Abstract
Genetic analyses aimed at identification of the pathways and downstream effectors of calorie restriction (CR) in the yeast Saccharomyces cerevisiae suggest the importance of central metabolism for the extension of replicative life span by CR. However, the limited gene expression studies to date are not informative, because they have been conducted using cells grown in batch culture which markedly departs from the conditions under which yeasts are grown during life span determinations. In this study, we have examined the gene expression changes that occur during either glucose limitation or elimination of nonessential amino acids, both of which enhance yeast longevity, culturing cells in a chemostat at equilibrium, which closely mimicks conditions they encounter during life span determinations. Expression of 59 genes was examined quantitatively by real-time, reverse transcriptase polymerase chain reaction (qRT-PCR), and the physiological state of the cultures was monitored. Extensive gene expression changes were detected, some of which were common to both CR regimes. The most striking of these was the induction of tricarboxylic acid (TCA) cycle and retrograde response target genes, which appears to be at least partially due to the up-regulation of the HAP4 gene. These gene regulatory events portend an increase in the generation of biosynthetic intermediates necessary for the production of daughter cells, which is the measure of yeast replicative life span.
Keywords: Aging, Calorie restriction, Saccharomyces cerevisiae, Central metabolism, Replicative life span, Retrograde response, Tricarboxylic acid cycle, Glyoxylate cycle
1. Introduction
Nutrient-limitation, which is also called CR, can delay aging and extend life span from lower eukaryotic organisms to mammals (Guarente, 2005). Early studies demonstrated that food restriction could extend the life span of laboratory rodents (Guarente, 2005). Later, researchers found that not only can CR extend life span, but it also can prevent various diseases in mammals (Guarente, 2005; Jolly, 2005). In the laboratory, limited food intake in rats and mice significantly increases longevity, retards aging, and delays or prevents age-associated diseases (Masoro, 1993). DNA microarray analyses have revealed that transcription of many genes is altered during aging and CR, in tissues such as skeletal muscle (Lee et al., 1999; Park et al., 2005), brain (Lee et al., 2000), and heart (Park et al., 2005). The effects of CR in nonhuman primates (monkeys) have also been investigated (Jolly, 2005). Masoro has suggested that CR is a long-term, low-intensity stressor (Masoro, 2000).
During the past decade, aging research has focused on molecular mechanisms of aging, and single gene mutations that extend lifespan have been identified. In the yeast model, it was shown that increased transcription of SIR2, which encodes an NAD-dependent protein deacetylase, extends life span (Kaeberlein et al., 1999), and SIR2 was needed for life extension during CR in S. cerevisiae, likely at least in part due to a reduction in formation of deleterious extrachromosomal ribosomal DNA circles (ERCs) (Lin et al., 2000; Lin et al., 2002). However, other studies dispute the role ofSIR2 in CR (Jiang et al., 2002; Kaeberlein et al., 2004). CR increased the respiration of yeast cells (Lin et al., 2002), and this was cited as the cause of increased longevity, although this conclusion has been called into question (Kaeberlein et al., 2005). Another gene that has been found to play a role in yeast longevity is RPD3, which encodes a histone deacetylase. Deletion of RPD3 can extend life span of yeast (Kim et al., 1999). SIR2 and RPD3 are highly conserved genes. In Drosophila, the SIR2 homolog (dSIR2) and RPD3 are involved in longevity regulation by CR (Rogina et al., 2002; Rogina et al., 2004), but this is not the case in yeast (Jiang et al., 2002). It was shown that transcription of mammalian Sir2 (SIRT1) is induced in rat and in human cells, which were treated with serum from CR rats (Cohen et al., 2004). Many single gene mutations in Caenorhabditis elegans, which can increase life span substantially have been found. Among these genes are daf-2, daf-16 (Kenyon et al., 1993), and age-1 (Friedman et al., 1988). An extra copy of sir-2.1, which is homologous in C. elegans to yeast SIR2, extended life span by up to 50% in a daf-16-dependent manner (Tissenbaum et al., 2001). sir-2.1 is required for life extension by CR in this worm, performing distinct but overlapping functions with daf-16 (Wang et al., 2006).
CR represents a metabolic mechanism in yeast longevity. Another such mechanism is the retrograde response (Kirchman et al., 1999). The retrograde response signals mitochondrial dysfunction to the nucleus resulting in numerous gene expression changes involving metabolic and stress response genes (Epstein et al., 2001). Although CR and the retrograde response operate in different pathways, they appear to share some overlapping effectors (Jiang et al., 2000).
Research on yeast aging and longevity shows that there are four main processes involved in the determination of lifespan: metabolism, stress resistance, chromatin-dependent gene regulation, and genome stability (Jazwinski, 2005). These four processes are linked by the retrograde response (Jazwinski, 2005). There are three proteins specific to this signaling pathway, Rtg2 and the Rtg1–Rtg3 heterodimer. Yeast ρ0 strains, which have lost their mitochondrial DNA and activate the retrograde response, show an extended mean and maximum lifespan (Kirchman et al., 1999). Deletion of the retrograde response genes, RTG2 and RTG3, abrogated the life extension (Kirchman et al., 1999; Borghouts et al., 2004). During the retrograde response, CIT2, encoding peroxisomal citrate synthase is highly induced (Liao et al., 1991). Microarray analysis showed that in ρ0 cells most genes encoding oxidative phosphorylation proteins in mitochondria were down-regulated, while most genes involved in intermediary metabolism, including CIT2 were up-regulated (Epstein et al., 2001). Mitochondrial dysfunction can also signal changes in metabolic gene expression in different human cell lines (Miceli et al., 2005), and its activation extends the clonal life span of mammalian cells (Passos et al., 2007).
CR is likely to affect metabolism in yeast cells. DNA microarray analysis showed that during CR (0.5%glucose) mitochondrial genes, cell wall genes, genes involved in intermediary metabolism, and ribosomal and nucleolar genes were up-regulated (Lin et al., 2002). The decrease in the glucose concentration in the growth medium extended yeast life span up to 35% (Lin et al., 2000). The gene expression changes associated with the reduction of glucose to 0.5% are likely to simply reflect a release from glucose repression. It has also been shown that decreasing the glucose concentration from 2% to 0.1% (or even lower) extended yeast life span up to 50% (Jiang et al., 2000; Jiang et al., 2002). This decrease in glucose concentration goes well beyond what is normally needed to relieve glucose repression, which is effected by the limitation of glucose to 0.5% (Van Hoek et al., 1998). Eliminating the non-essential amino acids caused an increase in life span up to 100% (Jiang et al., 2000; Jiang et al., 2002). This more profound effect on life span may be associated with additional effects on gene regulation. A genetic analysis of CR in yeast has pointed to the importance of gene expression changes that affect carbon metabolism and energy metabolism in life extension by this regimen (Jiang et al., 2002).
Here, we focus on changes in the expression of the genes of central metabolism during CR by using chemostat cultures rather than batch cultures. In chemostat cultures, the dilution rates, which are also known as the specific growth rates, culture pH, nutrients-limitation, and dissolved-oxygen concentration can be tightly controlled. These cultures also allow alteration of one parameter while keeping other conditions constant (Van den Berg et al., 1996; Van Hoek et al., 1998; Ter Linde et al., 1999; Boer et al., 2003). Chemostat culture is the most reliable way to perform gene expression research, because it assesses gene expression at steady state. In addition, chemostat cultures most closely approximate the situation encountered by individual yeast cells during the determination of their replicative life span on plates, allowing conclusions to be drawn concerning the possible mechanisms underlying the life-extending effects of CR.
2. Materials and methods
2.1. Strains
The S. cerevisiae strain YPK9 (MATa, ade2-101ochre, his3Δ-200, leu2Δ-1, lys2-801amber, trp1Δ-63, ura3-52) was used in this study (Kirchman et al., 1999). Modified, chemically defined (CM) medium was used (Jiang et al., 2000). Caloric restriction was imposed by reduction of either glucose levels (from 2 to 0.1%) or nonessential amino acids levels (from 100 to 0% of that normally present), leaving the required supplements constant (Jiang et al., 2000). The nonessential amino acids in the medium were arginine, aspartate, glutamate, methionine, phenylalanine, serine, threonine, tyrosine, and valine. The same modified CM medium was used for chemostat cultures and for life span determinations on plates, which contained 2% agar, and caloric restriction was performed in both cases as described above.
