Abstract
Reproductive aging in males is characterized by a diminution in sexual behavior beginning in middle age. We investigated the relationships among testosterone, androgen receptor (AR) and estrogen receptor alpha (ERα) cell numbers in the hypothalamus, and their relationship to sexual performance in male rats. Young (3 months) and middle-aged (12 months) rats were given sexual behavior tests, then castrated and implanted with vehicle or testosterone capsules. Rats were tested again for sexual behavior. Numbers of AR and ERα immunoreactive cells were counted in the anteroventral periventricular nucleus and the medial preoptic nucleus, and serum hormones were measured. Middle-aged intact rats had significant impairments of all sexual behavior measures compared to young males. After castration and testosterone implantation, sexual behaviors in middle-aged males were largely comparable to those in the young males. In the hypothalamus, AR cell density was significantly (5-fold) higher, and ERα cell density significantly (6-fold) lower, in testosterone- than vehicle-treated males, with no age differences. Thus, restoration of serum testosterone to comparable levels in young and middle-aged rats resulted in similar preoptic AR and ERα cell density concomitant with a reinstatement of most behaviors. These data suggest that age-related differences in sexual behavior cannot be due to absolute levels of testosterone, and further, the middle-aged brain retains the capacity to respond to exogenous testosterone with changes in hypothalamic AR and ERα expression. Our finding that testosterone replacement in aging males has profound effects on hypothalamic receptors and behavior has potential medical implications for the treatment of age-related hypogonadism in men.
Keywords: androgen receptor, estrogen receptor alpha, aging, male rat, anteroventral periventricular nucleus (AVPV), medial preoptic nucleus (MPN)
Introduction
In a variety of mammalian species including rats, monkeys and humans, there is an age-related decline in sexual performance that may be attributable to physiological or psychological changes (Chambers and Phoenix, 1983; Nicolosi et al., 2004; Smith et al., 1992). Much of the loss of male sexual function with aging has been correlated with a decline in serum testosterone. This change has been shown in humans (Harman et al., 2001), rhesus monkeys (Downs and Urbanski, 2006), and rats, including Sprague-Dawley (Roselli et al., 1986; Wu et al., 2009; Karpas et al., 1983), Wistar (Bernardi et al., 1998; Taylor et al., 1996), Brown Norway (Chen et al., 1994; Gruenewald et al., 2000), and Fischer 344 strains (Chambers et al., 1991; Luine et al., 2007).
However, not all studies show a clear relationship between testosterone, aging, and sexual behavior, a concept that is particularly important when considering the transitional life period of middle age. In fact, during this life stage, testosterone levels in rats may or may not differ from those in young animals depending up the absolute chronological age, strain, and/or other physiological, behavioral and experimental differences. Thus, whereas some studies have shown age-related declines in testosterone at middle age, others have not demonstrated such a loss (Gruenewald et al., 2000; Frankel and Mock, 1981; Wu and Gore, 2009). Furthermore, testosterone replacement to castrated aging rats does not effectively reinstate sexual performance as it does in young rats (Chambers and Phoenix, 1984; Chambers and Phoenix, 1986; Chambers et al., 1991; Sato et al., 1998), underscoring the point that absolute testosterone levels do not necessarily predict the capacity to perform sexual behavior, and that there are other changes to the aging brain that may explain this behavioral decline. Furthermore, much of this latter literature is primarily focused on old rats and information about effects of testosterone treatment to middle-aged rats is rather limited, raising the question of what hormonal and neurobiological changes may be occurring at this life stage.
We hypothesized that the loss of sexual behavior with age may not be due to levels of testosterone per se but rather to age-related changes in the actions of testosterone on its androgen receptor (AR) in brain regions involved in the control of masculine sexual behaviors and the hypothalamic-pituitary-gonadal axis (Vagell and McGinnis, 1998; Portillo et al., 2006). Circulating testosterone levels regulate expression of the AR, as in young males, castration can eliminate, and testosterone replacement can restore, AR expression in the brain (Iqbal et al., 1995; Lu et al., 1999; Lu et al., 1998; Wood and Newman, 1993). In comparing young and old male Fischer 344 rats (4 and 26 months, respectively), Chambers et al. showed that nuclear AR binding is very low in castrated rats, and elevated by testosterone treatment in both ages, albeit to a greater extent in the young group (Chambers et al., 1991). This suggests that the responsiveness of the aging brain to testosterone is diminished. We showed age-related increases in AR immunoreactive cells within several preoptic-hypothalamic regions between young and middle-aged rats (Wu and Gore, 2009; Wu et al., 2009). However, much more information is needed about the implications of this change at midlife.
It is also important to consider that some actions of testosterone on masculine behaviors and physiology are due to its aromatization to estradiol and subsequent actions on estrogen receptors (ER) (Clancy et al., 2000; Larsson et al., 1973; Morali et al., 1977; Vagell and McGinnis, 1997; Zumpe et al., 1993). Relatively little is known about the interactions among estradiol, aging, and behavior. Even the literature on serum estradiol is quite contradictory depending up rat strain and absolute age: levels of estradiol have been reported to undergo no change (Goya et al., 1990; Wu et al., 2009), decrease (an effect that was dependent upon prior sexual experience (Wu and Gore, 2009)), or increase (Fujita et al., 1990; Herath et al., 2001; Luine et al., 2007). The hypothalamic ERα may also change with age, although evidence to date shows little change in ERα gene expression or protein immunoreactivity with aging (Bottner et al., 2007; Madeira et al., 2000; Wu and Gore, 2009; Wu et al., 2009). Still, this question merits investigation in the context of sexual behavior and aging.
In the current study, we investigated the effects of aging, testosterone treatment, and their interactions on sexual behavior and expression of AR and ERα in the hypothalamus, focusing on the transition between young and middle-aged rats. Together, these studies were intended to provide novel information about the potential roles of these hormone receptors, their regulation by testosterone, and their relationship to sexual behavior during reproductive aging.
Materials and Methods
Animals
Male rats
Male Sprague-Dawley rats were purchased at 2-3 months (young, N=18) and 10-11 months (middle-aged, N=38) from Harlan Sprague-Dawley Inc (Indianapolis, Indiana; Stock/Strain: Hsd:Sprague Dawley®™ SD®™). The middle-aged rats were retired breeders. Young rats were given sexual experience by placing them with a receptive female rat (rotated among males) twenty consecutive nights and checking for sperm in the females' vaginas the next morning. At the end of this period, all young males had mated on average 15 times, estimated to be similar to what middle-aged breeders had experienced in the breeding colony.
Female rats
Female Sprague-Dawley rats (same stock) were purchased at 2-3 months (N=37). They were ovariectomized and estradiol-implanted [one silastic capsule (1.98-mm I.D. × 3.18-mm O.D. × 5-mm length; Dow Corning Corporation, Midland, MI) packed with 5% crystalline 17β-estradiol (Sigma-Aldrich, St. Louis, MO)]. These female rats received 500 μg progesterone (Sigma-Aldrich, St. Louis, MO) in sesame oil (0.1 ml) injection three hours before the mating trials. Only females who showed lordosis with male rats were used in mating trials.
Rats were housed in an AAALAC-approved facility (two same-sex animals per cage, cage dimensions 47 × 20 × 25 cm) with Rat Sterilizable Diet (Harlan Teklad LM-485 7012, Madison, WI) and water available ad libitum. The light cycle was 12 h light, 12 h dark cycle (lights on 2300h), and temperature was 21 ± 1°C. All animal procedures were approved by the UT-Austin Institutional Animal Care and Use Committee (Protocol number: 08030101) and studies were performed following the Guide for the Care and Use of Experimental Laboratory Animals.
Pre-tests for Mating Behaviors
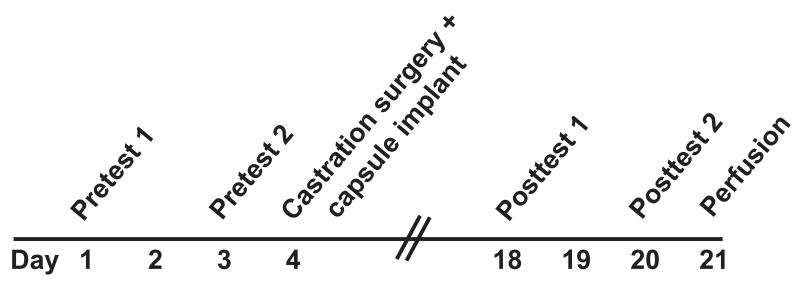
All the male rats were given two pre-tests (one day off between tests) to measure the baselines for each parameter of the mating behaviors, with different receptive females rotated among males. The experimental design is shown in Figure 1.
