Abstract
While CD4+CD25high regulatory T cells (Tregs) have garnered much attention for their role in the maintenance of immune homeostasis, recent findings have shown that subsets of CD8+ T cells (CD8+ Tregs) display immunoregulatory functions as well. Both CD4+ Tregs and CD8+ Tregs appear impaired in number and/or function in several autoimmune diseases and in experimental animal models of autoimmunity, suggesting the possibility of immunotherapeutic targeting of these cells for improved management of autoimmune conditions. Our group has developed a strategy to induce CD8+ Tregs in autoimmune mice through the use of a tolerogenic self-peptide, and new information has been gained on the phenotype, function and role of induced CD8+ Tregs in autoimmunity. Here we present an overview of the role and mechanisms of action of CD8+ Tregs in autoimmunity, with a special focus on lupus. We also discuss the potential role of CD8+ Tregs in other diseases, including chronic infection and cancer.
Keywords: Autoimmunity, Systemic Lupus Erythematosus, CD8+Tregs, Immune tolerance/ Suppression
1. Introduction
The first mention that CD8+ T cells were capable of suppressing other immune cells was reported in the 1970s [1]. After that, in the 1970s and early 1980s, the immunological research on cells with an inhibitory capacity favoring tolerance focused on CD8+ T cells, but this work fell out of favor due to methodological and technological limitations [2-6]. Renewed interest in regulatory T cells followed the discovery of CD4+CD25+ Tregs some decades later, as these cells were found to suppress effector T cells and inflammatory cytokines and thus play an important role in immune homeostasis and tolerance [7-11]. Since then, multiple subsets of CD4+ T cells, CD8+ T cells and B cells have been found to manifest immunoregulatory properties [12-16].
For CD8+ Tregs, multiple subsets have been identified and found to suppress through different mechanisms that include direct cell-cell contact with target cells, the secretion of cytokines, and the induction of anergy in antigen presenting cells. Despite significant advances, problems remain in the identification of specific marker(s) that could differentiate CD8+ Tregs from non-regulatory CD8+ T cell subsets. So far, CD8+ Tregs with immunosuppressive properties have been described in pathologies ranging from autoimmunity to cancer and viral infection [16-22]. In particular, CD8+ Tregs have been found impaired in numbers and/or function in patients with autoimmune diseases and in experimental animal models of autoimmunity [23, 24].
Work in our and other laboratories has focused on the induction of regulatory CD4+ and CD8+ T cell populations in lupus and in other autoimmune diseases, and it has been shown that such induction can be beneficial in alleviating disease. The development of autoimmunity is contingent on polygenic predisposition and the presence of environmental factors that can provoke an imbalanced immune response against self-components, with a resulting loss of immune tolerance. Delineating the role and function of immunoregulatory cell populations has the potential to open the door for immunotherapy in autoimmune diseases.
2. Natural and Induced Regulatory T cells
Tregs are often classified as either “natural” or “induced”. Natural Tregs are long-lived and develop in the thymus, while induced Tregs are thought to arise in the periphery following stimulation. Natural CD8+ Tregs include CD8+CD25+ T cells, which share functional and phenotypic similarities with CD4+ Tregs, expressing surface CTLA-4, GITR and intracellular Foxp3 [25]. CD8+CD25+ Tregs suppress CD4+CD25- T cells in a membrane-bound-TGFβ- and CTLA-4-mediated contact-dependent manner that induces a IL-2Rα downregulation in the target T cells [25]. Another subset of natural CD8+ Tregs are CD8+CD122+ T cells that have been shown to alleviate experimental autoimmune encephalomyelitis (EAE) upon adoptive transfer [26, 27]. Finally, naturally occurring human CD8+CXCR3+ T cells secrete IL-10 and suppress IFNγ production, in a manner similar to murine CD8+CD122+ Tregs [28].
Most of the reported CD8+ Tregs subtypes are induced. These include suppressor CD8+CD103+ T cells induced by allostimulation and augmented in cultures with IL-10, IL-4, and TGFβ [29], or CD8+CD103+ Foxp3+ cells expressing GITR and CTLA-4 and induced by stimulation with viral peptides [30]. One study reported that CD8+CD103- T cells could be induced to become regulatory CD8+CD103+ cells by alloantigen stimulation or treatment with TGFβ in vitro; these CD8+ Tregs suppressed in a cell-contact dependent manner that appears to play an important role in liver allograft tolerance [31]. In a study of kidney allograft survival, IL-10-producing CD8+ γδ Tregs were found to be responsible for the increased survival of transplanted mice that received repeated feeding of an oral alloantigen [32]. CD8+CD28- Treg cells were also induced by incubation with xenogeneic antigen presenting cells (APCs) [33] or allogeneic stimulation [34]. Non-antigen-specific CD8+CD28- Tregs have also been generated and suppress antigen-specific T lymphocytes and cytotoxic T lymphocytes after culture of PBMCs with GM-CSF, IL-10, IL-2, and autologous monocytes [35, 36]. Other types of induced Tregs include CD8+CD25+Foxp3+ suppressor T cells that can be induced through co-incubation with CD14+ monocytes and continuous antigen stimulation [37]; CD8+CD25+Foxp3+CTLA-4+ T cells arising in co-cultures with LPS-activated dendritic cells (DC) [38]; and CD8+CD25+Foxp3+LAG3+CCL4+ T cells arising upon antigen stimulation with Bacillus Calmette–Guerin [39]. Induced CD8 inhibitory populations also include CD8+CCR7+CD45RO+ T cells induced by plasmacytoid DCs from tumor ascites that suppress through IL-10 [40] and CD8+CD27+CD45RA+ cells induced in co-cultures with CD40 ligand–activated plasmacytoid DC that suppress through IL-10 [41]. Induction of alloantigen-specific CD45RO+CCR7- memory CD8+ Tregs expanded from CD8+CD25- T cells in vitro through CD40-activated B cells depends on IFNγ, IL-2, IL-4, and CTLA-4; these Tregs are CD8high and express Foxp3, CD25, CD27, CD28, and CD62L [42]. One subset of CD8+ Tregs suppressed T effector cell function in healthy human subjects after injection of immature DC pulsed with influenza matrix peptide [43]. Recently, in a model of Anterior Chamber-Associated Immune Deviation (ACAID), it has been found that ACAID-induced CD8+ Tregs secrete TGFβ and express CD94 and NKG2A [44]. While the role of IL-2 and TGFβ in the induction of CD4+CD25+ Tregs is well established [45], the methods of induction of CD8+ Tregs are as diverse as the subsets reported. Table 1 lists the CD8+ Tregs currently identified in the literature.
Table 1.
