Abstract
Pyruvate, which is produced in the final step of glycolysis, participates in anabolic metabolism, catabolic metabolism, and signal transduction. We have recently reported a new sensitive and selective fluorimetric assay for pyruvate measurement using Amplex Red as a fluorogenic dye. However, the fluorescence of the reaction product, resorufin, is pH-dependent, which limits the sensitivity of the assay at pH 6.7. In this note, we evaluate Invitrogen’s new dye, Amplex UltraRed, as a fluorogenic agent for pyruvate determination. Our results show that Amplex UltraRed improves the performance of the assay, providing brighter fluorescence and enhanced sensitivity.
Keywords: glycolysis, metabolite, fluorescence, pyruvate, Amplex UltraRed
Pyruvate is produced by pyruvate kinase (EC2.7.1.40), which converts phosphoenolpyruvate and ADP into ATP and pyruvate. As pyruvate production is associated with cell growth control, energy production, and intracellular signaling [1, 2], it is important to have sensitive assays to evaluate pyruvate concentrations. Recently, we reported a sensitive enzymatic fluorimetric assay for the determination of intracellular pyruvate based on the stoichiometric oxidization of non-fluorescent Amplex Red (10-acetyl-3, 7-dihydroxyphenoxazine) by H2O2 in the presence of horseradish peroxidase (HRP) to brightly fluorescent resorufin [3]. However, the absorption and fluorescence of resorufin are pH-dependent. At a pH below the pKa (~6.0), the absorption maximum shifts to ~480 nm and the fluorescence quantum yield is markedly reduced. For these reasons, the reactions should be performed at pH 7–8. On the other hand, two enzymes used in this assay, HRP and pyruvate oxidase (POX, EC 1.2.3.3), are more active in an acidic environment. The pH optimum of HRP is in the range of 6.0 to 6.5 [4]. For bacterial pyruvate oxidase, one unit will produce 1.0 μmole of H2O2 per min during the conversion of pyruvate and phosphate to acetylphosphate and CO2 at pH 5.7 at 37 °C. In addition, H2O2 has been reported to cause a nonenzymatic and stoichiometric decarboxylation of pyruvate [5, 6]. The side reaction between H2O2 and pyruvate can be effectively suppressed in acidic buffer (pH 5.6–6.0) and low temperature [5]. Therefore, the enzymatic reactions are favorable in acidic buffer, while the fluorogenic reaction is more efficient in a neutral or weakly basic solution. To overcome this problem, we examined this fluorimetric assay with the new dye Amplex UltraRed (Cat. No. A36006, Invitrogen, Carlsbad, CA) because the Amplex UltraRed reaction product has a lower pKa value than the reaction products of Amplex Red, thus giving Amplex UltraRed utility across a broader pH range. The results show that Amplex UltraRed improves the performance of pyruvate assay, providing enhanced signal sensitivity.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Fluorescence was measured with a FlexStation II plate reader (Molecular Devices, Sunnyvale, CA) using a 96-well black assay plate (Nunc Brand, Thermo Fisher Scientific, Rochester, NY). Calibration experiments were performed with 20 μl volumes of serially diluted pyruvate standards. Samples were pipetted into a 96-well plate then 180 μl of an assay cocktail was added into each well followed by incubation for 30 min. at room temperature. The final reaction solution contained 100 mM potassium phosphate with 1.0 mM EDTA, pH 6.7, 1.0 mM MgCl2, 10 μM FAD, 0.2 mM thiamine pyrophosphate, 0.2 U/ml pyruvate oxidase, 50 μM Amplex UltraRed and 0.5 U/ml HRP. Fluorescence excitation and emission were performed at 535 nm and 590 nm, respectively, for experiments requiring high sensitivity. The background intensity was subtracted using the value of the no pyruvate control for all sample readings.
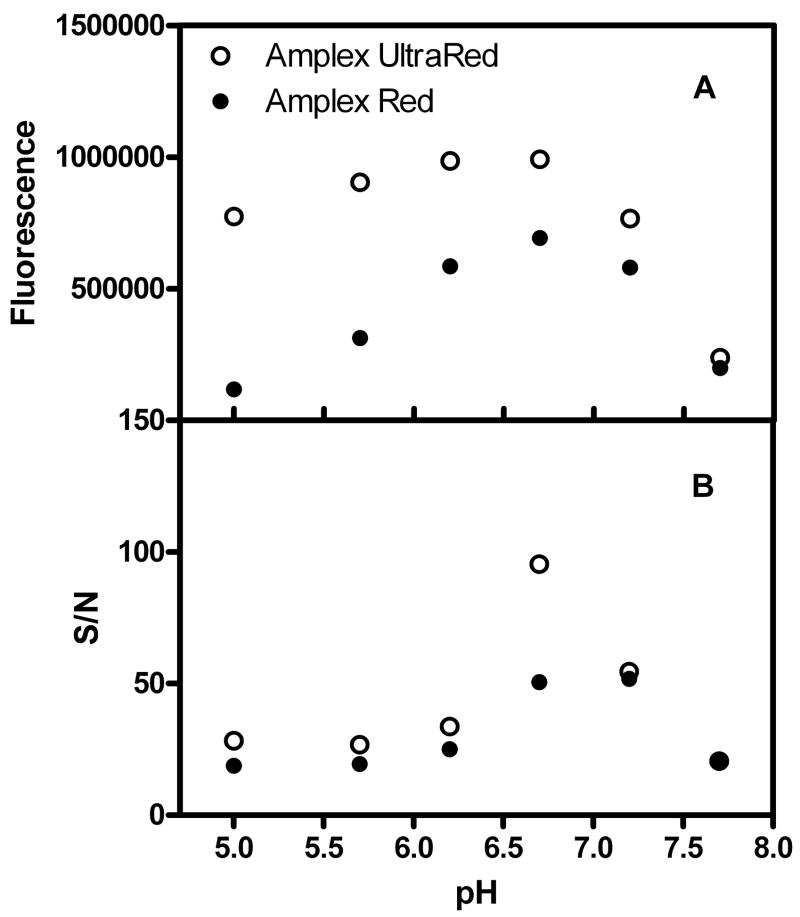
To assess the performance of Amplex UltraRed, we first performed pyruvate assays in various pH buffers with Amplex UltraRed and Amplex Red as indicator dyes. As shown in Fig. 1, the Amplex UltraRed reaction product displays brighter fluorescence in the pH range from 5.0 to 7.0 in comparison to resorufin. However, its background fluorescence is higher. For Amplex UltraRed, the signal to noise ratio (S/N) reaches its maximum at pH 6.7, which is about two-fold greater than that of the Amplex Red reagent.
