Abstract
Background
There are limited data assessing the relationship between fractional concentration of exhaled nitric oxide (FENO) and lung function or exacerbations in infants with recurrent wheezing.
Objectives
In a longitudinal pilot study of children < 2 years old we assessed whether baseline FENO was associated with lung function, bronchodilator responsiveness, changes in lung function, or subsequent exacerbations of wheezing.
Methods
Forced expiratory flows and volumes using the raised-volume rapid thoracic compression method were measured in 44 infants and toddlers (mean age 15.7 mos.) with recurrent wheezing. Single-breath exhaled nitric oxide (SB-eNO) was measured at 50 mL/sec. Lung function was again measured 6 months after enrollment.
Results
At enrollment FEV0.5, FEF25-75, and FEF75 z-scores for the cohort were significantly less than zero. There was no correlation between enrollment SB-eNO and enrollment lung function measures. SB-eNO was higher in infants with bronchodilator responsiveness (46.1 vs. 23.6 ppb, p<0.001), and was associated with a decline in FEV0.5 (r = -.54, P = 0.001), FEF25-75 (r = -0.6, P < 0.001), and FEF75 (r = -0.55, P = 0.001) over 6 months. A 10ppb increase in SB-eNO was associated with a 0.4 z-score decline in FEV0.5, a 0.4 z-score decline in FEF25-75, and a 0.42 z-score decline in FEF75. SB-eNO was superior to lung function and bronchodilator responsivenss in predicting subsequent wheezing treated with systemic steroids.
Conclusions
SB-eNO may predict changes in lung function and risk of future wheezing, and holds promise as a biomarker to predict asthma in wheezy infants and toddlers.
Keywords: exhaled nitric oxide, FENO, recurrent wheezing, infants, pulmonary function, raised-volume rapid thoracic compression
Introduction
Eighty percent of children with persistent asthma are symptomatic by age six years, with more than half symptomatic by age three years.1 There is evidence that children with an early onset of persistent asthma have diminished lung function by age six years,2 suggesting that early intervention may be important to alter the natural course of asthma. However, children under age three years with asthma coexist with a larger group of children with recurrent transient wheezing. Episodes of recurrent wheezing affect 20-30% of infants and toddlers, yet resolve in at least 50% of these children by age six years.2 Clinicians generally cannot distinguish young children with transient wheezing from those with early persistent asthma. Biomarkers to predict asthma in wheezy infants and toddlers are needed to assist in the timely diagnosis and treatment of asthma. NHLBI guidelines recommend daily inhaled corticosteroid therapy for children under age five years who have had > 3 episodes of wheezing and epidemiologic risk factors for asthma,3 yet many parents and clinicians are reluctant to chronically treat young children with inhaled steroids if the diagnosis of asthma is in doubt.
The fractional concentration of exhaled nitric oxide (FENO) is a proposed biomarker of airway inflammation in asthma.4 FENO has repeatedly been shown to be elevated in adults and children with allergic asthma,5 but has been minimally studied in infants and toddlers. In adults and school-age children with atopic asthma FENO is correlated with sputum and bronchoalveolar eosinophils, peak flow variability, and bronchial reactivity.6-13 FENO decreases following treatment with systemic or inhaled corticosteroids in adults and school-age children with asthma,14,15 and has been shown to have a superior diagnostic accuracy for asthma than conventional diagnostic approaches, including spirometry.16-19 In adults, FENO was shown to be a superior predictor of steroid responsiveness than was spirometry, bronchodilator responsiveness, methacholine challenge, or adenosine challenge.20
A flow-regulated single-breath exhaled nitric oxide (SB-eNO) measurement technique during infant lung function testing using the raised-volume rapid thoracoabdominal compression (RVRTC) technique has been described.21,22 However, the relationship between FENO and lung function has been minimally studied in infants and toddlers, and there are no reports of longitudinal data assessing the association between FENO and subsequent changes in lung function in this age group. We recruited a cohort of infants and toddlers with recurrent episodes of wheezing to prospectively evaluate associations between SB-eNO and lung function as well as exacerbations of wheezing over time in a longitudinal pilot study. We hypothesized that SB-eNO would be associated with measures of lung function, bronchodilator responsiveness, and subsequent exacerbations of wheezing. Some of these results have been reported in abstract form.23
Methods
Subjects
Children 6-24 months of age with ≥ 3 episodes of physician-diagnosed wheezing treated with bronchodilators or corticosteroids were recruited for a single-center longitudinal study. Subjects with birth < 36 weeks gestation, congenital heart disease, dysphagia, severe gastroesophageal reflux, or upper airway obstruction were excluded. Systemic or inhaled corticosteroid treatment was not permitted for 3 weeks prior to baseline lung function and FENO measurement. Thereafter, corticosteroid treatment was at the discretion of the subject's primary care or emergency department provider who did not have access to FENO data. Written consent was obtained from parents of subjects. The study was approved by the Seattle Children's Hospital Institutional Review Board.
