Abstract
The reduction of portal pressure in patients with early compensated cirrhosis may be more responsive to drugs increasing intrahepatic vasodilatation than those reducing portal venous inflow. The PDE-V inhibitor sildenafil can potentially reduce portal pressure by decreasing intrahepatic resistance, but its systemic vasodilatory effects may be deleterious.
Aim:
Evaluate the effect of sildenafil on systemic and portal hemodynamics in an open-label pilot study.
Methods:
Twelve patients with compensated cirrhosis and baseline hepatic venous pressure gradient (HVPG) >5 mmHg received 25mg of oral sildenafil. Mean arterial pressure(MAP), heart rate (HR) and HVPG were repeated after 30 and 60 minutes and in 9/12 patients at 90 minutes (after an additional 25mg of sildenafil). HVPG tracings were read by 3 blinded observers.
Results:
All 12 patients were Child A with median MAP 92 mm Hg(84-95) and median HVPG 10.4 mmHg(6.6-13.0). While MAP decreased significantly at all time points, sildenafil had no effect on HVPG.
Conclusion:
As shown with other vasodilators, in compensated cirrhotic patients, sildenafil at therapeutic doses for erectile dysfunction reduces MAP without reducing portal pressure. The search should continue for specific intrahepatic vasodilators.
Keywords: portal hypertension, phosphodiesterase-V inhibitor, HVPG
Portal hypertension is the major cause of morbidity and mortality in patients with cirrhosis. Portal hypertension is initially due to increased intrahepatic resistance and subsequently maintained by an increase in portal blood inflow. A reduction in portal pressure results in a lower rate of complications of cirrhosis(1-3).
Non-selective beta-blockers (BB) reduce portal pressure by reducing portal venous inflow. Although BB prevent variceal hemorrhage in patients with varices they do not prevent the development of varices(4), perhaps because portal hypertension in patients without varices (early disease) is more dependent on increased intrahepatic resistance than increased portal inflow. Experimental studies have demonstrated that cirrhotic rats without ascites already have impaired intrahepatic vasorelaxation(5).
Since portal pressure reduction in patients with early (compensated) cirrhosis is related to a decrease in outcomes(4), pharmacological therapy should be targeted at reducing intrahepatic resistance by increasing intrahepatic nitric oxide (NO). Available vasodilators not only reduce intrahepatic resistance but also have a systemic effect that reduces mean arterial pressure (MAP). This is potentially deleterious, particularly for patients with more advanced cirrhosis who are already vasodilated.
Increased phosphodiesterase-5 (PDE-V) expression is involved in the decreased vasodilator response to NO in cirrhotic rat livers(6). This has raised the possibility that PDE-V inhibitors, which also exert their effects through NO, may have a beneficial intrahepatic vasodilatory effect.
PDE-V inhibitors (PDEI) such as sildenafil, accentuate the vascular smooth muscle relaxation effects of NO by maintaining high levels of cyclic guanosine monophosphate (cGMP)(7;8). Studies of PDEI for portal hypertension have shown conflicting results, perhaps because they include patients with both compensated and decompensated cirrhosis(9-11). The objective of this study was to establish the portal and hemodynamic effects of sildenafil specifically in patients with compensated cirrhosis.
Patients and Methods
This was an open-label prospective single-center pilot study. Patients with compensated cirrhosis between 18 and 75 years with an HVPG >5 mmHg were eligible. Cirrhosis was established by non-invasive and/or histological criteria. Compensated cirrhosis was defined by the absence of ascites, variceal hemorrhage, jaundice or encephalopathy. Exclusion criteria were platelet count<50,000/mm3, portal vein thrombosis, hypersensitivity to sildenafil, use of nitrates or BB, MAP<60 mmHg, bacterial infection in the past 2 weeks or active alcohol use in the past 6 months. The study was approved by the Ethics Committee of Yale and the CT-VAHCS. All patients were studied at the CT-VAHCS and gave written informed consent.
Methods
Heart rate (HR), systolic (SBP), diastolic (DBP) and MAP were obtained using Welch Allyn, Propaq CS monitor (Model 242) every 5 minutes. Sedation was limited to ≤ 0.02 mg/kg of midazolam sedation, a dose known to have no effect on the HVPG(12;13). The HVPG was measured using the transjugular approach and a balloon tipped catheter as previously described(12). . Measurements of free hepatic venous pressure (FHVP) and wedged hepatic venous pressure (WHVP) were obtained in triplicate. HVPG was calculated by subtracting the FHVP from the WHVP. Permanent electronic tracings were obtained using the PowerLab data acquisition system (ADInstruments, Inc. Colorado Springs, CO).