Figure 1.
An experimental flowchart is shown. Young and middle-aged rats were given two pretests 2 days apart. One day later, they were anesthetized, and a blood sample was drawn to enable measurement of intact serum hormone levels. Then, rats were bilaterally castrated, and implanted with a Silastic capsule containing either testosterone or vehicle. Two weeks later, the post-tests were performed two days apart. One day after testing, rats were euthanized by perfusion; a blood sample collected at that time was used for measuring hormone levels following the castration and hormone treatment.
Mating tests were performed under dim red light, beginning 3 hours after lights out (1400h-1700h). Every female was habituated to the chamber for 10 minutes. Every male was habituated to the chamber for 20 minutes before the female was introduced. 20-minute mating trials took place in a transparent Plexiglas cage [76 cm (L) × 32 cm (W) × 46 cm (H)]. The following parameters of male sex behavior were recorded in each test (Agmo, 1997): latency to mount; frequencies of mount, intromission and ejaculation; and percentage of rats that intromit or ejaculate in the 20 minute test. The test was terminated at 20 minutes or after a second ejaculation, whichever came first. The choice of this 20-minute cut-off was based on the literature using trials of 15-30 minutes for similar measures (Damassa et al., 1977; Frankel, 1981; Chambers et al., 1991; Vagell & McGinnis 1998; Wu & Gore, 2009). Mating trials were recorded using a Sony Handycam Hi-Fi Stereo Video 8 XR camcorder (Sony Corporation of America, New York, NY). The videotaped trials were further analyzed by using JWatcher v1.0 computer software (www.jwatcher.ucla.edu, Dan Blumstein's Lab, UCLA & The Animal Behaviour Lab, Macquarie University, Sydney) to quantify the behavior of each experimental animal (Crews et al., 2007).
All the young males finished ejaculation in both of two pre-tests (n = 18). For middle-aged male rats, only those who ejaculated successfully in at least one of the two pre-tests were chosen to participate in further experiments (n = 27). Only these latter rats are described in the remaining sections, in order to focus comparisons on rats with residual capacity to mate and based on the paradigm of (Frankel 1981).
Castration and Hormone Implantation
The next day after the pretests, young and screened middle-aged males were randomly assigned to one of two experimental groups: gonadectomized with testosterone implants and gonadectomized with vehicle implants. Capsules were 1.5 c.m. Silastic tubing (1.98-mm I.D. × 3.18-mm O.D. × 5-mm length; Dow Corning Corporation, Midland, MI) filled with crystalline testosterone [4-Androsten-17β-ol-3-one (Sigma-Aldrich, St. Louis, MO)] or cholesterol (Sigma-Aldrich, St. Louis, MO). Capsule size was based on Vagell & McGinnis (1997) and Damassa et al. (1977), and confirmed by a pilot study in our laboratory showing that such capsules produced circulating total testosterone concentrations of ∼1 ng/ml in middle-aged male rats. The ends of implants were sealed with 100% silicone Household Adhesive (DAP Inc. Baltimore, MD). For the castration and implantation surgery, animals were anesthetized with isoflurane via a gas anesthesia machine. Bilateral orchidectomy was performed, after which a blood sample (∼ 0.5 ml) was collected from the rat's tail lateral vein to get a pre-castration hormone level. Then each rat was implanted subcutaneously either with two testosterone capsules or two vehicle capsules. Rats were treated post-operatively with 5 mg/kg Rimadyl. They were allowed to recover for two weeks.
Post-tests for Mating Behaviors
Two weeks after castration and capsule implantation, rats were given two mating tests (post-tests) to measure each parameter of the mating behaviors after treatment, each time with different receptive females (Figure 1). Behaviors were scored as described for the pre-tests. There was one day off between the two tests.
Perfusion
One day after post-tests at 0800-1100 h (0-3 hours before lights out at 1100h) rats were deeply anesthetized with ketamine (40 mg) and xylazine (8 mg). A 24 hour post-mating time point was chosen based on published work in this arena (Fernández-Guasti et al., 2003) and to enable us to make direct comparisons with our previous paper (Wu & Gore, 2009). The thoracic cavity was opened and a blood sample (8-9 ml) was drawn from the ventricle for subsequent hormone assays. Animals were perfused (48 ml/min) sequentially with 0.9% saline (24 ml), 0.9% saline with 10% heparin (24 ml), and 1% paraformaldehyde with 3.75% acrolein (48 ml), followed by 4% paraformaldehyde (480 ml) (Wu et al., 2009). PBSA (phosphate-buffered saline A: 0.122 M PO4, 0.077 M NaCl, pH=7.3) was used to dissolve all fixatives. We removed the brains from the skull and post-fixed them for 3 hours in 4% paraformaldehyde and then transferred the brains into PBSA with 0.05% sodium azide for storage at 4°C. Then brains were cut on a vibrating microtome (Leica VT 1000S, Leica Microsystems, Nussloch, Germany) to get tissue sections (40 μm-thick) for further experiments. Sections were stored in PBSA at 4°C until use.
Immunohistochemistry
Antibodies
For AR immunohistochemistry, sections were incubated in the rabbit polyclonal AR antibody N-20 (1:1500; Santa Cruz Biotechnology, Inc.) raised against a peptide mapping at the N-terminus (amino acids 299–315) of AR of human origin [Genetic locus: AR (human) mapping to Xq11.2-q12; AR (mouse) mapping to 9 A5.3]. Previous work has shown high specificity of this antibody for the AR based on preabsorption of this antibody with the antigen, western blots, and other controls (Chlenski et al., 2001; McKinnell et al., 2001). We also recently published a paper with this antibody and in that report (Wu and Gore, 2009) along with the current one, specific nuclear labeling was detected only in those brain regions that express the AR (Simerly et al., 1990).
The ERα antibody (Upstate Biotechnology, Waltham, MA, Ab C1355, used at 1:20,000 dilution) was a purified rabbit polyclonal antibody generated against the final 14 C-terminal amino acids (TYYIPPEAEGFPNTI) of the rat ERα. The validation of this antibody has been described in detail previously by others' laboratories and ours (Friend et al., 1997; Moffatt et al., 1998; Wu et al., 2009). Similar to the AR antibody, in this study and our previous ones (Chakraborty et al., 2003; Wu and Gore, 2009; Wu et al., 2009), labeling was exclusively nuclear and restricted to regions of the brain reported to express ERα (Simerly et al., 1990).
Immunohistochemistry procedure
All the sections were processed in two IHC runs per antibody. For each run, animals from each age and treatment group were equally represented. In order to make the experimenter blind to age and treatment group, sections IDs were then recoded. Sections containing the preoptic area were selected in a 1:2 series (AVPV region) or a 1:4 series (MPN region; see below for details) and rinsed in PBSB (phosphate-buffered saline B: 0.12 M PO4: 0.154 M NaCl, pH=7.3). DAB immunohistochemistry was performed as described previously in detail using either the AR or ERα antibody (Wu and Gore, 2009; Wu et al., 2009). Sections were mounted on slides, dehydrated and coverslipped.
Rationale for brain regions of analysis
The current study focused on AR and ERα immunoreactivity in the anteroventral periventricular nucleus (AVPV) and the medial preoptic nucleus (MPN). These regions were studied based on our initial pilot studies suggesting that these regions had among the highest density of both of these nuclear steroid hormone receptors throughout the entire hypothalamus-preoptic area (Wu & Gore, unpublished). We also wished to follow up on our two published reports studying relationships among age, hormone levels, and AR- and ERα-immunoreactivity in these same regions (Wu and Gore, 2009; Wu et al., 2009). Of interest, one of these studies showed a significant negative correlation between serum testosterone levels and AR-immunoreactivity in both the AVPV and the MPN of aging intact males (Wu et al., 2009). These two regions are also sexually dimorphic (Bloch and Gorski, 1988; Simerly, 1998): the MPN is a hub for the regulation of masculine sexual behavior (Hull and Dominguez, 2006) and the AVPV is believed to be involved in GnRH/gonadotropin release, although those results are based on female rats (Wiegand and Terasawa, 1982; Wintermantel et al., 2006). Finally, it was also reported that sexual behavior alters the volume of the AVPV in male rats (Prince et al., 1998). Therefore, although there are many other potential regions of interest, we chose to focus on AVPV and MPN.
