Abstract
Peripheral nerve injury leads to neuropathic pain, but the underlying mechanisms are not clear. The TRPV1 channel expressed by nociceptors is one receptor for noxious heat and inflammatory molecules. Lumbar 4 spinal nerve ligation (SNL) in mice induced persistent heat hyperalgesia 4–10 days following injury. The heat hypersensitivity was completely reversed by the TRPV1 antagonist A-425619. Furthermore, DRG neurons were isolated from the injured L4 ganglia or adjacent L3 ganglia 4–10 days after L4 SNL. Whole-cell patch clamp recordings were performed and heat stimuli (22–50°C/3 sec) were applied to the soma. Neurons were classified by soma size and isolectin-B4 (IB4) binding. Among directly injured L4 neurons, SNL increased the percentage of small-diameter IB4 positive neurons that were heat sensitive from 13% (naive controls) to 56% and conversely, decreased the proportion of small IB4-negative neurons that were heat sensitive from 66% (naive controls) to 34%. There was no change in IB4 binding in neurons from the injured ganglia. Surprisingly, in neurons from the adjacent L3 ganglia, SNL had no effect on the heat responsiveness of either IB4 positive or negative small neurons. Also, SNL had no effect on heat responses in medium-large diameter neurons from either the injured or adjacent ganglia.
Keywords: Neuropathic pain, heat, capsaicin, C fiber, patch clamp, hyperalgesia
Introduction
Neuropathic pain results from damage or dysfunction of the peripheral or central nervous system. Common causes include carpal tunnel syndrome, nerve compression by neuromas, tumors or herniated disks, and surgical procedures.38, 39 Patients with neuropathic pain frequently experience spontaneous pain as well as stimulus-evoked pain including heat or cold hyperalgesia, or mechanical allodynia.
Neuropathic pain is thought to be driven by functional changes in directly injured fibers as well as changes in fibers that lie adjacent to the injured axons.7, 15, 34, 57, 64 The injured distal axons undergo degeneration which recruits inflammatory processes around the adjacent intact fibers.42, 55 Nociceptors are broadly categorized as either myelinated A-fibers that transmit noxious mechanical and heat stimuli, or unmyelinated C-fibers that respond to intense heat, cold and/or mechanical stimuli. C-fiber nociceptors are further classified into peptidergic and non-peptidergic, and can be differentiated by isolectin B4 (IB4) binding, as non-peptidergic neurons primarily bind IB4.3, 53 These two groups appear to provide input to two distinct pain pathways from the skin to the brain.5, 69 Little is known about nerve injury-induced functional changes in peptidergic and non-peptidergic/IB4-binding nociceptors that may contribute afferent input toward neuropathic pain.
The molecular basis for neuropathic pain symptoms is largely unknown. One critical transducer in nociceptors is the TRPV1 channel, which is activated or sensitized by natural and endogenous stimuli including noxious heat (>43°C), protons, N-arachidonoyl-dopamine (NADA), leukotriene B4, anandamide, and 12- and 15-(S) HPETE lipoxygenase metabolites.9, 26, 59, 68 Thus, besides activation by heat, TRPV1 may also contribute to injury-induced hypersensitivity via its activation or sensitization by endogenous ligands. The involvement of TRPV1 in inflammatory pain is well established,8, 12 but its role in neuropathic pain is controversial. In some animal models of neuropathic pain, mice lacking the TRPV1 channel develop the full magnitude of behavioral mechanical allodynia and heat hyperalgesia.8 However, other studies show that TRPV1 mRNA and protein levels are substantially upregulated in neurons that course adjacent to injured axons, with the greatest increase occurring in adjacent myelinated A fiber neurons.18, 25, 41, 49 Furthermore, TRPV1 antagonists effectively block nerve injury-induced mechanical allodynia in animal models.24, 48
Previous studies have assessed the contribution of TRPV1 to neuropathic pain via analysis of protein or mRNA levels and pain behavior assays, but no study has investigated injury-induced changes in TRPV1 function in sensory neurons. On a functional level, TRPV1 may collaborate in heterotetramers with other TRP channels,11, 23 or exist in multiple splice variants,62 and these configurations could be missed by protein or mRNA analyses. Therefore, we used patch clamp recordings to investigate nerve injury-induced changes in membrane TRPV1 function to natural heat stimuli.
Methods
Animals
Experiments were performed in adult male wild type mice (C57BL/6J) and TRPV1-null mice (B6.129S4-TRPV1tm1Jul; Jackson Laboratories, Bar Harbor, ME). Ages ranged between 10–16 weeks, and body weight ranged 20–30 g. All procedures were approved by the Medical College of Wisconsin Animal Care and Use Committee and were performed in accordance with the policies of the International Association for the Study of Pain and the National Institutes of Health.
Spinal nerve ligation
Ligation and transection of the L4 spinal nerve was performed in mice with minor modifications to the widely-used Chung model for neuropathic pain in rats. 27 Adaptations were made to account for the neuroanatomical differences documented between rats and mice.50 Unlike rats where 98% of sciatic axons arise from the L4 and L5 spinal nerves,58 in mice, most fibers in the sciatic nerve arise from the L3 and L4 spinal nerves, with a smaller (< 20%) contribution from the L5 spinal nerve.50 Mice were randomly assigned to naive, SNL or sham groups. Under deep Avertin anesthesia, the paraspinal muscles (L3-S1 level) were bluntly dissected and the L5 transverse process was removed to expose the ventral ramus of the L3 and L4 spinal nerves. The L4 spinal nerve was tightly ligated with silk suture (6-0; Look, Surgical Specialties Corporation, Reading, PA) and transected distal to the ligation. In sham-operated mice, the L5 transverse process was exposed but not removed and the L4 spinal nerve was not touched. Naive, un-manipulated mice were included as controls to measure sham-surgery induced effects. The position of the ligature was examined post mortem by counting the dorsal root ganglia (DRG) embedded in the intervertebral foramen beginning with the DRG just caudal to the last rib as T13, the next caudal DRG as L1, etc. Neurons in the L4 DRG were considered directly injured and neurons in the L3 DRG were considered adjacent to the injury.