Study Visits
Study visits occurred a minimum of 3 weeks following resolution of an upper or lower respiratory infection, or acute exacerbation of wheezing. At enrollment medical history was reviewed, length and weight were measured with a calibrated stadiometer and digital scale, SB-eNO was measured, and lung function testing performed. Between enrollment and follow-up visits parents completed monthly symptom and medication diaries that recorded: wheezing episodes lasting ≥ 3 days treated with at least a bronchodilator, use of systemic (oral or intravenous) corticosteroids, and use of inhaled corticosteroids. Diaries were returned to study personnel on a monthly basis. Interval medical history and medication use was reviewed and lung function tests repeated at a follow-up visit 6 months after enrollment.
Lung Function Measurements
Forced expiratory volumes and flows (FVC, FEV0.5, FEF25-75, and FEF75) were measured using the RVRTC technique according to ATS/ERS guidelines for raised volume forced expirations in infants24 via the InSpire® Infant Pulmonary Lab (IPL). Additional detail on the methods for making these measurements is provided in the Online Repository. Lung function tests were performed at least 3 weeks after resolution of acute exacerbations of wheezing or respiratory illnesses. Lung function parameters were analyzed as z-scores calculated from published normative data.25 Measurement of forced expiratory flows and volumes were repeated 10 minutes after administration of 4 puffs of albuterol. Bronchodilator responsiveness was defined as ≥ 12% improvement in FEV0.5, or ≥ 25% improvement in FEF25-75.26
Exhaled Nitric Oxide Measurements
At least 3 SB-eNO measurements were performed in each subject immediately prior to lung function testing using a method modified from previous descriptions.21,22 The subject's lungs were inflated to 30 cm H2O 2-3 times to induce a respiratory pause, then a thoracoabdominal compression was performed using a jacket pressure of 25 cm H2O. Exhaled gas was collected using a full facemask. Sliding valves triggered following the last lung inflation diverted the IPL expiratory flow through a mass flow controller (Aerocine®, Sweden) that maintained flow at 50 mL/sec (+/- 5 mL/sec) when expiratory pressure was between 8-30cm H20 (see Figure E1 in the Online Repository), and bias flow away from the IPL circuit. Nitric oxide was measured using a Sievers® NOA 280 chemiluminescence analyzer (GE Analytical Instruments; Boulder, CO, USA). Additional detail on the methods for SB-eNO measurements is provided in the Online Repository.
Statistical Analysis
Forty subjects were estimated to provide 80% power to identify SB-eNO as a significant predictor of FEV0.5 if SB-eNO accounted for ≥15% of the total variance in FEV0.5 (i.e. r2=0.15). SB-eNO and lung function data was determined to be normally distributed by the D'Agostino and Pearson as well as Kolmogorov-Smirnov tests.
The coefficient of variation (CV%) was calculated for SB-eNO measurements from each subject. The mean intra-individual CV% and intraclass correlation coefficient (ICC) for SB-eNO measurements were calculated. The Pearson coefficient was used to estimate correlations between enrollment SB-eNO and lung function measures. Multiple variable linear regression was used to assess associations between enrollment SB-eNO, baseline subject characteristics, and changes in lung function during follow-up. A receiver operating characteristics (ROC) analysis was performed to determine the enrollment SB-eNO level that best predicted a decline in FEV0.5.
Multiple variable logistic regression was used to assess associations between enrollment SB-eNO and exacerbations of wheezing (defined as an acute episode of wheezing lasting ≥ 3 days, treated with albuterol and systemic corticosteroids for ≥ 3 days) during the follow-up period, adjusting for enrollment FEV0.5, bronchodilator responsiveness, enrollment age, gender, family history of asthma, eczema, sustained inhaled corticosteroid treatment, and tobacco smoke exposure. ROC analyses were performed to determine the enrollment SB-eNO, FEV0.5, and FEF25-75 values that best predicted systemic corticosteroid treatment for exacerbations of wheezing during the follow-up period. Using the best cutoff points for SB-eNO and FEV0.5, the sensivity, specificity, positive predictive value, and negative predictive value of enrollment SB-eNO, FEV0.5, bronchodilator responsiveness, and the combination of SB-eNO with FEV0.5 or bronchodilator responsiveness, were calculated. Data analyses were conducted using GraphPad® Prism 4.0 (LaJolla, CA, USA) and SPSS for Windows® version 11.5 (Chicago, IL, USA). Additional detail regarding statistical analyses is provided in the Online Repository.
