Abstract
Mitochondrial superoxide production is an important source of reactive oxygen species in cells, and may cause or contribute to ageing and the diseases of ageing. Seven major sites of superoxide production in mammalian mitochondria are known and widely accepted. In descending order of maximum capacity they are the ubiquinone binding sites in complex I (site IQ) and complex III (site IIIQo), glycerol 3-phosphate dehydrogenase, the flavin in complex I (site IF), the electron transferring flavoprotein:Q oxidoreductase (ETFQOR) of fatty acid beta oxidation, and pyruvate and 2-oxoglutarate dehydrogenases. None of these sites is fully characterized and for some we only have sketchy information. The topology of the sites is important because it determines whether or not a site will produce superoxide in the mitochondrial matrix and be able to damage mitochondrial DNA. All sites produce superoxide in the matrix; site IIIQo and glycerol 3-phosphate dehydrogenase also produce superoxide to the intermembrane space. The relative contribution of each site to mitochondrial reactive oxygen species generation in the absence of electron transport inhibitors is unknown in isolated mitochondria, in cells or in vivo, and may vary considerably with species, tissue, substrate, energy demand and oxygen tension.
Keywords: Reactive oxygen species, ROS, electron transport, semiquinone, complex I, complex III, glycerol phosphate dehydrogenase, ETFQOR
1. Introduction
In the mitochondrial free radical theory of ageing (Harman, 1972), mitochondrial reactive oxygen species (ROS) generation is an inevitable consequence of oxidative ATP production, and the primary cause of macromolecular damage. Some damage is not repaired, causing progressive failure of cellular machinery, aging-related diseases, and ageing. There is considerable evidence both in favour and against the theory (Beckman and Ames, 1998; Finkel and Holbrook, 2000; Golden et al., 2002; Muller et al., 2007; Sanz et al., 2006). Most authors think that mitochondria-generated oxidative stress is important, particularly in ageing-related diseases, but is not the sole cause of ageing. According to a recent review (Muller et al., 2007), mitochondrial ROS convincingly determines lifespan in fungi (hyphal senescence in Podospora and chronological aging in Saccharomyces) and reasonably convincingly in Caenorhabditis elegans. The case is tentative in Drosophila but inconclusive in mouse and human, and better tests are required.
Seven specific sites that are involved in ROS generation have been defined in isolated mammalian mitochondria using electron transport chain inhibitors (Andreyev et al., 2005; Brand et al., 2004; Jezek and Hlavata, 2005; Murphy, 2009; Raha and Robinson, 2000; Turrens, 2003), but we lack reliable measurements of their rates in the absence of such inhibitors, and there is no consensus on their relative importance. In cells our knowledge of which sites are important is even worse. It is based mostly on measurements using inhibitors of electron transport. Typically, if rotenone (a complex I inhibitor) raises ROS production in cells, endogenous ROS generation is inferred to be from complex I, but this inference is unjustifiable, and new approaches are needed.
2. The mitochondrial free radical theory of ageing
An association between mitochondrial ROS generation and age-related disease is generally accepted, although it is also agreed that the mitochondrial free radical theory's explanation of ageing is incomplete (Beckman and Ames, 1998; Finkel and Holbrook, 2000; Golden et al., 2002; Muller et al., 2007; Sanz et al., 2006). Since age is the primary risk factor for many diseases, understanding the mechanisms of ageing may allow us to significantly reduce the burden of disease and increase human healthspan. It is therefore crucial to fully understand mitochondrial ROS production to assess its role in age-related diseases and ageing, and ultimately to allow rational design of beneficial therapies.
Age-related disease can be caused by overproduction of superoxide, resulting in molecular damage. Mitochondrial superoxide is detoxified to H2O2 by superoxide dismutases (matrix Mn-SOD, cytosolic Cu/Zn-SOD), then to O2 and H2O by antioxidant defences, including catalase or glutathione peroxidase. However, these antioxidants are imperfect, and superoxide that evades them damages proteins, lipids and DNA. H2O2 is relatively unreactive, but in the presence of Fe(III) it forms reactive hydroxyl radicals, initiating membrane lipid peroxidation (Halliwell and Gutteridge, 1999). The products of sugar, protein and lipid oxidation cause secondary damage to proteins (Shigenaga et al., 1994; Sohal and Weindruch, 1996). Thus, mitochondrial superoxide causes ‘oxidative stress’. The toxicity of matrix superoxide is shown by Mn-SOD knockout mice, which live only 10–20 days even in the presence of antioxidants (Lebovitz et al., 1996; Li et al., 1995). In contrast, cytosolic superoxide is not lethal in Cu/ZnSOD knockout mice (although they are sensitive to oxidative stress (Ho et al., 1998)) showing that extramitochondrial superoxide is less toxic. Many pathologies are related to oxidative stress, including atherosclerosis, hypertension, ischemia-reperfusion, inflammation, cancer, diabetes, Parkinson’s and Alzheimer’s disease (Halliwell and Gutteridge, 1999).