Behavioral testing
The radiant heat paw withdrawal test was used to assess heat hypersensitivity.6, 22 Mice were allowed to freely move on a glass floor surrounded by a plexiglass chamber. Radiant noxious heat was applied to the plantar hind paw. Ipsilateral and contralateral sides were tested in alternation and 4–5 measurements were obtained for each paw at each time point. Paw withdrawal latencies were assessed approximately 30 minutes after intraperitoneal injection of a selective TRPV1 antagonist, A-42561916 (Abbott Laboratories, Abbott Park, IL), or vehicle. The vehicle contained 34% weight by volume hydroxypropyl beta cyclodextrin in 10% sterile DMSO/dH20.
Isolation of neurons from the L3 and L4 DRGs
The L3 and L4 ganglia were removed from the operated side 4–10 days (median 7 days) after SNL or sham surgery. For naive controls, the L3 and L4 ganglia were removed bilaterally from a non-manipulated mouse and dissociated separately. Cells were isolated as previously described.13 Briefly, DRGs were incubated with collagenase followed by trypsin (Sigma-Aldrich, St. Louis, MO) and mechanically dissociated into single cells. Cells were plated onto poly-L-lysine coated coverslips. No exogenous growth factors were added. Recordings were performed 2–24 hr after plating.
Electrophysiology
Whole-cell patch-clamp recording was performed as described.13 Briefly, fire-polished glass electrodes (3–6 MΩ resistance) were filled with solution containing (in mM): KCl, 135; NaCl, 10; MgCl2, 1; EGTA, 1; NaGTP, 0.2; ATPNa2, 2.5; HEPES, 10; pH 7.2; osmolarity = 290 mOsm. The recording chamber was superfused with solution containing (in mM): NaCl, 140; KCl, 5; CaCl2, 2; MgCl2, 1; HEPES, 10; glucose, 10; pH 7.4; osmolarity = 310 mOsm. All recordings were performed at room temperature (23 ± 1°C). Soma size was estimated by a calibrated eyepiece reticle. Only neurons isolated from neighboring cells were used.
Membrane voltage or current was clamped using an EPC-9 amplifier run by Pulse software (version 8.78; HEKA Electronic, Lambrecht, Germany). Neurons were included if they formed a tight seal (> 1 gigaohm), had a stable resting membrane potential <−40 mV, and exhibited an action potential (AP) overshoot. Series resistance was compensated by > 60%. The presence of an inflection on the falling phase of the AP was determined by taking the first derivative (dV/dt) of the AP waveform as described13 and a late negative component indicated an inflection.
To measure whole-cell currents, membrane voltage was clamped at −70 mV. Capsaicin (1 μM; Sigma-Aldrich) was dissolved in 1-methyl-2-pyrrolidinone 13, 46 and applied for 10 s using a nonmetallic needle placed 50–100 μm from the soma.13 The vehicle had no effect on control, injured L4 or adjacent L3 neurons (data not shown). The criterion for a capsaicin response was an inward current ≥ 40 pA.6
Heat stimuli were applied as a fast ramp of heated buffer (22°C to 50°C in 3 sec) 50–80 μm from the soma using a custom-made heat stimulator designed by Dr. Ivan Dittert.14 The peak stimulus temperature was set at 50 °C because higher temperatures can induce irreversible damage to the membrane.36 The temperature was measured at the tip of the heat stimulator and the actual temperature at the soma membrane was approximately 1–2°C lower.
For pharmacological inhibition of TRPV1, neurons were first tested with a heat ramp in normal buffer. Following a 2 min superfusion with buffer containing the TRPV1 antagonist A-425619, a second heat stimulus was applied. After a 2 min washout with normal buffer, a third heat ramp was applied.
Immediately after recording, neurons were incubated with 10 μg/ml isolectin B4 (Bandeiraea Simplicifolia) conjugated to fluorescein isothiocyanate (IB4-FITC; Sigma-Aldrich) for 10 min and the IB4-FITC staining was visualized. A neuron was considered IB4 positive if it had a continuous green ring around the perimeter.13 Neurons with a soma diameter ≤ 26 μm were considered “small” and neurons >26 μm in diameter were considered “medium-large.” The majority of IB4-positive neurons are ≤ 26 μm diameter and 95% of these IB4-positive neurons are negative for the 200 kD neurofilament antibody N52, a marker for myelinated neurons.13 Medium-large neurons were classified into those with- and without a somal AP inflection because a tight association between a somal AP inflection and nociceptive response properties has been described for myelinated neurons.17, 28, 51 For the naive and sham-surgery control groups, the response properties of neurons from the L3 and L4 ganglia were not different and thus, were combined.