Results
Forty-seven infants and toddlers were recruited with a mean age of 15.6 (SD ± 5.2) months at enrollment. Technically acceptable RVRTC flow-volume curves were obtained from 44 subjects at enrollment. Baseline characteristics of the cohort are presented in Table I. The mean FEV0.5, FEF25-75, and FEF75 z-scores for the cohort at enrollment were significantly less than zero (a z-score equal to zero represents the mean value of published normative data). Four subjects were lost to follow-up, and 8 subjects who completed technically acceptable lung function measurements at enrollment were unable to do so at the 6-month follow-up visit (Figure E2). There were no significant differences in baseline characteristics between the subjects completing lung function testing at both visits and the subjects who did not (Table I). Enrollment of subjects was evenly distributed across seasons (Figure E3). Eight of the 32 subjects completing lung function testing at both visits used inhaled corticosteroids on a sustained basis during the follow-up period.
TABLE I. Baseline Characteristics of Subjects Completing Lung Function Testing.
| All Subjects Completing Enrollment Lung Function Testing | Subjects Completing Enrollment and Follow-up Lung Function Testing | Subjects Completing only Enrollment Lung Function Testing | |
|---|---|---|---|
| Subjects (n) | 44 | 32 | 12 |
| Gender (male) | 29 (66%) | 22 (69%) | 7 (58%) |
| Race (Caucasian) | 32 (73%) | 26 (81%) | 6 (50%) |
| Mean age at enrollment (months)* | 15.7 ± 5.2 | 15.2 ± 4.9 | 15.5 ± 5.4 |
| Eczema (physician-diagnosed and steroid treated) | 16 (36%) | 10 (31%) | 6 (50%) |
| Family history asthma (1st degree relative) | 23 (52%) | 14 (44%) | 9 (75)% |
| Enviromental tobacco smoke exposure | 12 (27%) | 9 (28%) | 3 (25%) |
| Prior treatment with systemic steroids for wheezing | 33 (75%) | 25 (78%) | 8 (67%) |
| History of ED visit for wheezing | 33 (75%) | 24 (75%) | 9 (75%) |
| History of hospitalization for wheezing | 16 (36%) | 10 (31%) | 6 (50)% |
| History of wheezing apart from viral infections | 16 (36%) | 12 (38%) | 4 (33%) |
| Enrollment SB-eNO (ppb)* | 28.7 ppb ± 16.1 | 27.4 ppb ± 15.3 | 31.2± 19.5 |
| Enrollment FVC (z-score)** | -0.04 (-0.42 to 0.35) | -0.01 (-0.51 to 0.49) | -0.24 (-0.65 to 0.17) |
| Enrollment FEV0.5 (z-score)** | -0.42 (-0.78 to -0.07) | -0.41 (-0.80 to -0.04) | -0.21 (-0.46 to -0.05) |
| Enrollment FEF25-75 (z-score)** | -0.67 (-1.10 to -0.25) | -0.82 (-1.33 to -0.30) | -0.45 (-1.15 to 0.27) |
| Enrollment FEF75 (z-score)** | -0.72 (-1.14 to -0.31) | -0.84 (-1.31 to -0.43) | -0.56 (-1.22 to -0.12) |
Definition of Abbreviations: SB-eNO = single-breath exhaled nitric oxide; ED = emergency department.
Data are presented as the mean ± SD
Data are presented as the mean ± 95% CI
Enrollment visit SB-eNO was measured in 45 subjects, including all 44 infants with enrollment lung function measurements. The mean intra-individual CV% of SB-eNO for the cohort was 6.2% (range 0.9-13.1%) and the ICC was 0.92. The mean enrollment SB-eNO for the cohort was 28.7ppb (range 3 – 76ppb). There was a small but significant association between increasing age and enrollment SB-eNO (Table EI). A history of eczema was also associated with enrollment SB-eNO. There was no association between SB-eNO and gender, family history of asthma, environmental tobacco smoke exposure, wheezing apart from viral respiratory infections, hospitalization for wheezing, or oral steroid treatment.
There was no correlation between enrollment SB-eNO and enrollment FVC (r = -0.07, P = .7), FEV0.5 (r = 0.05, P = .7), FEF25-75 (r = 0.2, P = .2), or FEF75 (r = 0.2, P = .1) before or after adjustment for gender, eczema, family history of asthma, and tobacco smoke exposure using multiple variable linear regression analyses.