2.2. Culture conditions
YPK9 was grown at 30°C in a 2.5-liter chemostat (BioFlow 3000, New Brunswick Scientific) with a working volume of 1.5 liters. Cultures were fed with CM medium that limited growth by either glucose or nonessential amino acids with all other growth gradients, Yeast Nitrogen Base and essential amino acids, in excess and at a constant residual concentration. The dilution rate was set at 0.20 h−1. The pH was measured by the pH electrode and kept constant at 5.5 by the automatic addition of 1 M NaOH with a feeding pump which was controlled by the chemostat. Agitation speed was 500 rpm, and the airflow was 0.4 liters·min−1. Dissolved oxygen tension was measured by the DO probe. The off-gas was cooled by a condenser connected to a thermostat set at 12°C, and oxygen and carbon dioxide were measured with an EX-2000 off-gas analyzer (New Brunswick Scientific). Steady-state samples were harvested after ~10–12 volume changes (Boer et al., 2003). Glucose concentration, dry weight, dissolved oxygen and gas profiles had to be constant over at least 3 volume changes before sampling for RNA extraction (Boer et al., 2003).
2.3. Sample collection
Samples were collected on ice in 1.5-ml microfuge tubes with screw-caps. Cells were centrifuged at 4°C using a benchtop centrifuge at maximum speed. The pellets were washed once with distilled water. The cells were frozen in liquid nitrogen, and then stored at −80°C if not used for RNA preparation immediately. The entire harvesting of samples was accomplished in less than 5 minutes to prevent metabolic changes in the cells. Dry weight measurements were performed as described by Postma et al. (1989). Nitrocellulose filters (pore size 0.45 μm; Pall Corporation, Ann Arbor, Mich.) were used.
2.4. Analysis of glucose concentration
Samples for measuring glucose concentration were obtained by centrifugation of cultures at 4°C, as above. The supernatants were used for analysis immediately, or they were stored at 4°C no more than 16 hours. In this assay, the Amplex Red Glucose/Glucose Oxidase Assay Kit (Molecular Probes, Inc.) was used. The reaction volume was 100 μl. A serial dilution of culture supernatant in 1× reaction buffer was done to determine the optimal amount of sample in the assay. The final glucose concentration in the reaction was lower than 100 μM, to prevent quenching by nonfluorescent products.
2.5. RNA isolation
RNA was isolated by using the hot, acidic-phenol method (Ausubel et al., 1993). RNA was dissolved in distilled-water. RNA concentrations were measured by absorbance at 260 nm. The A260/A280 ratios of the preparations ranged from 1.9 to 2.0.
2.6. qRT-PCR
qRT PCR was performed on a Prism 7000 Sequence Detection real-time PCR system (Applied Biosystems, Inc) in a two-step reaction as described (Miceli et al., 2005). Total RNA was reversed-transcribed in a 25-μl volume using RT reagents from ABI; random hexamers were used as primers. For real-time PCR, primers were designed by using the ABI Primer Express software and purchased from Integrated DNA Technologies Inc. (Coralville, IA). All primer pairs were checked by BLAST search of the Saccharomyces Genome Database to assure homology only against the target genes. A 20-μl reaction volume was used, which contained 0.02 μg cDNA template, 0.2 μM each of the forward and reverse primers, and 10 μl 2× SYBR green PCR Master Mix (ABI). All reactions were performed in triplicate on at least two independent samples. The amplification conditions are described in Miceli and Jazwinski (2005). The housekeeping gene ACT1 was used as a control for template loading.
2.7. Life span determination
Determination of replicative life span has been described previously (Kim et al., 1998). Briefly, cells were precultured in medium containing glycerol as a carbon source to eliminate petites. The cells were spotted on agar plates, and individual, budding cells were micromanipulated to isolated spots on the plate. The buds were removed when they were mature to initiate the life span determination, while the mother cells were discarded. Every bud produced by these isolated cells (40 for each determination) was removed and discarded. This was continued until these cells stopped dividing and lysed or lost refractility. The total number of these buds was counted to yield the life span in generations.
2.8. Statistical analysis
Tests of statistical significance of the qRT-PCR data were performed using StatMost statistical software (DataMost Corporation, Sandy, UT). Analysis of variance was performed on the raw values corrected for DNA loading. P values of <0.05 were taken as the criterion of significance after correction for multiple comparisons. Statistical significance of differences between life spans was assessed using the Mann-Whitney test.
3. Results
3.1. Physiological changes in S. cerevisiae in nutrient-limited culture
Adaptation to environmental changes often entails metabolic alterations, many of which are regulated transcriptionally. Fig. 1 shows the pathways of central carbon metabolism in yeast and all of the genes investigated in this study. When glucose is the carbon source, metabolic conditions can be completely different depending on its concentration. Above a critical glucose concentration, metabolism becomes increasingly anaerobic. The respiratory quotient (RQ) begins to increase, and the growth yield declines. The glucose concentration at which this metabolic shift occurs varies substantially from strain to strain (Van Hoek et al., 1998). In glucose rich media, glycolysis/fermentation is the primary source of energy, with ethanol produced as a byproduct. Genes encoding enzymes for gluconeogenesis, the tricarboxylic acid (TCA) cycle, and respiratory metabolism are repressed. Glucose repression and de-repression involve several signaling pathways (Johnston, 1999; Kaniak et al., 2004). In glucose-limited, aerobic culture, this glucose repression is released, and respiration in mitochondria is the major energy-yielding pathway. Reduction of glucose concentration beyond the point at which glucose repression no longer operates likely results in further metabolic changes, as evidenced by changes in the rate of cell production (Sierkstra et al., 1992) and replicative life span (Jiang et al., 2000). We have investigated the expression of genes of central carbon metabolism under two different nutrient limitation conditions during steady-state culture in a chemostat. To verify equilibrium was reached under each condition, glucose concentration, biomass yields, and oxygen consumption and carbon dioxide production were measured (Table 1). At the 0.2 h−1 dilution rate obtained in glucose-limited culture (0.1% glucose), the cells mainly respired (RQ=0.88) and a high biomass yield was obtained. This RQ was lower than the RQ of 1.0 found in glucose-limited cultures at dilution rates less than 0.3h−1 (Van Hoek et al., 1998). However, the glucose concentration in that study was 0.75%, compared to the 0.1% used here. In our glucose-limited cultures, fatty acid oxidation and other metabolic processes, such as the glyoxylate cycle, are activated, judging by the RQ (Table. 1), which was 0.88 and significantly lower than 1.0 (P<0.05, from 4 separate experiments). The glucose was used efficiently, as evidenced by the yield. On the other hand, the glucose consumption, RQ, and the biomass yield were similar on 2% glucose with or without the nonessential amino acids in the medium, suggesting that metabolic changes under these conditions may be more subtle.
Fig. 1.
Schematic of central metabolism pathways in Saccharomyces cerevisiae. Variations in gene expression found in this study are highlighted. Regular font indicates no changes under both CR conditions. Bold font shows changes under 0.1% glucose limitation conditions. Italic font depicts changes in 2% glucose without nonessential amino acids conditions. Bold Italic font highlights changes that were found under both nutrient limitation conditions. Decreased expression is indicated by underlines. All other changes in expression are increases.
Table 1.
Glucose concentration and the physiological parameters of the steady-state cultures under different culture conditions.
| Growth conditions | Residual glucose (g/L) | Y(g/g)a | qglucoseb | qCO2c (× 10−4) | qO2d (× 10−4) | RQe |
|---|---|---|---|---|---|---|
| 2%glucose | 14.45 ± 0.25 | 0.127 ± 0.0025 | 8.79 ± 0.16 | 60.6 + 2.9 | 25.6 + 4.4 | 2.43 + 0.32 |
| 0.1%glucose | 0.041 ± 0.005 | 0.66 ± 0.01 | 1.68 ± 0.025 | 22.9 + 3.3 | 26.2 + 3.9 | 0.88 + 0.12 |
| 2%glucose without nonessential amino acids | 13.0 ± 0.85 | 0.0915 ± 0.0175 | 12.0 ± 1.55 | 59.5 + 0.3 | 23.9 + 3.6 | 2.55 + 0.37 |
Data for the glucose concentration, biomass and glucose consumption rate present the average and S.D. of two separate chemostat steady state cultures. Data for the carbon dioxide production rate, oxygen consumption rate and the respiratory quotient present the average and S.D. of at least four separate chemostat steady state cultures.
yield of biomass (g/g of glucose consumed)
mmol glucose consumed/g biomass/hour
mmol carbon dioxide produced/g biomass/hour
mmol oxygen consumed/g biomass/hour
respiratory quotient (qCO2/qO2)
3.2. Differential regulation of low- and high-affinity hexose tranporters
Hexose transport into the cell is the first step in carbon metabolism. We examined four hexose transporter genes: HXT1 and HXT3, which are low-affinity transporters; HXT2, a high-affinity transporter repressed at high glucose concentration; and HXT4, which is a moderate-affinity transporter. Table 2 shows that under glucose-limited conditions, the transporter pairs HXT1/HXT3 and HXT2/HXT4 had similar patterns of transcription, respectively. Both HXT1 and HXT3 genes were significantly down-regulated; on the contrary, HXT2 and HXT4 were up-regulated significantly, even though HXT4 was induced nearly 5-fold higher than HXT2. All four genes were up-regulated when nonessential amino acids were eliminated from the medium. The changes for HXT2 and HXT4 were over 1.5-fold. These results suggest that the regulation of hexose transport genes depends not only on the glucose in the culture, but also on nitrogen sources.