Calcium Imaging
DRG neurons were loaded with 2.5 μg/ml Fura-2 AM (Invitrogen, Eugene, OR) in extracellular buffer containing 2% BSA. Images of Fura-2-loaded neurons (excitation at 340 and 380 nm) were captured with a cooled LCD camera (CoolSNAP fx, Photometrics, Tucson, AZ; exposure time 2 sec). The ratio of fluorescence intensity (340 nm/380 nm) was analyzed using MetaFluor (version 7.1.4.0, Molecular Devices, Sunnyvale, CA). Background fluorescence was corrected by subtracting an image taken from a region of the coverslip devoid of cells or debris. The criterion for a response was ≥ 20% increase above the baseline ratio. A test solution of 50 mM K+ was used to depolarize neurons, increase intracellular Ca2+ and thereby, differentiate neurons from non-neuronal cells. After imaging, cells were incubated with IB4-FITC as above; IB4-FITC staining was visualized in neurons that responded to 50 mM K+.
Statistical analysis
Values are given as either mean ± SEM or median and quartile range. Differences in the current densities between different groups were compared using a Kruskal-Wallis test. Percentages of neurons responding to heat were compared using a Chi-square test followed by Fisher’s exact test. For behavioral experiments, the overall main effect was assessed by either one-way ANOVA or two-way repeated measures ANOVA, followed by paired t-test comparisons at each time point.
Results
Pharmacological inhibition of TRPV1 reverses SNL-induced heat hypersensitivity
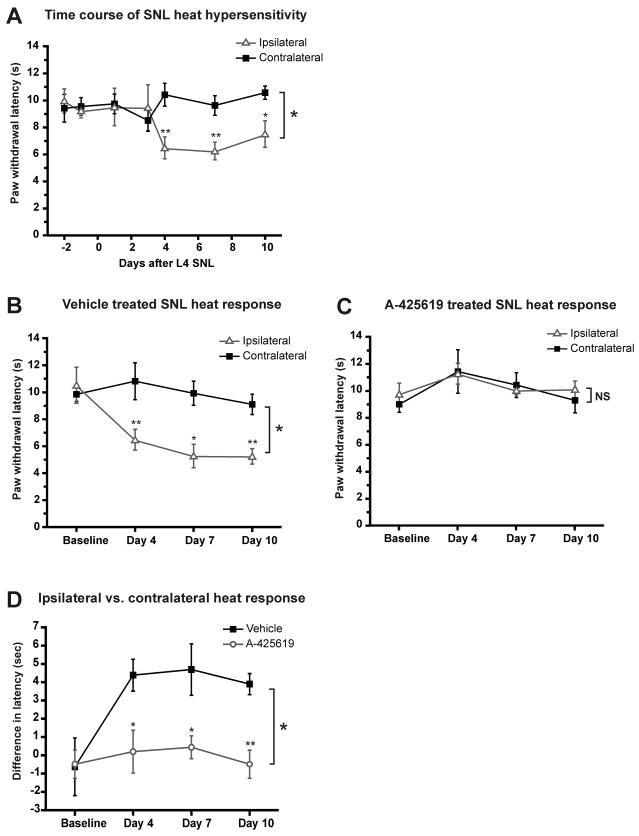
First we performed an initial time course to determine the onset and extent of behavioral heat hypersensitivity induced by L4 spinal nerve ligation (SNL). By day 4 after SNL, mice developed prominent heat hypersensitivity in the paw ipsilateral to SNL compared to either the contralateral side or baseline before surgery. The heat hypersensitivity persisted through day 10 (Fig. 1A). In order to determine the contribution of the TRPV1 receptor to the heat hypersensitivity, we treated separate groups of SNL-injured mice with either the TRPV1 antagonist A-425619 (200 μmol/kg, i.p.) or vehicle 30 min before each behavioral test on days 4, 7 and 10. Vehicle-treated mice with SNL exhibited behavioral heat hyperalgesia over the 4–10 day time course that was similar to the initial L4 SNL group (Fig. 1B). In contrast, acute pharmacological inhibition of TRPV1 with A-425619 completely reversed the SNL-induced heat hypersensitivity at all time points (Fig. 1C and 1D). Heat responses in the contralateral paw were not affected by A-425619. These data indicate that acute pharmacological inhibition of TRPV1 completely reverses nerve injury-induced heat hyperalgesia.
Figure 1. Heat hypersensitivity after L4 SNL is mediated by TRPV1.
Paw withdrawal latencies to heat in mice after L4 SNL. A. Mice developed heat hypersensitivity in the ipsilateral paw 4 days after SNL; the hypersensitivity persisted through 10 days. The ipsilateral (SNL side) paw was compared to the contralateral paw. One-way ANOVA main effect for all time points, both sides was p<0.05. Right and left paws were compared for individual time points using paired t-tests (**p<0.01; *p<0.05; n ≥ 4 for each time point). B. Vehicle treatment (i.p.) 30 min prior to the behavioral test on each day did not alleviate the SNL-induced heat hypersensitivity. Two-way repeated measures ANOVA main effect for all time points, both sides was p< 0.05. Right and left paws were compared for individual time points using paired t-tests (**p<0.01; *p<0.05; n=5). C. Treatment with A-425619 (200 μmol/kg, i.p.) 30 min prior to the behavioral test on each day completely blocked the heat hypersensitivity in the ipsilateral paw. Two-way repeated measures ANOVA (p>0.1; n=5). D. In addition, the effect of A-425619 versus vehicle was compared by calculating the difference between the ipsilateral and contralateral paw withdrawal responses in the A-425619-treated group to that in the vehicle-treated group (two-way repeated measures ANOVA main effect p < 0.05; a t-test compared the difference at each time point between A-425619- and vehicle-treated mice (**p<0.01; * p<0.05).