In ageing, there is good evidence for the importance of mitochondrial ROS generation (Cadenas and Davies, 2000). Beckman and Ames (1998) discuss 14 lines of evidence for the free radical theory, most implicating mitochondrial ROS. However, many are correlative and do not prove causality. One intriguing inverse correlation, between maximum lifespan and mitochondrial ROS production during reverse electron transport through complex I in different species (Barja et al., 1994; Ku et al., 1993; Lambert et al., 2007), is hard to explain if complex I ROS production does not contribute to ageing. More direct tests involve altering antioxidant defences, but give ambiguous results. Overexpression of Cu/Zn-SOD in Drosophila either has no effect (Seto et al., 1990), or increases lifespan (Sun et al., 2002). SOD/catalase mimetics increase lifespan in C.elegans (Melov et al., 2000), but only under specific conditions (Keaney and Gems, 2003). Overexpression of antioxidant defences in mice does not generally increase lifespan (Perez et al., 2009). In principle, the best test is to show that decreasing mitochondrial ROS production slows ageing. A striking result from RNAi screens is that knockdown of most mitochondrial electron transport proteins extends life in C. elegans (Dillin et al., 2002; Lee et al., 2003). However, because we do not know which sites in the electron transport chain produce the most ROS, it is hard to know how to alter ROS production without also altering ATP synthesis.
3. Mitochondria as a source of ROS
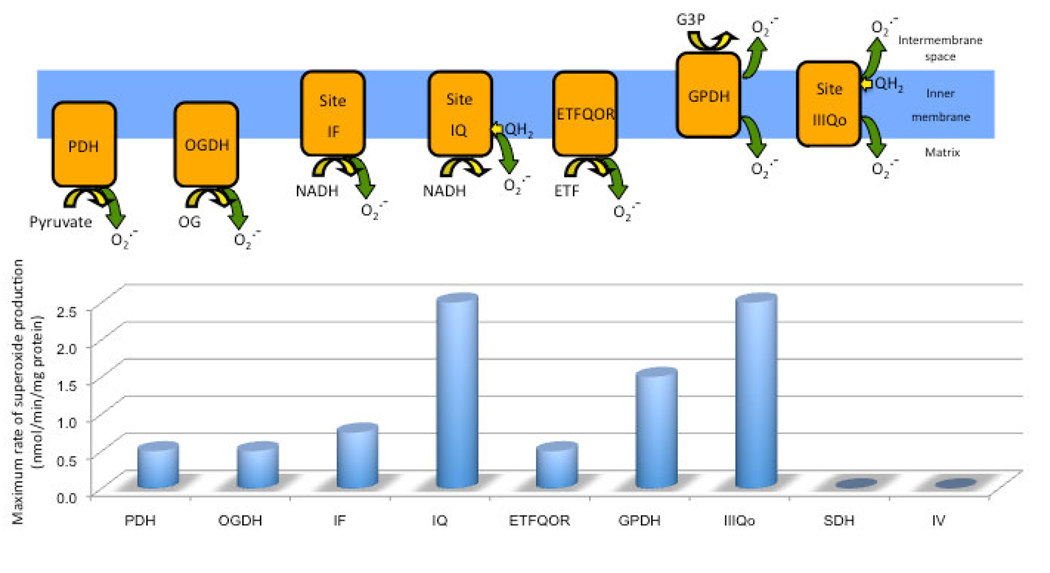
The respiratory chain produces superoxide when single electrons leak to O2 as electron pairs flow down the chain (Chance et al., 1979). Interest in mitochondrial ROS began more than 40 years ago (Boveris and Chance, 1973; Boveris et al., 1972; Hinkle et al., 1967; Jensen, 1966) and has been well-reviewed (Andreyev et al., 2005; Brand et al., 2004; Jezek and Hlavata, 2005; Murphy, 2009; Raha and Robinson, 2000; Turrens, 2003). There are currently seven separate sites of mammalian mitochondrial ROS production that have been identified and widely accepted (Fig. 1) and these are discussed in more depth below. The sites with the greatest maximum capacities to produce ROS are at complex I (site IQ) and complex III (site IIIQo) (Barja, 1999; Chen et al., 2003; Kudin et al., 2004; Liu et al., 2002; Raha and Robinson, 2000; St-Pierre et al., 2002; Votyakova and Reynolds, 2001), although the other five sites also have significant maximum rates (Andreyev et al., 2005) (Fig. 1). The topology may be vital, given the location of mitochondrial DNA in the matrix and its susceptibility to oxidative damage. Complex I produces superoxide to the matrix, whereas complex III produces it to the matrix and intermembrane space at about equal rates under de-energized conditions (Fig. 1) (Han et al., 2003; Han et al., 2001; Miwa and Brand, 2005; Miwa et al., 2003; Muller et al., 2004; St-Pierre et al., 2002).
Figure 1. The sites and topology of mitochondrial superoxide production.
The seven identified sites of superoxide production in mitochondria are shown in the upper diagram, with an indication of the location of their substrate-binding sites and the topology of their superoxide production. PDH, pyruvate dehydrogenase; OGDH, 2-oxoglutarate dehydrogenase; site IF, the FMN-containing NADH binding site of complex I; site IQ, ubiquinone reduction site of complex I; ETFQOR, electron transferring flavoprotein ubiquinone oxidoreductase – the entry point of electrons from flavin-linked beta-oxidation of fatty acids via ETF (electron transferring flavoprotein) to ubiquinone in the electron transport chain; GPDH, glycerol 3-phosphate dehydrogenase; site IIIQo, the outer quinone-binding site of the Q-cycle in complex III. PDH and OGDH may also produce hydrogen peroxide directly.
The lower panel shows representative values of the maximum superoxide production rate from each site, and from SDH (succinate dehydrogenase; complex II) and IV (complex IV). Rates are for rat skeletal muscle mitochondria, from Lambert et al., 2007; Lambert and Brand, 2004a, b; Lambert et al., 2008a; Lambert et al., 2008b; St-Pierre et al., 2002 and Jason Treberg, Casey Quinlan and Martin D Brand (unpublished observations), and are greatest from sites IQ and IIIQo, followed by GPDH, then sites IF, EFTQOR, PDH and OGDH. There is insignificant superoxide production from complex II and complex IV (or from pool Q and cytochrome c, not shown).