CD8+ Tregs Subtypes
| Table 1: CD8+ Tregs Subtypes | ||||
|---|---|---|---|---|
| Subset | Natural/Induced | Cells/Model Studied | Mechanism of Suppression | Citation |
| CD8+ CD28- | Natural | Human cancer cells | IL-10 secretion | [61] |
| CD8+CCR7+ CD45RO+ | Induced | Human ovarian cancer | IL-10 secretion | [40] |
| CD8+ CD28- Foxp3+ | Natural | Human prostate cancer | Cell-cell contact dependent | [62] |
| CD8+ TGFβ+ CD25- Foxp3- | Induced | Human HIV patient PBMC | Secretion of TGFβ | [136] |
| CD8+ IL10+ CD25- Foxp3- | Induced | Human HIV patient PBMC | Secretion of IL-10 | [130-132] |
| CD8+ CD28- | Induced | Human lupus, lancer, HIV, Hashimoto's thyroiditis, and Graves’ Disease patients | IL-10 secretion | [35, 36, 112] |
| CD8+CD103high LAPhigh Foxp3+ | Induced | Human lupus | TGFβ secretion | [149] |
| CD8+ CD25+ Foxp3+ LAG3+ | Induced | Human MTB patients and healthy donors | Secretion of CCL4 | [39] |
| CD8+CD25+ D69+ CTLA-4+ Foxp3+ | Induced | Human PBMC from AS patients | CTLA-4 contact dependent | [38] |
| CD8+CTLA4+Foxp3+ GITR+ | Induced | Human PBMC from HCV patients | Cell-cell contact dependent | [30] |
| CD8+ CD28- | Induced | Human PBMC from healthy honors | Induction of ILT3/ILT4 on APC | [33] |
| CD8+ CD103+ | Induced | Human PBMC from healthy donors | Cell-cell contact dependent | [29] |
| CD8+ CD25+ CD28+ Foxp3+ | Induced | Human PBMC from healthy donors | Cell-cell contact dependent | [37] |
| CD8+ IL-10+ | Induced | Human PBMC from healthy donors | Secretion of IL-10 | [41] |
| CD8+ CD25+ CTLA-4+ Foxp3+ | Induced | Human PBMC from Type 1 diabetes and healthy patients | Cell-cell contact dependent | [46] |
| CD8+ CD25+ CTLA-4+ Foxp3+ GITR+ | Natural | Human postnatal thymus specimens | TGFβ and CTLA-4 contact dependent | [25] |
| CD8+ CD28- CD56+ | Induced | Human synovial tissue from RA Patients/Human synovium-SCID mouse chimeras | Down-regulation of CD80 and CD86 on synovial fibroblasts | [75] |
| CD8+ IL-16+ | Induced | Human synovium-SCID mouse chimeras | Secretion of IL-16 | [70] |
| CD8+ CD28- | Induced | IBD induced by injection of CD4+CD45RBhigh cells into (RAG)-deficient mice | Secretion of IL-10, | [60] |
| CD8+ Foxp3+ PD-1+ | Induced | BWF1 lupus-prone Mice | Secretion of TGFβ | [47-49] |
| CD8+ CD25+ Foxp3+ | Natural | Cynomolgus Macaques and African Green Monkey SIV PBMC | Not described | [133, 134] |
| CD8+ CD28- Foxp3+ | Induced | Experimental murine model of Autoimmune Myasthenia Gravis | Reduction of IFNγ | [122] |
| CD8+ CD11c+ | Induced | Murine CII-induced arthritis | IFNγ mediated induction of IDO in CD11+b monocytes and CD11c+ dendritic cells | [69] |
| CD8+ | Induced | Murine stimulatory graft vs. host Disease | Partly TGFβ dependent | [66] |
| CD8+ CD122+ | Natural | Normal mice and CD122 deficient mice | Secretion of IL-10 | [26, 27, 64, 118, 119, 150] |
| CD8+CD62L+ TGFβ+ GITR+ | Induced | SNF1 lupus-prone mice | Secretion of TGFβ | [68] |
| CD8+ NKG2A+ CD90+ | Induced | ACAID mice | Secretion of TGFβ | [44] |
| CD8+ LAP+ | Natural | Experimental Autoimmune Encaphomyelitis | TGFβ and IFNγ Dependent | [121] |
| CD8+ CD45RO+ CD101+ CD103+ | Induced | TNF ARE mice | Cell-cell contact dependent | [67] |
3. Markers of CD8+ Tregs
A specific marker for identification of CD8+ Tregs is still elusive. Many of the markers for subsets of CD8+ Tregs overlap with markers for CD4+ Tregs, e.g. surface CD25 [25, 30, 38, 39, 46] and intracellular Foxp3 [27, 30, 37, 39, 46-49]. While Foxp3 expression has been suggested as a unique marker for the identification of both CD4+ Tregs and CD8+ Tregs, the finding that TCR activation also upregulates Foxp3 expression in cells without significant regulatory capacity [10, 50-52] diminishes enthusiasm for this idea. It has been argued that non-regulatory Foxp3+ T cells could represent a dormant reservoir with the potential to become regulatory cells after homeostatic expansion [53]. Additionally, Foxp3 is expressed in human and murine non-lymphoid cells [54-56], and in humans non-regulatory cells can display transient upregulation of Foxp3+ [57, 58]. It has been debated in the literature whether CD28 is present [47, 48] or absent [33, 35, 59-62] on the surface of CD8+ Tregs. Data from our lab suggest that Foxp3 expression might represent a better indicator of a suppressive phenotype because both CD28- and CD28+ CD8+ Tregs that express Foxp3 can mediate suppression [47-49].
4. Mechanisms of Suppression
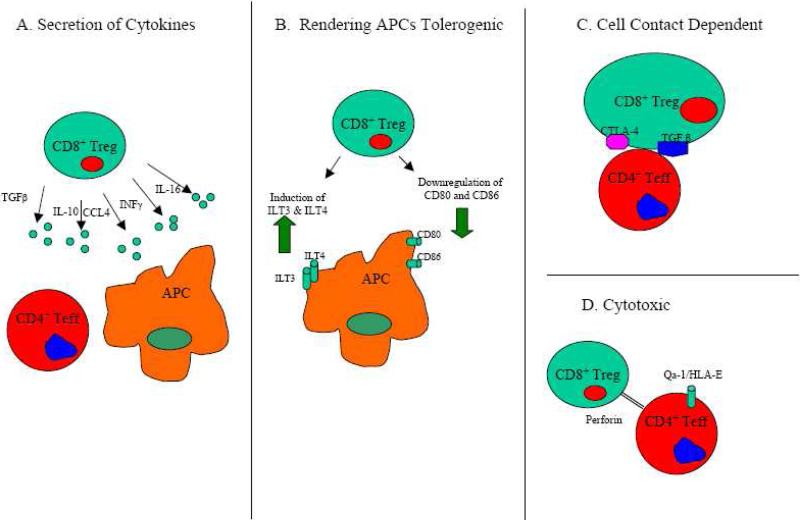
Not unlike their CD4+ Treg counterparts [63], CD8+ Tregs suppress through a variety of mechanisms that include secretion of cytokines, cell-to-cell contact, induction of a tolerogenic phenotype in APCs that can then induce regulatory CD4+ T cells, and cytotoxic activity (Figure 1). In the case of suppression through cytokine secretion, different subsets of CD8+ Tregs are reported to suppress through the secretion of different cytokines. Among the cytokines and chemokines reported to play a suppressive role are IL-10 [26, 27, 40, 41, 59, 60, 64, 65], TGFβ [25, 47-49, 66-68], IFNγ [69], IL-16 [70], and CCL4 [39]. Some CD8+ Treg subsets, in a manner similar to Tregs, can suppress through a cell contact dependent mechanism [29, 30, 37, 46, 60]. Additionally, membrane bound TGFβ and CTLA-4 play a role in cell-cell contact dependent mechanisms of CD8+ Treg-mediated suppression [25, 38].