Stereological Analysis
A stereological analysis was performed according to methods described in detail previously (Chakraborty et al., 2005; Wu et al., 2009). For the AVPV, a 1:2 series (5-6 sections) and for MPN a 1:4 series (8-9 sections) was used. A wet-mount of fresh tissue showed that average tissue thickness was 40.89 μm. The sections were carefully matched for rostral-caudal landmarks among all the animals, and the regions were identified in Nissl-stained sections by comparing anatomical landmarks to an atlas of the rat brain (Swanson, 1998). Quantitative analyses were done as previously described (Wu et al., 2009). The number and density (# immunoreactive cells/volume of each nucleus) of AR-immunoreactive (AR-ir) or ERα-immunoreactive (ERα-ir) nuclei falling within the regions was quantified. The coefficients of error (Cruz-Orive/Geiser) and variation of the estimates were calculated as described by Schmitz and Hof (Schmitz and Hof, 2000) and were between 0.03 and 0.10 per group. Photomicrographs were taken to produce the figures, and images were subjected to only minor adjustments of contrast using Adobe Photoshop 7.0 (Adobe, San Jose, CA). In order to avoid any bias, any adjustments were applied equally to tissues from rats of different ages.
Serum Hormone Concentrations
Blood samples collected at the time of castration surgery or perfusion were centrifuged (8000 rpm, 5 min) and the serum collected and stored at −80 °C for hormone assays.
Enzyme immunoassay (EIA) of serum testosterone
Testosterone concentrations were measured by a single testosterone enzyme immunoassay using the EIA kit DSL-10-4000 according to the method described by Diagnostic Systems Laboratories, Inc. (Webster, TX). Duplicate samples were run at a volume of 50 μl each. The minimum detectable level of testosterone was 0.04 ng/ml per tube. Intra-assay variability was 2.60%. The cross-reactivity percentage of the testosterone antiserum with 5α-dihydrotestosterone is 6.6%. However, the relatively low concentration of 5α-dihydrotestosterone in male rats predicts that it will have a minimal effect on assay results.
Enzyme immunoassay (EIA) of serum free testosterone
Free testosterone concentrations were measured by two testosterone enzyme immunoassays using the EIA kit DSL-10-4900 according to the method described by Diagnostic Systems Laboratories, Inc. Duplicate samples were run at a volume of 50 μl each. The minimum detectable level of testosterone was 0.19 pg/ml per tube. Intra-assay variability was 1.95% and 1.65%. Inter-assay variability was 8.82%. The cross-reactivity of free testosterone antiserum with 5α-dihydrotestosterone is negligible.
Radioimmunoassay (RIA) of serum estradiol
Estradiol concentrations were measured by a single ultra-sensitive estradiol RIA using the DSL-4800 RIA kit according to the method described by Diagnostic Systems Laboratories, Inc. Duplicate samples were run at a volume of 200 μl each. The minimum detectable level of estradiol was 2.2 pg/ml per tube. Intra-assay variability was 1.86%. The estradiol antiserum has a high affinity for estradiol and low cross-reactivity to other naturally-occurring estrogens.
Statistical Analysis
Statistical analysis was done with each rat as the unit of analysis. Using SPSS statistical software (version 15.0; SPSS Inc., Chicago, Illinois), effects of age and treatments were evaluated on the following endpoints: regional AR-ir and ERα-ir density (calculated as cell numbers/volume), serum hormone levels, and behavioral parameters. First, datasets were tested for homogeneity of variance and normality. For datasets that met these criteria, comparisons were made ANOVA (see below for details) followed by post hoc analysis when indicated by a significant interaction. Otherwise data were square root transformed to normalize variation across a broad range of values and then analyzed. Repeated measures ANOVA was used for behavioral parameters, and two-way ANOVA was used for AR- and ERα-ir density. For calculation of percentage of males showing intromissions and ejaculations, Fisher's Exact Test was used (McGinnis et al., 1996; Vagell & McGinnis, 1998). The criterion for statistical significance was p < 0.05.
Results
Sexual Behavior of Male Rats
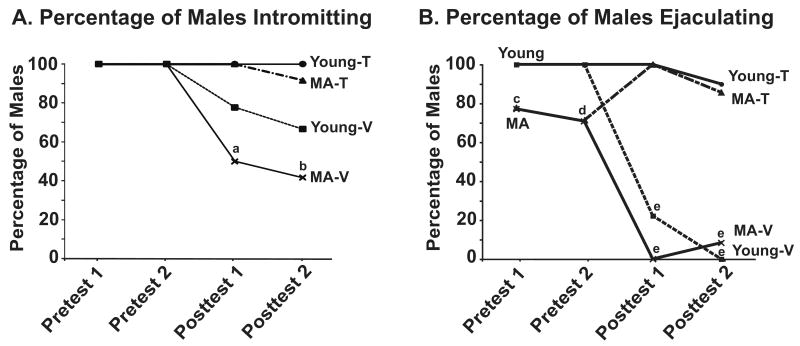
All of the young males (n = 18) mated to ejaculation in the two pre-tests. Twenty-seven of the original 38 MA males mated to ejaculation in at least one pre-test, and subsequent data shown are from those 27 males. The percentage of males intromitting or ejaculating in each trial (Figure 2A and 2B) was calculated, and comparisons were made using Fisher's Exact test (df = 1, 45 for all). For the percentage of males intromitting, the young-T group and MA-T groups were significantly higher than the MA-V group in both post-test 1 (young-T vs. MA-V: P = 0.017; MA-T vs. MA-V: P = 0.00686) and in post-test 2 (young-T vs. MA-V: P = 0.00681; MA-T vs. MA-V: P = 0.0129; Figure 2A). For the percentage of males ejaculating, the young group was significantly different from the MA group in both pre-test 1 (P = 0.0316) and in pre-test 2 (P = 0.0085). Following castration and hormone treatment, the percentage of ejaculations was significantly lower in the young-V and MA-V rats in both post-tests compared to the testosterone groups (P < 0.001 for all comparisons of vehicle to testosterone treated rats; Figure 2B).
Figure 2.
Behavioral analyses of the two pre-tests and two post-tests are shown. The mean percentages of males intromitting and ejaculating are shown in panel A and B. Data for the pre-tests are shown combined for each age. For the post-tests, data for the four groups (young-T, young-V, MA-T, MA-V) are shown separately. In post-test 1 and post-test 2, the percentage of males intromitting were significantly higher in the young-T and MA-T compared to the MA-V group (a: P < 0.05 and P < 0.001 compared to the corresponding young-T group and MA-T respectively; b: P < 0.001 and P < 0.05 compared to the corresponding young-T group and MAT respectively. The percent of males ejaculating was significantly higher for young than middle-aged males (c: P < 0.05; d: P < 0.01). In Post-test 1 and Post-test 2, Fisher's Exact test was used with the young-T and MA-T groups as the bases of comparison. These groups were significantly different than the young-V and MA-V groups (e: P < 0.001 for all comparisons). Sample sizes were: Young-T (n=9), Young-V (n=9), MA-T (n=14), and MA-V (n=13). Abbreviations: MA, middle-aged; T, testosterone; V, vehicle.
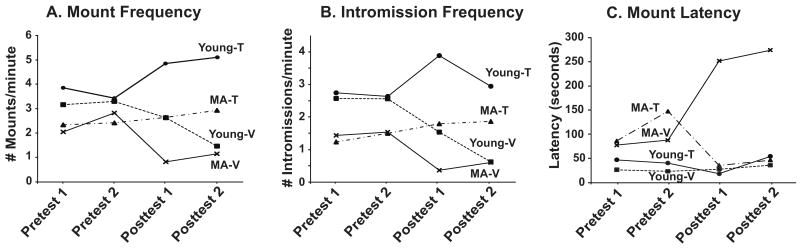
The results on behavioral measures of mount frequency, intromission frequency and mount latency are shown in Figure 3 (panels A, B and C, respectively, and in Supplementary Table 1). Time was the within-subjects factor and age and treatment were the between-subjects factors in this repeated measures design. The effects of time were not significant for any of these parameters. However, the interaction effects of time and treatment were significant for mount and intromission frequencies (mount frequency: F3,102=9.031, P < 0.001, Figure 3A; intromission frequency: F3,102=14.175, P < 0.001, Figure 3B). The main effect of age was significant for mount latency (F1,35=7.463, P < 0.05; Figure 3C), mount frequency (F1,34=13.757, P < 0.01) and intromission frequency (F1,34=21.693, P < 0.001) with young groups having shorter mount latency and higher mount and intromission frequencies. A main effect of hormone treatment was detected for mount frequency (F1,34=16.086, P < 0.001) and intromission frequency (F1,34=16.738, P < 0.001). Testosterone-treated animals had significantly higher frequencies than V-treated groups for both mounts and intromissions. There were no significant interactions between treatment and age on the three measurements (Mount latency: F1,35=2,483, P = 0.124; Mount frequency: F1,34=1.761, P = 0.194; Intromission frequency: F1,34=1.759, P = 0.194).