In isolated cells, or in vivo, it is thought that mitochondria produce significant amounts of ROS (Skulachev, 1996), although the evidence is not fully compelling. The main indirect evidence comes from the lifespan-shortening effect of Mn-SOD knockout in mice (Lebovitz et al., 1996; Li et al., 1995). Fluorescent probes such as dichlorodihydrofluorescein or dihydrorhodamine have been used in hundreds of studies to infer mitochondrial ROS production in cells, but suffer from interpretational problems (Wardman, 2007). Hydroethidine and its mitochondria-targeted form, mitoSOX, may be more reliable (Jekabsons and Nicholls, 2006; Johnson-Cadwell et al., 2007; Mukhopadhyay et al., 2007; Song et al., 2007).
The frequent assertion that 1–4% of mitochondrial O2 consumption is diverted to ROS in vivo is wrong. It is based on the maximum rate of superoxide production from complex III in mitochondria in the presence of antimycin, at saturating substrate and O2 (Chance et al., 1979). Less unrealistic conditions predict lower values, only 0.15% (Hansford et al., 1997; St-Pierre et al., 2002). Which of the specific sites of mitochondrial ROS production is most active in cells in the absence of inhibitors of the electron transport chain is unclear.
4. Measurement of ROS production in isolated mitochondria
Superoxide does not cross the mitochondrial inner membrane, so bulk phase assays using isolated mitochondria do not register matrix superoxide. However, matrix Mn-SOD converts most of the matrix superoxide to H2O2, which diffuses to the medium for assay. The standard assay of extramitochondrial H2O2 is to add horseradish peroxidase (to reduce H2O2 to H2O) and a substrate (e.g. amplex red) that is oxidized to a fluorescent product (resorufin). The method underestimates matrix superoxide production if significant amounts evade Mn- SOD in the matrix, or if significant amounts of H2O2 are scavenged by peroxidases such as glutathione peroxidase or catalase. Early work used homovanillic acid for detection (Barja, 2002). However, this suffers from high background rates and low accuracy, and the native rates of superoxide production during oxidation of pyruvate and malate in skeletal muscle or heart mitochondria are at or below the detection limit (St-Pierre et al., 2002). Amplex red or amplex ultra red are better probes, giving a greater signal and low backgrounds, although it is still necessary to control for fluorescence quenching and to calibrate carefully (Lambert et al., 2007; Lambert et al., 2008a; Lambert et al., 2008b). With amplex red, native rates of superoxide production of less than 0.1 nmol.min−1.(mg protein)−1 can be measured (Lambert et al., 2007). This compares to 3 to 6 nmol.min−1.(mg protein)−1 during reverse electron transport, or forward electron transport plus antimycin in mammalian heart or skeletal muscle mitochondria or Drosophila mitochondria (Lambert and Brand, 2004b; Lambert et al., 2008b; Miwa et al., 2003). Matrix aconitase can also be used to assay matrix superoxide under different conditions (Talbot et al., 2004). Aconitase has a labile active site Fe-S centre, and is sensitive to superoxide. If calibrated using known rates of superoxide production, e.g. from site IF, aconitase inactivation kinetics can report matrix ROS production in absolute units (Miwa and Brand, 2005).
5. Sites of mitochondrial ROS production
The relative importance of each site to total superoxide production in isolated mitochondria is contentious, partly because of different assays, different substrates and different sources of mitochondria. Most assays of superoxide production from defined sites measure maximal capacities for superoxide production, and the actual rate from each site in the absence of inhibitors is not known. During reverse electron transport from succinate to NAD±, complex I can produce superoxide at high rates (Han et al., 2003; Hansford et al., 1997; Kushnareva et al., 2002; Lambert and Brand, 2004a, b; Liu et al., 2002; Turrens and Boveris, 1980; Votyakova and Reynolds, 2001), although the physiological relevance is unclear (Muller et al., 2008). During forward electron transport from NAD-linked substrates (which may be more physiological), mostmitochondria produce superoxide at high rates after addition of inhibitors such as rotenone (for complex I) (Han et al., 2003; Hansford et al., 1997; Lambert and Brand, 2004a, b; Liu et al., 2002; St-Pierre et al., 2002; Votyakova and Reynolds, 2001) or antimycin A (for complex III) (Chen et al., 2003; Gyulkhandanyan and Pennefather, 2004; Herrero and Barja, 1997; Kwong and Sohal, 1998; Liu et al., 2002; McLennan and Degli Esposti, 2000; Raha et al., 2000; St-Pierre et al., 2002; Turrens et al., 1985). Other physiologically-relevant substrates, such as fatty acids and glycerol 3-phosphate, may cause superoxide production from sites that are less active during pyruvate oxidation, such as ETF–Q oxidoreductase and glycerol 3-phosphate dehydrogenase.
5.1. ROS production at Complex I: centre IF
Several pathologies are associated with elevated production of superoxide by defective complex I (Raha and Robinson, 2000), and it is important to identify the sites of superoxide production within this large multi-subunit complex. The redox centres are flavin mononucleotide (FMN), eight Fe-S clusters (N1a, N1b, N2, N3, N4, N5, N6a and N6b), and Q. The complex has a hydrophobic arm and a matrix-protruding hydrophilic arm (containing FMN and the Fe-S clusters). The Q binding site is at the junction between the arms. How electron transfer is coupled to proton pumping is a major unresolved question (Sharma et al., 2009).