Figure 1. Mechanisms of Suppression of CD8+ Tregs.
A) CD8+ Tregs secrete cytokines/chemokines such as IFNγ, TGFβ, IL-16, IL-10, and CCL4 that suppress immune responses. B) CD8+ Tregs render APCs tolerogenic by downregulating stimulatory molecules, such as CD80 and CD86, and upregulating inhibitory receptors, such as ILT3 and ILT4. C) CD8+ Tregs can suppress in a contact-dependent fashion that may be dependent on surface expression of molecules such as membrane-bound TGFβ or CTLA-4. D) MHC Class I restricted CD8+ Tregs are capable to kill activated CD4+ T effectors that express Qa-1/HLA-E.
CD8+CD28- Tregs can also render APCs tolerogenic through the upregulation of inhibitory receptors such as immunoglobulin-like transcript (ILT)-3 and ILT-4 on APCs [71, 72]. Tolerogenic APCs can then have an anti-inflammatory function and induce anergy and possible regulatory functions in CD4+ T cells [71, 73, 74]. One study has shown a CD8+CD28--mediated downregulation of the costimulatory ligands CD80 and CD86 on APCs as important in the suppression of CD4+ T cell responses [75], and another study has reported that CD80 and CD86 play an important role in the suppressive activity of CD8+CD122+ T cells [76]. Finally, another mechanism of suppression for CD8+ Tregs is cytolysis of antigen activated CD4+ Th cells, which is dependent on the expression of the MHC class 1b molecule Qa-1 (HLA-E in humans) [77-80].
5. CD8+ Tregs and Lupus
5.1 Lupus-prone Murine Models
We and others have developed strategies to tolerize lupus-prone mice with either histone-based peptides or peptides based on complementary determining regions of anti-dsDNA antibodies [68, 81-85]. We have developed a tolerogenic 15-amino acid peptide – called pConsensus (pCons) – that is based on MHC class I and class II T-cell determinants in the VH region of murine anti-DNA Ig [83-85]. Our model of SLE utilizes New Zealand black/New Zealand White F1 female (BWF1) mice, which spontaneously develop a disease that clinically and immunologically resembles human systemic lupus erythematosus (SLE). The administration of a high dose of pCons (1 mg i.v.) to BWF1 lupus-prone mice increased survival, delayed the onset of nephritis, decreased CD4+ T cell-mediated secretion of IFNγ, and decreased serum levels of autoantibodies against dsDNA, nucleosomes, and cardiolipin [83, 86]. This tolerance, and alleviation of disease, is mediated in large part by pCons-induced CD4+ Tregs [87] and CD8+ Tregs [49]. Initial studies [49] showed that following tolerization with pCons, BWF1 mice have an expansion of CD8+ T cells and developed CD8+ Tregs capable of suppressing anti-DNA IgG production (both in vivo and in vitro) and CD4+ Th cell production of IFNγ in vitro [49]. The CD8+ Treg cells isolated from spleens of tolerized mice suppressed target cells through the secretion of TGFβ. Additionally, silencing of Foxp3 with siRNA abrogated the ability of CD8+ Tregs to suppress anti-DNA antibodies. We later showed that CD8+ T cells from tolerized mice are capable to suppress CD4+ Th cell proliferation from naïve mice in vitro [47, 48]. Additionally, CD8+ cells (both CD28+ or CD28-) from pCons-treated mice have upregulated Foxp3 expression, and the inhibitory function of tolerized CD8+ T cells lasts for up to eight weeks after tolerization [47]. Interestingly, while both tolerized CD8+CD28+ and CD8+CD28- T cell subsets are Foxp3+, the CD8+CD28- T cells have greater Foxp3 expression that also lasts for a longer period of time [47]. In either subset, gene-silencing of Foxp3 yields a decline of suppression and TGFβ production [47, 48]. Incidentally, some tolerized CD8+ T cells also display an increased expression of the cytotoxic effector molecule granzyme B and are weakly cytotoxic against B cells from nephritic mice [48].
Our most recent work has focused on programmed death 1 (PD-1), a membrane protein, that upon binding to its ligand PD-L1 inhibits positive signaling pathways, causing cell anergy and cytotoxicity [88-91]. We have shown that there is reduced mRNA and protein expression of surface PD-1 in CD8+ T cells from pCons-tolerized mice [48]. Foxp3 expression decreased upon PD-1/PDL-1 blockade in T cells from tolerized mice. However, PD-1/PD-L1 blockade increased the expression Foxp3 in naïve T cells [48]. One possible explanation could be that downregulation of PD-1 is needed for the initial acquisition of suppressive capacity, but the complete elimination of this molecule leads to the disabling of CD8+ Treg suppressive capacity and autoimmunity [48, 92]. Therefore, PD-1 expression should be low to intermediate for CD8+ Tregs to retain suppressive function. In support of this possibility, studies of chronic viral infection suggest that high expression of PD-1 is associated with increased rates of apoptosis and anergy along with the inability to be rescued by growth cytokines. In contrast, cells with low to intermediate expression of PD-1 could be converted to cells capable of cytotoxicity for virus-infected cells [93-100].
Using the BWF1 SLE mouse model, Mozes’ group studied Tregs in mice treated with a tolerogenic peptide based on the light chain complementarity determining region 1 (hCDR1) of human anti-dsDNA antibodies [81, 101]. Tolerization with hCDR1 induced CD4+CD25high Tregs and CD8+CD28- Tregs in mice, which suppressed lymphocyte proliferation and autoantibody production, increased TGFβ and decreased IFNγ and IL-10 production in cultured splenocytes, and decreased lymphocyte apoptosis with down regulation of caspase-2, -8 and JNK and up-regulation of Bcl-xL [82, 101-105]. The CD8+ Tregs appear temporally essential in that model for the suppressive function, Foxp3 upregulation, and the expansion of CD4+CD25+ Tregs [105], suggesting that there is crosstalk between the two Treg subsets and that CD8+ Tregs are necessary for the optimal function of CD4+CD25high Tregs.
Kang et al. [68] conducted studies with a histone-derived tolerizing peptide (H471-94) in (SWR X NZB) SNF1 lupus-prone mice. SNF1 mice treated with a biweekly, subcutaneous low dose (1μg) of histone peptides had longer survival, a 50% decrease in anti-dsDNA autoantibodies, and reduced anti-nucleosome and anti-histone autoantibody production after three months of treatment. The attenuation of disease was concomitant with the expansion of CD4+CD25+ Tregs and CD8+ Tregs. CD8+ Tregs from the spleens of treated mice expressed TGFβ, CD62L, and GITR. The TGFβ secreted from CD8+ Tregs was the mechanism primarily responsible for the observed decrease in autoantibody production.
Zheng et al. explored the role of CD8+ and CD4+ Tregs in murine Stimulatory Graft-vs. -Host Disease (SGVHD), a lupus-like disease [66]. SGVHD was induced in (DBA/2 × C57BL/6) F1 mice following the injection of T cells from DBA/2 mice. CD4+ and CD8+ T cells with inhibitory capacity favored an increased survival of mice and required IL-2 and TGFβ. The transfer of TGFβ-primed cells to diseased animals with high anti-DNA Ab levels increased survival and decreased anti-DNA autoantibodies and proteinuria in host mice.