After baseline measurements, patients received an oral dose of 25mg of sildenafil and measurements were repeated 30 and 60 minutes later. If the MAP had not decreased by >15% from baseline and the patient remained asymptomatic, a second dose of 25mg of sildenafil was administered with measurements 30 minutes later, and the study was terminated. A dose of 50mg of Sildenafil was felt to be sufficient as it is the dose commonly used for erectile dysfunction.
Data Analysis
As this was a pilot proof-of-concept study, formal sample size calculations were not performed. Since a previous study(9) using the PDE-V inhibitor vardenafil demonstrated a median reduction in the HVPG in 5 patients of 16%, we assumed that investigating 10-12 patients would be sufficient to test whether this effect was reproducible and whether proceeding to a placebo-controlled trial would be warranted.
All HVPG tracings were read by three independent observers (II, GGT, RJG) who were blinded to the patient and sequence of tracings.
Baseline measurements of hemodynamic parameters were compared to 30, 60 and 90 minutes and expressed as percent (%) change from baseline. Statistical comparisons were made using the Friedman test. The Wilcoxon test was used to compare each time point to baseline if the Friedman was significant. Statistical significance was considered a p-value of <0.05. Statistical analysis was done using SPSS 14.0 (SPSS Inc, Chicago, IL). All results are expressed as medians ± interquartile ranges, or as ranges for the percent changes in Table 1.
Table 1.
Percent (%) change from baseline at each time point
| Variables | Baseline (IQR) (N=12) |
Median % change at 30min [range](N=11) |
Median % change at 60min [range] (N=12) |
Median % change at 90min (N=9) [range] |
p |
|---|---|---|---|---|---|
|
Systolic blood pressure |
134 (124- 149) |
− 6%* [−16% to 0%] |
−6%* [−18% to 4%] |
−8%* [−12% to − 16%] |
0.008 |
|
Diastolic blood pressure |
70 (60-76) | −8%* [−23% to 16%] |
−5%* [−40% to 9%] |
−8% [−27% to 4%] |
0.040 |
|
Mean arterial pressure |
92 (84-95) | −8%* [−16% to 13%] |
−7%* [−22% to 8%] |
−9%* [−17% to − 2%] |
0.003 |
| Heart rate | 67 (60-73) | 0 [−13% to 13%] |
+2% [−12% to 15%] |
+3% [−11% to 16%] |
0.056 |
|
WHVP (mmHg) |
21.4 (18.0- 27.0) |
0 [−12% to 30%] |
+2% [−12% to 31%] |
−1% [−8% to 31%] |
0.162 |
|
FHVP (mmHg) |
11.2 (8.5- 13.3) |
+ 1% [−28% to 16%] |
+4% [−32% to 38%] |
+11% [−21% to 53%] |
0.007 |
|
HVPG (mmHg) |
10.4 (6.6- 13.0) |
+1% [−26% to 48%] |
−4% [−19% to 28%] |
−5% [−30% to 40%] |
0.506 |
significantly different from baseline
Results
Between September 2006 and March 2007 18 patients were enrolled and had baseline hemodynamic measurements performed. Of these, 5 were excluded because of an HVPG < 5 mmHg and 1 because of software malfunction. All patients were male with a median age of 55(52–59) years. All patients were Child Pugh class A with a median baseline albumin of 3.8(3.3–4.2) g/dL, bilirubin of 1.0(0.8–1.3) mg/dL, INR of 1.2(1.0–1.4) and creatinine of 0.9(0.8–1) mg/dL. Cirrhosis was biopsy proven in 11/12 patients and 58% had an alcoholic etiology. Baseline MAP was 92 (84 – 95) mmHg and baseline HVPG was 10.4(6.6–13.0) mmHg.
Thirty-minute HVPG measurements were performed in 11 of 12 patients because the position of the hepatic vein catheter had to be readjusted in one patient. All patients had 60-minute measurements (expected peak effect of sildenafil). The 90-minute measurement was performed in only 9 patients.
Effect of sildenafil on systemic hemodynamics
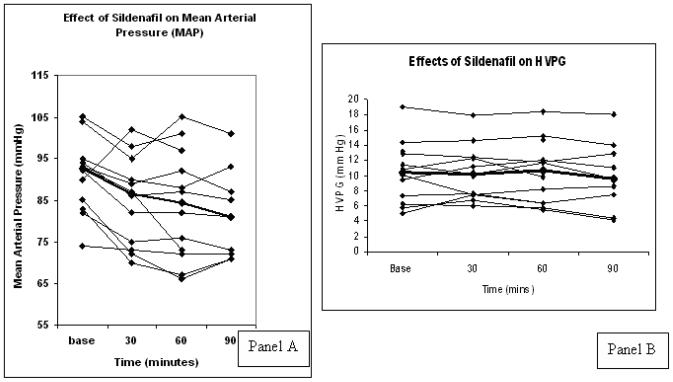
As shown in Table 1, SBP, DBP and MAP significantly decreased at all time points after sildenafil administration. None of the patients were symptomatic. The median decrease in MAP was 7 (4.3 – 9.8) mm Hg at 30 minutes, 6.5 (1.3 – 14.5) mm Hg at 60 minutes, and 8 (2.3 – 11.8) at 90 minutes. Heart rate did not change significantly (Table 1).