In isolated complex I, the fully reduced flavin (site IF) dominates superoxide production (Kussmaul and Hirst, 2006). In isolated mitochondria, substrates for forward electron transport that feed into complex I (e.g. pyruvate plus malate) give low rates of superoxide production (Gyulkhandanyan and Pennefather, 2004; Hansford et al., 1997; Kudin et al., 2004; Kushnareva et al., 2002; Kwong and Sohal, 1998; Lambert and Brand, 2004b; Liu et al., 2002; Votyakova and Reynolds, 2001). Under these 'physiological' conditions, site IF is relatively oxidized, and the balance between superoxide from this site and the remaining sites in the electron transport chain is currently unknown. Rotenone blocks the quinine-binding site of complex I, causing electrons to back up and fully reduce the upstream redox centres. This increases the low rate several-fold, suggesting that complex I has the capacity to make superoxide at significant rates during forward electron transport from a site upstream of the rotenone block. This site is usually identified as site IF. However, it may also include matrix dehydrogenases (pyruvate dehydrogenase and oxoglutarate dehydrogenase) (Chinta et al., 2009; Gazaryan et al., 2002; Starkov et al., 2004; Tretter and Adam-Vizi, 2004). Site IF has been championed from inhibitor studies (Johnson et al., 2003; Liu et al., 2002; Young et al., 2002) and on thermodynamic grounds (Kudin et al., 2004). Fe-S clusters (Herrero and Barja, 2000), specifically N1a (Kushnareva et al., 2002) or N2 (Genova et al., 2001), have also been proposed. However, there is now general agreement that superoxide production from rotenone-inhibited mitochondria in the presence of substrates that reduce matrix NAD is primarily from site IF (and from upstream dehydrogenases).
5.2. ROS production at Complex I: centre IQ
The maximum rate of superoxide production by complex I in mitochondria varies considerably. Succinate or glycerol 3-phosphate give the highest rates (Gyulkhandanyan and Pennefather, 2004; Hansford et al., 1997; Kushnareva et al., 2002; Lambert and Brand, 2004b; Liu et al., 2002; Miwa et al., 2003; Ohnishi et al., 2005; Votyakova and Reynolds, 2001). These substrates reduce ubiquinone (Q) to QH2 and generate a protonmotive force, driving electrons thermodynamically uphill through complex I to reduce NAD± to NADH. Rotenone (the complex I Q site inhibitor) markedly diminishes superoxide production (Gyulkhandanyan and Pennefather, 2004; Hansford et al., 1997; Kushnareva et al., 2002; Lambert and Brand, 2004b; Liu et al., 2002; Ohnishi et al., 2005; Votyakova and Reynolds, 2001), showing that large amounts of superoxide are generated by complex I during reverse electron transport from QH2 to NAD±. This superoxide appears to come from the quinine-binding site in complex I, site IQ. Maximum superoxide production from complex I during reverse electron transport is much faster than the maximum rate from the flavin during forward electron transport, so site IF has a lower maximum capacity and contributes little to the high rate during reverse electron transport (Fig. 1).
There is some controversy about the presence of two separate sites of superoxide production in complex I (sites IF and IQ) rather than a single site (Hirst et al., 2008; Lambert et al., 2008a; Lambert et al., 2008b), and more work needs to be done to fully resolve this issue. Complicating the analysis, some of the inhibitors used to identify sites in complex I are poorly characterized. They include 'FMN-specific' diphenyleneiodonium, Fe-S centre inhibitors such as p-chloromercuribenzoate and ethoxyformic anhydride, and a variety of Q site inhibitors (Degli Esposti, 1998). In our hands, (except for rotenone and piericidin) these inhibitors also uncouple and lack site-specificity, making them unreliable for identifying ROS sites. In our current model of superoxide production during reverse electron transport, the majority originates from site IQ. Site IF produces superoxide at modest rates that only dominate when site IQ is inactive and the flavin is fully reduced, such as during forward electron transport in the presence of rotenone and lack of a pH gradient (Lambert and Brand, 2004a). Site IQ is not normally very active during forward electron transport. It only generates ROS during forward electron transport when very specific conditions are met: the presence of both a large pH gradient and a specific inhibitor of the Q-binding site, such as myxothiazol, which may distort the site to allow ROS generation and mimic the state that occurs during reverse electron transport (Lambert and Brand, 2004a). Rotenone has a similar, but less marked, effect in the presence of a pH gradient. In the absence of a pH gradient it gives the same ROS production as stigmatellin, a complex III inhibitor, so ROS production during forward electron transport in the presence of rotenone or stigmatellin comes from site IF (plus upstream sites).
We have tested these assignments of IQ and IF in mitochondria. Previous evidence for the involvement of site IF during reverse electron transport, based on the use of a 'flavin-site specific' inhibitor, diphenyleneiodonium, is flawed because the inhibitor works not at site IF but at site IQ in mitochondria in short-term incubations (Lambert et al., 2008a). We have also disproved suggestions that ROS production from complex I during reverse electron transport is from site IF (or upstream) and can be explained by excessive reduction of the flavin under these conditions – we find that the NAD(P)H pool (which reports the flavin redox state) during reverse electron transport is not more, but less reduced than during forward electron transport with rotenone (Lambert et al., 2008b).