Table 2.
Gene expression changes during glucose limitation and during elimination of nonessential amino acids.
| Name | 0.1% glucose/2% glucose | 2% glucose without nonessential amino acids/2% glucose | |||||
|---|---|---|---|---|---|---|---|
| Mean | S.E. | P valuea | Mean | S.E. | P valuea | ||
| Hexose transporter genes | HXT1 | 0.06 | 0.001 | 1.3×10−24 | 1.4 | 0.094 | 8.7×10−5 |
| HXT2 | 2.2 | 0.211 | 0.0001 | 1.7 | 0.117 | 7.9×10−13 | |
| HXT3 | 0.013 | 0.001 | 8.5×10−15 | 1.4 | 0.075 | 3.7×10−8 | |
| HXT4 | 11 | 0.587 | 3.5×10−14 | 1.5 | 0.038 | 0.0002 | |
| Genes involved in the glycolysis pathway | HXK1 | 28 | 5.210 | 9.3×10−25 | 1.8 | 0.329 | 4.9×10−6 |
| HXK2 | 3.0 | 0.173 | 4.5×10−16 | 1.1 | 0.016 | 0.0084 | |
| GLK1 | 8.7 | 0.194 | 2.3×10−26 | 2.0 | 0.147 | 2.3×10−11 | |
| PFK1 | 1.1 | 0.062 | 0.044 | 1.2 | 0.024 | 5.2×10−6 | |
| PFK2 | 1.2 | 0.194 | 9.2×10−5 | 1.1 | 0.097 | 0.029 | |
| FBA1 | 0.78 | 0.022 | 3.3×10−7 | 1.3 | 0.032 | 2.5×10−7 | |
| TDH1 | 0.56 | 0.036 | 5.6×10−13 | 0.82 | 0.051 | 3.8×10−7 | |
| CDC19 | 0.78 | 0.074 | 0.059 | 1.4 | 0.142 | 0.0002 | |
| Genes involved in glycogen and trehalose synthesis | TPS1 | 1.5 | 0.105 | 1.2×10−7 | 1.3 | 0.094 | 4.6×10−5 |
| GSY1 | 8.8 | 2.21 | 3.9×10−16 | 2.3 | 0.561 | 1.1×10−5 | |
| GSY2 | 3.0 | 0.055 | 1.9×10−17 | 1.1 | 0.065 | 0.3795 | |
| Genes involved in the TCA cycle | CIT1 | 6.4 | 0.684 | 4.8×10−20 | 1.9 | 0.175 | 5.0×10−5 |
| ACO1 | 4.0 | 0.254 | 1.0×10−19 | 1.7 | 0.098 | 1.3×10−10 | |
| IDH1 | 3.2 | 0.305 | 1.2×10−12 | 2.0 | 0.174 | 3.1×10−9 | |
| IDH2 | 3.3 | 0.404 | 2.8×10−13 | 1.7 | 0.196 | 6.2×10−8 | |
| KGD1 | 5.4 | 0.435 | 2.1×10−8 | 1.1 | 0.059 | 0.0026 | |
| SDH1 | 15 | 0.275 | 3.1×10−27 | 1.3 | 0.059 | 2.3×10−5 | |
| MDH1 | 7.4 | 1.19 | 4.8×10−20 | 1.1 | 0.239 | 0.013 | |
| ATP synthase | ATP2 | 4.2 | 0.228 | 1.7×10−22 | 0.97 | 0.077 | 0.025 |
| Genes involved in the mitochondrial respiratory chain | CYT1 | 4.5 | 0.381 | 8.7×10−13 | 1.2 | 0.024 | 1.4×10−5 |
| CYC1 | 2.3 | 0.170 | 6.6×10−9 | 1.2 | 0.070 | 0.13 | |
| NDI1 | 11 | 1.07 | 7.4×10−10 | 1.6 | 0.055 | 3.3×10−9 | |
| Genes involved in the gluconeogenesis pathway | FBP1 | 25 | 3.13 | 3.6×10−13 | 1.0 | 0.056 | 0.067 |
| PCK1 | 13 | 3.61 | 2.0×10−7 | 1.3 | 0.095 | 0.0007 | |
| MDH2 | 27 | 4.95 | 1.2×10−10 | 2.1 | 0.327 | 7.4×10−8 | |
| Genes involved in the glyoxylate cycle | CIT2 | 2.4 | 0.241 | 1.0×10−12 | 1.5 | 0.134 | 6.8×10−7 |
| ICL1 | 13 | 0.984 | 7.4×10−20 | 1.4 | 0.058 | 1.0×10−5 | |
| MLS1 | 20 | 2.31 | 2.4×10−12 | 1.2 | 0.073 | 0.38 | |
| Genes involved in the pentose phosphate pathway | ZWF1 | 0.98 | 0.031 | 0.2463 | 2 | 0.06 | 1.4×10−9 |
| GND2 | 2.9 | 0.312 | 5.3×10−12 | 1.1 | 0.022 | 0.017 | |
| Genes involved in pyruvate metabolism | PDC1 | 0.49 | 0.031 | 4.6×10−8 | 1.0 | 0.045 | 0.10 |
| PDA1 | 1.3 | 0.075 | 0.0003 | 1.1 | 0.073 | 0.029 | |
| PDB1 | 1.3 | 0.041 | 0.0001 | 1.0 | 0.022 | 0.018 | |
| PYC1 | 2.8 | 0.272 | 7.7×10−12 | 2.3 | 0.239 | 2.4×10−10 | |
| ADH1 | 1.4 | 0.057 | 7.7×10−9 | 1.4 | 0.056 | 2.3×10−7 | |
| ALD2 | 1.7 | 0.172 | 2.6×10−9 | 1.5 | 0.117 | 1.5×10−5 | |
| ACS1 | 366 | 61.6 | 2.7×10−16 | 1.9 | 0.106 | 1.4×10−7 | |
| ACS2 | 1.5 | 0.077 | 3.4×10−8 | 1.2 | 0.070 | 0.003 | |
| Genes encoding amino acid permeases | AGP1 | 4.7 | 0.092 | 7.3×10−23 | 2.9 | 0.189 | 1.3×10−11 |
| BAP2 | 1.4 | 0.018 | 1.4×10−10 | 0.82 | 0.023 | 0.0011 | |
| GAP1 | 4.6 | 0.138 | 1.7×10−21 | 7.6 | 0.961 | 8.4×10−13 | |
| Ammonium transporter | MEP2 | 1.5 | 0.085 | 7.6×10−7 | 4.0 | 0.354 | 9.1×10−13 |
| Allantoate permease | DAL5 | 1.6 | 0.106 | 4.3×10−5 | 1.8 | 0.112 | 0.0001 |
| Glutamine synthetase | GLN1 | 0.95 | 0.091 | 0.50 | 1.7 | 0.131 | 8.9×10−10 |
| Genes involved in antioxidant reactions | SOD1 | 1.3 | 0.155 | 0.27 | 1.9 | 0.274 | 2.0×10−5 |
| SOD2 | 3.5 | 0.904 | 8.2×10−14 | 1.6 | 0.136 | 1.4×10−5 | |
| Transcription factor | HAP4 | 5.5 | 0.874 | 3.2×10−12 | 2.7 | 0.181 | 5.2×10−6 |
| Amino acid sensor genes | SSY1 | 1.7 | 0.091 | 2.6×10−6 | 1.8 | 0.029 | 1.4×10−8 |
| TOR1 | 0.94 | 0.026 | 0.42 | 1.7 | 0.091 | 1.6×10−5 | |
| TOR2 | 1.1 | 0.030 | 0.054 | 1.5 | 0.026 | 2.2×10−5 | |
| Glucose sensor genes | SNF1 | 0.98 | 0.016 | 0.71 | 1.4 | 0.076 | 0.022 |
| RGT2 | 2.3 | 0.049 | 3.3×10−7 | 1.4 | 0.048 | 4.6×10−5 | |
| SNF3 | 3.8 | 0.113 | 2.3×10−11 | 1.0 | 0.049 | 0.38 | |
| Genes encoding histone deacetylases | SIR2 | 1.6 | 0.118 | 5.9×10−5 | 1.8 | 0.148 | 4.6×10−5 |
| RPD3 | 1.1 | 0.096 | 0.022 | 1.5 | 0.133 | 3.2×10−5 | |
Raw P values for individual comparisons. Significance of changes described in the text was determined after Bonferroni correction.