Of note, many studies in lupus-prone mice share numerous similarities in their findings on induced CD8+ Tregs despite the differences in the type of model used and the method of induction. Many times the suppression was mediated by TGFβ and decreased IFNγ, and the induced regulatory populations suppressed proliferation of responder CD4+CD25- T cells in vitro. In the case of Foxp3 expression, our group [47-49] and Mozes’ group [105] found that Foxp3 expression was upregulated in CD8+ T cells following treatment with tolerizing peptides that were based on anti-dsDNA IgG. However, Kang et al. [68], found that only CD4+CD25+ T cells and not CD8+ Tregs contained upregulated Foxp3 following tolerization with peptides based on histone sequences. While groups have defined CD8+ Tregs as cells lacking surface CD28 (104), our studies [47] and Kang et al. [68] suggest that both CD28+ and CD28- T cells can mediate suppression.
5.2 In Human SLE
Studies on T cells from lupus patients have shown conflicting results on the possible impairment of the regulatory compartment in SLE. Several studies have shown that CD4+CD25high Tregs are reduced in number and/or have impaired function in SLE patients compared to controls [9, 15, 106, 107], while other studies have shown the contrary [108, 109]. Treatment of PBMCs isolated from SLE patients with anti-DNA IgG peptides has been shown to increase the number and suppressive function of CD4+CD25high Tregs in vitro [110, 111]. Similarly, some studies on CD8+ Tregs in lupus patients have reported defective and/or reduced numbers [112, 113], while another study found no difference in the number of CD8+ Tregs in SLE patients [114]. Tulunay et al. [113] found that CD8+CD28- T cells from healthy patients had higher mRNA expression of TGFβ when compared to CD8+ CD28+ T cells.
Filaci et al. [112] generated CD8+ Tregs following incubation of purified CD8+ T from SLE patients and healthy patients with IL-2 and granulocyte monocyte colony stimulating factor (GM-CSF). While there were no major phenotypic differences between CD8+ Tregs from each group, CD8+ Tregs from SLE patients with active disease showed no ability to suppress, while CD8+ Tregs from patients in remission had a suppressive capacity similar to healthy controls. There were no differences in IL-10, IL-4, IFNγ, or TGFβ production between CD8+ Tregs from subjects with and without disease, but CD8+ Tregs from SLE patients produced lower levels of IL-6 and higher levels of IL-12 compared to healthy subjects. Regulatory function was dependent on IFNγ and IL-6. The authors hypothesized that the dysfunction of CD8+ Tregs in SLE patients was possibly due to an imbalance between inhibitory (IL-6) and stimulatory (IL-12) cytokines.
In a recent study on the effects of autologous hematopoietic stem cell transplantation (HSCT) in refractory lupus patients, Zhang et al. [115] showed that post-transplant patients who improved had a definable difference in T cell function from non-responders. Specifically, there was an abrogation of pathogenic T cell activity (as indicated by a reduced IFNγ response following culture with histone peptides) and an increased IL-13 response. Importantly, there was also an increase in the number of CD4+CD25highFoxp3+ T cells, CD8+Foxp3+ T cells, and CD8+CD103+ T cells. In short-term cell lines from post-transplant patients, CD8+Foxp3+ T cells had increased expression of Latency Associated Peptide (LAP), CD103, PD-1, PD-L1, and CTLA4. CD8+ Tregs from post-transplant patients suppressed through the secretion of TGFβ and had a stronger suppressive function on the proliferation of CD4+CD25- cells than CD4+CD25+ Tregs from patients. Suppression was contact-independent and both non-specific and autoantigen specific.
6. CD8+ T Cells in Other Autoimmune Diseases
6.1 Rheumatoid Arthritis
Using a chimera model of RA (where synovial tissues from RA patients are grafted onto severe combined immunodeficiency (SCID) mice), Klimiuk et al. [70] found a marked reduction in lesional immune activity and tissue production of IFNγ, IL-1β, and TNF-α following adoptive transfer of autologous synovial tissue CD8+ T cells into human synovium-SCID chimeras. Blocking experiments and in vitro culture experiments revealed that these CD8+ Tregs suppressed through the secretion of IL-16. Davila et al. [75], working in a similar model, generated CD8+ CD28- CD56+ T cells with a suppressive capacity by stimulating PBMCs of healthy HLA-A2- donors with irradiated HLA-A2 myelomonocytic THP-1 cells. The CD8+CD28- T cells suppressed in a contact-dependent fashion, and not through cytokine secretion. The adoptive transfer of CD8+CD28-CD56+ T cells resulted in decreases in CXCL10 and Mig chemokines and transcription of IFNγ and TNF-α. Additionally, the CD8+ Tregs induced tolerogenic cells by down regulating the costimulatory molecules CD80 and CD86 on APCs and synovial fibroblasts, respectively.
Seo et al. [69] showed that treatment with anti-4-1BB monoclonal antibody reduced serum antibodies to collagen type II and CD4+ T cell recall responses to collagen type II in a murine collage type II (CII)-induced arthritis (CIA) disease model. Treatment with the antibody was followed by the induction of antigen-dependent CD8+CD11c+ T cells that secreted IFNγ but not TGFβ, IL-10, or IL-4. The secretion of IFNγ by these CD8+CD11c+ cells increased indoleamine 2,3-dioxygenase (IDO) in CD11b+ monocytes and CD11c+ DC, and the accumulation of IDO in these cells led to subsequent suppression of antigen-specific CD4+ T cells in the Th1 cell-mediated model of CIA.
More recently, Notley et al. [116] showed that CIA mice treated with a single injection of anti-CD3 antibodies experienced a marked decrease in disease severity as well as reduced joint damage and inflammation concomitantly with an expansion of CD8+ T cells in lymph nodes and increased percentages of peripheral CD4+CD25+Foxp3+ Tregs and CD8+CD25+Foxp3+ Tregs. Of note, the CD4+CD25+Foxp3+ Tregs from treated mice did not display any differential ability to suppress effector T cells, IFNγ production or IL-17 production, while induced CD8+CD25+Foxp3+ T cells in the anti-CD3 treated mice were capable of suppressing effector T cells, IFNγ production, and IL-17 production in vitro.
6.2 Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory disease that leads to the demyelination of the central nervous system (CNS) and serves as an experimental model for human multiple sclerosis (MS) [117]. Working in a model of EAE induced by the MOG35-55 peptide, Lee et al. [118] found that depletion of CD122+ cells in EAE mice caused an increase in clinical measures of EAE, an increase in IFNγ production, and a decrease in IL-10 production. CD8+CD122+ cells from naïve mice were capable of suppressing CD4+CD25--mediated production of IFNγ in vitro, and adoptive transfer of CD8+CD122+ Tregs to EAE mice alleviated disease, increased IL-10 production, and suppressed inflammatory cytokine production and CNS infiltration. Zozulya et al. [119] have also explored the role of CD8+CD122+ T cells in the alleviation of EAE. It was known that treatment of mice with DC loaded with self peptide lead to an acceleration and exacerbation of disease that was paralleled by an accumulation of CD4+ and CD8+ T cells in the CNS [120]. However, treating mice with DC deficient for PD-L1 lead to amelioration of EAE concomitantly with the recruitment of CD8+CD122+ Tregs into the CNS, restating the link between PD-L1 and DC with the recruitment of CD8+CD122+ Tregs [119].