Fig. 1.
Comparison of fluorescence signals and signal-to-noise ratios (S/N) for assays using Amplex UltraRed and Amplex Red as indicator dyes. Each point is the mean of triplicate measurements. Error bars are not shown because their sizes are smaller than the symbols. The pyruvate concentration is 10 μM in each well. An excitation wavelength of 535 nm is used.
At higher pyruvate concentrations (10 μM, 2000 pmol/well) the plate reader’s detection capability becomes a limiting factor in performing the assay. In this case, the measured intensity is reduced by changing the excitation wavelength to 490 nm where the linearity of the standard curve is improved in comparison to 535 nm (Fig. 2A and B) with no effect on the S/N ratio.
Fig 2.
Properties of the Amplex UltraRed assay. Calibration curves for Amplex UltraRed using excitation wavelengths of 490 nm (A) and 535 nm (B) are shown. The linearity of these data are excellent, as judged by the coefficients of determination of 0.993 and 0.980 for experiments conducted at 490 nm and 535 nm, respectively. The specificity of the assay is shown in panel C. The final concentrations of test compounds in reaction solutions are 10 μM. The NaClO4 concentration is 12.5 mM. Data are expressed as the mean ± SD of triplicate wells. The excitation wavelength is 490 nm.
The z′ value has been widely accepted as a means of evaluating assay quality [7]. As we reported in a previous paper [3], the z′ value of the Amplex Red assay is 0.89; we now find the z′ value of the Amplex UltraRed assay to be 0.93. The S/N ratio and z′ value demonstrate the enhancement of the fluorimetric pyruvate assay with Amplex UltraRed.
We next evaluated the selectivity of the method by performing the same assay with phosphoenolpyruvate (PEP), lactate, acetoacetate, ketoglutarate, 2-oxobutyrate, oxaloacetate as samples. As shown in Fig. 2C, PEP, lactate, acetoacetate, ketoglutarate, and 2-oxobutyrate do not interfere with pyruvate measurement, whereas oxaloacetate generates a significant signal, as previously noted [3, 8]. In many cases this may not be important because pyruvate concentrations have been reported to be ~20-fold higher than oxaloacetate in cells [9]. As HClO4 is commonly used as a deproteinizing reagent in pyruvate sample preparation, we tested its effect on this assay. The first column in Fig. 2C shows that ClO4− concentrations of up to 12.5 mM do not interfere with the assay, thus confirming its utility in studies of blood or cell extractions.
We also tested the stability of Amplex UltraRed. We did not find changes in color or reaction activity for six months with storage in solid form at −20°C or in DMSO solution at −80°C. However, we observed the formation of colorless precipitates in 1 mM H2O or PBS buffer (pH 7.4) solution within 30 min. of preparation. Therefore, we recommend fresh preparations of Amplex UltraRed aqueous solutions prior to assays.
Acknowledgments
Research Funding: This work was supported, in part, by the Division of Intramural Research of the Eunice Kennedy Schriver National Instutute of Child Health and Human Development, NIH, DHHS, contract number N01-HD-2-3342 and subcontract WSU04055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mazurek S, Boschek CB, Eigenbrodt E. The role of phosphometabolites in cell proliferation, energy metabolism, and tumor therapy. J Bioenerg Biomembr. 1977;29:315–30. doi: 10.1023/a:1022490512705. [DOI] [PubMed] [Google Scholar]
- 2.Bakowski D, Parekh AB. Regulation of store-operated calcium channels by the intermediary metabolite pyruvic acid. Curr Biol. 2007;17:1076–1081. doi: 10.1016/j.cub.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Zhu A, Romero R, Petty HR. A sensitive fluorimetric assay for pyruvate. Anal Biochem. 2010;396:146–51. doi: 10.1016/j.ab.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schomburg D, Salzmann M, Stephan D. Enzyme handbook. Springer-Verlag; Berlin; New York: 1990. [Google Scholar]
- 5.Giandomenico AR, Cerniglia GE, Biaglow JE, Stevens CW, Koch CJ. The importance of sodium pyruvate in assessing damage produced by hydrogen peroxide. Free Radic Biol Med. 1997;23:426–434. doi: 10.1016/s0891-5849(97)00113-5. [DOI] [PubMed] [Google Scholar]
- 6.Desagher S, Glowinski J, Premont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–9067. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 8.Lamprecht W, Heinz F. Pyruvate. In: Bergmeyer HU, Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. Verlag Chemie; Weinheim; Deerfield Beach, Fla: 1983. pp. 570–577. [Google Scholar]
- 9.Williamson DH, Brosnan JT. Concentrations of metabolites in animal tissues. In: Bergmeyer VH, editor. Methods of enzymatic analysis. Academic Press; New York: 1971. pp. 2266–2302. [Google Scholar]