Enrollment SB-eNO was significantly higher in subjects whose lung function subsequently declined between enrollment and the 6 month follow-up visit (Figure E5). There were significant correlations between enrollment SB-eNO and decline in FEV0.5 (r = -.54, P = .001), FEF25-75 (r = -0.6, P < .001), and FEF75 (r = -0.55, P = .001) (Figure E4). After adjustment for age at enrollment, gender, family history of asthma, history of eczema, sustained use of inhaled corticosteroids during follow-up, and environmental tobacco smoke exposure, each additional 10ppb increment of enrollment SB-eNO (0-10, 11-20, 21-30ppb, etc) was associated with a 0.4 z-score decline in FEV0.5, a 0.4 z-score decline in FEF25-75, and a 0.42 z-score decline in FEF75 (Table II). Sustained use of inhaled corticosteroids was associated with an increase in forced expiratory flows. An ROC analysis showed that an enrollment SB-eNO level > 29ppb predicted a decline in FEV0.5 during follow-up with a sensitivity of 67% and a specificity of 86%, with an area under the curve (AUC) of 0.89 (95% CI: 0.77 – 1.0) (Figure E6).
TABLE II. Associations Between Enrollment SB-eNO And Changes In Lung Function.
| Δ FEV0.5 z-scores | Δ FEF25-75 z-score | Δ FEF75 z-score | ||||
|---|---|---|---|---|---|---|
| ß Coefficient | P value | ß Coefficient | P value | ß Coefficient | P value | |
|
Enrollment SB-eNO (10ppb increments) |
-0.40 | 0.01 | -0.40 | 0.01 | -0.42 | 0.003 |
|
Eczema (yes/no; physician-diagnosed and steroid treated)) |
-0.34 | 0.3 | -0.73 | 0.1 | -0.3 | 0.4 |
|
Sustained ICS use during follow-up (yes/no) |
0.84 | 0.1 | 1.3 | 0.03 | 1.1 | 0.04 |
| Gender | -0.08 | 0.8 | -0.11 | 0.8 | 0.07 | 0.9 |
|
Age at enrollment (1 month increments) |
-0.03 | 0.4 | -0.06 | 0.2 | -0.04 | 1.0 |
|
FH Asthma (yes/no) |
0.24 | 0.5 | 0.32 | 0.5 | -0.04 | 0.9 |
|
Tobacco smoke exposure (yes/no) |
-0.56 | 0.2 | -0.62 | 0.2 | -0.47 | 0.3 |
Definition of Abbreviations: SB-eNO = single-breath exhaled nitric oxide; ICS = inhaled corticosteroids; FH = family history. N=32 subjects. ß Coefficient represents change in lung function z-score associated with each variable or increment of a variable (e.g 10ppb increment of SB-eNO). Bold type denotes a statistically significant association.
Thirty-seven subjects completed forced expiratory volume and flow measurements following administration of albuterol at either enrollment or follow-up visits. SB-eNO levels were significantly higher in subjects with bronchodilator responsiveness than in subjects without bronchodilator responsiveness (46.1 ppb vs. 23.6 ppb, P < .001; Figure 1).
FIGURE 1.
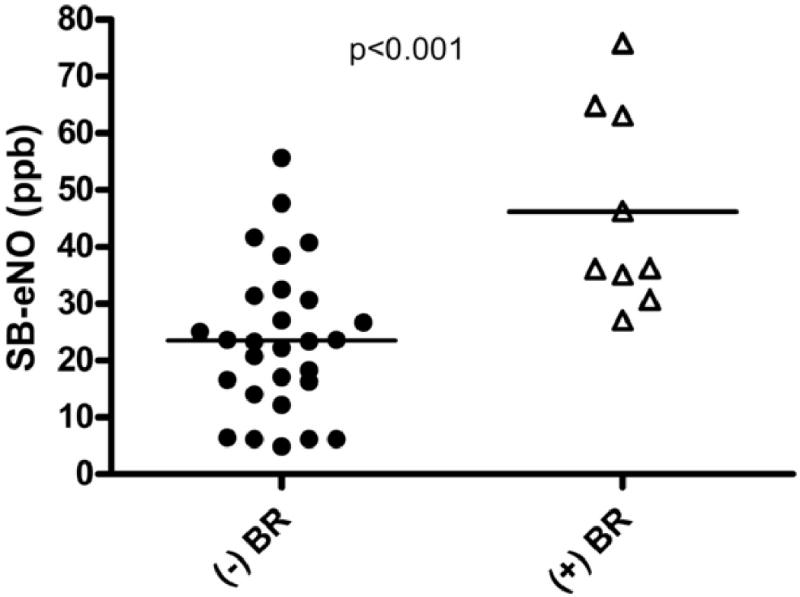
Relationship between single-breath exhaled nitric oxide (SB-eNO) levels and bronchodilator responsiveness (BR). Bronchodilator responsiveness defined as a ≥12% improvement in FEV0.5 or ≥25% improvement in FEF25-75 following administration of albuterol (open triangles).