Chen et al. [121] described the existence of CD8+ Tregs that express the latency-associated peptide (LAP) on their cell surface and suppress in a TGFβ dependent manner in vivo in EAE. CD8+LAP+ cells from naïve mice were able to suppress CD4+CD25-LAP- T cells in vitro and alleviated EAE in vivo. While only a small percentage of CD8+LAP+ cells expressed Foxp3, the overall expression of Foxp3 in CD8+LAP+ T cells was higher than in CD8+LAP3- T cells. In addition, CD8+LAP+ cells had increased expression of CTLA-4 and decreased expression of CD45 and CD25 when compared to CD8+LAP- cells.
Tennakoon et al. [79] showed that MS patients treated with Glatiramer Acetate (GA), a synthetic peptide shown to be effective in reducing the number of new lesions in relapsing remitting MS, have an expansion of GA-specific CD8+ T cells with suppressive capacity, as compared to untreated patients. These CD8+ Tregs from GA-treated patients suppressed CD4+ T cells in a contact-dependent manner, expressed perforin, and exhibited HLA-E-restricted cytotoxicity.
6.3 Inflammatory Bowel Disease (IBD)
Intestinal Bowel Disease (IBD) describes a group of inflammatory diseases that afflict the small intestine and large intestine, including Crohn's disease (CD) and ulcerative colitis (UC). Menager-Marcq et al. [60] studied CD8+ Tregs in a murine experimental model of IBD-like disease generated by the injection of CD4+ CD45RBhigh cells into RAG-2 deficient mice. CD8+CD28- T cells from naïve mice prevented disease upon adoptive transfer. Interestingly, neither CD8+CD28+ T cells from unmanipulated mice nor CD8+CD28- T cells from IL-10-deficient mice had any suppressive effect in this model. Moreover, when disease was induced with CD4+CD45RBhigh T cells from mice without the ability to respond to TGFβ, CD8+CD28- T cells were unable to attenuate disease, restating the importance of TGFβ in the mechanisms of CD8+CD28- Treg-mediated suppression. Ho et al. [67] used a mouse model of Crohn's Disease that featured over expression of tumor necrosis factor to show that CD8+ Tregs expressing CD44-CD103+ secreted TGFβ, inhibited in vitro CD4+ T cell proliferation, and attenuated ileitis in immunodeficient RAG-/- mice upon adoptive transfer. These Tregs did not secrete IL-10 or IFNγ.
6.4 Myasthenia Gravis
Mysathenia Gravis (MG) is an autoimmune disease in which weakness and fatigue are caused by the disruption of neurotransmission due to autoantibodies against the acetylcholine receptor (AchR). Ben-David et al. [122] found that mice with experimental autoimmune MG (EAMG) treated with a dual altered peptide ligand (made of two tandemly arranged single amino acid analogs of two myasthenogenic peptides) decreased the clinical manifestations of EAMG, MG-lymph node T cell proliferation, and IFNγ secretion concomitantly with the induction of Foxp3-expressing CD8+CD28- T cells. Experiments with CD8 knockout mice suggested that CD8+ Tregs were necessary for the decreased lymph node T cell proliferation and the secretion of IFNγ.
6.5 Autoimmune Diabetes
Evidence for a role for CD8+ Tregs in Non Obese Diabetic (NOD) mice has been available for more than a decade. Harrison et al. [123] found that treatment of NOD mice with aerosol insulin after onset of clinical disease alleviated the disease concomitantly with the induction of CD8+ γδ T cells with regulatory properties. Wang et al. [124] found that adoptive transfer of LPS-stimulated splenocytes retrovirally transduced with a IgG fusion construct of glutamate decarboxylate 65 (GAD) had an alleviation of disease paralleled by the rise of CD4+ Tregs and CD8+Foxp3+ Tregs. In vivo transfer of retrovirally-transduced splenocytes that were either depleted of CD8+ T cells or CD8+CD25+ T cells led to a decrease in CD8+Foxp3+ Tregs as well as a worsening of disease.
Bisikirska et al. [46] showed that type 1 diabetes patients treated with an anti-CD3 mAb had an attenuation of disease that corresponded with an increase in the number of CD8+CD25+ T cells and an expansion of the CD8+ T cell compartment. Similar results were found in in vitro culture experiments that showed an expansion of CD8+ T cells as well as the development of CD8+ Tregs that expressed GITR and Foxp3 and suppressed CD4+ T helper proliferation and antigen-specific responses.
7. CD8 Tregs in Cancer and Infectious Disease
The induction of CD4+ Tregs and CD8+ Tregs might be beneficial in attenuating autoimmunity and transplantation, but the same is not the case in cancer and chronic infection. In these diseases, induction of CD8+ Tregs may not be beneficial and can instead be harmful to the host.
7.1 CD8+ Tregs and infectious disease
The role of CD8+ Tregs in infectious diseases is complex. While the suppression of immune responses can be harmful to the host by allowing for pathogenic persistence, regulatory T cell activity can limit tissue damage and pernicious inflammation. Chronic viral infection is a result of viral persistence resulting from inappropriate virus specific T-cell responses. CD8+ Tregs mediate the persistence of infection by suppressing cytotoxic T cell responses through the secretion of anti-inflammatory cytokines [125-127]. CD8+ Tregs subsets play a role in the persistence of infectious diseases such as Hepatitis C (HCV) [30, 128, 129], human immunodeficiency virus (HIV) [130-134] and Herpes Simplex Virus (HSV-2) infection [135]. It has been hypothesized that CD8+ Tregs can both play an important role in viral persistence and serve an important function in protection from pathogenic tissue damage due to overwhelming anti-viral cytotoxic T cell responses [18]. Studies on PBMCs from HIV patients found increased IL-10-secreting CD8+ Tregs that decreased the CD8+ T cytotoxic function in patients with progressive or active disease as compared to patients with non-progressing disease [130]. Stimulation with HIV antigens resulted in increased numbers of IL-10-producing CD8+ T cells that suppressed through a cell-cell contact-dependent mechanism [130, 131]. Another study on HIV patients found that PBMCs stimulated with HIV antigens increased TGFβ-producing CD8+ Tregs that suppressed IFNγ production [136]. In animal models of Simian Immunodeficiency Virus (SIV), increased CD4+ Tregs and CD8+CD25+ Tregs following infection have also been reported [133, 134].
Furthermore, studies on PBMCs from HCV patients stimulated with HCV antigens found an IL-10-dependent induction of CD8+CD25+ Tregs expressing GITR and CTLA-4 that were capable to suppress IFNγ production in a contact-dependent fashion [30]. Of note, in many of these instances, IL-10 has a key role in allowing viral persistence through the suppression of anti-viral immune responses, and the blockade of IL-10 results in the increase of CD8+ T cell production of IFNγ [128] and viral clearance [137, 138].