Since the paw withdrawal test measures a reflex that includes DRG neurons as the sensory component, we next determined whether functional upregulation of TRPV1 in DRG neurons contributes to the nerve injury-induced heat hypersensitivity.
Heat sensitivity of naive DRG neurons
First, we assessed heat responses in neurons from naive mice. A subpopulation of neurons exhibited a robust inward current that rapidly returned to baseline immediately after the heat ramp (Fig. 2A). All other neurons exhibited a small non-specific leak current during the heat ramp (Fig. 2B). Thus, the criteria for an authentic heat response was a clear heat threshold evident by onset of a fast rising inward current as previously documented.36 Repeated heat-evoked currents exhibited little desensitization when applied at 2 min intervals (Fig. 2A).
Figure 2. Responsiveness of naive small-diameter neurons to heat and capsaicin.
A. Example of a small-diameter neuron responding to heat (22–50 °C in 3 sec) with a clear threshold of 46 °C. Repeated heat responses showed little sensitization or desensitization. B. Example of the non-specific background current in a heat-insensitive neuron.
CD. Percentage of small-diameter (≤ 26 μm) neurons that responded to heat (C) or 1 μM capsaicin (D). Many IB4 negative neurons responded to both heat and capsaicin, whereas few IB4 positive neurons responded to either stimulus. E. Percentage of medium-large neurons (>26 μm in diameter) that responded to heat or capsaicin (1 μM).
A total of 32% (31/98) neurons of all sizes from naive mice responded to heat. Among small-diameter neurons, 51% (27/53; range: 16.3–25.3μm diameter) responded to heat compared to only 9% (4/45) of medium-large neurons (range: 26.9–47.3μm diameter). Heat activation thresholds in all naive neurons ranged from 42 to 47°C, with a mean threshold of 44.9 ± 0.2°C.
Further stratification of the heat-sensitive small neurons into IB4 positive and negative subgroups revealed that the large majority (92.6%; n=38) of heat-sensitive small neurons were IB4 negative, whereas only 7.4% (n=15) were IB4 positive. Hence, among all small neurons tested, significantly more IB4 negative neurons responded to heat (65.8%), compared to just 13.3% of IB4 positive neurons (Fig. 2C; P = 0.0007; Fisher’s exact test). This differential heat-sensitivity between IB4 positive and negative small neurons paralleled the sensitivities the two subgroups to capsaicin (1 μM; Fig. 2D). Neither the average magnitude of the heat current (IB4−: 141 ± 33.8 pA/pF; IB4+: 208.6 pA/pF) nor the heat thresholds (IB4−: 45.11 ± 0.5°C; IB4+: 44.5°C) were different between the two groups. The low percentage of IB4 positive neurons responding to heat in this study (13.3%) is different than our previous report where 49% responded to heat.54 This difference is likely due to the shorter time in culture (on average less than 12 hr in the current study versus 27 hr in the previous study) and possibly to a shorter duration heat ramp (3 sec in current study versus 10 sec in previous study). The low heat-sensitivity of medium-large neurons corresponded with their low capsaicin sensitivity (Fig. 2E).
TRPV1 is critical for heat responses in naive DRG somata
Since DRG neurons are known to express heat sensitive channels other than TRPV1,10, 19, 33, 53 we investigated the proportion of heat-evoked responses due to activation of TRPV1 channels by using both acute pharmacological inhibition of TRPV1 and genetic ablation of TRPV1. The TRPV1 antagonist A-425619 (1μM) completely and reversibly inhibited heat-evoked currents in small diameter neurons (Fig. 3A, B), leaving only a small, residual non-specific current. Second, none of the small-diameter DRG neurons isolated from TRPV1 −/− mice (n=29) responded to heat, compared to 51% of small neurons from C57BL6 wild type mice (p < 0.0001; Fisher’s exact test). In medium-large neurons, all heat currents had kinetics identical with the heat currents from small neurons and the percentages of heat- and capsaicin-responsive neurons were the same. Together with our observation that the heat thresholds in naive neurons are characteristic of TRPV1,9 our findings indicate that at the level of the cell soma, all heat-evoked currents in naive DRG neurons are mediated via activation of TRPV1.
Figure 3. TRPV1 inhibition by A-425619 blocks all heat responses in DRG neurons.
A. Example of a heat-evoked current in a small-diameter neuron that is completely blocked by A-425619 (1 μM) and recovers by 2 min after washout. B. Quantification of the magnitude of heat responses in naive small neurons before, during and after inhibition with A-425619 (n=6). After the first heat test, neurons were superfused with A-425619 (1 μM) for 2 min and then tested with heat in the presence of the antagonist. Note that the heat response shows almost complete recovery by 2 min after washout of A-425619. Error bars represent SEM.
Nerve injury-induced changes in heat response in small neurons
In mice, the sciatic nerve receives the majority of its axons from the L3 and L4 spinal nerves, and a small contribution from the L5 nerve.50 Therefore, after L4 SNL, the L4 DRG contains all directly injured neurons whereas the L3 DRG contains somata that have intact axons, and some of these axons course adjacent to injured axons. Since pain following nerve injury involves damage to nerve fibers, degeneration of the injured nerve fibers, and local inflammation, 61 we compared directly injured and adjacent neurons to naive neurons. Because sham surgery involves local inflammation caused by the skin and muscle dissection, we also compared neurons from sham operated mice to SNL mice to identify the specific contributions of the nerve injury component.