Figure 3.
Mean mount frequency (A), intromission frequency (B) and mount latency (C) are shown. Repeated measures analyses showed significant effects of age (mount frequency: P < 0.01; intromission frequency: P < 0.001; mount latency: P < 0.05) and treatment (mount frequency: P < 0.001; intromission frequency: P < 0.001). See text for further analytical details on statistical results, which could not be plotted due to the repeated measures design, and Supplementary Table 1 for reporting of the means ± standard errors.
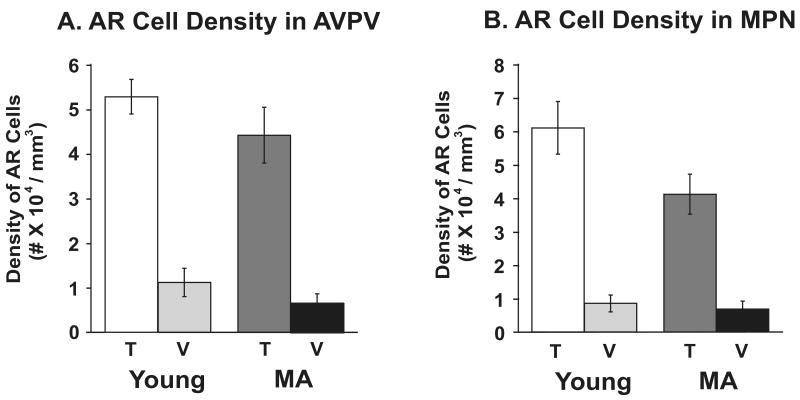
AR Immunoreactivity in AVPV and MPN
Representative photomicrographs of AR-immunoreactivity in the AVPV and MPN are provided in Figure 4. Quantitative stereologic analyses of AR-ir cell densities were performed, and results shown in Figure 5. There was no effect of age on AR cell density. A significant main effect of treatment in both AVPV and MPN was found (AVPV: F1,21=78.43, P < 0.0001; MPN: F1,21=91.97, P < 0.0001), with significantly greater density of AR cells in the testosterone than the vehicle treated groups of both ages.
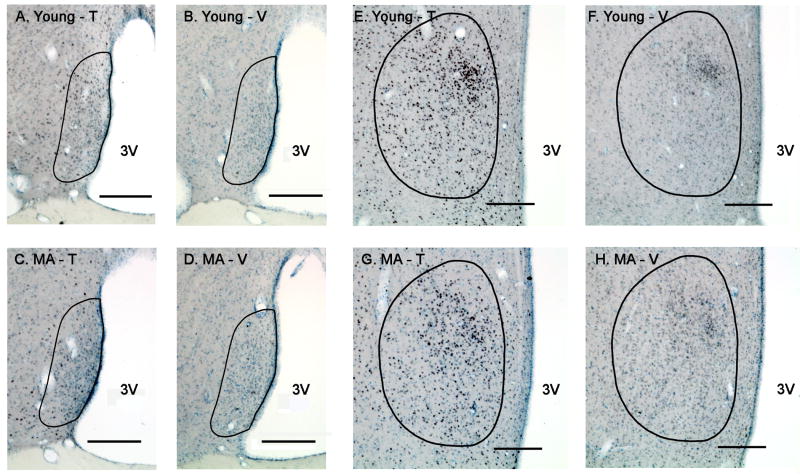
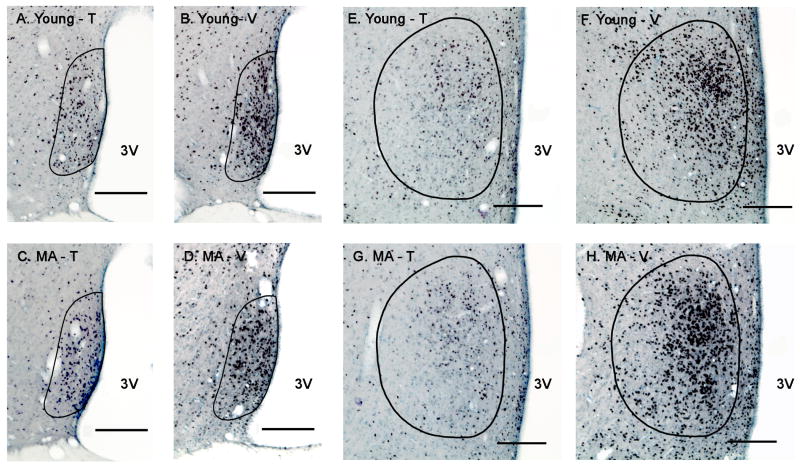
Figure 4.
Photomicrographs of AR immunoreactivity in the AVPV (left) and MPN (right) of young and middle-aged rats are shown. Rats were perfused at the completion of the two post-tests, 17 days after castration and hormone (testosterone or vehicle) treatment. Representative sections are shown for the AVPV and MPN, respectively, of young-T (A, E), young-V (B, F), middle-aged-T (C, G) and middle-aged-V (D, H) male rats. The contours of the AVPV or MPN are drawn based on Nissl staining and according to Swanson's rat brain atlas (1998). Scale bar = 200 μm. Abbreviations: MA, middle-aged; T, testosterone; V, vehicle; 3V, third ventricle.
Figure 5.
Stereologic analysis results of AR-immunoreactive cell density (number of immunoreactive cells/regional volume) in the AVPV (panel A, n=8 rats per group) and MPN (panel B, n=8 rats per group). The bars represent the mean ± SEM. AR cell density was significantly higher in T- than in V-treated males of both ages (P < 0.0001). Abbreviations: AVPV, anteroventral periventricular nucleus; MPN, medial preoptic nucleus; T, testosterone; V, vehicle; MA, middle-aged.
ERα Immunoreactivity in AVPV and MPN
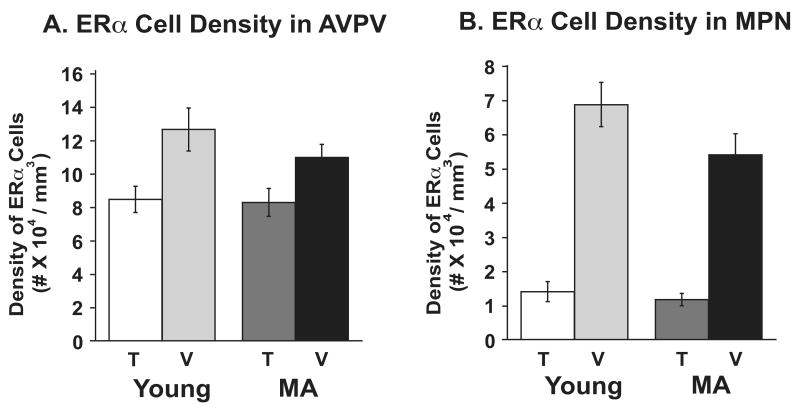
Representative photomicrographs of ERα-immunoreactivity in the AVPV and MPN are shown in Figure 6. Stereologic analyses of ERα -ir cell densities were performed (Figure 7). There was a significant main effect of treatment on ERα cell density in AVPV (F1,17=12.69, P < 0.01) and MPN (F1,17=125.97, P < 0.0001), with ERα cell density being significantly lower in the testosterone than the vehicle groups of both ages. There was no effect of age on ERα cell density.
Figure 6.
Photomicrographs of ERα immunoreactivity in the AVPV (left) and MPN (right) of the same young and middle-aged rats are shown. Representative sections from the AVPV or MPN, respectively, of young-T (A, E), young-V (B, F), middle-aged-T (C, G) and middle-aged-V (D, H) male rats are presented. The contours of the AVPV or MPN are drawn based on Nissl staining and according to Swanson's rat brain atlas (1998). Scale bar = 200 μm. Abbreviations: MA, middle-aged; T, testosterone; V, vehicle; 3V, third ventricle.
Figure 7.
Stereologic analysis results of ERα-immunoreactive cell density (number of immunoreactive cells/regional volume) are shown for the AVPV (panel A, n=6 rats per group) and the MPN (panel B, n=6 rats per group). The bars represent the mean ± SEM. ERα-cell density was significantly lower in T-treated rats of both ages in AVPV (P < 0.01) and MPN (P < 0.0001). Abbreviations: AVPV, anteroventral periventricular nucleus; MPN, medial preoptic nucleus; T, testosterone; V, vehicle; MA, middle-aged.