7.2 CD8+ Tregs and Cancer
While much work has been done on the role of CD4+CD25+ Tregs in cancer [139-142], the role of CD8+ Tregs has only recently started to garner attention [143, 144]. CD8+ Tregs appear to be of detriment in cancer, as they may allow for the suppression of anti-tumor immunity that can lead to tumor growth [144]. Both CD4+ Tregs and CD8+ Tregs are found at higher percentages in tumor microenvironments [144-147]. Tumors secrete cytokines that create a milieu favorable for the recruitment and induction of CD8+ Tregs [61, 62]. Furthermore, plasmacytoid DC induce CD8+ Tregs in human tumors [40]. CD8+CD28- Tregs found among Tumor Infiltrating Lymphocytes have an increased functionality and suppressor activity that appears correlated with advanced stages of the disease [61], and in human colorectal cancer and prostate cancer, increased numbers of CD8+CD25+Foxp3+ Tregs can better suppress effector T cells in the tumor microenvironment compared to regular tissue [62, 145]. Interestingly, CD8+CD25+ T cells in prostate tumors can have their suppressive function silenced by using Toll-like Receptor 8 ligands [62].
8. Concluding Remarks
A multiplicity of different CD8+ Tregs subsets appears to have different mechanisms of suppression, markers, and modalities of induction. This diversity is one of the field's most salient characteristics because there is so far no single molecule that can specifically identify CD8+ Tregs, either naturally occurring or induced. On the other hand, the differences among subsets could be more than superficial and instead result from unique characteristics of the model studied and/or the methods of induction. Further research on surface markers, mRNA expression, and cytokine secretion is needed to identify unique and divergent characteristics of the multiple CD8+ Treg subtypes. For now, the reemergence of CD8+ Tregs in recent years after their original discovery over three decades ago has created an exciting new field of investigation. Of note, it has become clearer that CD4+ and CD8+ Tregs have overlapping and independent inhibitory roles. In vivo and in vitro experiments in murine lupus [105], murine MG [122], and human SLE [115] seem to suggest that CD8+ Tregs might be necessary for the proper function of Tregs, indicating that a crosstalk between CD4+ Tregs and CD8+ Tregs might be in play in the mechanisms of immune tolerance and that bidirectional interactions between T cells and DC are responsible for the functionality of regulatory T cell populations [148].
Presently, a major problem in the study of CD8+ Treg is the lack of a specific marker that unequivocally defines these cells as suppressors —a problem that also exists to a lesser degree for CD4+ Tregs. Some of the best-described subsets of CD8+ Tregs include induced and natural CD8+CD28- T cells and CD8+CD25+ T cells, as well as naturally occurring CD8+CD122+ T cells. Foxp3 expression has been looked at as a possible marker but it appears that different subsets may have not only diverse sets of markers but also very diverse methods of suppression. Some CD8+ Tregs can suppress through cell-cell contact dependent mechanisms and others by secretion of cytokines/chemokines such as IL-10, TGFβ, and CCL4. Other mechanisms of suppression include the induction of tolerogenic APC or the exertion of cytotoxicity on target cells. It is not clear whether the different subsets may be completely distinct from each other or whether they may derive from a common precursor.
Here we outline the important consideration that CD8+ Tregs, along with CD4+ Tregs, play crucial roles in autoimmune diseases, infectious diseases, and cancer. The opposing nature of autoimmunity and proliferative diseases such as cancer suggest that therapies designed to target these cells and exploit their suppressive characteristics should be carefully designed to obtain clinical benefits. Considering that work in experimental models has shown that antigen-specific Tregs can prevent, attenuate, or reverse the course of autoimmune disease, further research is now required to finely tune the current information towards acquisition of the specific roles and function of CD8+ Tregs for their use in settings with translational significance to humans.
Acknowledgments
Supported by NIH grants AR54034, AI 083894, AI65645 to RPS, Rheuminations, Inc. and AI46776 to BHH, UCLA Senate Core Grant and Southern California Arthritis Foundation (SCAF) grant to BHH and RPS, and gifts from Jeanne Rappaport and the Maltz and Horchow families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- The most common CD8+ Treg surface markers reported are CD25 and a lack of CD28; others are Foxp3, CD103, CD122, CTLA-4, and GITR.
- CD8+ Tregs suppress through a variety of mechanisms that include secretion of cytokines, cell-cell contact, induction of anergy in APC, and cytotoxicity.
- Tolerization using peptide therapy has been shown to be successful in alleviating murine lupus. Studies suggest that CD8+ Tregs could be important for the proper function of Tregs.
- The suppressive function of CD8+ Tregs isolated from SLE patients appears impaired.
- Studies on patients with refractory lupus who have undergone hematopoietic stem cell transplantation (HSCT) indicate that the induction of a population of CD8+ Tregs plays a major role in the attenuation of disease.
- The role of induced CD8+ Tregs in a variety of other murine models of autoimmune conditions has been detailed. These Tregs vary in their mechanisms of suppression and markers. Further research on CD8+ Tregs as therapeutic targets is likely to lead to findings with a great potential for translational significance.
References
- 1.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 2.Baker PJ, Stashak PW, Amsbaugh DF, Prescott B, Barth RF. Evidence for the existence of two functionally distinct types of cells which regulate the antibody response to type 3 pneumococcal polysaccharide. J Immunol. 1970;105:1581–3. [PubMed] [Google Scholar]
- 3.Barth RF, Singla O, Liu C. Suppressor T cells and host resistance to type III pneumococcus after treatment with antilymphocyte serum. Infect Immun. 1975;12:1307–12. doi: 10.1128/iai.12.6.1307-1312.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391–40. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantor H, Hugenberger J, McVay-Boudreau L, Eardley DD, Kemp J, Shen FW, et al. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978;148:871–7. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eardley DD, Hugenberger J, McVay-Boudreau L, Shen FW, Gershon RK, Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978;147:1106–15. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 8.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 9.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 10.Banham AH, Powrie FM, Suri-Payer E. FOXP3+ regulatory T cells: Current controversies and future perspectives. Eur J Immunol. 2006;36:2832–6. doi: 10.1002/eji.200636459. [DOI] [PubMed] [Google Scholar]
- 11.Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Regulatory T cells and human disease. Clin Dev Immunol. 2007;2007:89195. doi: 10.1155/2007/89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang XL, Smith TR, Kumar V. Specific control of immunity by regulatory CD8 T cells. Cell Mol Immunol. 2005;2:11–9. [PubMed] [Google Scholar]
- 13.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–14. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 14.Lemoine S, Morva A, Youinou P, Jamin C. Regulatory B cells in autoimmune diseases: how do they work? Ann N Y Acad Sci. 2009;1173:260–7. doi: 10.1111/j.1749-6632.2009.04651.x. [DOI] [PubMed] [Google Scholar]
- 15.La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17:421–5. doi: 10.1177/0961203308090028. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–9. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billerbeck E, Thimme R. CD8+ regulatory T cells in persistent human viral infections. Hum Immunol. 2008;69:771–5. doi: 10.1016/j.humimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–23. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Joosten SA, Ottenhoff TH. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol. 2008;69:760–70. doi: 10.1016/j.humimm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costantino CM. Baecher-Allan CM and Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921–4. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang HK, Datta SK. Regulatory T cells in lupus. Int Rev Immunol. 2006;25:5–25. doi: 10.1080/08830180500544480. [DOI] [PubMed] [Google Scholar]