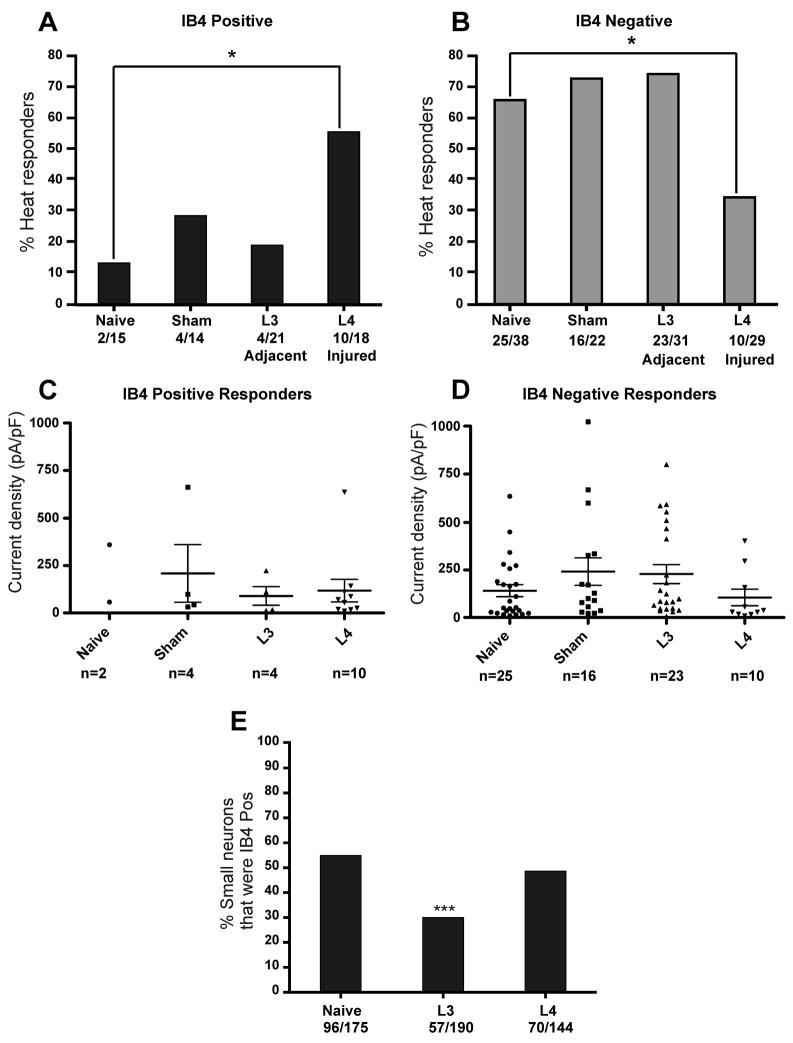
Spinal nerve ligation affected the heat responsiveness of small neurons from the injured L4 ganglia. The percentage of IB4 positive neurons that were heat responsive increased by 4-fold, from 13% in naive controls to 56% in injured L4 neurons (Fig 4A). In contrast, SNL decreased the percentage of IB4 negative neurons that were heat sensitive from 66% in naive controls to 34% in injured L4 neurons (Fig. 4B). However, SNL had no effect on the heat-responsiveness of either IB4 positive or negative small neurons from the adjacent L3 ganglia (Fig. 4A, B). Sham surgery had no effect on the heat responsiveness of either population of small neurons. The average amplitude of the heat-evoked currents did not change after injury in any group (Kruskal-Wallis test, Fig. 4C, D).
Figure 4. Nerve injury-induced changes in heat responsiveness in small neurons.
A. Percentage of IB4 positive small-diameter (≤ 26 μm) neurons (putative C fiber type neurons) that respond to heat in naive, sham control, L3 adjacent or L4 injured groups. Lumbar 4 spinal nerve ligation (SNL) induced a significant increase in the percentage of IB4 positive neurons from the directly injured L4 ganglia that responded to heat (* p = 0.027; Fisher’s exact test). There was no significant change in either the adjacent L3 neurons or the sham surgery controls. B. Percentage of IB4 negative small-diameter neurons that respond to heat in naive, sham control, L3 adjacent or L4 injured groups. L4 SNL induced a significant decrease in the percentage of IB4 negative neurons from the directly injured L4 ganglia that responded to heat (* p = 0.014; Fisher’s exact test). CD. Neither SNL nor sham surgery induced changes in the average magnitude of the heat-evoked currents in any group for either IB4 positive small neurons (C) or IB4 negative neurons (D) (long and short horizontal bars represent the mean and SEM, respectively). E. Percentage of small-diameter neurons (≤ 26 μm) that stained positively for IB4-FITC in live cultures of lumbar 3–4 DRG neurons. Prior to IB4-FITC staining, cells were tested for responsiveness to 50 mM K+ using ratiometric calcium imaging. Only cells that responded to 50 mM K+ were considered healthy neurons and quantified. Following L4 SNL, the percentage of small neurons that were IB4 positive decreased in the adjacent L3 group but not in the directly injured L4 group. (*** p<0.0001, Fisher’s exact test).
To determine whether the injury-induced increase in heat-responsive IB4 positive neurons was due to TRPV1 or another heat-sensitive channel, we used the TRPV1 antagonist A-425619 (1μM). Treatment with A-425619 completely and reversibly blocked all heat-evoked currents in IB4 positive L4 neurons after SNL (n=5), IB4 negative L4 neurons after SNL (n= 8), and all adjacent L3 neurons after SNL (n=2 IB4 positive; n =10 IB4 negative). Furthermore, A-425619 blocked all heat responses in sham controls (n=4 IB4 positive; n=16 IB4 negative). Thus, all heat-evoked currents after SNL were mediated entirely by TRPV1.
