] [Google Scholar]
- Floyd M., Ayyar D. R., Barwick D. D., Hudgson P., Weightman D. Myopathy in chronic renal failure. Q J Med. 1974 Oct;43(172):509–524. [PubMed] [Google Scholar]
- HEDMAN R. The available glycogen in man and the connection between rate of oxygen intake and carbohydrate usage. Acta Physiol Scand. 1957 Oct 22;40(4):305–321. doi: 10.1111/j.1748-1716.1957.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Hands L. J., Bore P. J., Galloway G., Morris P. J., Radda G. K. Muscle metabolism in patients with peripheral vascular disease investigated by 31P nuclear magnetic resonance spectroscopy. Clin Sci (Lond) 1986 Sep;71(3):283–290. doi: 10.1042/cs0710283. [DOI] [PubMed] [Google Scholar]
- Hermansen L., Osnes J. B. Blood and muscle pH after maximal exercise in man. J Appl Physiol. 1972 Mar;32(3):304–308. doi: 10.1152/jappl.1972.32.3.304. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Honig C. R., Connett R. J., Gayeski T. E. O2 transport and its interaction with metabolism; a systems view of aerobic capacity. Med Sci Sports Exerc. 1992 Jan;24(1):47–53. [PubMed] [Google Scholar]
- LAWRIE R. A. The activity of the cytochrome system in muscle and its relation to myoglobin. Biochem J. 1953 Sep;55(2):298–305. doi: 10.1042/bj0550298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro R. P., Kirshner H. S. Proximal muscle weakness in uremia. Case reports and review of the literature. Arch Neurol. 1980 Sep;37(9):555–558. doi: 10.1001/archneur.1980.00500580051007. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G., Cook J. D., Nunnally R. L. Muscle fatigue in McArdle's disease studied by 31P-NMR: effect of glucose infusion. J Appl Physiol (1985) 1985 Dec;59(6):1991–1994. doi: 10.1152/jappl.1985.59.6.1991. [DOI] [PubMed] [Google Scholar]
- Lim V. S., DeGowin R. L., Zavala D., Kirchner P. T., Abels R., Perry P., Fangman J. Recombinant human erythropoietin treatment in pre-dialysis patients. A double-blind placebo-controlled trial. Ann Intern Med. 1989 Jan 15;110(2):108–114. doi: 10.7326/0003-4819-110-2-108. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Renaud J. M. The effect of acid-base balance on fatigue of skeletal muscle. Can J Physiol Pharmacol. 1985 May;63(5):403–416. doi: 10.1139/y85-072. [DOI] [PubMed] [Google Scholar]
- Marie P. Y., Escanye J. M., Brunotte F., Robin B., Walker P., Zannad F., Robert J., Gilgenkrantz J. M. Skeletal muscle metabolism in the leg during exercise in patients with congestive heart failure. Clin Sci (Lond) 1990 May;78(5):515–519. doi: 10.1042/cs0780515. [DOI] [PubMed] [Google Scholar]
- Massie B. M., Conway M., Rajagopalan B., Yonge R., Frostick S., Ledingham J., Sleight P., Radda G. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation. 1988 Aug;78(2):320–326. doi: 10.1161/01.cir.78.2.320. [DOI] [PubMed] [Google Scholar]
- Massie B., Conway M., Yonge R., Frostick S., Ledingham J., Sleight P., Radda G., Rajagopalan B. Skeletal muscle metabolism in patients with congestive heart failure: relation to clinical severity and blood flow. Circulation. 1987 Nov;76(5):1009–1019. doi: 10.1161/01.cir.76.5.1009. [DOI] [PubMed] [Google Scholar]
- Mayer G., Thum J., Cada E. M., Stummvoll H. K., Graf H. Working capacity is increased following recombinant human erythropoietin treatment. Kidney Int. 1988 Oct;34(4):525–528. doi: 10.1038/ki.1988.213. [DOI] [PubMed] [Google Scholar]
- Metcoff J., Lindeman R., Baxter D., Pederson J. Cell metabolism in uremia. Am J Clin Nutr. 1978 Sep;31(9):1627–1634. doi: 10.1093/ajcn/31.9.1627. [DOI] [PubMed] [Google Scholar]
- Minotti J. R., Johnson E. C., Hudson T. L., Zuroske G., Murata G., Fukushima E., Cagle T. G., Chick T. W., Massie B. M., Icenogle M. V. Skeletal muscle response to exercise training in congestive heart failure. J Clin Invest. 1990 Sep;86(3):751–758. doi: 10.1172/JCI114771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Painter P. L. Exercise in end-stage renal disease. Exerc Sport Sci Rev. 1988;16:305–339. [PubMed] [Google Scholar]
- Painter P., Messer-Rehak D., Hanson P., Zimmerman S. W., Glass N. R. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron. 1986;42(1):47–51. doi: 10.1159/000183632. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Holloszy J. O. Augmentation of skeletal muscle myoglobin by a program of treadmill running. Am J Physiol. 1967 Sep;213(3):783–785. doi: 10.1152/ajplegacy.1967.213.3.783. [DOI] [PubMed] [Google Scholar]
- Robertson H. T., Haley N. R., Guthrie M., Cardenas D., Eschbach J. W., Adamson J. W. Recombinant erythropoietin improves exercise capacity in anemic hemodialysis patients. Am J Kidney Dis. 1990 Apr;15(4):325–332. doi: 10.1016/s0272-6386(12)80079-5. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Radda G. K., Gadian D. G., Rocker G., Esiri M., Falconer-Smith J. Examination of a case of suspected McArdle's syndrome by 31P nuclear magnetic resonance. N Engl J Med. 1981 May 28;304(22):1338–1342. doi: 10.1056/NEJM198105283042206. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Hultman E. Creatine kinase equilibrium and lactate content compared with muscle pH in tissue samples obtained after isometric exercise. Biochem J. 1975 Nov;152(2):173–180. doi: 10.1042/bj1520173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Nylind B., Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976 Dec 28;367(2):143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Muscle carnitine metabolism during incremental dynamic exercise in humans. Acta Physiol Scand. 1990 Mar;138(3):259–262. doi: 10.1111/j.1748-1716.1990.tb08845.x. [DOI] [PubMed] [Google Scholar]
- Savica V., Bellinghieri G., Di Stefano C., Corvaja E., Consolo F., Corsi M., Maccari F., Spagnoli L. G., Villaschi S., Palmieri G. Plasma and muscle carnitine levels in haemodialysis patients with morphological-ultrastructural examination of muscle samples. Nephron. 1983;35(4):232–236. doi: 10.1159/000183087. [DOI] [PubMed] [Google Scholar]
- Siami G., Clinton M. E., Mrak R., Griffis J., Stone W. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron. 1991;57(3):306–313. doi: 10.1159/000186280. [DOI] [PubMed] [Google Scholar]
- Wagner P. D. Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc. 1992 Jan;24(1):54–58. [PubMed] [Google Scholar]