3.3. Regulation of genes in the glycolytic pathway
Table 2 shows the response of major glycolytic pathway genes to different nutrient-limited conditions. Hexoses can be used by the cell only if they are phosphorylated, a reaction catalyzed by the hexokinases. There are three hexokinases in yeast, HXK1, HXK2, and GLK1 (glucokinase). As shown in the table, these three genes were all highly induced in glucose-limited growth. Under nonessential amino acids limitation, HXK1 and GLK1 were up-regulated significantly; HXK2 showed no significant change compared to the control. Transcription of HXK1 and GLK1 is repressed by glucose in batch culture (Herrero et al., 1995). Unlike the cytoplasmic enzymes Hxk1p and Glk1p, Hxk2p is also partially localized in the nucleus; it plays an important role in glucose repression (Entian, 1980; Fernández et al., 1986). Most of the enzymes in the glycolytic pathway normally localize to the cytoplasm; however, Hxk2p can interact with nuclear proteins directly to regulate the transcription of the HXK1 and GLK1 genes and to autoregulate its own transcription (Rodríguez et al., 2001).
There are two other key enzymes in the glycolytic pathway which catalyze reactions utilizing ATP. They are phosphofructokinase, encoded by PFK1 and PFK2, and pyruvate kinase, encoded by CDC19. Two other major genes involved in the glycolytic pathway were also selected for analysis, FBA1 encoding fructose-bisphosphate adolase and TDH1 encoding glyceraldehyde-3-phosphate dehydrogenase. These genes showed changes less than 1.5-fold in expression except TDH1, whose expression was down-regulated 1.8-fold on glucose limitation.
3.4. Transcriptional induction of genes encoding enzymes of glycogen and trehalose synthesis under glucose-limited conditions
It is well known that glycogen and trehalose participate in many important processes in the cell, such as stress resistance and regulation of cell growth and division. Table 2 shows significant up-regulation of GSY1 encoding glycogen synthase under both of the nutrient-limitation conditions. GSY2 encoding another glycogen synthase was induced in glucose-limited conditions but showed no change in the absence of nonessential amino acids. GSY2 encodes the predominant glycogen synthase in yeast. Deletion of the GSY2 gene reduces glycogen synthase activity by about 90% (Farkas et al., 1991). TPS1 encoding trehalose-6-phosphate synthase was up-regulated 50% in glucose-limited conditions only. The promoter regions of all of these genes contain stress response elements (STRE), and all of them are induced by heat shock (Winderickx et al., 1996; Parrou et al., 1997; Unnikrishnan et al., 2003). The results suggest that CR may be a stressor in yeast, especially when imposed by glucose limitation.
3.5. Regulation of TCA cycle genes
Table 2 shows the effects of nutrient limitation on the regulation of TCA cycle genes. This cycle is localized in the mitochondrial matrix, and it plays an important role in energy production. All major genes involved in the TCA cycle as well as ATP2 encoding F1, F0-ATPase complex β subunit were up-regulated significantly in glucose-limited growth. These results are consistent with the finding that in aerobic, glucose-limited conditions, yeast cells respire completely (Postma et al., 1989). In the absence of nonessential amino acids, CIT1 encoding citrate synthase, ACO1 encoding aconitase, and IDH1 and IDH2, which both encode isocitrate dehydrogenase, were induced about 2-fold. These results suggest that in the absence of nonessential amino acids, the TCA cycle is not a major source of energy. The gene expression changes observed here are similar to those that occur on activation of the retrograde response (Epstein et al., 2001), suggesting part of the TCA cycle is induced to provide biosynthetic precursors, in particular glutamate.
The mitochondrial respiratory chain plays an important role in energy production during aerobic metabolism. Table 2 shows the changes in the expression of three genes of the respiratory chain. In glucose-limited conditions, these genes were highly induced. These changes were consistent with those found for the TCA cycle genes. In the absence of nonessential amino acids, CYT1 encoding cytochrome c1 and CYC1 encoding iso-1-cytochrome c were not substantially different from the control, and NDI1 encoding NADH dehydrogenase was only 60% induced. These results support the conclusion that the TCA cycle may not play a major role in energy production during growth in medium lacking the nonessential amino acids.
The induction of genes encoding respiratory chain components raises the possibility that increased respiration may lead to enhanced production of reactive oxygen species during glucose-limited growth. The activities of antioxidant enzymes are significantly up-regulated in the livers of mice and rats which are fed a CR diet (Semsei et al., 1989; Lee et al., 1990; Sohal et al., 1994). The SOD1 gene, encoding cytoplasmic Cu/Zn superoxide dismutase, is induced in rat liver under CR (Lee et al., 1990); however, the same study found no obvious changes in the activities of this antioxidant enzyme. As shown in Table 2, SOD2, which codes for Mn superoxide dismutase in mitochondria, was induced over 3-fold during glucose-limited growth; SOD1 showed no significant changes. In the absence of nonessential amino acids, SOD2 and SOD1 were induced 60% and 90%, respectively. These results suggest that CR may retard aging by decreasing production of reactive oxygen species. Recent studies have shown that mitochondrial reactive oxygen species production was reduced during CR in yeast even as respiratory activity was increased (Barros et al., 2004).
3.6. Genes involved in gluconeogenesis and the glyoxylate cycle are highly expressed in glucose-limited culture
During glucose-limited growth, the genes involved in the gluconeogenesis pathway were all induced at least 10-fold. These included FBP1, encoding fructose-1,6-bisphosphatase, PCK1 encoding phosphoenolpyruvate carboxykinase and MDH2 encoding malate dehydrogenase (Table 2). This suggests that C6 carbon sources are insufficient to support the growth of cells, and that this deficit is corrected by gluconeogenesis. In the absence of nonessential amino acids, the transcription levels of FBP1 and PCK1 were similar to the control, whereas the transcription levels of MDH2 were up-regulated 2-fold.
In yeast, there is another gluconeogenesis pathway, which is called the glyoxylate cycle. It produces oxaloacetate, which can be decarboxylated by phosphoenolpyruvate carboxykinase to phosphoenolpyruvate, the common intermediate in both glycolysis and gluconeogenesis. Thus, the glyoxylate cycle can be used to produce energy as well as glucose. It also produces TCA cycle intermediates to replenish those drawn off in biosynthetic reactions. Citrate synthase encoded by CIT2 catalyzes the first step of the glyoxylate cycle. The transcription level of CIT2 was induced 2 to 3-fold in glucose-limited growth and 50% during growth in the absence of nonessential amino acids, respectively (Table 2). The other two main genes ICL1 and MLS1, which encode isocitrate lyase and malate synthase, respectively, were up regulated over 10-fold in glucose-limited growth. CIT2 is a nuclear gene (Rosenkrantz et al., 1986). Cit2p and other glyoxylate enzymes are located in peroxisomes (Lewin et al., 1990). CIT2 is the only gene in the glyoxylate cycle that is activated in the retrograde response, suggesting that Cit2p may play an important role in the metabolic connection between the glyoxylate cycle and mitochondria (Chelstowska et al., 1995). Thus, elimination of nonessential amino acids has an effect similar to the retrograde response on the glyoxylate cycle.
3.7. Transcriptional changes in the pentose phosphate pathway
The pentose phosphate pathway is not a major component of carbohydrate metabolism in yeast. The contribution of this pathway to glucose metabolism is 0.9% when cells are grown in a rich medium; it can be up to 2.5% when grown in a synthetic medium (Zimmermann et al., 1997). It is known that the pentose phosphate pathway is the major source of NADPH in yeast. Recent research shows that this pathway is connected to some other biological processes. Deletion of the genes involved in amino acid sensing, such as SSY1, STP1 and STP2, results in the derepression of the pentose phosphate pathway (Eckert-Boulet et al., 2004). Disruption of GRR1, which prevents the induction of the amino acid permease gene AGP1 and glucose repression, induces the transcription of pentose phosphate pathway genes (Eckert-Boulet et al., 2005). Strains with deleted pentose phosphate pathway genes are menadione-sensitive; NADPH probably plays a significant role in the detoxification of the superoxide ion (Jensen et al., 2004). Table 2 shows the transcriptional changes in selected pentose phosphate pathway genes. ZWF1, encoding glucose-6-phosphate dehydrogenase, which is one of the key enzymes in the pentose phosphate pathway, showed no change during glucose-limited growth. In the absence of nonessential amino acids, ZWF1 transcription was increased 2-fold. GND2, which encodes another key enzyme in the pentose phosphate pathway, 6-phosphogluconate dehydrogenase, was 2.9-fold up-regulated in glucose-limited growth, whereas no change was found during growth in the absence of nonessential amino acids. Thus, the pentose phosphate pathway may be induced under both growth conditions, but the regulation may differ.