These issues have raised the question of whether the major complex I ROS-producing site (IQ) is active in vivo. The answer is unknown, but is crucial to testing and fully formulating the mitochondrial free radical theory of ageing. Some in vitro evidence suggests that it may be active (Muller et al., 2008; St-Pierre et al., 2002). The strongest indirect evidence that the site is physiologically active is that the activity of site IQ is inversely correlated with longevity in mitochondria from different species (Barja et al., 1994; Ku et al., 1993) even when confounding effects of body mass are eliminated (Lambert et al., 2007). However, better and more direct evidence of the importance (or not) of site IQ, and indeed of all the mitochondrial sites, is required, firstly in mitochondria oxidizing 'physiological' substrates and secondly in cells.
The components of protonmotive force (particularly the pH gradient (Lambert and Brand, 2004b)) dramatically affect superoxide production by site IQ (Gyulkhandanyan and Pennefather, 2004; Hansford et al., 1997; Korshunov et al., 1997; Lambert and Brand, 2004a, b; Liu, 1997; Liu et al., 2002; Votyakova and Reynolds, 2001). Therefore mild uncoupling, which slightly lowers both components, greatly decreases the high superoxide production during reverse electron transport. Mild uncoupling also tends to oxidize other electron carriers and decrease the local oxygen tension, decreasing superoxide production from the other sites as well. This has led to concept of "uncoupling to survive", which proposes that lowering protonmotive force by mild uncoupling lowers superoxide production whilst allowing sufficient ATP production. This might lower oxidative damage and increase lifespan (Brand, 2000; Skulachev, 1996).
5.3. ROS production at Complex III: centre IIIQo
Superoxide is produced by the single electron reduction of O2 by electron carriers. In complex III these are cytochromes b566, b562 and c1, the Rieske Fe-S centre, and the quinones at centres ‘i’ and ‘o’ (Zhang et al., 2000). The main reductant is the semiquinone at centre o (IIIQo) (Boveris and Chance, 1973; Cadenas et al., 1977; Turrens et al., 1985), consistent with a Q cycle mechanism (Turrens, 2003). In mitochondria, complex III produces superoxide at high rates only in the presence of antimycin (Chen et al., 2003; Gyulkhandanyan and Pennefather, 2004; Herrero and Barja, 1997; Kwong and Sohal, 1998; Liu et al., 2002; McLennan and Degli Esposti, 2000; Raha et al., 2000; St-Pierre et al., 2002; Turrens et al., 1985). This inhibitor blocks electron transfer from b566to the quinone at centre i, causing a small build-up of semiquinone at centre o, which readily reduces O2 to superoxide (Cape et al., 2007). Centre o inhibitors of complex III (e.g. myxothiazol or stigmatellin), stop electrons accessing centre o, and prevent these high rates (Ksenzenko et al., 1983; Raha et al., 2000; Turrens et al., 1985), suggesting that the main superoxide site at complex III is the semiquinone at centre o, with little from neighbouring sites, such as centre i, pool ubiquinone (QH2/Q) or complex II (Miwa et al., 2003; St-Pierre et al., 2002). Over-reduction of site IIIQo decreases the concentration of the reactive semiquinone at centre o, and lowers superoxide production from this site below the maximum rate obtainable under slightly more oxidizing conditions (Drose and Brandt, 2008; Erecinska and Wilson, 1976).
The superoxide production rate from site IIIQo in coupled mitochondria oxidizing NAD-linked substrates in the absence of antimycin is unknown, because addition of myxothiazol or stigmatellin to block this site also indirectly perturbs the complex I sites by collapsing the protonmotive force and altering the redox state of the Q and NAD pools. Some authors propose that site IIIQo is the main site under physiological conditions (Chen et al., 2003); others argue that its role is minor (Kushnareva et al., 2002; Lambert and Brand, 2004a; Liu et al., 2002; Votyakova and Reynolds, 2001). Definitive experiments are needed.
5.4. ROS production at other mitochondrial sites
Complex II mutations and consequent overproduction of ROS causes disease in humans (Rustin et al., 2002) and C. elegans (Senoo-Matsuda et al., 2001). Compared to the maximum rates of superoxide production at complexes I or III, rates at complex II (succinate dehydrogenase) are normally negligible (Chen et al., 2003; Gyulkhandanyan and Pennefather, 2004; Hansford et al., 1997; McLennan and Degli Esposti, 2000; Miwa et al., 2003; Turrens et al., 1985) (Fig. 1) because the enzyme suppresses the formation of a flavin radical in the active site. The rates during succinate oxidation by fumarate reductase, which catalyses the reverse reaction in Escherichia coli and lacks this mechanism, are much greater (Messner and Imlay, 2002).
Glycerol 3-phosphate dehydrogenase generates ROS in mitochondria (Drahota et al., 2002; Gyulkhandanyan and Pennefather, 2004; Kwong and Sohal, 1998; Miwa et al., 2003; Sekhar et al., 1987; Tretter et al., 2007). Topological measurements show that superoxide is produced to both sides of the membrane (Miwa et al., 2003). Since the crystal structure shows that the flavin is well outside the plane of the membrane (in the intermembrane space) (Yeh et al., 2008), the membrane-buried quinone-binding site of this dehydrogenase may be the main site of superoxide production. Further study on this site is needed.
During oxidation of fatty acids the electron transferring flavoprotein Q oxidoreductase (ETFQOR) may produce superoxide at significant rates to the matrix side of the membrane (St-Pierre et al., 2002), but this site remains poorly characterized.
ROS production from sites downstream of complex III (cytochrome c, complex IV) can be measured by difference when these sites are fully reduced by addition of cyanide (subtracting off measured rates from upstream); they make little contribution (Miwa et al., 2003).