Serum Hormone Concentrations
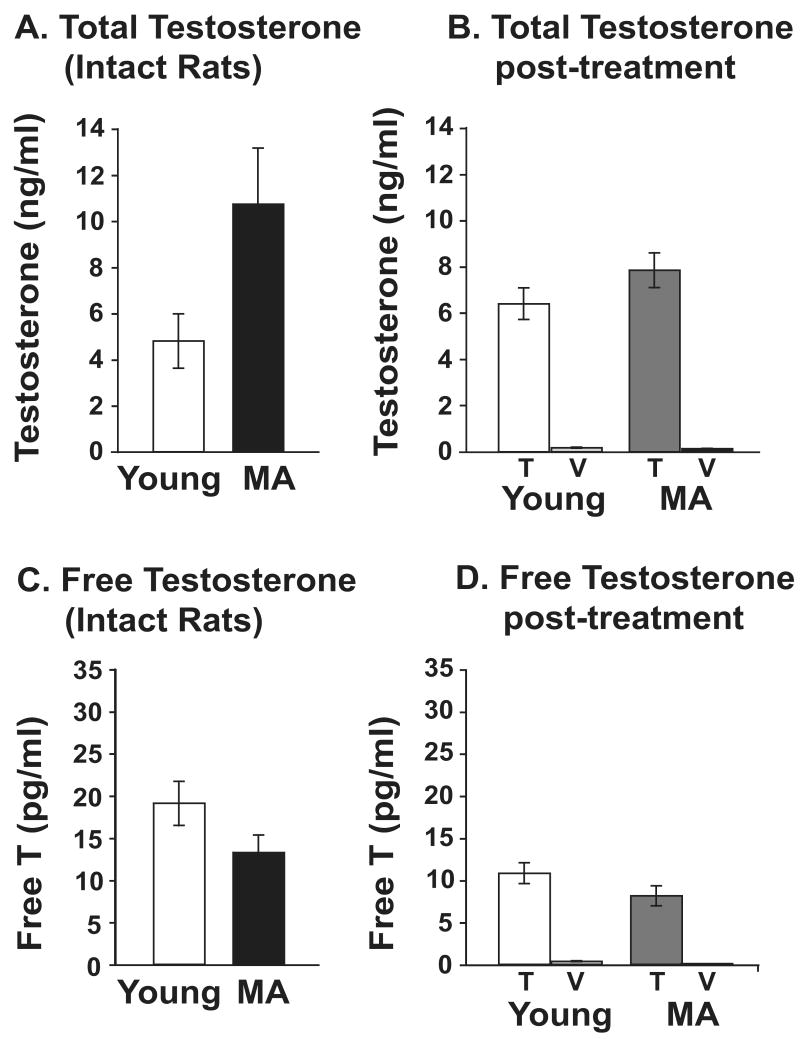
In intact rats (Figure 8A), serum total testosterone levels tended to be higher in the middle-aged than the young group but this was not significant (P = 0.113). Seventeen days after castration and hormone (T or V) treatment, there was no effect of age (P = 0.198) but a significant effect of treatment (P < 0.001). Vehicle animals had significantly lower testosterone levels than the testosterone group. In addition, the testosterone levels in the T-replaced group were in a similar range to pre-castration levels (c.f. Figures 8A and 8B).
Figure 8.
Serum total and free testosterone were assayed in young and middle-aged male rats before and after castration and hormone treatment. Hormones from intact young and middle-aged rats are shown in panels A and C. Hormones in the four groups 17 days after castration and treatment are shown in panels B and D. There was a significant main effect of testosterone treatment on total and free testosterone in samples collected at euthanasia (P < 0.001 for both). The bars represent the mean ± SEM. Abbreviations: T, testosterone; V, vehicle; MA, middle-aged.
Free testosterone concentrations in intact rats did not differ between the middle-aged and the young group (P = 0.087; Figure 8C). Following castration and hormone (T or V) treatment, there was no effect of age (P = 0.131) and a significant effect of treatment (P < 0.001), with free testosterone levels higher in the testosterone than the vehicle groups.
Serum estradiol levels were measured in intact rats, with levels similar between the young and middle-aged groups (Young: 8.96 ± 1.62; MA: 6.77 ± 1.33). Due to a technical error, serum estradiol levels could not be measured in the terminal sample.
Discussion
In these studies, we examined the effects of testosterone on masculine sexual behavior and on androgen receptor and estrogen receptor α expression in the preoptic area of the brain in young and middle-aged male rats. We made the following observations: 1) Aging was associated with a significant decline in sexual behavior in intact males, a finding that is in agreement with previous studies (Chambers and Phoenix, 1983; Smith et al., 1992). 2) Testosterone given to young or middle-aged rats at the time of castration was associated with the maintenance or even improvement of most masculine sexual behaviors, at similar levels to these behaviors in the young pre-test (intact) rats. The exceptions were mount and intromission frequencies, which were consistently lower in the middle-aged than the young group. 3) Rats receiving the vehicle at the time of castration had a loss of sexual behaviors, with the exception of mount latency in the young group. 4) In the POA of gonadectomized adult male rats, AR density was significantly higher, and ERα density lower, in testosterone than vehicle-treated males, an effect that was similar in the two age groups. 5) Serum testosterone and estradiol levels, in general, were similar between the two ages. As a whole, these data show that absolute serum hormone levels cannot explain age differences in sexual behavior. Further, the aging brain has the continued capacity to respond to exogenous testosterone treatment with increased AR, and decreased ERα, in a manner similar to the young brain.
Masculine Sexual Behavior, Hormones, and Aging
In the current study, we observed an age-associated decline in sexual behavior, but little difference in testosterone concentrations. Although testosterone is important for masculine sexual behavior, a causal relationship between testosterone concentrations and sexual behavior is controversial, and our results and others' suggest that at least some of these processes are dissociated. For example, it was reported in young rats that administration of less than one-third the normal intact levels of testosterone is needed in castrated male rats to maintain normal sexuality (Damassa et al., 1977). Intact male rats that could complete copulatory behavior showed no correlation between the behavioral measures and testosterone levels (Smith et al., 1992). In other words, more testosterone is not “better” than less testosterone in maintaining masculine sexual behaviors, as long as some threshold level of testosterone is achieved (Damassa et al., 1977; Frankel, 1981).
The ability of testosterone to regulate sexual behavior becomes even further dissociated with aging. Declines in plasma testosterone concentrations have typically been associated with a loss of sexual performance in aging males across many species, although most of this work utilized males of more advanced age than the middle-aged rats in the current study (Bernardi et al., 1998; Chambers et al., 1991; Chen et al., 1994; Downs and Urbanski, 2006; Gruenewald et al., 2000; Harman et al., 2001; Luine et al., 2007; Roselli et al., 1986; Taylor et al., 1996; Wu et al., 2009). When testosterone levels in aging male rats were replaced to levels similar to those in young rats, the decline of sexual arousal (Gray et al., 1981) and copulatory activity [current results and (Chambers et al., 1991; Hsu et al., 1986)] could not be restored or maintained. Together, these findings indicate that low levels of serum testosterone in aging males are not exclusively responsible for the diminution of sexual behaviors.
The one behavior that was not regulated in a predictable way by testosterone was mount latency. Castrated young male rats had similar mount latencies whether replaced with vehicle or testosterone. Middle-aged rats had longer mount latencies in the pre-test, and after castration, this behavior was facilitated by testosterone but not vehicle. These results suggest that mount latency is testosterone-independent only at the young age, and indicates a potential age-related change in the neural circuitry underlying this motivational behavior. Previously, mount latency was shown to be modulated by testosterone in mice (James and Nyby, 2002). Beyer et al. (1981) reported that effects of hormone treatment (either testosterone or estradiol) in castrated male rats differed for mounts compared to intromissions and ejaculations, with a greater restorative effect in the former than the latter. Thus, the neural mechanisms controlling mounting behavior probably differ somewhat from those involved in intromission and ejaculation (reviewed in Hull and Dominguez, 2007). It is also notable that there were only two behaviors that differed between the two ages in the post-tests: mount frequency and intromission frequency, both of which were lower in middle-aged than young rats. Again, because hormone levels were largely comparable between these age groups, the differences are presumably due to changes in neural circuits with aging.