- 25.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–14. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 27.Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–34. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Z, Okuno Y, Rifa'i M, Endharti AT, Akane K, Isobe K, et al. Human CD8+CXCR3+ T cells have the same function as murine CD8+CD122+ Treg. Eur J Immunol. 2009;39:2106–19. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- 29.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177:2775–83. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 30.Billerbeck E, Blum HE, Thimme R. Parallel expansion of human virus-specific FoxP3- effector memory and de novo-generated FoxP3+ regulatory CD8+ T cells upon antigen recognition in vitro. J Immunol. 2007;179:1039–48. doi: 10.4049/jimmunol.179.2.1039. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Yu Y, Li G, Pu L, Zhang F, Zheng S, et al. CD8(+)CD103 (+) regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9::546–8. doi: 10.1016/j.intimp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Appleton SE, Stadnyk A, Lee TD, Nashan BA. CD8+ gammadelta T regulatory cells mediate kidney allograft prolongation after oral exposure to alloantigen. Transpl Int. 2008;21:679–87. doi: 10.1111/j.1432-2277.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 33.Ciubotariu R, Colovai AI, Pennesi G, Liu Z, Smith D, Berlocco P, et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28- regulatory T cells. J Immunol. 1998;161:5193–202. [PubMed] [Google Scholar]
- 34.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- T cells. Int Immunol. 1998;10:775–83. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 35.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibits both T-cell proliferation and CTL function. Hum Immunol. 2004;65:142–56. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Filaci G, Rizzi M, Setti M, Fenoglio D, Fravega M, Basso M, et al. Non-antigen-specific CD8 (+) T suppressor lymphocytes in diseases characterized by chronic immune responses and inflammation. Ann N Y Acad Sci. 2005;1050:115–23. doi: 10.1196/annals.1313.013. [DOI] [PubMed] [Google Scholar]
- 37.Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, et al. Generation of highly suppressive adaptive CD8 (+) CD25 (+) FOXP3 (+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008;38:640–6. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 38.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35:2896–908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 39.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007;104:8029–34. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 41.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam KT, et al. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J Immunol. 2009;183:3742–50. doi: 10.4049/jimmunol.0901329. [DOI] [PubMed] [Google Scholar]
- 43.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8 (+) regulatory T cells in vivo in humans. Blood. 2002;100:174–7. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 44.He H, Yang P, Jiang L, Zhang J, Zhao C, Chen L, et al. Upregulation of CD94 on CD8+T cells in anterior chamber-associated immune deviation. BMC Immunol. 2008;9:53. doi: 10.1186/1471-2172-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La Cava A. Tregs are regulated by cytokines: implications for autoimmunity. Autoimmun Rev. 2008;8:83–7. doi: 10.1016/j.autrev.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904–13. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007;178:7649–57. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- 48.Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB x NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J Immunol. 2008;180:2069–80. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- 49.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005;175:7728–37. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 50.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 52.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci U S A. 2005;102:4091–6. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–50. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 55.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–86. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen GY, Chen C, Wang L, Chang X, Zheng P, Liu Y. Cutting edge: Broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol. 2008;180:5163–6. doi: 10.4049/jimmunol.180.8.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 58.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Filaci G, Fravega M, Fenoglio D, Rizzi M, Negrini S, Viggiani R, et al. Non-antigen specific CD8+ T suppressor lymphocytes. Clin Exp Med. 2004;4:86–92. doi: 10.1007/s10238-004-0042-3. [DOI] [PubMed] [Google Scholar]
- 60.Menager-Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. CD8+CD28- regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775–85. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–34. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 62.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–58. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 63.Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008;7:370–5. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Endharti AT, Rifa IMs, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 65.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology. 2007;148:6040–6. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 66.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr., Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–9. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 67.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573–80. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174:3247–55. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 69.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–94. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 70.Klimiuk PA, Goronzy JJ, Weyand CM. IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. J Immunol. 1999;162:4293–9. [PubMed] [Google Scholar]
- 71.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 72.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, et al. Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055–68. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 73.Suciu-Foca N, Cortesini R. Central role of ILT3 in the T suppressor cell cascade. Cell Immunol. 2007;248:59–67. doi: 10.1016/j.cellimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 74.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, et al. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005;174:7292–301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 76.Shi Z, Rifa'i M, Lee YH, Shiku H, Isobe K, Suzuki H. Importance of CD80/CD86-CD28 interactions in the recognition of target cells by CD8+CD122+ regulatory T cells. Immunology. 2008;124:121–8. doi: 10.1111/j.1365-2567.2007.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang H, Ware R, Stall A, Flaherty L, Chess L, Pernis B. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing V beta 8 TCR: a role of the Qa-1 molecule. Immunity. 1995;2:185–94. doi: 10.1016/s1074-7613(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 78.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218–21. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119–29. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 80.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–23. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 81.Eilat E, Dayan M, Zinger H, Mozes E. The mechanism by which a peptide based on complementarity-determining region-1 of a pathogenic anti-DNA auto-Ab ameliorates experimental systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2001;98:1148–53. doi: 10.1073/pnas.98.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharabi A, Haviv A, Zinger H, Dayan M, Mozes E. Amelioration of murine lupus by a peptide, based on the complementarity determining region-1 of an autoantibody as compared to dexamethasone: different effects on cytokines and apoptosis. Clin Immunol. 2006;119:146–55. doi: 10.1016/j.clim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 83.Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K, Ebling FM. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum. 2001;44:432–41. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 84.Ohnishi K, Ebling FM, Mitchell B, Singh RR, Hahn BH, Tsao BP. Comparison of pathogenic and non-pathogenic murine antibodies to DNA: antigen binding and structural characteristics. Int Immunol. 1994;6:817–30. doi: 10.1093/intimm/6.6.817. [DOI] [PubMed] [Google Scholar]
- 85.Tsao BP, Ebling FM, Roman C, Panosian-Sahakian N, Calame K, Hahn BH. Structural characteristics of the variable regions of immunoglobulin genes encoding a pathogenic autoantibody in murine lupus. J Clin Invest. 1990;85:530–40. doi: 10.1172/JCI114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh RR, Ebling FM, Albuquerque DA, Saxena V, Kumar V, Giannini EH, et al. Induction of autoantibody production is limited in nonautoimmune mice. J Immunol. 2002;169:587–94. doi: 10.4049/jimmunol.169.1.587. [DOI] [PubMed] [Google Scholar]