One explanation for the divergent changes in heat responsiveness of IB4 positive versus negative neurons after axotomy (L4 SNL group) may be a change in the IB4 staining. For example, some former IB4 negative neurons may have gained IB4 staining after injury, thereby increasing the apparent heat responsiveness of IB4 positive neurons. Thus, we quantified IB4 staining in small-diameter L3 and L4 DRG neurons from naive mice, or from mice 7 days after SNL. To verify that all cells examined were viable neurons, cells were tested by depolarization with 50 mM K+ using Fura-2 calcium imaging, prior to staining with IB4-FITC. Cells that responded with an increase in Fura-2 ratio > 20% over baseline during exposure to 50 mM K+ were considered healthy neurons. In contrast to findings in rat, 21, 32 the frequency of IB4 binding among small neurons did not change in injured L4 neurons compared to naive controls in mouse (Fig. 4E). This suggests that the increase in heat-responsive IB4 positive injured neurons and decrease in heat-responsive IB4 negative neurons was not due to changes in IB4 binding. However, IB4 binding decreased in neurons adjacent to injury from 54% in naive L3 neurons to 30% in adjacent L3 neurons after L4 SNL (p<0.0001, Fisher’s exact test).
Nerve injury had no effect on heat responsiveness in medium-large neurons
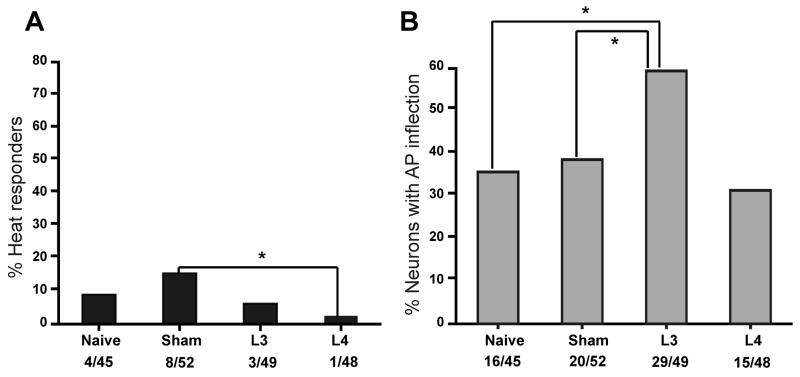
Among medium-large diameter neurons, SNL had no significant effect on heat response in either the injured L4 neurons or adjacent L3 neurons compared to naive neurons (Fig. 5A). However, injured L4 neurons were significantly less responsive to heat compared to sham controls (Fig. 5A). The difference between sham neurons and injured L4 SNL neurons may be because sham neurons are exposed to some inflammation from surgery whereas the L4 SNL neurons are exposed to axotomy and inflammation.
Figure 5. Nerve injury-induced changes in medium-large neurons.
A. Percentage of medium-large (>26 μm) diameter neurons (putative A fiber type neurons) that responded to heat after L4 SNL. There was no change in the percentage of heat-responsive neurons in the adjacent L3 or injured L4 groups compared to naive controls. However, the injured L4 neurons are less responsive to heat than the sham control neurons (* p = 0.032; Fisher’s exact test). B. Percentage of medium-large neurons that exhibited an inflection on the descending phase of the somal action potential in naive, sham control, L3 adjacent or L4 injured groups. SNL increased the percentage of L3 adjacent neurons that expressed an action potential inflection (* p<0.05, Fisher’s exact test).
One criterion to identify putative nociceptors among the medium-large somata after isolation is the presence of an inflection on the falling phase of the action potential.17, 28, 51 All four heat-sensitive neurons in the medium-large naive group exhibited a prominent inflection on the somal action potential. After L4 SNL, significantly more L3 adjacent neurons expressed an inflection compared to either naive or sham neurons (Fig. 5B). This finding agrees with changes reported by others37 and indicates that the presence of an inflection on the somal action potential is not a stable criterion for defining myelinated nociceptors in injury models.
Discussion
Here we show that peripheral nerve injury in mice differentially regulates the heat response properties of directly-injured small neurons during the same time course of behavioral hypersensitivity to noxious heat. Furthermore, TRPV1 inhibition completely blocks the heat hypersensitivity at both the sensory neuron membrane and the behavioral levels, indicating that in mice, TRPV1 mediates the heat hyperalgesia induced by spinal nerve ligation.