Steroid Hormones in Intact Aging Male Rats
It may initially seem puzzling that testosterone and free testosterone concentrations did not differ significantly between the young and middle-aged intact males of the present study, as many previous publications showing an age-related decline in serum testosterone (Bernardi et al., 1998; Chambers et al., 1991; Chen et al., 1994; Downs and Urbanski, 2006; Gruenewald et al., 2000; Harman et al., 2001; Luine et al., 2007; Roselli et al., 1986; Taylor et al., 1996; Wu et al., 2009). By contrast, there is a body of literature that demonstrates little or no difference in total testosterone concentrations between middle-aged and young male rats (Frankel, 1981; Frankel and Mock, 1981; Wu and Gore, 2009; Gruenewald et al., 2000). It is difficult to reconcile these disparate results, but testosterone concentrations are known to vary based on rat strain, time of day (Gruenewald et al., 2000), and sexual experience (Wu and Gore, 2009; Edinger and Frye, 2007). In addition, testosterone is released in a pulsatile manner, with more than 5-fold fluctuations from nadir to peak (Steiner et al., 1984). When these factors are put into the context of chronological age, they further complicate the interpretation of the results. Our concentrations in young males are in a similar range (from 1-10 ng/ml) as reported in the literature (Sprague-Dawley: Steiner et al., 1984; Frankel 1981; Karpas et al, 1983; Wu et al., 2009; Wu and Gore, 2009; Fischer 344: Chambers et al., 1991; Brown-Norway: Gruenewald et al., 2000; Gruenewald & Matsumoto 1991; Long-Evans: Damassa et al., 1977; Wistar: Herrera-Pérez et al., 2008; Fernández-Guasti et al., 2003; Romano-Torres et al 2006; Taylor et al., 1996). For middle-aged rats, testosterone levels have been reported to range from 0.5 to 6 ng/ml, with results from the literature overlapping with our current findings (Sprague-Dawley: Frankel 1981; Frankel & Mock 1981; Wu et al., 2009; Wu and Gore, 2009; Brown-Norway: Gruenewald et al., 2000; Wistar: Herrera-Pérez et al., 2008).
Free testosterone concentrations, which represent the bioavailable pool, were similar between our young and middle-aged rats. We previously reported that free testosterone increased between young and middle-aged rats (Wu and Gore, 2009). Few published studies have addressed levels of free testosterone in an aging rat model. Andersen et al. (2002) reported that free testosterone levels in Wistar rats decreased from 3 to 22 months in Wistar rats, but no intermediate ages were studied. Shen et al. (2000) found that free testosterone concentrations were similar in Sprague-Dawley rats at 2- and 13-months of age, similar to our current results. Thus, future studies would help to resolve some differences in results.
We also found no difference in serum estradiol concentrations in our intact young and middle-aged animals. The relationship between serum estradiol and aging is unclear, as the literature in rats reports increases (Luine et al., 2007; Herath et al., 2001), decreases (Wu and Gore, 2009; Herrera-Pérez et al., 2008), or no change (Wu et al., 2009; Goya et al., 1990; Fujita et al., 1990) depending upon the model and the ages studied. Of these reports, to our knowledge only four include middle-aged animals: in two there was a decrease with aging (Wu and Gore, 2009; Herrera-Pérez et al., 2008) and in two there was no change (Wu et al., 2009; Fujita et al., 1990). There are experimental differences in rat strains, the ages of analysis, and the specific assay used to measure hormone levels. The range of estradiol levels in the current study, 5-10 pg/ml, is similar to that in the previously published literature from our laboratory [Sprague-Dawley rats, range 5-35 pg/ml; (Wu and Gore, 2009)], and others [young Wistar rats, range 10-15 pg/ml; (Phillips-Farfán et al., 2007); Wistar rats: young (19 pg/ml) and middle-aged (4 pg/ml) (Herrera-Pérez et al., 2008)]. Again, future work is needed to provide much-needed reconciliation of these results.
Role of Sexual Experience and/or Satiety
The behavioral status, sexual experience, or satiety of the experimental animals may have an effect on hormones and their hypothalamic receptors. In the current study, the middle-aged rats were retired breeders and had not bred for several months, whereas young rats were given extensive experience and then used in our tests shortly thereafter. Several studies in male rats (both young and aging) has shown that animals that have mated have higher testosterone levels than non-mated counterparts (Frankel, 1984). Edinger and Frye (2007) showed that young male rats with sexual experience had higher testosterone concentrations than naïve males. A recent study from our laboratory also supports and extends that result by showing that animals with sexual experience, irrespective of age, have higher total and free testosterone than naïve counterparts (Wu and Gore, 2009). The timing and amount of the latest sexual activity may also influence the results (reviewed in Fernández-Guasti and Rodriguez-Manzo, 2003). We chose a 24-hour post-testing interval for euthanasia for comparison with our previous study (Wu & Gore, 2009) and that of other laboratories (Phillips-Farfán et al., 2007). In experienced young male rats, testosterone concentrations did not differ with time relative to the last sexual experience (Romano-Torres et al., 2007) or with sexual exhaustion (Fernández-Guasti et al., 2003). Estradiol levels also did not differ between experienced rats given a range of sexual behavior (Phillips-Farfán et al., 2007).
Steroid Hormones and Behavior in Castrated Male Rats
Our study showed that castration and testosterone implantation resulted in concentrations of total and free testosterone that were in a similar range as intact levels. Nevertheless, exogenous testosterone maintained or even facilitated aspects of sexual behavior. This is interpreted to mean that exogenous tes tosterone may be more behaviorally effective than the same mean level of endogenous testosterone in intact males, especially in shortening mount and intromission latencies. There are three interpretations for these differences between serum endogenous and exogenous testosterone: 1) circulating testosterone concentrations may not be completely representative of the testosterone concentration in regions of the brain, including preoptic area, that control sexual behavior; 2) Circulating serum testosterone in intact males includes peaks and troughs (Bartke et al., 1973) whereas testosterone capsules probably provide more continuous release. The consequences of pulsatile vs. continuous testosterone may include a difference in neural actions of testosterone. In support of this, Gruenewald et al. showed that whereas basal testosterone concentrations did not differ between young and middle-aged rats, the circadian rhythm of testosterone release was dampened with age (Gruenewald et al., 2000). 3) Our rats were given testosterone implantation at the time of castration, thereby maintaining hormone levels. Other studies have evaluated effects of testosterone treatment following a longer post-castration delay (Chambers and Phoenix, 1984; Chambers et al., 1991; Sato et al., 1998). These varying experimental paradigms may differentially up- or down-regulate neural steroid hormone receptors, thereby producing behavioral differences. This hypothesis is consistent with the observation that the antiandrogen, Sch 16423, was able to block the restoration of masculine sexual behavior but had a comparatively weak effect on the maintenance of copulatory behavior (McGinnis and Mirth, 1986). Moreover, considerably higher amounts of testosterone are required for restoration of sexual behavior in castrates, as opposed to its maintenance when treatment is initiated immediately (Damassa et al., 1977).
AR and ERα Immunoreactivity in Aging Males
The expression of AR mRNA and protein in the hypothalamus varies with age and hormone status, with most published work focused on developing or young adult animals (Iqbal et al., 1995; Lu et al., 1999; Lu et al., 1998; Walker et al., 2009; Wood and Newman, 1993). We previously reported that AR immunoreactive cell density increased with age in the preoptic area of intact male rats (Wu and Gore, 2009; Wu et al., 2009). By contrast, Chambers et al. (1991) showed that androgen binding was decreased in the preoptic area of aged compared to young male rats, a difference that may be attributable to the more advanced age of the animals of Chambers et al. (1991) compared to our rats. A relationship between testosterone concentrations and hypothalamic AR expression is well-established: castration diminishes AR expression while testosterone treatment can restore it (Krey and McGinnis, 1990; Wood and Newman, 1993). Our current results add to both of these lines of research. They show that the age difference in AR is not detectable in castrated male rats; further, we show that testosterone treatment at the time of castration elevates AR immunoreactive cell density similarly in young and middle-aged rats. We conclude that the preoptic area continues to be responsive to testosterone as manifested by expression of AR protein immunoreactivity in middle aged animals.