During the follow-up period 16 subjects experienced an exacerbation of wheezing for which a primary care or emergency department provider treated them with systemic corticosteroids for ≥ 3 days. Enrollment SB-eNO levels were significantly higher in subjects who were subsequently treated with systemic steroids for an acute episode of wheezing than in those subjects who were not (39.3 ppb vs. 22 ppb, P < .001; Figure 2). The odds of an exacerbation of wheezing treated with systemic steroids during the follow-up period increased over threefold (OR 3.3; 95% CI 1.2 – 10.1) for every 10ppb increase in enrollment SB-eNO (Table E2). An ROC analysis showed that an enrollment SB-eNO level > 29ppb predicted treatment with systemic corticosteroids for wheezing exacerbations during follow-up with a sensitivity of 88%, a specificity of 82%, and an area under the curve (AUC) of 0.83 (95% CI: 0.69 – 0.96) (Figure E7). The sensitivity, specificity, positive predictive value, and negative predictive value of enrollment SB-eNO for wheezing exacerbations during follow-up was superior to enrollment FEV0.5, enrollment FEF25-75, or bronchodilator responsiveness (Table III). The predictive value of a combination of enrollment SB-eNO with enrollment lung function measures or bronchodilator responsiveness was not superior to enrollment SB-eNO alone.
FIGURE 2.
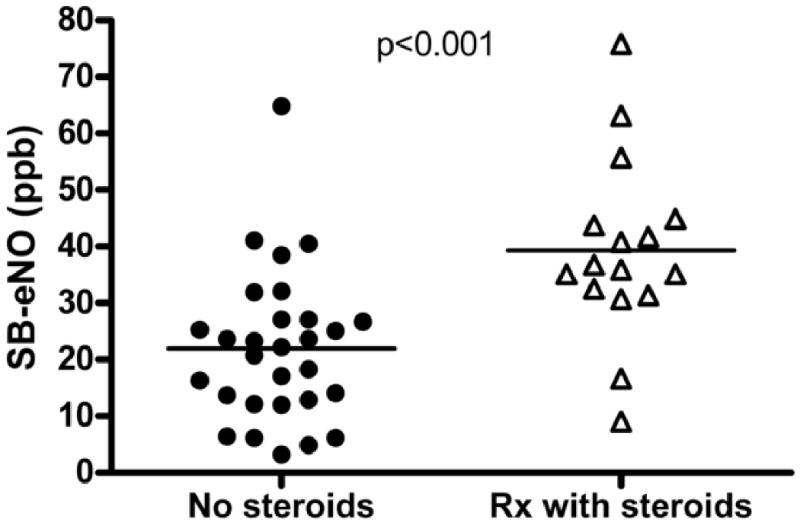
Enrollment single-breath exhaled nitric oxide (SB-eNO) levels in subjects treated with systemic (oral or intravenous) steroids for an acute episode of wheezing during the follow-up period (open triangles) vs. SB-eNO levels in subjects not treated with systemic steroids (closed circles).
TABLE III. Sensitivity, Specificity, PPV, and NPV of Enrollment SB-eNO, FEV0.5, FEF25-75 and Bronchodilator Responsiveness for Wheezing Exacerbations Treated With Systemic Corticosteroids During Follow-Up.
| Cutoff | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| SB-eNO | >29ppb | 88% | 82% | 70% | 92% |
| Enrollment FEV0.5 | z-score < -0.53 | 31% | 59% | 29% | 61% |
| Enrollment FEF25-75 | z-score < -1.0 | 31% | 58% | 29% | 60% |
| BR | ≥ 12% improvement FEV0.5, or ≥ 25% improvement FEF25-75 | 33% | 78% | 44% | 69% |
| SB-eNO and FEV0.5 | >29ppb; z-score < -0.53 | 25% | 89% | 57% | 68% |
| SB-eNO and FEF25-75 | >29ppb; z-score < -1.0 | 38% | 93% | 75% | 73% |
| SB-eNO > 29ppb and BR | >29ppb; BR | 33% | 91% | 67% | 72% |
Definition of Abbreviations: PPV = positive predictive value; NPV = negative predictive value; SB-eNO = single-breath exhaled nitric oxide; BR = bronchodilator responsive
After excluding subjects who used inhaled corticosteroids during the study (n=8), there was a significant direct correlation between enrollment and follow-up SB-eNO levels (r = 0.5, P = .01; Figure E8), with slightly higher SB-eNO at the follow-up visit (Figure E9; mean 29.3 vs. 22.5ppb, P = .01). There was no correlation between intra-individual changes in SB-eNO levels and change in z-scores for FEV0.5 or forced expiratory flows between enrollment and follow-up visits (Figure E10).