Whereas IB4 positive neurons from non-injured mice rarely respond to noxious heat, spinal nerve ligation increased their heat responsiveness 4-fold, such that after injury over 50% of the injured IB4 positive neurons responded to noxious heat. In contrast, IB4 negative neurons which are normally very responsive to noxious heat, were decreased in heat sensitivity by spinal nerve ligation. These differential effects of injury on functionally distinct subpopulations of small neurons were not likely due to underlying changes in IB4 binding because the percentage of neurons binding IB4 after injury was unchanged in mice. Other studies in rats21, 32 have shown a marked decrease in IB4 staining in DRG neurons after injury. Glial cell line-derived neurotrophic factor (GDNF) is critical for the survival and function of IB4 positive neurons.2, 43, 67 The differential effect of injury on IB4 binding in mice verses rats may be because mice and rats express different profiles for GDNF production by Schwann cells and satellite cells after nerve injury. 20, 45
Three pieces of evidence indicate that the heat-evoked current in the somata of all neurons from either naive mice or injured mice is mediated entirely by TRPV1. First, a selective TRPV1 antagonist, A-425619, completely and reversibly inhibited all heat-evoked inward currents in naive, sham, SNL injured and SNL adjacent neurons. Second, none of the neurons from TRPV1-mutant mice responded to noxious heat. Third, the heat threshold for all neurons ranged between 42 and 47 °C, which is consistent with the documented threshold for TRPV1 in an expression system.9 Thus, in isolated somata, heat-evoked inward currents in neurons from naive, nerve injured and inflamed mice were entirely due to activation of TRPV1.6 In contrast to the soma, heat responses at the peripheral terminal are less dependent on TRPV1. Recordings from skin-nerve preparations, where heat is applied at the peripheral terminal and responsiveness is recorded in the peripheral axon, indicate that heat-sensitive C fibers with thresholds ~ 42 °C remain partly intact in TRPV1 deficient mice8, 30, 63, 66 (and Stucky C.L., unpublished data), albeit to different degrees in different laboratories. The residual heat responses in peripheral axons may be mediated by heat-sensitive ion channels expressed only at the terminal region of the neuron or by non-neuronal cells in the skin. For example, keratinocytes express functional heat-sensitive channels including TRPV3 and TRPV419, 47 and recent evidence suggest that keratinocytes confer heat responsiveness to nearby sensory terminals via release of ATP.31, 40 Together, these findings indicate that future studies searching for heat transduction molecules should include a component where the sensory terminal remains in its native milieu such that interactions between non-neuronal cells in the target tissue and the sensory neuron membrane can be appropriately modeled and investigated.
When using isolated DRG somata as a model for the sensory terminal, the dissociation and culture procedure may intrinsically injure the neuron, thereby altering the composition and density of ion channels normally expressed by the soma membrane in vivo.29, 65 Nonetheless, using DRG somata as a model provides an important measure of the overall function of TRPV1 channels in the plasma membrane. Indeed, TRPV1 is expressed by many membrane regions of the sensory neuron, including central terminals in the spinal cord,44 peripheral axons of passage,4 the cell body and the peripheral terminals. In our study, DRG somata from all groups were processed in an identical manner, and therefore, the differences observed were likely due to the injury in vivo.
The somata of directly injured neurons no longer have axons that reach peripheral targets. Injured neurons frequently form neuromas at the cut end of an injured nerve. Increased expression of ion channels at the neuroma in the setting of an inflammatory milieu may contribute to spontaneous pain via spontaneous input to the spinal cord and/or sensitization of spinal cord neurons. It has been hypothesized that increased TRPV1 function may contribute to spontaneous activity via a reduction in the thermal threshold, such that in the setting of nerve or tissue injury, TRPV1 becomes activated at body temperature.52, 56
Our data add to a growing body of evidence that IB4 positive C fiber neurons play important roles in injury. The majority of cutaneous C fiber neurons that innervate the epidermis bind IB4.35 They are known to be nociceptors and respond to mechanical stimuli in situ.1, 17, 60 A variety of animal models of persistent pain indicate that IB4 positive neurons are highly sensitive and malleable to injury and upregulate their functional response properties in response to injury. For example, IB4 positive neurons increase TRPV1 function after peripheral inflammation.6 Interestingly, evidence now indicates that IB4 positive neurons provide input to a special pathway to the brain that is involved in modulating the emotional processing of pain.5, 69 Together, these data suggest that IB4 positive nociceptors may be involved in providing enhanced input to the emotional aspects of neuropathic pain.
Nerve injury pain is due to a mixture of effects from axon injury and inflammation. Inflammation is well-documented to increase TRPV1 function.8, 12 In our study, neurons from animals with sham surgery were very likely exposed to some degree of inflammation. This is suggested by a trend for increased TRPV1-mediated heat responsiveness in medium-large neurons from sham animals compared to naive controls (Fig 5A). In animals with SNL, the L3 adjacent neurons were exposed to minor injury and inflammation, whereas L4 neurons were axotomized and exposed to inflammation. We speculate that these differences in inflammation and axon injury between groups underlies our finding that TRPV1 function was significantly decreased in injured L4 medium-large neurons compared to sham surgery controls, and that the net effect of direct injury on myelinated neurons is decreased TRPV1 function. Thus, the overall change in TRPV1 function in myelinated neurons is due to the combination of inflammation and nerve injury. In different animal models of nerve injury, if inflammation is predominant, TRPV1 function increases; if injury is predominant, TRPV1 function decreases.
In behavioral experiments, L4 SNL induced heat hypersensitivity in the hind paw that was completely reversed by acute intraperitoneal injection with the TRPV1 inhibitor A-425619. In the days following L4 SNL, the plantar paw presumably loses innervation by the L4 afferents but retains innervation by L3 afferent terminals. The heat hypersensitivity is not likely due to sensitization of L3 adjacent neurons because we found no change in TRPV1 function in neurons from the L3 ganglia. Instead, we observed increased TRPV1-mediated heat responses in IB4 positive neurons from the injured L4 ganglia. We propose that increased TRPV1 activity in injured IB4 positive neurons, possibly due to reduced TRPV1 heat threshold and activation by body temperature, contributes to enhanced spontaneous activity in these neurons that subsequently sensitizes spinal cord neurons to respond more to input from intact L3 afferents activated by peripheral heat stimuli.