3.8. Regulation of pyruvate metabolism genes
As the final product of glycolysis, pyruvate can take part in either respiration or fermentation, depending on culture conditions. In aerobic, glucose-limited growth conditions, most of the pyruvate is converted to acetyl-CoA by the pyruvate dehydrogenase complex encoded by PDA1 and PDB1 (Pronk et al., 1994), and then acetyl-CoA is completely degraded to carbon dioxide through the TCA cycle, coupled to the production of ATP. Acetyl-CoA can also be produced via the so-called bypass, which requires pyruvate decarboxylase encoded by PDC1, acetaldehyde dehydrogenase encoded by ALD2, and acetyl-CoA synthetase encoded by ACS1 and ACS2. The bypass is necessary for growth on glucose (Van den Berg et al., 1995; Flikweert et al., 1996). Deletion of ACS2 prevents growth on glucose due to glucose repression of ACS1, and an ACS1 and ACS2 double mutant strain is not viable (Van den Berg et al., 1995). In fermentation, the reduction of acetaldehyde to ethanol serves to regenerate NAD, a reaction catalyzed by alcohol dehydrogenase encoded by ADH1. Pyruvate carboxylase, encoded by PYC1 and PYC2, catalyzes the conversion of pyruvate to oxaloacetate, a reaction that requires carbon dioxide. Oxaloacetate is a key intermediate in the TCA cycle and in gluconeogenesis.
As seen in Table 2, in aerobic, glucose-limited chemostat culture, expression of PDC1 was 50% decreased. This result suggests that the first step of the bypass pathway for acetyl-CoA synthesis was partially repressed by limitation of glucose in the medium. ALD2, ACS2 and PYC1 were up-regulated from 50% to 2.8-fold. ACS1 was induced over 300-fold. ACS1 is the aerobic form of acetyl-CoA synthetase in yeast and is repressed by high glucose concentration, whereas ACS2 is the anaerobic form (Van den Berg et al., 1996) and necessary for growth on glucose in batch cultures (Van den Berg et al., 1995). ACS2 plays a role in the transcriptional regulation of ACS1 (Van den Berg et al., 1996). In the absence of nonessential amino acids, PDC1, PDA1, PDB1 and ACS2 displayed no significant changes. ALD2, PYC1 and ACS1 were induced 50% to 2.3-fold. These results show that the genes involved in pyruvate metabolism show more profound regulation by glucose limitation compared to elimination of nonessential amino acids from the growth medium. ACS1, ALD4, and PYC1 are also up-regulated in the retrograde response (Epstein et al., 2001).
3.9. Genes involved in nitrogen metabolism are up-regulated during nutrient limitation
Changes in availability of amino acids in the environment result in the transcriptional induction of amino acid permease genes, such as AGP1, BAP2 and GAP1. GAP1 is the general amino acid permease, it can transport all L-amino acids in proteins, D-amino acids and toxic amino acid analogues (Grenson et al., 1970; Jauniaux et al., 1990). Table 2 shows that AGP1 and GAP1 were highly induced in both glucose-limited growth and in the absence of nonessential amino acids. The transcription of MEP2, the ammonium sensor and transporter was induced 50% and 4-fold in glucose-limited growth and in the absence of nonessential amino acids, respectively (Table 2). DAL5 encoding the allantoate permease was up-regulated less than 2-fold in both growth conditions (Table 2). GLN1 encoding glutamine synthetase showed no change in glucose-limited growth and a 70% increase in the absence of nonessential amino acids (Table 2). The results demonstrate that the genes involved in nitrogen metabolism were not only up-regulated in the absence of nonessential amino acids but also during glucose-limited growth. These results suggest overlap in the transcriptional regulation of metabolic pathways under various nutrient-limitation conditions. Most amino acid permeases and the ammonium transporter, which were investigated in this study, were up-regulated in glucose-limited conditions, suggesting that insufficiency of glucose promotes uptake and utilization of amino acids.
3.10. Regulatory gene expression
Genes encoding the glucose sensors, RGT2 and SNF3, were up-regulated significantly during glucose-limited growth (Table 2). This may be an adaptation to the low glucose levels, and it could be sufficient to explain the changes in hexose transporter gene expression. No substantial changes were observed in the absence of nonessential amino acids, as might be expected. On the other hand, no gene expression changes were observed for SNF1 under either nutrient limitation condition (Table 2). This may seem surprising given the role of the Snf1 protein kinase in the release from glucose repression (Hardie et al., 1998), which encompasses many of the genes whose expression is induced on glucose-limitation and found here. The metabolic signal that inactivates Snf1 kinase is generated by the hexokinase Hxk2, whose expression was induced on glucose limitation (Table 2), suggesting that the origin of the metabolic gene expression changes we have observed is complex.
Surprisingly given their role in signaling the presence of plentiful nutrients, growth in the absence of the nonessential amino acids resulted in mild induction of both TOR1 and TOR2, though no changes were observed on glucose limitation (Table 2). The TOR genes are involved in nutrient responses involving both nitrogen and carbon sources (Beck et al., 1999). The plasma membrane amino acid sensor gene, SSY1, was significantly induced under both nutrient limitation conditions (Table 2), suggesting some common features of both nutrient limitation regimes. The induction of these three genes suggests that the signaling pathways activated by nutrient limitation are distinct from the retrograde response (Jazwinski, 2002), as demonstrated previously in genetic experiments (Jiang et al., 2000).
The histone deacetylases Sir2p and Rpd3p both play a role in determining life span in yeast (Kaeberlein et al., 1999; Kim et al., 1999). Rpd3p binds upstream of numerous genes, many of which perform functions in similar anabolic processes (Kurdistani et al., 2002). Overexpression of the SIR2 gene or decreased expression of RPD3 extends the life span in both yeast and Drosophila (Kaeberlein et al., 1999; Kim et al., 1999; Rogina et al., 2002; Rogina et al., 2004). In the latter, RPD3 negatively regulates expression of SIR2 (Rogina et al., 2002); however, this is not the case in yeast (Jiang and Jazwinski, unpublished). On glucose limitation or in the absence of nonessential amino acids, SIR2 showed a 60%–80% increase in expression, while RPD3 was induced 50% in the absence of nonessential amino acids only (Table 2). These gene expression changes are not directly consistent with the lack of effect of deletion of SIR2 on life extension by CR in two of the three studies in yeast (Jiang et al., 2002; Kaeberlein et al., 2004), but they are consistent with the effects of RPD3 deletion (Jiang et al., 2002).
The gene expression changes observed during CR were clearly more extensive on glucose limitation. The profiles of gene expression in central metabolism were distinct on glucose-limitation and nonessential amino acids elimination, but they showed some overlap. HAP4, which encodes a transcriptional activator and a component of the Hap2-3-4-5 complex in yeast, was highly induced under both CR conditions (Table 2). Hap4p regulates the genes involved in mitochondrial function, especially those affecting respiration and biogenesis. DNA microarray analysis showed that of a total of 324 genes whose expression was altered by HAP4 overexpression or CR (0.5% glucose in batch culture), 55 genes were up-regulated under both conditions (Lin et al., 2002). Most of these genes are involved in mitochondrial function, implementing the role of the HAP4 gene in the metabolic switch from fermentation to respiration.
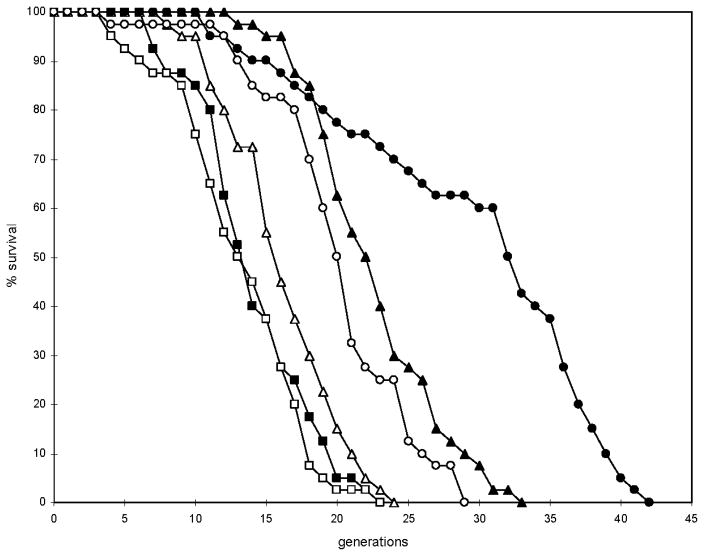
HAP4 overexpression extends life span significantly on 2% glucose, but this was not observed on 0.5% glucose, which was taken as evidence that CR and HAP4 operate the same mechanism of life extension (Lin et al., 2002). However contrariwise, HAP4 expression was not induced during CR (0.5% glucose) (Lin et al., 2002). In this study, the transcription of the HAP4 gene was highly induced (over 2.7-fold) by both our CR conditions (Table 2). In 0.1% glucose, all genes involved in respiratory metabolism and mitochondrial function were highly induced, as were the glyoxylate cycle genes. This raises the possibility that HAP4 induction is necessary for the regulation of metabolism by CR. Consistent with this, deletion of HAP4 suppressed the life span extension 30% and 33% on 0.1% glucose and on elimination of nonessential amino acids, respectively (Fig. 2). These results suggest that HAP4 is partly, though not completely, responsible for life extension by CR.