Discussion
In a cohort of infants and toddlers with a history of recurrent episodes of wheezing we found that enrollment SB-eNO measurements were not associated with baseline forced expiratory volumes or flows. However, enrollment SB-eNO was significantly higher in subjects with a subsequent decline in lung function than in subjects with an increase in lung function over 6 months of follow-up. SB-eNO values were significantly higher in subjects with bronchodilator responsiveness. Higher enrollment SB-eNO was associated with an increased risk of treatment with systemic corticosteroids for acute exacerbations of wheezing during the 6 month follow-up period. Furthermore, enrollment SB-eNO was superior to enrollment lung function and bronchodilator responsiveness as a predictor of wheezing exacerbations during follow-up. Finally, we have shown that SB-eNO measurements during RVRTC maneuvers are feasible and highly reproducible utilizing an automated technique to control expiratory and standardized criteria to determine SB-eNO plateau acceptability.
Other groups have reported SB-eNO measurements in infants and toddlers obtained during RVRTC maneuvers against an expiratory resistance.22,22 Wildhaber et al demonstrated by measuring nasal end-tidal CO2 that this technique results in closure of the velum thereby minimizing contamination of SB-eNO measurements by nasal nitric oxide.22 Studies by both Wildhaber et al22 and Franklin et al27 et al found that SB-eNO levels were higher in wheezy infants than in healthy infants. Our findings are consistent with those of Wildhaber el al who reported no association between SB-eNO and FEV0.5 in a cross-sectional study of wheezy infants, as well as with results from studies of older children where no association between FENO and lung function was found at a single time point.28,29 SB-eNO in our subjects was similar to results reported from wheezy infants by Wildhaber et al using an expiratory flow of 50 ml/sec and Franklin et al using an expiratory flow of 40 ml/sec. To our knowledge, our study is the first to perform SB-eNO measurements using an automated method to regulate expiratory flow, apply rigorous a priori criteria to the determination of the SB-eNO plateau during single-breath measurements, and to obtain longitudinal lung function measurements, in a cohort of infants and toddlers with recurrent physician-diagnosed wheezing.
Follow-up of this cohort into school-age will be essential to determine if SB-eNO predicts which children will have asthma and which will experience resolution of wheezing. It is also possible that the intra-individual change in SB-eNO between infancy and school-age may be more predictive of asthma than a single measurement. Findings from the Tucson Children's Respiratory Study2 suggest that infants with transient wheezing have diminished lung function shortly after birth that may predispose them to wheeze with viral infections, yet experience improvement in lung function by age 6 years, while infants who continue to wheeze into the school age years have normal lung function in early infancy yet experience a decline in lung function by age 6 years. It is possible that in our cohort a low SB-eNO may identify a transient wheezing phenotype, while an elevated SB-eNO, which in older children and adults is associated with lower airway eosinophilic inflammation, may identify infants and toddlers at risk for atopic asthma. In adults and school-age children FENO has been shown to be a superior adjunctive diagnostic test for asthma than conventional objective diagnostic strategies, including spirometry, peak flow measurement, exercise challenge, or methacholine challenge.16-19 The lower age limit at which this applies has yet to be determined.
Infants and toddlers with recurrent wheezing are a heterogeneous group. The majority of school-age asthmatics experience their initial symptoms during the first several years of life, typically during viral respiratory infections. However, the majority of wheezy infants and toddlers do not have asthma, but rather wheeze for a variety of anatomic and/or pathophysiologic reasons, including small airway caliber, post-viral airway inflammation and injury, tracheomalacia, and dysphagia. Episodic wheezing resolves in most of these children by school-age, leading to the epidemiologic characterization of this phenomenon as transient wheezing. Discriminating between infants with transient wheezing and infants with asthma is a major challenge for clinicians. A predictive biomarker for asthma does not exist for infants or toddlers, greatly complicating efforts to prevent thousands of asthma exacerbations, emergency department visits, and hospitalizations in young children each year.30 Castro-Rodriquez et al proposed an index of epidemiologic risk factors to assist in predicting asthma in wheezy infants and toddlers,31 however, this index has not been assessed in a prospective manner, and when applied to children in the cohort from which it was developed had a positive predictive value for asthma at age 8 years of only 29%. Our data suggest that SB-eNO may hold promise as an adjunctive diagnostic test for asthma in wheezy infants and toddlers. We found that SB-eNO was a superior predictor of subsequent exacerbations of wheezing than measurement of forced expiratory flows and volumes or bronchodilator responsiveness. Furthermore, the combination of lung function measurements and SB-eNO was not superior to SB-eNO alone as predictors of subsequent exacerbations of wheezing. Our results are consistent with the recent finding by Beigelman et al in a subgroup of preschool age children with recurrent wheezing from the Acute Intervention Management Strategies clinical trial of the Childhood Asthma Research and Education Network that FENO was associated with subsequent respiratory tract illnesses.32 Diagnosing asthma at an early age using an objective test such as FENO could greatly facilitate more targeted use of asthma therapies, prevent under- and over-treatment with corticosteroids, and reduce preventable emergency department visits and hospitalization for asthma exacerbations.