Effect of sildenafil on portal hemodynamics
Compared to baseline, there were no significant changes in HVPG or in WHVP. There was a statistically significant but mild and unexplained increase in the FHVP.
Discussion
This proof-of concept study shows that the PDEI sildenafil does not reduce portal pressure in patients with compensated cirrhosis. In keeping with the widespread distribution of PDE-V(6;14-17), although mild, the vasodilatory action of sildenafil was not liver-specific, resulting in a significant reduction in MAP. Other studies of PDEI in cirrhosis have also demonstrated a mild and asymptomatic reduction in MAP(10) and even an impact on the effective circulating blood volume as supported by an increase in plasma renin activity and aldosterone levels(18).
The published experience of the effects of PDE-V inhibitors on portal hemodynamics has been variable(9-11). In a study using vardenafil, a significant reduction in HVPG was demonstrated in only 5 patients(9). However, another 2 small series using sildenafil in patients with both compensated and decompensated cirrhosis, showed no effect on portal hemodynamics(10;11).
Our study had the advantage of including a homogeneous series of patients with early, compensated cirrhosis, as shown by a median HVPG of only 10.4(6.6-13.0) with only 4/12 (33.3%) having a baseline HVPG > 12 mm Hg. It is in this unique group of patients, in whom we assume that the hyperdynamic splanchnic circulation is not well developed, that we aimed to assess the therapeutic effect of an intrahepatic vasodilator. Additional advantages of our study include that hepatic vein pressures were recorded in the form of electronic tracings and that the final results were obtained through a blinded and independent analysis of pressure tracings by three observers who were unaware of the patient and the sequence of tracings (i.e. before or after sildenafil).
The reason for the lack of an HVPG response in our study is likely due to insufficient intrahepatic vasodilation. Like other vasodilating agents, there was a reduction in MAP, suggesting a physiological effect of the 50mg dose of sildenafil. Furthermore, this dose has been previously shown to alter hepatic venous NO and cGMP(11). Given these issues and our small sample size, the lack of response includes the potential for Type 2 error.
It is unclear why the results of our study differ from a smaller study using vardenafil in patients with compensated cirrhosis(9). In that study, 4/5 patients had a significant reduction in HVPG that was associated with a significant (albeit asymptomatic) reduction in SBP. The 10mg dose of vardenafil is roughly equivalent to the total dose of 50mg of sildenafil administered to our patients(19) and both drugs have a similar half-life. Furthermore, although our results can only be applied to compensated patients with cirrhosis, the 2 larger series(10 and 13 patients) of mixed compensated and decompensated patients concur with our results in that they demonstrated no effect of sildenafil on portal pressure(10;11)
In summary, PDE-V inhibitors do not have a beneficial effect on portal pressure reduction in patients with compensated cirrhosis. If utilized for erectile dysfunction, the MAP should be followed. The previous demonstration of an increased PDE-V expression in rat cirrhotic livers(6) suggests that the development of more specific liver PDE-V inhibitors could be effective in reducing HVPG without inducing systemic effects. The search should also continue for alternate vasodilators that are specifically targeted to the intrahepatic circulation and lack a systemic effect.
Figure 1.
Panel A: Effects of Sildenafil on Mean Arterial Pressures (MAP). The thick line represent median values for each time-point. MAP decreased significantly at 30, 60 and 90 minutes as compared to baseline (p<0.005). Panel B: Effects of Sildenafil on HVPG. The thick line represent medians. HVPG did not change at 30, 60 and 90 minutes as compared to baseline (p=0.506).