Knowledge about nerve injury-induced changes in TRPV1 function has important clinical implications. First, injury-induced upregulation of TRPV1 function provides rationale for treatment strategies that inhibit or desensitize TRPV1. Second, identifying specific neuronal subtypes that exhibit increased TRPV1 function may lead to treatments that specifically target these neuronal subtypes. For example, selective inhibition or elimination of IB4 positive C fiber-type neurons during neuropathic pain may help alleviate negative emotional aspects of chronic pain, but leave intact the acute, protective nociceptive responses necessary for safe interaction with the environment.
Acknowledgments
This work was funded by NIH grant NS40538 to CLS. We thank Dr. Aniko Szabo, Ph.D. in the Biostatistical Consulting Center at the Medical College of Wisconsin for assistance with statistical analyses.
Footnotes
Perspective: TRPV1 function is upregulated in IB4 positive sensory neurons and TRPV1 is responsible for the behavioral heat hypersensitivity in the spinal nerve ligation model. Since IB4 positive neurons may contribute to the emotional perception of pain, TRPV1 antagonists, targeting both sensory and affective pain components, could have broad analgesic effects.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardini N, Neuhuber W, Reeh PW, Sauer SK. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126:585–590. doi: 10.1016/j.neuroscience.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 11.Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J Gen Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 13.Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB(4)- positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89:513–524. doi: 10.1152/jn.00371.2002. [DOI] [PubMed] [Google Scholar]
- 14.Dittert I, Benedikt J, Vyklicky L, Zimmermann K, Reeh PW, Vlachova V. Improved superfusion technique for rapid cooling or heating of cultured cells under patch-clamp conditions. J Neurosci Methods. 2006;151:178–185. doi: 10.1016/j.jneumeth.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Kouhen R, Surowy CS, Bianchi BR, Neelands TR, McDonald HA, Niforatos W, Gomtsyan A, Lee CH, Honore P, Sullivan JP, Jarvis MF, Faltynek CR. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1 receptor antagonist, blocks channel activation by vanilloids, heat, and acid. J Pharmacol Exp Ther. 2005;314:400–409. doi: 10.1124/jpet.105.084103. [DOI] [PubMed] [Google Scholar]
- 17.Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565:927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 19.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- 21.Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- 22.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 23.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 24.Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El Kouhen R, Lee CH, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- 25.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 28.Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- 29.LaMotte RH. Acutely dissociated sensory neurons: normal or neuropathic? Focus on: “Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP”. J Neurophysiol. 2007;97:1–2. doi: 10.1152/jn.01021.2006. [DOI] [PubMed] [Google Scholar]
- 30.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Caterina MJ. TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch. 2005;451:160–167. doi: 10.1007/s00424-005-1438-y. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Zhou XF. Pericellular Griffonia simplicifolia I isolectin B4-binding ring structures in the dorsal root ganglia following peripheral nerve injury in rats. J Comp Neurol. 2001;439:259–274. doi: 10.1002/cne.1349. [DOI] [PubMed] [Google Scholar]
- 33.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Zhou XF, Rush RA. Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neurosci Res. 2001;41:355–363. doi: 10.1016/s0168-0102(01)00293-0. [DOI] [PubMed] [Google Scholar]
- 36.Lyfenko A, Vlachova V, Vyklicky L, Dittert I, Kress M, Reeh PW. The effects of excessive heat on heat-activated membrane currents in cultured dorsal root ganglia neurons from neonatal rat. Pain. 2002;95:207–214. doi: 10.1016/S0304-3959(01)00401-8. [DOI] [PubMed] [Google Scholar]
- 37.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- 38.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 39.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- 40.Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009 doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 44.Nakatsuka T, Furue H, Yoshimura M, Gu JG. Activation of central terminal vanilloid receptor-1 receptors and alpha beta-methylene-ATP-sensitive P2X receptors reveals a converged synaptic activity onto the deep dorsal horn neurons of the spinal cord. J Neurosci. 2002;22:1228–1237. doi: 10.1523/JNEUROSCI.22-04-01228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 46.Nicol GD, Cui M. Enhancement by prostaglandin E2 of bradykinin activation of embryonic rat sensory neurones. J Physiol. 1994;480 (Pt 3):485–492. doi: 10.1113/jphysiol.1994.sp020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 48.Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. in vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:387–393. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- 49.Rashid MH, Inoue M, Kondo S, Kawashima T, Bakoshi S, Ueda H. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J Pharmacol Exp Ther. 2003;304:940–948. doi: 10.1124/jpet.102.046250. [DOI] [PubMed] [Google Scholar]
- 50.Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136:188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- 52.Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol. 2007;581:631–647. doi: 10.1113/jphysiol.2006.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 54.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur J Neurosci. 2000;12:457–466. doi: 10.1046/j.1460-9568.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 56.Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- 57.Sukhotinsky I, Ben-Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur J Pain. 2004;8:135–143. doi: 10.1016/S1090-3801(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 58.Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exp Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- 59.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 60.Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- 61.Wall P, Melzack R. Textbook of Pain. 5. Churchill Livingstone; 2006. pp. 705–727. [Google Scholar]
- 62.Wang C, Hu HZ, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem. 2004;279:37423–37430. doi: 10.1074/jbc.M407205200. [DOI] [PubMed] [Google Scholar]
- 63.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng JH, Walters ET, Song XJ. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J Neurophysiol. 2007;97:15–25. doi: 10.1152/jn.00559.2006. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann K, Leffler A, Fischer MM, Messlinger K, Nau C, Reeh PW. The TRPV1/2/3 activator 2-aminoethoxydiphenyl borate sensitizes native nociceptive neurons to heat in wildtype but not TRPV1 deficient mice. Neuroscience. 2005;135:1277–1284. doi: 10.1016/j.neuroscience.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 67.Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 69.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]