Some effects of testosterone on sexual behavior may be mediated through its aromatization to estradiol and subsequent actions on estrogen receptors. Further, estradiol facilitates masculine sexual behavior (Baum and Vreeburg, 1973; Larsson et al., 1976; Vagell and McGinnis, 1997). Effects of castration, hormone treatment, and aging on the expression of the ERα in male rats have been investigated, although not within a single study. Castration was reported to increase ERα mRNA in the MPN of young male rats (Handa et al., 1996). By direct contrast, Roselli and Resko (1993) reported that estradiol binding in the preoptic area was significantly lower in castrated compared to intact male rats at both young and old ages. Treatment of male rats with hormones also affects ERα binding and immunoreactivity. Clancy et al. (2000) reported that counts of ER-immunoreactive cells in the medial preoptic area were significantly lower in estradiol- compared to control-treated male rat, and this was region specific, as a difference was not detected in the bed nucleus of the stria terminalis or the lateral septum. Effects of testosterone treatment on ERα expression were studied in the current study: in the MPN, ERα cell density was substantially higher in the vehicle compared to the testosterone treated rats of both ages. In the AVPV, testosterone also decreased ERα cell density but to a lesser extent. Finally, the effects of aging on estrogen receptor have been investigated. No significant effect of age in AVPV and MPN was found in this study; similarly, Madeira et al. (2000) showed no difference in ERα immunoreactive cell numbers in the MPN. In further support, Bottner et al. (2007) showed no difference in ERα mRNA levels in the preoptic area. We speculate that testosterone replacement in the current study may regulate ERα levels either indirectly through binding to AR-expressing cells, or directly through its aromatization to estradiol. As some cells may co-express AR and ERα (Greco et al., 1998), these actions are not necessarily mutually exclusive.
Sexual experience and/or satiety may influence levels of hypothalamic hormone receptors. Phillips-Farfán et al. (2007) showed that ERα immunoreactive cell density was increased in the medial preoptic area of rats that mated to satiety, but not rats given a single ejaculation, compared to unmated controls. We previously found no difference in either AR- or ERα immunoreactive cell density in the AVPV or MPN of sexually naïve or experienced male rats (Wu and Gore, 2009). In experienced male rats, time after mating to satiety was related to AR density in several hypothalamic and preoptic brain regions (Romano-Torres et al., 2007). In the MPN, AR density was decreased 24 hours after males were allowed to mate to one ejaculation and further decreased in males mated to exhaustion (Fernández-Guasti et al., 2003). In comparing young males that were sorted into copulators or non-copulators, Portillo et al. (2006) reported no difference between these groups in AR density in the medial preoptic area, and lower numbers of ERα cells in non-copulators compared to copulators. As a whole, these data show the importance of consideration of behavioral status in relationship to the endpoint of AR and ERα cells, as aging, experience, time since sexual activity, and amount of activity, are important factors in their expression.
Conclusion
We had initially hypothesized that the age-related behavioral decline in masculine behaviors may be due to changes in androgen and estrogen receptor numbers in the hypothalamus, and/or a diminished responsiveness of these receptors to testosterone treatment in animals of increasing age. The current results do not support that hypothesis, as both young and middle-aged castrated males responded similarly to testosterone replacement with an up-regulation of the AR and a down-regulation of the ERα. Therefore, we postulate that other neurotransmitter systems involved in the control of sexual behaviors in male rats are responsible for the pre-castration differences between the two age groups. In addition, other brain regions involved in the neural circuitry that controls masculine behaviors merit investigation. Future studies are necessary to delineate the nature of these circuits in middle-aged males.
Supplementary Material
Acknowledgments
We thank Deena Walker for running RIAs of steroid hormones.
Footnotes
This work was supported by NIH AG028051 to ACG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A. Male rat sexual behavior. Brain Res Brain Res Protoc. 1997;1:203–9. doi: 10.1016/s1385-299x(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Bignotto M, Borges Machado R, Turik S. Does paradoxical sleep deprivation and cocaine induce penile erection and ejaculation in old rats? Addiction Biol. 2002;7:285–290. doi: 10.1080/13556210220139497. [DOI] [PubMed] [Google Scholar]
- Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92:1223–8. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Vreeburg JT. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973;182:283–5. doi: 10.1126/science.182.4109.283. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S, Purdy RH, Petraglia F, Genazzani AR. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol. 1998;138:316–21. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- Beyer C, Contreras JL, Morali G, Larsson K. Effects of castration and sex steroid treatment on the motor copulatory pattern of the rat. Horm Behav. 1981;27:727–730. doi: 10.1016/0031-9384(81)90247-x. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the size of several components of the medial preoptic area in the male rat. J Comp Neurol. 1988;275:613–22. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- Bottner M, Leonhardt S, Wuttke W, Jarry H. Changes of expression of genes related to the activity of the gonadotrophin-releasing hormone pulse generator in young versus middle-aged male rats. J Neuroendocrinol. 2007;19:779–87. doi: 10.1111/j.1365-2826.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–21. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Rajendren G, Gore AC. Expression of estrogen receptor α in the anteroventral periventricular nucleus of hypogonadal mice. Exp Biol Med (Maywood) 2005;230:49–56. doi: 10.1177/153537020523000106. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Phoenix CH. Sexual behavior in response to testosterone in old long-term-castrated rhesus males. Neurobiol Aging. 1983;4:223–7. doi: 10.1016/0197-4580(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Phoenix CH. Testosterone and the decline of sexual behavior in aging male rats. Behav Neural Biol. 1984;40:87–97. doi: 10.1016/s0163-1047(84)90194-8. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Phoenix CH. Testosterone is more effective than dihydrotestosterone plus estradiol in activating sexual behavior in old male rats. Neurobiol Aging. 1986;7:127–132. doi: 10.1016/0197-4580(86)90151-x. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Thornton JE, Roselli CE. Age-related deficits in brain androgen binding and metabolism, testosterone, and sexual behavior of male rats. Neurobiol Aging. 1991;12:123–30. doi: 10.1016/0197-4580(91)90050-t. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15:551–7. [PubMed] [Google Scholar]
- Chlenski A, Nakashiro K, Ketels KV, Korovaitseva GI, Oyasu R. Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate. 2001;47:66–75. doi: 10.1002/pros.1048. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D, Michael RP. Estrogen in the medial preoptic area of male rats facilitates copulatory behavior. Horm Behav. 2000;38:86–93. doi: 10.1006/hbeh.2000.1602. [DOI] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–6. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damassa DA, Kobashigawa D, Smith ER, Davidson JM. Negative feedback control of LH by testosterone: a quantitative study in male rats. Endocrinology. 1976;99:736–42. doi: 10.1210/endo-99-3-736. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm Behav. 1977;8:275–86. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Aging-related sex-dependent loss of the circulating leptin 24-h rhythm in the rhesus monkey. J Endocrinol. 2006;190:117–27. doi: 10.1677/joe.1.06745. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Sexual experience of male rats influences anxiety-like behavior and androgen levels. Physiol Behav. 2007;92:443–453. doi: 10.1016/j.physbeh.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Rodriguez-Manso G. Pharmacological and physiological aspects of sexual exhaustion. Scand J Psychol. 2003;44:257–263. doi: 10.1111/1467-9450.00343. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Swaab D, Rodriguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinol. 2003;28:501–512. doi: 10.1016/s0306-4530(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Frankel AI. Hormone release during computer-monitored sexual behavior in mature and aged male rats. Horm Behav. 1981;15:312–320. doi: 10.1016/0018-506x(81)90020-9. [DOI] [PubMed] [Google Scholar]
- Frankel AI. Plasma testosterone levels are higher in old mating rats than in old non-mating rats. Exp Gerontol. 1984;19:345–8. doi: 10.1016/0531-5565(84)90042-1. [DOI] [PubMed] [Google Scholar]
- Frankel AI, Mock EJ. Time course of hormonal response to sexual behavior in aging male rats. Exp Gerontol. 1981;16:363–369. doi: 10.1016/0531-5565(81)90057-7. [DOI] [PubMed] [Google Scholar]