There are several limitations to this study. First, change in lung function was estimated from only two measurements separated by 6 months. Infant lung function testing using the RVRTC method is time consuming, highly specialized, and requires sedation, limiting the feasibility of more frequent assessment. We were careful to only obtain measurements when subjects were at their baseline without acute respiratory infections or exacerbations within 3 weeks of testing. Multiple variable linear regression estimated declines of 0.4 z-scores for FEV0.5, FEF25-75, and FEF75 for each 10ppb incremental increase of enrollment SB-eNO. Data from large longitudinal pediatric cohort studies suggest that declines in lung function in asthmatics occur early in childhood, with little decline from age 6 years onward.33-35 While our results clearly demonstrate an association between FENO and change in lung function, we do not believe that the magnitude of decline we observed over 6 months of follow-up can be extrapolated to predict a sustained rate of decline over many years.
Another limitation is that only 73% of subjects completed acceptable lung function testing at both time points. Of the 87 attempts to perform RVRTC lung function measurements at both visits, 76 were successful (87%). Although, a higher percentage of subjects completing follow-up measurements would be preferable, the failure rate of sedation with chloral hydrate increases with age.36 Chloral hydrate is used to sedate infants for lung function testing by nearly all centers using the RVRTC method, and our overall success rate is comparable to other centers.37,38 Although lung function measurements in our subjects were expressed and analyzed as z-scores based on published normative data,25 our study lacks a within-center healthy control group. U.S. regulations and ethical guidelines for research involving children require that subjects not be exposed to procedures involving greater than minimal risk without the prospect of direct benefit to individual subjects.39 The interpretation of these guidelines by the majority of pediatric institutional review boards in the United States precludes the use of infant pulmonary function testing in healthy infants given the need for sedation without the prospect of direct benefit to normal subjects.
Finally, an important limitation of this pilot study is that we did not perform skin or radioallergosorbent testing of subjects to food or inhalant allergens at enrollment. Kulig et demonstrated in a birth cohort that development of allergic sensitization to inhalant allergens occurs mostly beyond age 2 years40, however, ideally we would have objective data characterizing the sensitization status of our subjects at enrollment. Future studies evaluating the predictive value of FENO must include prospective serial measures of allergic sensitization. We chose the very general enrollment criterion of ≥ 3 episodes of physician-diagnosed and treated wheezing episodes because we felt that assessing the predictive value of SB-eNO for changes in lung function and future wheezing in such a population was a relevant clinical question commonly faced by pediatric practioners as well as academic researchers. Until this and other cohorts of wheezy infants and toddlers can be followed into the school-age years it is important not to over-interpret our results by concluding that SB-eNO predicts asthma in this population.
To be consistent with ATS recommendations for FENO measurements in older children and adults,41 we chose an expiratory flow of 50 ml/sec to obtain SB-eNO measurements. However, 50 ml/sec may not be an ideal flow to measure FENO in infants and toddlers given their small airway caliber. A positive association between age and FENO have been reported in several large studies of healthy school age children.42-44 Possible mechanisms for the age dependency of FENO include increased lung volume and airway surface area, changes in NO diffusion coefficients, changes in nitric oxide synthase expression with age, or changes in S-nitrosoglutathione (GSNO) kinetics.45 In addition, a fixed flow of 50 ml/sec represents a higher relative flow in young subjects with smaller airways compared to older subjects with larger airways, resulting in lower FENO due the flow dependence of FENO. Future studies in this age group should also assess FENO at several lower flows.
In conclusion, we have shown that baseline SB-eNO measurements in a cohort of infants and toddlers with a history of recurrent episodes of physician-diagnosed wheezing did not correlate with baseline forced expiratory volumes or flows. However, higher enrollment SB-eNO levels were associated with a decline in lung function and increased risk of acute exacerbations of wheezing treated with systemic corticosteroids during the follow-up period. Furthermore, we found that SB-eNO levels were higher in subjects with bronchodilator responsiveness. Although follow-up into the school-age years with a larger sample size will be essential to definitely evaluate its predictive value for asthma in wheezy infants and toddlers, these data suggest that SB-eNO measurement in this population is a promising objective biomarker that may prove very useful clinically, but may also increase our understanding of the evolution of asthma in young children.
Clinical Implications.
In wheezy infants single-breath exhaled nitric oxide levels are associated with subsequent changes in lung function over time, bronchodilator responsiveness, and subsequent exacerbations of wheezing.
Acknowledgments
Supported by: NHLBI: K23HL077626; CTSA Grant Number I ULI RR025014-01; Seattle Children's Hospital.