Acknowledgments
Grant Support/Funding: Clinical Core, Yale Liver Center NIH P30 DK34989
Abbreviations
- BB
Non-selective-beta-blockers
- cGMP
cyclic guanosine monophosphate
- FHVP
free hepatic venous pressure
- HVPG
hepatic venous pressure gradient
- MAP
mean arterial pressure
- NO
nitric oxide
- PDE-V
phosphodiesterase-V
- WHVP
wedged hepatic venous pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Co-first authorship
Study concept and design
acquisition of data
analysis and interpretation of data
drafting of the manuscript
critical revision of the manuscript for important intellectual content
Disclosures: Dr. Garcia-Tsao is consultant for Dong Pharmaceuticals that makes udenafil (a long-acting PDE-V inhibitor)
Writing assistance: None
Reference List
- 1.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodes J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003 Apr;37(4):902–8. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. HVPG reduction and prevention of variceal bleeding in cirrhosis. A systematic review. Gastroenterology. 2006;131:1611–24. doi: 10.1053/j.gastro.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell'era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol. 2006 Mar;101(3):506–12. doi: 10.1111/j.1572-0241.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 4.Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–61. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 5.Gupta TK, Chung MK, Toruner M, Groszmann RJ. Endothelial dysfunction in the intrahepatic microcirculation of the cirrhotic rat. Hepatology. 1998;28(4):926–31. doi: 10.1002/hep.510280405. [DOI] [PubMed] [Google Scholar]
- 6.Loureiro-Silva MR, Iwakiri Y, Abraldes JG, Haq O, Groszmann RJ. Increased phosphodiesterase-5 expression is involved in the decreased vasodilator response to nitric oxide in cirrhotic rat livers. J Hepatol. 2006 May;44(5):886–93. doi: 10.1016/j.jhep.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000 Sep;184(3):409–20. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Mullershausen F, Russwurm M, Thompson WJ, Liu L, Koesling D, Friebe A. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J Cell Biol. 2001 Oct 15;155(2):271–8. doi: 10.1083/jcb.200107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rossle M, et al. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver -- results of a pilot study. Aliment Pharmacol Ther. 2006 Jan 1;23(1):121–8. doi: 10.1111/j.1365-2036.2006.02735.x. [DOI] [PubMed] [Google Scholar]
- 10.Clemmesen JO, Giraldi A, Ott P, Dalhoff K, Hansen BA, Larsen FS. Sildenafil does not influence hepatic venous pressure gradient in patients with cirrhosis. World J Gastroenterol. 2008 Oct 28;14(40):6208–12. doi: 10.3748/wjg.14.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KC, Yang YY, Wang YW, Hou MC, Lee FY, Lin HC, et al. Acute administration of sildenafil enhances hepatic cyclic guanosine monophosphate production and reduces hepatic sinusoid resistance in cirrhotic patients. Hepatol Res. 2008 Dec;38(12):1186–93. doi: 10.1111/j.1872-034X.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- 12.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: Anything worth doing should be done right. Hepatology. 2004 Feb;39(2):280–3. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 13.Steinlauf AF, Garcia-Tsao G, Zakko MF, Dickey K, Gupta TK, Groszmann RJ. Low-dose midazolam sedation: an option for patients undergoing serial hepatic venous pressure measurements. Hepatology. 1999;29:1070–3. doi: 10.1002/hep.510290421. [DOI] [PubMed] [Google Scholar]
- 14.Sampson LJ, Hinton JM, Garland CJ. Evidence for expression and function of phosphodiesterase type 5 (PDE-V) in rat resistance arteries. Br J Pharmacol. 2001 Jan;132(1):13–7. doi: 10.1038/sj.bjp.0703831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbin JD, Francis SH. Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction. J Androl. 2003 Nov;24(6 Suppl):S38–S41. doi: 10.1002/j.1939-4640.2003.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 16.Ahn HS, Foster M, Cable M, Pitts BJ, Sybertz EJ. Ca/CaM-stimulated and cGMP-specific phosphodiesterases in vascular and non-vascular tissues. Adv Exp Med Biol. 1991;308:191–7. doi: 10.1007/978-1-4684-6015-5_15. [DOI] [PubMed] [Google Scholar]
- 17.Medina P, Segarra G, Martinez-Leon JB, Vila JM, Aldasoro M, Otero E, et al. Relaxation induced by cGMP phosphodiesterase inhibitors sildenafil and zaprinast in human vessels. Ann Thorac Surg. 2000 Oct;70(4):1327–31. doi: 10.1016/s0003-4975(00)01914-7. [DOI] [PubMed] [Google Scholar]
- 18.Thiesson HC, Jensen BL, Jespersen B, Schaffalitzky de Muckadell OB, Bistrup C, Walter S, et al. Inhibition of cGMP-specific phosphodiesterase type 5 reduces sodium excretion and arterial blood pressure in patients with NaCl retention and ascites. Am J Physiol Renal Physiol. 2005 May;288(5):F1044–F1052. doi: 10.1152/ajprenal.00142.2004. [DOI] [PubMed] [Google Scholar]
- 19.Potency Bischoff E. selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004 Jun;16(Suppl 1):S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]