Fig. 2.
Deletion of HAP4 partially suppresses life extension by CR. Mean life spans (maximum life spans) for YPK9 (wild type) on 2% glucose (control) (■), 0.1% glucose (▲), and 2% glucose without nonessential amino acids (●) were 14 (23), 23 (33), and 30 (42) generations, respectively. The life extension due to glucose limitation (P<0.00001) and elimination of nonessential amino acids (P<0.00001) was significant. For the YPK9 hap4Δ strain on 2% glucose (control) (□), 0.1% glucose (△), and 2% glucose without nonessential amino acids (○), the life spans were 13 (23), 16 (24) and 20 (29) generations, respectively. There was no significant difference in life span between the wild type and mutant on 2% glucose (P=0.18). Deletion of HAP4 significantly suppressed the life extension on glucose limitation (P<0.00001) and elimination of nonessential amino acids (P<0.00001). The suppression on 0.1% glucose (P<0.02) and on medium lacking nonessential amino acids (P<0.00001) was not complete, as indicated by comparison with the life span on 2% glucose. The results were reproduced in the three separate experiments carried out.
4.0. Discussion
In this study, we investigated 59 genes, of which 48 genes are involved in central metabolism. Two of them are antioxidant genes, SOD1 and SOD2; three of them are glucose sensor genes, SNF1, SNF3 and RGT2; three of them are nitrogen sensor genes, SSY1, TOR1 and TOR2. The other three are regulatory genes, HAP4, a transcription factor, and SIR2 and RPD3, which are histone deacetylases. We compared genes differentially regulated at least 1.5-fold. The changes in gene expression investigated in this study are summarized in Fig. 1.
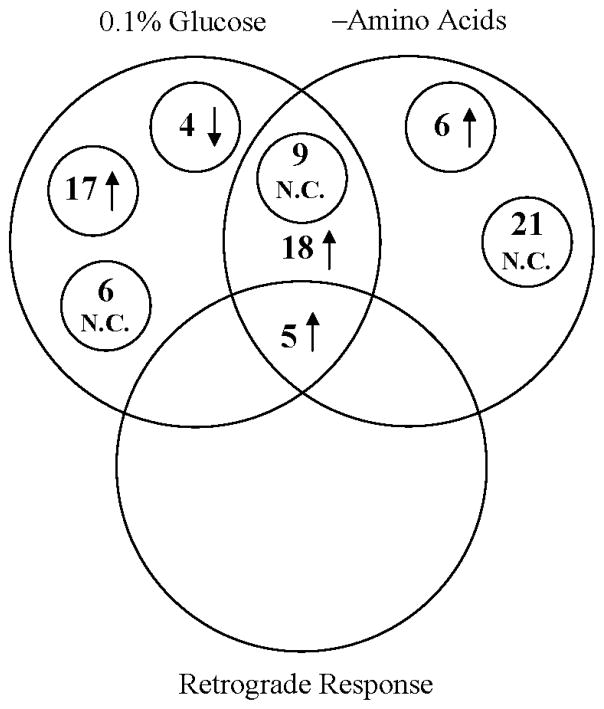
As Fig. 3 shows, in glucose-limited, chemostat culture, 4 genes were significantly down-regulated. 40 genes were up-regulated markedly. 15 genes did not show significant changes compared to the 2% glucose control culture. In nonessential amino acid-limited, chemostat culture, 29 genes were highly induced. 30 genes showed no significant differences compared to the 2% glucose control culture. 23 genes were up-regulated significantly in both CR regimes, and 5 of these were shared with the retrograde response. 9 genes did not show significant changes under either CR regime.
Fig. 3.
Comparison of changes in gene expression between glucose-limitation, nonessential amino acids-elimination conditions, and the retrograde response. N.C., no change.
We compared our data with those of Boer et al. (2003), who researched genome-wide transcription using a chemostat under four different nutrient limitation conditions, in which glucose-limited and nitrogen-limited conditions were involved. In their research, in glucose-limited culture, BAP2 was substantially induced, whereas HXT4 and AGP1 were down-regulated. In our hands, BAP2 was only 40% induced. HXT4 and AGP1 were highly up-regulated. In nitrogen-limited culture, their data showed that BAP2 and HXT2 were substantially down-regulated. However, our data showed that BAP2 was less than 20% down-regulated. HXT2 was up-regulated markedly. The differences between our results and those of Boer et al. (2003) may rest on three factors. First, the glucose and ammonia concentrations were very different in the two studies, and we eliminated the non-essential amino acids. Second, there was a 2-fold difference in the specific growth rates. Third, the yeast strains were different. Our conditions were chosen to coincide with those found to extend yeast replicative life span (Jiang et al., 2000).
In this study, we have found that most genes of central metabolism are co-regulated under certain conditions. The regulation of gene expression in yeast largely involves two transcriptional co-activator complexes, SAGA (Spt-Ada-Gcn5 acetyltransferase) and TFIID, which is directly associated with RNA polymerase II. These two co-activators dominate in particular genome regions but do not mutually exclude each others influence throughout the genome. Microarray data show that TFIID regulates about 90% of the genome, including most housekeeping genes. SAGA regulates the remaining 10% of the genome, including most stress related genes (Huisinga et al., 2004). Glycolysis genes, which were selected in this study are SAGA dominated except for HXK2, which is TFIID dominated (Huisinga et al., 2004). The genes involved in the first three steps of the TCA cycle are SAGA dominated; KGD1, SDH1 and MDH1 are TFIID dominated (Huisinga et al., 2004). This alternating list of SAGA and TFIID dominated genes in this study goes on and on. Masoro has suggested that CR is a long-term, low-intensity stressor (Masoro, 2000). It is not surprising that most of the genes in central metabolism are SAGA dominated. It has been found that the promoters of genes such as CIT1, MDH1, PYC1, ZWF1, HXK1, GLK1, TPS1, GSY1, GSY2 and AGP1 contain stress response elements (STRE), which can interact with transcriptional activators to induce the expression of specific genes to protect cells from various stresses (Moskvina et al., 1998; Zahringer et al., 2000).
CIT2 is involved in the glyoxylate cycle and is the diagnostic gene of the retrograde response. A novel SLIK (SAGA-like) histone acetyl-transferase complex functions in the retrograde response pathway (Pray-Grant et al., 2002). Rtg2p is one of the central components in this pathway in yeast, which regulates the expression of some nuclear genes when mitochondria are dysfunctional. Rtg2p is a component of the SLIK complex, and it has been shown that Rtg2p is bound to chromatin at the promoter of the activated CIT2 gene (Pray-Grant et al., 2002). This suggests that SLIK rather than SAGA regulates CIT2 transcription. Another glyoxylate cycle gene, ICL1, is TFIID dominated.
CR usually arises from the limitation of nutrients. However, it has been shown that deletion of HXK2 can mimic the effect of CR in yeast (Lin et al., 2000). Surprisingly, HXK2 was induced over 2-fold in 0.1%glucose-limited culture and no changes occurred under amino acids deprivation compared to the 2% glucose control. Deletion of HXK2 releases yeast from glucose repression (Diderich et al., 2001), which may extend life span. However, this may not be equivalent to the life extension nutrient limitation effects in a normal, wild-type yeast cell.