- Friend KE, Resnick EM, Ang LW, Shupnik MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol. 1997;131:147–55. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Fujita S, Chiba M, Ohta M, Kitani K, Suzuki T. Alteration of plasma sex hormone levels associated with old age and its effect on hepatic drug metabolism in rats. J Pharmacol Exp Ther. 1990;253:369–74. [PubMed] [Google Scholar]
- Goya RG, Lu JK, Meites J. Gonadal function in aging rats and its relation to pituitary and mammary pathology. Mech Ageing Dev. 1990;56:77–88. doi: 10.1016/0047-6374(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Gray GD, Smith ER, Dorsa DM, Davidson JM. Sexual behavior and testosterone in middle-aged male rats. Endocrinology. 1981;109:1597–604. doi: 10.1210/endo-109-5-1597. [DOI] [PubMed] [Google Scholar]
- Greco B, Edwards DA, Zumpe D, Clancy AN. Androgen receptor and mating-induced fos immunoreactivity are co-localized in limbic and midbrain neurons that project to the male rat medial preoptic area. Brain Res. 1998;781:15–24. doi: 10.1016/s0006-8993(97)01136-0. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, M A. Age-related decreases in serum gonadotropin levels and gonadotropin-releasing hormone gene expression in the medial preoptic area of the male rat are dependent upon testicular feedback. Endocrinology. 1991;129:2442–2450. doi: 10.1210/endo-129-5-2442. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl. 2000;21:72–84. [PubMed] [Google Scholar]
- Handa RJ, Kerr JE, DonCarlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Brain Res Mol Brain Res. 1996;39:57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Herath CB, Watanabe G, Wanzhu J, Noguchi J, Akiyama K, Kuramoto K, Groome NP, Taya K. Elevated levels of inhibin-A and immunoreactive inhibin in aged male Wistar rats with testicular Leydig cell tumor. J Androl. 2001;22:838–46. [PubMed] [Google Scholar]
- Herrera-Pérez JJ, Martinez-Mota L, Fernández-Guasti A. Aging increases the susceptibility to develop anhedonia in male rats. Prog Neuro-Psychopharmacol Biol Psychiatr. 2008;32:1798–1803. doi: 10.1016/j.pnpbp.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Hsu C, Yu JY, Peng MT. Effects of long-term testosterone replacement on copulatory activity in old male rats. Gerontology. 1986;32:10–7. doi: 10.1159/000212760. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Swanson JJ, Prins GS, Jacobson CD. Androgen receptor-like immunoreactivity in the Brazilian opossum brain and pituitary: distribution and effects of castration and testosterone replacement in the adult male. Brain Res. 1995;703:1–18. doi: 10.1016/0006-8993(95)00983-3. [DOI] [PubMed] [Google Scholar]
- James PJ, Nyby JG. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus) Physiol Behav. 2002;75:287–94. doi: 10.1016/s0031-9384(01)00666-7. [DOI] [PubMed] [Google Scholar]
- Karpas AE, Bremner WJ, Clifton DK, Steiner RA, Dorsa DM. Diminished luteinizing hormone pulse frequency and amplitude with aging in the male rat. Endocrinology. 1983;112:788–792. doi: 10.1210/endo-112-3-788. [DOI] [PubMed] [Google Scholar]
- Krey LC, McGinnis MY. Time-courses of the appearance/disappearance of nuclear androgen + receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: relationships with gonadotropin secretion. J Steroid Biochem. 1990;35:403–8. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- Larsson K, Sodersten P, Beyer C. Induction of male sexual behaviour by oestradiol benzoate in combination with dihydrotestosterone. J Endocrinol. 1973;57:563–4. doi: 10.1677/joe.0.0570563. [DOI] [PubMed] [Google Scholar]
- Larsson K, Sodersten P, Beyer C, Morali G, Perez-Palacios G. Effects of estrone, estradiol and estriol combined with dihydrotestosterone on mounting and lordosis behavior in castrated male rats. Horm Behav. 1976;7:379–90. doi: 10.1016/0018-506x(76)90009-x. [DOI] [PubMed] [Google Scholar]
- Lu S, Simon NG, Wang Y, Hu S. Neural androgen receptor regulation: effects of androgen and antiandrogen. J Neurobiol. 1999;41:505–12. doi: 10.1002/(sici)1097-4695(199912)41:4<505::aid-neu6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–51. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Paula-Barbosa MM. Hypertrophy of the ageing rat medial preoptic nucleus. J Neurocytol. 2000;29:173–97. doi: 10.1023/a:1026598906739. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Mirth MC. Inhibition of cell nuclear androgen receptor binding and copulation in male rats by an antiandrogen, Sch 16423. Neuroendocrinol. 1986;43:63–8. doi: 10.1159/000124510. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Williams GW, Lumia AR. Inhibition of male sex behavior by androgen receptor blockade in preoptic area or hypothalamus, but not amygdala or septum. Physiol Behav. 1996;60:783–9. doi: 10.1016/0031-9384(96)00088-1. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction. 2001;122:419–29. doi: 10.1530/rep.0.1220419. [DOI] [PubMed] [Google Scholar]
- Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. J Neurosci. 1998;18:9556–63. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morali G, Larsson K, Beyer C. Inhibition of testosterone-induced sexual behavior in the castrated male rat by aromatase blockers. Horm Behav. 1977;9:203–13. doi: 10.1016/0018-506x(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Nicolosi A, Laumann EO, Glasser DB, Moreira ED, Jr, Paik A, Gingell C. Sexual behavior and sexual dysfunctions after age 40: the global study of sexual attitudes and behaviors. Urology. 2004;64:991–7. doi: 10.1016/j.urology.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Phillips-Farfán BV, Lemus AE, Fernández-Guasti A. Increased estrogen receptor alpha immunoreactivity in the forebrain of sexually satiated rats. Horm Behav. 2007;51:328–334. doi: 10.1016/j.yhbeh.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Portillo W, Diaz NF, Cabrera EA, Fernández-Guasti A, Paredes RG. Comparative analysis of immunoreactive cells for androgen receptors and oestrogen receptor α in copulating and non-copulating male rats. J Neuroendocrinol. 2006;18:168–176. doi: 10.1111/j.1365-2826.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- Prince KN, Prince JS, Kinghorn EW, Fleming DE, Rhees RW. Effects of sexual behavioral manipulation on brain plasticity in adult rats. Brain Res Bull. 1998;47:349–355. doi: 10.1016/s0361-9230(98)00118-x. [DOI] [PubMed] [Google Scholar]
- Romano-Torres M, Phillips-Farfán BV, Chavira R, Rodriguez-Manzo G, Fernández-Guasti A. Relationship between sexual satiety and brain androgen receptors. Neuroendocrinol. 2006;85:16–26. doi: 10.1159/000099250. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Kaler LW, Resko JA. Hypothalamic aromatase activity in young and old male rats. Neurobiol Aging. 1986;7:121–5. doi: 10.1016/0197-4580(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44:499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- Sato Y, Shibuya A, Adachi H, Kato R, Horita H, Tsukamoto T. Restoration of sexual behavior and dopaminergic neurotransmission by long term exogenous testosterone replacement in aged male rats. J Urol. 1998;160:1572–5. [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Shen ZJ, Lu YL, Chen ZD, Chen F, Chen Z. Effects of androgen and ageing on gene expression of vasoactive intestinal polypeptide in rat corpus cavernosum. BJU International. 2000;86:133–137. doi: 10.1046/j.1464-410x.2000.00721.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992;26:110–35. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Steiner RA, Bremner WJ, Clifton DK, Dorsa DM. Reduced pulsatile luteinizing hormone and testosterone secretion with aging in the male rat. Biol Reprod. 1984;31:251–258. doi: 10.1095/biolreprod31.2.251. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; 1998. [Google Scholar]
- Taylor G, Bardgett M, Farr S, Humphrey W, Womack S, Weiss J. Aging of the brain-testicular axis: reproductive systems of healthy old male rats with or without endocrine stimulation. Proc Soc Exp Biol Med. 1996;211:69–75. doi: 10.3181/00379727-211-43953. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY. The role of aromatization in the restoration of male rat reproductive behavior. J Neuroendocrinol. 1997;9:415–21. doi: 10.1046/j.1365-2826.1997.00598.x. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY. The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats. Horm Behav. 1998;33:163–79. doi: 10.1006/hbeh.1998.1445. [DOI] [PubMed] [Google Scholar]
- Walker DM, Juenger TE, Gore AC. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology. 2009;150:2308–2316. doi: 10.1210/en.2008-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinol. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Intracellular partitioning of androgen receptor immunoreactivity in the brain of the male Syrian hamster: effects of castration and steroid replacement. J Neurobiol. 1993;24:925–38. doi: 10.1002/neu.480240706. [DOI] [PubMed] [Google Scholar]
- Wu D, Gore AC. Sexual experience changes sex hormones but not hypothalamic steroid hormone receptor expression in young and middle-aged male rats. Horm Behav. 2009;56:299–308. doi: 10.1016/j.yhbeh.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumpe D, Bonsall RW, Michael RP. Effects of the nonsteroidal aromatase inhibitor, fadrozole, on the sexual behavior of male cynomolgus monkeys (Macaca fascicularis) Horm Behav. 1993;27:200–15. doi: 10.1006/hbeh.1993.1015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.