- 87.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black x New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173:3542–8. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 88.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PDL1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–14. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 91.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 92.Skaggs BJ, Singh RP, Hahn BH. Induction of immune tolerance by activation of CD8+ T suppressor/regulatory cells in lupus-prone mice. Human Immunology. 2008;69:790–6. doi: 10.1016/j.humimm.2008.08.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 94.Golden-Mason L, Klarquist J, Wahed AS, Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol. 2008;180:3637–41. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 95.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–70. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 96.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 97.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 98.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 99.Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, et al. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215–25. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-beta. Proc Natl Acad Sci U S A. 2006;103:8810–5. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharabi A, Luger D, Ben-David H, Dayan M, Zinger H, Mozes E. The role of apoptosis in the ameliorating effects of a CDR1-based peptide on lupus manifestations in a mouse model. J Immunol. 2007;179:4979–87. doi: 10.4049/jimmunol.179.8.4979. [DOI] [PubMed] [Google Scholar]
- 103.Sharabi A, Azulai H, Sthoeger ZM, Mozes E. Clinical amelioration of murine lupus by a peptide based on the complementarity determining region-1 of an autoantibody and by cyclophosphamide: similarities and differences in the mechanisms of action. Immunology. 2007;121:248–57. doi: 10.1111/j.1365-2567.2007.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rapoport MJ, Sharabi A, Aharoni D, Bloch O, Zinger H, Dayan M, et al. Amelioration of SLE-like manifestations in (NZBxNZW)F1 mice following treatment with a peptide based on the complementarity determining region 1 of an autoantibody is associated with a down-regulation of apoptosis and of the pro-apoptotic factor JNK kinase. Clin Immunol. 2005;117:262–70. doi: 10.1016/j.clim.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 105.Sharabi A, Mozes E. The suppression of murine lupus by a tolerogenic peptide involves foxp3-expressing CD8 cells that are required for the optimal induction and function of foxp3-expressing CD4 cells. J Immunol. 2008;181:3243–51. doi: 10.4049/jimmunol.181.5.3243. [DOI] [PubMed] [Google Scholar]
- 106.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 107.Gerli R, Nocentini G, Alunno A, Bocci EB, Bianchini R, Bistoni O, et al. Identification of regulatory T cells in systemic lupus erythematosus. Autoimmun Rev. 2009;8:426–30. doi: 10.1016/j.autrev.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 108.Yates J, Whittington A, Mitchell P, Lechler RI, Lightstone L, Lombardi G. Natural regulatory T cells: number and function are normal in the majority of patients with lupus nephritis. Clin Exp Immunol. 2008;153:44–55. doi: 10.1111/j.1365-2249.2008.03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang B, Zhang X, Tang FL, Zhu LP, Liu Y, Lipsky PE. Clinical significance of increased CD4+CD25-Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann Rheum Dis. 2008;67:1037–40. doi: 10.1136/ard.2007.083543. [DOI] [PubMed] [Google Scholar]
- 110.Sthoeger ZM, Sharabi A, Dayan M, Zinger H, Asher I, Sela U, et al. The tolerogenic peptide hCDR1 downregulates pathogenic cytokines and apoptosis and upregulates immunosuppressive molecules and regulatory T cells in peripheral blood mononuclear cells of lupus patients. Hum Immunol. 2009;70:139–45. doi: 10.1016/j.humimm.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 111.Hahn BH, Anderson M, Le E, La Cava A. Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2488–97. doi: 10.1002/art.23609. [DOI] [PubMed] [Google Scholar]
- 112.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166:6452–7. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 113.Tulunay A, Yavuz S, Direskeneli H, Eksioglu-Demiralp E. CD8+CD28-, suppressive T cells in systemic lupus erythematosus. Lupus. 2008;17:630–7. doi: 10.1177/0961203308089400. [DOI] [PubMed] [Google Scholar]
- 114.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–8. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183:6346–58. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Notley CA, McCann FE, Inglis JJ, Williams RO. ANTI-CD3 therapy expands the numbers of CD4+ and CD8+ treg cells and induces sustained amelioration of collagen-induced arthritis. Arthritis Rheum. 62:171–8. doi: 10.1002/art.25058. [DOI] [PubMed] [Google Scholar]
- 117.Lassmann H. Experimental models of multiple sclerosis. Rev Neurol (Paris) 2007;163:651–5. doi: 10.1016/s0035-3787(07)90474-9. [DOI] [PubMed] [Google Scholar]
- 118.Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–32. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 119.Zozulya AL, Ortler S, Fabry Z, Sandor M, Wiendl H. The level of B7 homologue 1 expression on brain DC is decisive for CD8 Treg cell recruitment into the CNS during EAE. Eur J Immunol. 2009;39:1536–43. doi: 10.1002/eji.200839165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zozulya AL, Ortler S, Lee J, Weidenfeller C, Sandor M, Wiendl H, et al. Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. J Neurosci. 2009;29:140–52. doi: 10.1523/JNEUROSCI.2199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen ML, Yan BS, Kozoriz D, Weiner HL. Novel CD8(+) regulatory T cells suppress experimental autoimmune encephalomyelitis by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol. 2009;34:3423–35. doi: 10.1002/eji.200939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ben-David H, Sharabi A, Dayan M, Sela M, Mozes E. The role of CD8+CD28 regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand. Proc Natl Acad Sci U S A. 2007;104:17459–64. doi: 10.1073/pnas.0708577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996;184:2167–74. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang R, Han G, Song L, Wang J, Chen G, Xu R, et al. CD8+ regulatory T cells are responsible for GAD-IgG gene-transferred tolerance induction in NOD mice. Immunology. 2009;126:123–31. doi: 10.1111/j.1365-2567.2008.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 127.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 128.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, et al. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–72. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882–92. doi: 10.1128/JVI.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elrefaei M, Barugahare B, Ssali F, Mugyenyi P, Cao H. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J Immunol. 2006;176:1274–80. doi: 10.4049/jimmunol.176.2.1274. [DOI] [PubMed] [Google Scholar]
- 131.Elrefaei M, Ventura FL, Baker CA, Clark R, Bangsberg DR, Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007;178:3265–71. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 132.Elrefaei M, Baker CA, Jones NG, Bangsberg DR, Cao H. Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. J Immunol. 2008;180:7757–63. doi: 10.4049/jimmunol.180.11.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–91. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, et al. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol. 2007;81:13444–55. doi: 10.1128/JVI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nordstrom I, Nurkkala M, Collins LV, Eriksson K. CD8+ T-cells suppress antigen-specific and allogeneic CD4+ T-cell responses to herpes simplex virus type 2-infected human dendritic cells. Viral Immunol. 2005;18:616–26. doi: 10.1089/vim.2005.18.616. [DOI] [PubMed] [Google Scholar]
- 136.Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–54. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 137.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–72. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 140.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A. 2007;104:12087–92. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Voo KS, Peng G, Guo Z, Fu T, Li Y, Frappier L, et al. Functional characterization of EBV-encoded nuclear antigen 1-specific CD4+ helper and regulatory T cells elicited by in vitro peptide stimulation. Cancer Res. 2005;65:1577–86. doi: 10.1158/0008-5472.CAN-04-2552. [DOI] [PubMed] [Google Scholar]
- 142.Wang HY, Peng G, Guo Z, Shevach EM, Wang RF. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174:2661–70. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 143.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 144.Wang RF. CD8+ regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol. 2008;69:811–4. doi: 10.1016/j.humimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 145.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–9. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 146.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 147.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 148.Vlad G, Cortesini R, Suciu-Foca N. License to heal: bidirectional interaction of antigen-specific regulatory T cells and tolerogenic APC. J Immunol. 2005;174:5907–14. doi: 10.4049/jimmunol.174.10.5907. [DOI] [PubMed] [Google Scholar]
- 149.Zhang L, Jarvis LB, Baek HJ, Gaston JS. Regulatory IL4+CD8+ T cells in patients with ankylosing spondylitis and healthy controls. Ann Rheum Dis. 2009;68:1345–51. doi: 10.1136/ard.2008.088120. [DOI] [PubMed] [Google Scholar]
- 150.Rifa'i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–47. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]