Other mitochondrial sites can generate ROS (Andreyev et al., 2005), including 2- oxoglutarate and pyruvate dehydrogenases (Chinta et al., 2009; Gazaryan et al., 2002; Starkov et al., 2004; Tretter and Adam-Vizi, 2004) and non-mammalian NADH-Q oxidoreductases (Fang and Beattie, 2003). Their ROS production is poorly characterized and deserves further work.
6. Topology of mitochondrial superoxide production
The topology of ROS production is crucial for which sites might most damage mtDNA. In intact mitochondria superoxide that is produced to the matrix is converted by matrix Mn-SOD to H2O2, which diffuses out and is assayed in the extramitochondrial medium. However, superoxide that is produced to the intermembrane space requires added SOD to be fully converted to H2O2 for assay, so H2O2 production that is dependent on added SOD is produced to the intermembrane space not to the matrix. This assay accurately identifies external superoxide production from particular sites, and enables estimation of the topology of superoxide production, although the quantitative estimate of sidedness is crude because of effects of endogenous external dismutases and internal peroxidases (Miwa and Brand, 2005; Miwa et al., 2003; St-Pierre et al., 2002; Muller et al., 2004). Using this assay, the topology of superoxide production from each of the electron transport chain sites has been determined (Fig. 1). Because of their matrix localization, pyruvate dehydrogenase and oxoglutarate dehydrogenase are presumed to make superoxide exclusively in the matrix. All of the electron transport chain sites also produce superoxide exclusively to the matrix, except for complex III and glycerol 3-phosphate dehydrogenase (Miwa and Brand, 2005; Miwa et al., 2003; St-Pierre et al., 2002).
The original view was that ROS production by complex III was directed towards the matrix, but the crystal structure (Iwata et al., 1998) showed that site IIIQo faces the cytoplasmic side of the membrane, making it unclear how this site could generate matrix superoxide. We found that ROS from IIIQo was generated partly outside and partly inside the mitochondria (St-Pierre et al., 2002). The same held in Drosophila mitochondria, where superoxide from both site IIIQo and glycerol 3-phosphate dehydrogenase was directed about equally to each side of the membrane (Miwa and Brand, 2005; Miwa et al., 2003). Approximately equal production of site IIIQo superoxide to each side of the membrane was also shown in an elegant study in mammalian mitochondria (Muller et al., 2004). The mechanism by which these sites generate superoxide to both sides of the membrane is not fully resolved, but it probably involves release of semiquinone or hydroperoxyl radicals within the membrane bilayer, followed by diffusion and reaction (with oxygen or by deprotonation, respectively) to form superoxide which spills out to both sides of the membrane (Muller et al., 2004).
7. ROS production by mitochondria in cells
Mitochondria are frequently asserted to be the main producers of ROS in cells (Skulachev, 1996), but this conclusion is not thoroughly established. In some cells, other sources, such as cytochrome P450 (Caro and Cederbaum, 2004) or plasma membrane NADPH oxidases (Babior et al., 2002), produce large amounts of ROS (Jezek and Hlavata, 2005), and it remains to be established what proportion of cellular ROS production is mitochondrial in different cells. Since mitochondrial ROS production may depend hyperbolically on oxygen tension, with different oxygen affinities at different sites (Hoffman and Brookes, 2009), the contribution of different sites may vary as oxygen tension in a cell rises and falls.
Two problems hinder identification of the specific mitochondrial sites of ROS production in cells. The first is the methods to quantify ROS production. Fluorescent probes such as dichlorodihydrofluorescein or dihydrorhodamine have interpretational problems – their specificity is often unclear and they may themselves cause ROS production (Wardman, 2007). Hydroethidine and mitoSOX may be more reliable (Jekabsons and Nicholls, 2006; Johnson-Cadwell et al., 2007; Mukhopadhyay et al., 2007; Song et al., 2007). The second is the use of electron transport inhibitors to identify sites of ROS production, which poses the same problems as with isolated mitochondria. It is unknown whether reverse electron transport and the associated high superoxide production occur in cells. Some studies (Aon et al., 2003; Chandel et al., 1998; Li and Trush, 1998; Parthasarathi et al., 2002; Quillet-Mary et al., 1997; Schuchmann and Heinemann, 2000; Vrablic et al., 2001; Zamzami et al., 1995) find that rotenone decreases cellular ROS formation, suggesting that site IQ (or IIIQo) is active in cells. Conversely, many studies report that rotenone increases ROS production in cells (Barrientos and Moraes, 1999; Li et al., 2003; Nakamura et al., 2001; Siraki et al., 2002). The latter result is often taken to imply the involvement of site IF in the cells, however, this conclusion is erroneous, because rotenone addition reduces site IF and increases ROS generation, regardless of whether it was sufficiently reduced to produce significant ROS before inhibition. Therefore, the observation of increased ROS production in cells following addition of rotenone cannot be used to infer the identity of the native mitochondrial site of ROS production, and other methods need to be developed.
8. Conclusion
Mitochondrial ROS production is clearly involved in ageing and the diseases of ageing, but its exact roles and importance remain ambiguous. We now know the identity of seven sites within mitochondria that generate superoxide, and have a good idea of their topology and their relative maximum capacities (Fig. 1). What we do not know is the rate at which these sites run under different physiological conditions of substrate supply and energy demand in isolated mitochondria, in intact cells, and in vivo. Working out ways to make such measurements is an important future challenge, but very necessary if we are to fully understand mitochondrial ROS production and its role in ageing and disease.