In this study, we investigated the histone deacetylase genes SIR2 and RPD3, which can mediate transcriptional silencing. Transcriptional silencing has been shown to affect yeast life span (Kim et al., 1999). CR increased yeast lifespan apparently by activating SIR2 (Lin et al., 2000). Deletion of RPD3 markedly extended lifespan in yeast (Kim et al., 1999). Glucose-limitation and elimination of nonessential amino acids may share common downstream effectors for life extension with RPD3 deletion based on epistasis analysis (Jiang et al., 2002). On the other hand, SIR2 and CR act on distinct effectors for life extension (Jiang et al., 2002). According to other research, SIR2 is not necessary for life extension by CR (Kaeberlein et al., 2004). Our data show that SIR2 transcription levels were up-regulated by both nutrient-limitation conditions. RPD3 expression levels showed no change or were up-regulated in glucose-limited and amino acids deprivation conditions, respectively. SIR2 represses amino acid biosynthesis genes (Bernstein et al., 2000; Hughes et al., 2000). Sir2p also inhibits the formation of ERCs, extrachromosomal rDNA circles, which accumulate in old cells (Sinclair et al., 1997). Rpd3p, a corepressor of transcription, binds the upstream regions of genes that have high transcription activity (Kurdistani et al., 2002), playing an antagonistic role to SAGA (Huisinga et al., 2004). Statistical analysis of functional categories of genes whose upstream regions were bound by Rpd3p shows that Rpd3p may be important in the regulation of anabolic genes (Kurdistani et al., 2002). Rpd3p induces some amino acid biosynthesis genes, showing an opposite effect to Sir2p (Bernstein et al., 2000).
The transcription factor HAP4 plays an important role in respiratory growth of yeast. Overexpression of HAP4 results in up-regulation of many mitochondrial protein genes, and overexpression of HAP4 extends life span (Lin et al., 2002). There was some overlap in gene expression profiles between HAP4 overexpression and CR (0.5% glucose in batch culture) (Lin et al., 2002). It seemed that CR extended lifespan of yeast by increasing respiration (Lin et al., 2002). This conclusion was challenged by recent research, which shows that respiration was not required for life extension by CR in yeast (Kaeberlein et al., 2005). In our research, HAP4 was highly induced under both nutrient-limitation conditions. All the genes involved in the TCA cycle were significantly induced in 0.1% glucose. Genes involved in the first three step of TCA cycle were also induced markedly when non-essential amino acids were eliminated from the growth medium. This coupled to the fermentative production of energy points to the biosynthetic nature of the induction of this portion of the TCA cycle under these conditions. These results together suggest that the induction of the TCA cycle plays a role in life extension by CR. Indeed, the effect of deletion of HAP4 on the life extension by CR shown here indicates that that this is the case, although it does not appear that HAP4 regulated gene expression explains the entire CR effect on life span.
CIT2, CIT1, ACO1, IDH1 and IDH2 were significantly induced under both CR conditions. They are all diagnostic genes of the retrograde response (Liao et al., 1991; Liu et al., 1999). Even more genes are regulated in common if the retrograde response and glucose limitation alone are compared. The extension of life span by CR did not require RTG2, however, deletion of RTG3 was additive to the life extension by CR (Jiang et al., 2000). It is not surprising that CIT1, ACO1, IDH1 and IDH2, which are involved in the TCA cycle, were highly induced in glucose-limited conditions, in which mitochondrial function was strongly increased, because these genes are controlled by the Hap4 transcription factor during respiratory growth. On the other hand, their expression is controlled by RTG genes when the mitochondria are dysfunctional (Liu et al., 1999). CR can increase lifespan in ρ0 cells (Kaeberlein et al., 2005). This places the emphasis in CR on aspects of central metabolism that remain intact in ρ0 cells and on genes that are controlled by the RTG complex and the HAP complex, depending on the circumstances. These may be genes co-activated by the SAGA and SLIK complexes, as suggested by the patterns of gene expression as discussed earlier. All in all, the gene expression changes point to the replenishment of TCA cycle and biosynthetic intermediates. In addition, the utilization of acetate in gluconeogenesis is suggested by the gene expression changes during glucose limitation.
Our results suggest that the induction of the TCA cycle and the glyoxylate cycle play an important role in CR under glucose-limited conditions. On elimination of nonessential amino acids, the changes are more subtle. However, in both conditions substantial overlap with the retrograde response is evident. This is consistent with the results of genetic studies (Jiang et al., 2000; Jiang et al., 2002), which indicate that there is overlap between the downstream effectors of CR and the retrograde response even though the signaling pathways differ.
The key to the evaluation of the patterns of gene expression during CR in the context of replicative life span is the use of the chemostat. In the chemostat unlike batch culture, the cells are at equilibrium for nutrients and biomass. This mimics life span determinations on individual cells growing in a film of medium on the surface of an agar plate. We propose the model for life extension by CR shown in Fig. 4. In this model, CR represses ERC formation by up-regulation of SIR2 expression, an increase in TCA cycle by up-regulation of HAP4 expression, and the induction of retrograde response target genes. These metabolic changes lead to the generation of more daughter cells, which are the measure of replicative life span. A major difference between the two CR regimes discussed here is the induction of the back portion of the TCA cycle, which is needed during respiratory growth at low glucose concentrations.
Fig. 4.
Model for the relationship between nutrient-limitation (CR) and life extension.
Acknowledgments
This work was supported by grants from the National Institute on Aging of the National Institutes of Health (U.S.P.H.S.) and by donations from Heinz Keller from Tasmania (Australia).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, Albright LM, Coen DM, Varki A, Janssen K, editors. Current Protocols in Molecular Biology. Wiley InterScience; New York: 1993. [Google Scholar]
- Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Tong JK, Schreiber SL. Genomewide studies of histone deacetylase function in yeast. Proc Natl Acad Sci USA. 2000;97:13708–13713. doi: 10.1073/pnas.250477697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer VM, De Winde JH, Pronk JT, Piper MD. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J Biol Chem. 2003;278:3265–3274. doi: 10.1074/jbc.M209759200. [DOI] [PubMed] [Google Scholar]
- Borghouts C, Benguria A, Wawryn J, Jazwinski SM. Rtg2 protein links metabolism and genome stability in yeast longevity. Genetics. 2004;166:765–777. doi: 10.1534/genetics.166.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelstowska A, Butow RA. RTG genes in yeast that function in communication between mitochondria and the nucleus are also required for expression of genes encoding peroxisomal proteins. J Biol Chem. 1995;270:18141–18146. doi: 10.1074/jbc.270.30.18141. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, De Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Diderich JA, Raamsdonk LM, Kruckeberg AL, Berden JA, Van Dam K. Physiological properties of Saccharomyces cerevisiae from which hexokinase II has been deleted. Appl Environ Microbiol. 2001;67:1587–1593. doi: 10.1128/AEM.67.4.1587-1593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert-Boulet N, Nielsen PS, Friis C, Dos Santos MM, Nielsen J, Kielland-Brandt MC, Regenberg B. Transcriptional profiling of extracellular amino acid sensing in Saccharomyces cerevisiae and the role of Stp1p and Stp2p. Yeast. 2004;21:635–648. doi: 10.1002/yea.1120. [DOI] [PubMed] [Google Scholar]
- Eckert-Boulet N, Regenberg B, Nielsen J. Grr1p is required for transcriptional induction of amino acid permease genes and proper transcriptional regulation of genes in carbon metabolism of Saccharomyces cerevisiae. Curr Genet. 2005;47:139–149. doi: 10.1007/s00294-004-0553-1. [DOI] [PubMed] [Google Scholar]
- Entian KD. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in carbon catabolite repression in yeast. Mol Gen Genet. 1980;178:633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- Epstein CB, Waddle JA, Hale W, 4th, Dave V, Thornton J, Macatee TL, Garner HR, Butow RA. Genome-wide responses to mitochondrial dysfunction. Mol Biol Cell. 2001;12:297–308. doi: 10.1091/mbc.12.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Hardy TA, Goebl MG, Roach PJ. Two glycogen synthase isoforms in Saccharomyces cerevisiae are coded by distinct genes that are differentially controlled. J Biol Chem. 1991;266:15602–15607. [PubMed] [Google Scholar]
- Fernández R, Herrero P, Fernández MT, Moreno F. Mechanism of inactivation of hexokinase PII of Saccharomyces cerevisiae by D-xylose. J Gen Microbiol. 1986;132:3467–3472. doi: 10.1099/00221287-132-12-3467. [DOI] [PubMed] [Google Scholar]