Acknowledgements
Supported by grants from the National Institutes of Health (P01 AG025901, PL1 AG032118, P30 AG025708 and R01 AG033542) and the Keck Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg. Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- Barja G. The quantitative measurement of H2O2 generation in isolated mitochondria. J Bioenerg. Biomembr. 2002;34:227–233. doi: 10.1023/a:1016039604958. [DOI] [PubMed] [Google Scholar]
- Barja G, Cadenas S, Rojas C, Perez-Campo R, Lopez-Torres M. Low mitochondrial free radical production per unit O2 consumption can explain the simultaneous presence of high longevity and high aerobic metabolic rate in birds. Free Rad. Res. 1994;21:317–327. doi: 10.3109/10715769409056584. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Moraes CT. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J. Biol. Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem. J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Rad. Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977;180:248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Rad. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Cape JL, Bowman MK, Kramer DM. A semiquinone intermediate generated at the Qo site of the cytochrome bc1 complex: importance for the Q-cycle and superoxide production. Proc. Nat. Acad. Sci.USA. 2007;104:7887–7892. doi: 10.1073/pnas.0702621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharm. Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Nat. Acad. Sci. USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Rane A, Yadava N, Andersen JK, Nicholls DG, Polster BM. Reactive oxygen species regulation by AIF- and complex I-depleted brain mitochondria. Free Rad. Biol. Med. 2009;46:939–947. doi: 10.1016/j.freeradbiomed.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli Esposti M. Inhibitors of NADH-ubiquinone reductase: an overview. Biochim. Biophys. Acta. 1998;1364:222–235. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Drahota Z, Chowdhury SK, Floryk D, Mracek T, Wilhelm J, Rauchova H, Lenaz G, Houstek J. Glycerophosphate-dependent hydrogen peroxide production by brown adipose tissue mitochondria and its activation by ferricyanide. J. Bioenerg. Biomembr. 2002;34:105–113. doi: 10.1023/a:1015123908918. [DOI] [PubMed] [Google Scholar]
- Drose S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Wilson DF. The effect of antimycin A on cytochromes b561, b566, and their relationship to ubiquinone and the iron-sulfer centers S-1 (+N-2) and S-3. Arch. Biochem. Biophys. 1976;174:143–157. doi: 10.1016/0003-9861(76)90333-7. [DOI] [PubMed] [Google Scholar]
- Fang J, Beattie DS. External alternative NADH dehydrogenase of Saccharomyces cerevisiae: a potential source of superoxide. Free Rad. Biol. Med. 2003;34:478–488. doi: 10.1016/s0891-5849(02)01328-x. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Gazaryan IG, Krasnikov BF, Ashby GA, Thorneley RN, Kristal BS, Brown AM. Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J. Biol. Chem. 2002;277:10064–10072. doi: 10.1074/jbc.M108264200. [DOI] [PubMed] [Google Scholar]
- Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001;505:364–368. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1:117–123. doi: 10.1046/j.1474-9728.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Gyulkhandanyan AV, Pennefather PS. Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J. Neurochem. 2004;90:405–421. doi: 10.1111/j.1471-4159.2004.02489.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 1999. [Google Scholar]
- Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol. Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J. Am. Geriat. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G. Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon. Mech. Ageing Dev. 1997;98:95–111. doi: 10.1016/s0047-6374(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G. Localization of the site of oxygen radical generation inside the complex I of heart and nonsynaptic brain mammalian mitochondria. J. Bioenerg. Biomembr. 2000;32:609–615. doi: 10.1023/a:1005626712319. [DOI] [PubMed] [Google Scholar]
- Hinkle PC, Butow RA, Racker E, Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J. Biol. Chem. 1967;242:5169–5173. [PubMed] [Google Scholar]
- Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J. Biol. Chem. 1998;273:7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J. Biol. Chem. 2009;284:16236–16245. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- Jekabsons MB, Nicholls DG. Bioenergetic analysis of cerebellar granule neurons undergoing apoptosis by potassium/serum deprivation. Cell Death Differentiation. 2006;13:1595–1610. doi: 10.1038/sj.cdd.4401851. [DOI] [PubMed] [Google Scholar]
- Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. I. pH dependency and hydrogen peroxide formation. Biochim. Biophys. Acta. 1966;122:157–166. doi: 10.1016/0926-6593(66)90057-9. [DOI] [PubMed] [Google Scholar]
- Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Jr, Choksi K, Widger WR. NADH-Ubiquinone oxidoreductase: substrate-dependent oxygen turnover to superoxide anion as a function of flavin mononucleotide. Mitochondrion. 2003;3:97–110. doi: 10.1016/S1567-7249(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. 'Mild uncoupling' does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J. Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- Keaney M, Gems D. No increase in lifespan in Caenorhabditis elegans upon treatment with the superoxide dismutase mimetic EUK-8. Free Rad. Biol. Med. 2003;34:277–282. doi: 10.1016/s0891-5849(02)01290-x. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Ksenzenko M, Konstantinov AA, Khomutov GB, Tikhonov AN, Ruuge EK. Effect of electron transfer inhibitors on superoxide generation in the cytochrome bc1 site of the mitochondrial respiratory chain. FEBS Lett. 1983;155:19–24. doi: 10.1016/0014-5793(83)80200-2. [DOI] [PubMed] [Google Scholar]
- Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Rad. Biol. Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J. Biol. Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussmaul L, Hirstq J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Nat. Acad. Sci. USA. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch. Biochem. Biophys. 1998;350:118–126. doi: 10.1006/abbi.1997.0489. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J. Biol. Chem. 2004a;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 2004b;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Buckingham JA, Boysen HM, Brand MD. Diphenyleneiodonium acutely inhibits reactive oxygen species production by mitochondrial complex I during reverse, but not forward electron transport. Biochim. Biophys. Acta. 2008a;1777:397–403. doi: 10.1016/j.bbabio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Buckingham JA, Brand MD. Dissociation of superoxide production by mitochondrial complex I from NAD(P)H redox state. FEBS Lett. 2008b;582:1711–1714. doi: 10.1016/j.febslet.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Nat. Acad. Sci. USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Gen. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys. Res. Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- McLennan HR, Degli Esposti M. The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J. Bioenerg. Biomembr. 2000;32:153–162. doi: 10.1023/a:1005507913372. [DOI] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Messner KR, Imlay JA. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J. Biol. Chem. 2002;277:42563–42571. doi: 10.1074/jbc.M204958200. [DOI] [PubMed] [Google Scholar]
- Miwa S, Brand MD. The topology of superoxide production by complex III and glycerol 3-phosphate dehydrogenase in Drosophila mitochondria. Biochim. Biophys. Acta. 2005;1709:214–219. doi: 10.1016/j.bbabio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Miwa S, St-Pierre J, Partridge L, Brand MD. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Rad. Biol. Med. 2003;35:938–948. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protocols. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem. J. 2008;409:491–499. doi: 10.1042/BJ20071162. [DOI] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Rad. Biol. Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Bindokas VP, Kowlessur D, Elas M, Milstien S, Marks JD, Halpern HJ, Kang UJ. Tetrahydrobiopterin scavenges superoxide in dopaminergic neurons. J. Biol. Chem. 2001;276:34402–34407. doi: 10.1074/jbc.M103766200. [DOI] [PubMed] [Google Scholar]
- Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J. Bioenerg. Biomembr. 2005;37:1–15. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- Parthasarathi K, Ichimura H, Quadri S, Issekutz A, Bhattacharya J. Mitochondrial reactive oxygen species regulate spatial profile of proinflammatory responses in lung venular capillaries. J. Immunol. 2002;169:7078–7086. doi: 10.4049/jimmunol.169.12.7078. [DOI] [PubMed] [Google Scholar]
- Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J. Biol. Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- Raha S, McEachern GE, Myint AT, Robinson BH. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase. Free Rad. Biol. Med. 2000;29:170–180. doi: 10.1016/s0891-5849(00)00338-5. [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Rustin P, Munnich A, Rotig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur. J. Hum. Genet. 2002;10:289–291. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antiox. Redox Sign. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Heinemann U. Increased mitochondrial superoxide generation in neurons from trisomy 16 mice: a model of Down's syndrome. Free Rad. Biol. Med. 2000;28:235–250. doi: 10.1016/s0891-5849(99)00226-9. [DOI] [PubMed] [Google Scholar]
- Sekhar BS, Kurup CK, Ramasarma T. Generation of hydrogen peroxide by brown adipose tissue mitochondria. J. Bioenerg. Biomembr. 1987;19:397–407. doi: 10.1007/BF00768542. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H, Hartman PS, Ishii N. A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J. Biol. Chem. 2001;276:41553–41558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- Seto NO, Hayashi S, Tener GM. Overexpression of Cu-Zn superoxide dismutase in Drosophila does not affect life-span. Proc. Nat. Acad. Sci.USA. 1990;87:4270–4274. doi: 10.1073/pnas.87.11.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma LK, Lu J, Bai Y. Mitochondrial respiratory complex I: structure, function and implication in human diseases. Curr. Med. Chem. 2009;16:1266–1277. doi: 10.2174/092986709787846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc. Nat. Acad. Sci. USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraki AG, Pourahmad J, Chan TS, Khan S, O'Brien PJ. Endogenous and endobiotic induced reactive oxygen species formation by isolated hepatocytes. Free Rad. Biol. Med. 2002;32:2–10. doi: 10.1016/s0891-5849(01)00764-x. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Du Y, Prabhu SD, Epstein PN. Diabetic cardiomyopathy in OVE26 mice shows mitochondrial ROS production and divergence between in vivo and in vitro contractility. Rev. Diabet. Stud. 2007;4:159–168. doi: 10.1900/RDS.2007.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot DA, Lambert AJ, Brand MD. Production of endogenous matrix superoxide from mitochondrial complex I leads to activation of uncoupling protein 3. FEBS Lett. 2004;556:111–115. doi: 10.1016/s0014-5793(03)01386-3. [DOI] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J. Neurosci. 2004;24:7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Takacs K, Hegedus V, Adam-Vizi V. Characteristics of alpha-glycerophosphate-evoked H2O2 generation in brain mitochondria. J. Neurochem. 2007;100:650–663. doi: 10.1111/j.1471-4159.2006.04223.x. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. Δψm-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- Vrablic AS, Albright CD, Craciunescu CN, Salganik RI, Zeisel SH. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001;15:1739–1744. doi: 10.1096/fj.00-0300com. [DOI] [PubMed] [Google Scholar]
- Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Rad. Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Yeh JI, Chinte U, Du S. Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism. Proc. Nat. Acad. Sci. USA. 2008;105:3280–3285. doi: 10.1073/pnas.0712331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TA, Cunningham CC, Bailey SM. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch. Biochem. Biophys. 2002;405:65–72. doi: 10.1016/s0003-9861(02)00338-7. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Berry EA, Huang LS, Kim SH. Mitochondrial cytochrome bc1 complex. Subcell. Biochem. 2000;35:541–580. [PubMed] [Google Scholar]