- Flikweert MT, Van Der Zanden L, Janssen WM, Steensma HY, Van Dijken JP, Pronk JT. Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast. 1996;12:247–257. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C247::AID-YEA911%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M, Hou C, Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV Evidence for a general amino acid permease. J Bacteriol. 1970;103:770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Calorie restriction and SIR2 genes--towards a mechanism. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Herrero P, Galíndez J, Ruiz N, Martínez-Campa C, Moreno F. Transcriptional regulation of the Saccharomyces cerevisiae HXK1, HXK2 and GLK1 genes. Yeast. 1995;11:137–144. doi: 10.1002/yea.320110205. [DOI] [PubMed] [Google Scholar]
- Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, Bennett HA, Coffey E, Dai H, He YD, Kidd MJ, King AM, Meyer MR, Slade D, Lum PY, Stepaniants SB, Shoemaker DD, Gachotte D, Chakraburtty K, Simon J, Bard M, Friend SH. Functional discovery via a compendium of expression profiles. Cell. 2000;102:109–126. doi: 10.1016/s0092-8674(00)00015-5. [DOI] [PubMed] [Google Scholar]
- Huisinga KL, Pugh BF. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol Cell. 2004;13:573–585. doi: 10.1016/s1097-2765(04)00087-5. [DOI] [PubMed] [Google Scholar]
- Jauniaux JC, Grenson M. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae. Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur J Biochem. 1990;190:39–44. doi: 10.1111/j.1432-1033.1990.tb15542.x. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. Growing old: metabolic control and yeast aging. Annu Rev Microbiol. 2002;56:769–792. doi: 10.1146/annurev.micro.56.012302.160830. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. The retrograde response links metabolism with stress responses, chromatin-dependent gene activation, and genome stability in yeast aging. Gene. 2005;354:22–27. doi: 10.1016/j.gene.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Jensen LT, Sanchez RJ, Srinivasan C, Valentine JS, Culotta VC. Mutations in Saccharomyces cerevisiae iron-sulfur cluster assembly genes and oxidative stress relevant to Cu,Zn superoxide dismutase. J Biol Chem. 2004;279:29938–29943. doi: 10.1074/jbc.M402795200. [DOI] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Jiang JC, Wawryn J, Shantha Kumara HM, Jazwinski SM. Distinct roles of processes modulated by histone deacetylases Rpd3p, Hda1p, and Sir2p in life extension by caloric restriction in yeast. Exp Gerontol. 2002;37:1023–1030. doi: 10.1016/s0531-5565(02)00064-5. [DOI] [PubMed] [Google Scholar]
- Johnston M. Feasting, fasting and fermenting. Glucose sensing in yeast and other cells. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- Jolly CA. Diet manipulation and prevention of aging, cancer and autoimmune disease. Curr Opin Clin Nutr Metab Care. 2005;8:382–387. doi: 10.1097/01.mco.0000172577.56396.7a. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, Dang N, Fields S, Kennedy BK. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2005;1:E69. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniak A, Xue Z, Macool D, Kim JH, Johnston M. Regulatory network connecting two glucose signal transduction pathways in Saccharomyces cerevisiae. Eukaryot Cell. 2004;3:221–231. doi: 10.1128/EC.3.1.221-231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kim S, Kirchman PA, Benguria A, Jazwinski SM. Experimentation with the yeast model. In: Yu BP, editor. Methods in Aging Research. CRC Press; Boca Raton: 1998. pp. 191–213. [Google Scholar]
- Kim S, Benguria A, Lai CY, Jazwinski SM. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:3125–3136. doi: 10.1091/mbc.10.10.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman PA, Kim S, Lai CY, Jazwinski SM. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics. 1999;152:179–190. doi: 10.1093/genetics/152.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdistani SK, Robyr D, Tavazoie S, Grunstein M. Genome-wide binding map of the histone deacetylase Rpd3 in yeast. Nat Genet. 2002;31:248–254. doi: 10.1038/ng907. [DOI] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lee DW, Yu BP. Modulation of free radicals and superoxide dismutases by age and dietary restriction. Aging (Milano) 1990;2:357–362. doi: 10.1007/BF03323951. [DOI] [PubMed] [Google Scholar]
- Lewin AS, Hines V, Small GM. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990;10:1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao XS, Small WC, Srere PA, Butow RA. Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:38–46. doi: 10.1128/mcb.11.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Liu Z, Butow RA. A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol Cell Biol. 1999;19:6720–6728. doi: 10.1128/mcb.19.10.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Dietary restriction and aging. J Am Geriatr Soc. 1993;41:994–999. doi: 10.1111/j.1532-5415.1993.tb06767.x. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Miceli MV, Jazwinski SM. Common and cell type-specific responses of human cells to mitochondrial dysfunction. Exp Cell Res. 2005;302:270–280. doi: 10.1016/j.yexcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Moskvina E, Schuller C, Maurer CT, Mager WH, Ruis H. A search in the genome of Saccharomyces cerevisiae for genes regulated via stress response elements. Yeast. 1998;14:1041–1050. doi: 10.1002/(SICI)1097-0061(199808)14:11<1041::AID-YEA296>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Park SK, Prolla TA. Gene expression profiling studies of aging in cardiac and skeletal muscles. Cardiovasc Res. 2005;66:205–212. doi: 10.1016/j.cardiores.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Parrou JL, Teste MA, Francois J. Effects of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiology. 1997;143:1891–1900. doi: 10.1099/00221287-143-6-1891. [DOI] [PubMed] [Google Scholar]
- Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schauble K, Birch-Machin MA, Kirkwood TBL, von Zglinicki T. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:E110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma E, Verduyn C, Scheffers WA, Van Dijken JP. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray-Grant MG, Schieltz D, McMahon SJ, Wood JM, Kennedy EL, Cook RG, Workman JL, Yates JR, III, Grant PA. The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol Cell Biol. 2002;22:8774–8786. doi: 10.1128/MCB.22.24.8774-8786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk JT, Wenzel TJ, Luttik MA, Klaassen CC, Scheffers WA, Steensma HY, Van Dijken JP. Energetic aspects of glucose metabolism in a pyruvate-dehydrogenase-negative mutant of Saccharomyces cerevisiae. Microbiology. 1994;140:601–610. doi: 10.1099/00221287-140-3-601. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A, De la Cera T, Herrero P, Moreno F. The hexokinase 2 protein regulates the expression of the GLK1, HXK1 and HXK2 genes of Saccharomyces cerevisiae. Biochem J. 2001;355:625–631. doi: 10.1042/bj3550625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrantz M, Alam T, Kim KS, Clark BJ, Srere PA, Guarente LP. Mitochondrial and nonmitochondrial citrate synthases in Saccharomyces cerevisiae are encoded by distinct homologous genes. Mol Cell Biol. 1986;6:4509–4515. doi: 10.1128/mcb.6.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsei I, Rao G, Richardson A. Changes in the expression of superoxide dismutase and catalase as a function of age and dietary restriction. Biochem Biophys Res Commun. 1989;164:620–625. doi: 10.1016/0006-291x(89)91505-2. [DOI] [PubMed] [Google Scholar]
- Sierkstra LN, Nouwen NP, Verbakel JM, Verrips CT. Analysis of glucose repression in Saccharomyces cerevisiae by pulsing glucose to a galactose-limited continuous culture. Yeast. 1992;8:1077–1087. doi: 10.1002/yea.320081210. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Ter Linde JJ, Liang H, Davis RW, Steensma HY, van Dijken JP, Pronk JT. Genome-wide transcriptional analysis of aerobic and anaerobic chemostat cultures of Saccharomyces cerevisiae. J Bacteriol. 1999;181:7409–7413. doi: 10.1128/jb.181.24.7409-7413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Unnikrishnan I, Miller S, Meinke M, LaPorte DC. Multiple positive and negative elements involved in the regulation of expression of GSY1 in Saccharomyces cerevisiae. J Biol Chem. 2003;278:26450–26457. doi: 10.1074/jbc.M211808200. [DOI] [PubMed] [Google Scholar]
- Van den Berg MA, Steensma HY. ACS2, a Saccharomyces cerevisiae gene encoding acetyl-coenzyme A synthetase, essential for growth on glucose. Eur J Biochem. 1995;231:704–713. doi: 10.1111/j.1432-1033.1995.tb20751.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg MA, De Jong-Gubbels P, Kortland CJ, van Dijken JP, Pronk JT, Steensma HY. The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J Biol Chem. 1996;271:28953–28959. doi: 10.1074/jbc.271.46.28953. [DOI] [PubMed] [Google Scholar]
- Van Hoek P, Van Dijken JP, Pronk JT. Effect of specific growth rate on fermentative capacity of baker’s yeast. Appl Environ Microbiol. 1998;64:4226–4233. doi: 10.1128/aem.64.11.4226-4233.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Winderickx J, De Winde JH, Crauwels M, Hino A, Hohmann S, Van Dijck P, Thevelein JM. Regulation of genes encoding subunits of the trehalose synthase complex in Saccharomyces cerevisiae: novel variations of STRE-mediated transcription control? Mol Gen Genet. 1996;252:470–482. doi: 10.1007/BF02173013. [DOI] [PubMed] [Google Scholar]
- Zahringer H, Thevelein JM, Nwaka S. Induction of neutral trehalase Nth1 by heat and osmotic stress is controlled by STRE elements and Msn2/Msn4 transcription factors: variations of PKA effect during stress and growth. Mol Microbiol. 2000;35:397–406. doi: 10.1046/j.1365-2958.2000.01706.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann FK, Entian KD. Yeast Sugar Metabolism: Biochemistry, Genetics, Biotechnology, and Application. Technomic Publishing Co; Lancaster: 1997. [Google Scholar]