Abbreviations
- FENO
fractional concentration of exhaled nitric oxide
- SB-eNO
single-breath exhaled nitric oxide
- FEV0.5
forced expiratory volume in 0.5 seconds
- FEF25-75
forced expiratory flow 25-75% of expiration
- RVRTC
raised volume rapid thoracic compression
- ICC
intraclass correlation coefficient
- CV
coefficient of variation
- ROC
receiver operating characteristics
- AUC
area under the curve
- NHLBI
National Heart Lung Blood Institute
- ATS
American Thoracic Society
- ERS
European Respiratory Society
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109(2):362–367. [PubMed] [Google Scholar]
- 2.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel Report 3 (EPR3) Guidelines for the Diagnosis and Management of Asthma. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.html.
- 4.Kharitonov SA. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DR, Pijnenburg MW, Smith AD, De Jongste JC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61(9):817–27. doi: 10.1136/thx.2005.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharitonov SA. Changes in the dose of inhaled steroid affect exhaled nitric oxide levels in asthmatic patients. Eur Respir J. 1996;9:196–201. doi: 10.1183/09031936.96.09020196. [DOI] [PubMed] [Google Scholar]
- 8.Lim S, Jatakanon A, John M, Gilbey T, O'Connor BJ, Chung KF, Barnes PJ. Effect of inhaled budesonide on lung function and airway inflammation. Am J Respir Crit Care Med. 1999;159:22–30. doi: 10.1164/ajrccm.159.1.9706006. [DOI] [PubMed] [Google Scholar]
- 9.Jatakanon A, Kharitonov SA, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54:108–114. doi: 10.1136/thx.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson PG, Henry RL, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J. 2000;16:1008–1015. [PubMed] [Google Scholar]
- 11.Dupont LJ, Rochette F, Demedts MG, Verleden GM. Exhaled nitric oxide correlates with airway hyper-responsiveness in steroid-naïve patients with mild asthma. Am J Respir Crit Care Med. 1998;157:894–8. doi: 10.1164/ajrccm.157.3.9709064. [DOI] [PubMed] [Google Scholar]
- 12.Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JDM, Ennis M, Shields MD. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57:383–87. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grootendorst DC. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clin Exp Allergy. 1997;27:769–779. [PubMed] [Google Scholar]
- 14.Massoaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med. 1995;152:800–803. doi: 10.1164/ajrccm.152.2.7633745. [DOI] [PubMed] [Google Scholar]
- 15.Yates DH, Kharitonov SA, Robbins RA, Thomas PS, Barnes PJ. Effect of a nitric oxide synthase inhibitor and a glucocorticosteroid on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:892–896. doi: 10.1164/ajrccm.152.3.7663801. [DOI] [PubMed] [Google Scholar]
- 16.Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest. 2003;123(3):751–6. doi: 10.1378/chest.123.3.751. [DOI] [PubMed] [Google Scholar]
- 17.Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, Taylor DR. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 2004;169(4):473–8. doi: 10.1164/rccm.200310-1376OC. [DOI] [PubMed] [Google Scholar]
- 18.Malmberg L, Pelkonen A, Haahtela T, Turpeinen M. Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax. 2003;58(6):494–499. doi: 10.1136/thorax.58.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivan Y, Gadish T, Fireman E, Soferman R. The use of exhaled nitric oxide in the diagnosis of asthma in school children. J Pediatr. 2009;155(2):211–6. doi: 10.1016/j.jpeds.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005;172(4):453–9. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 21.Martinez T, Weist A, Williams T, Clem C, Silkoff P, Tepper RS. Assessment of exhaled nitric oxide kinetics in healthy infants. J Appl Physiol. 2003;94(6):2384–90. doi: 10.1152/japplphysiol.00758.2002. [DOI] [PubMed] [Google Scholar]
- 22.Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med. 1999;159(1):74–8. doi: 10.1164/ajrccm.159.1.9805021. [DOI] [PubMed] [Google Scholar]
- 23.Debley J, Cochrane E, Stamey S, Worrell K, Redding G. Exhaled Nitric Oxide (eNO), Lung Function, and Exacerbations in Wheezy Infants. Am J Respir Crit Care Med. 2009;179:A5370. [Google Scholar]
- 24.ATS/ERS Statement: Raised Volume Forced Expirations in Infants Guidelines for Current Practice. Am J Respir Crit Care Med. 2005;172:1463–1471. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 25.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants. Am J Respir Crit Care Med. 2000;161:353–359. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein AB, Castile RG, Davis SD, Filbrun DA, Flucke RL, McCoy KS, Tepper RS. Bronchodilator responsiveness in normal infants and young children. Am J Respir Crit Care Med. 2001;164:447–454. doi: 10.1164/ajrccm.164.3.2005080. [DOI] [PubMed] [Google Scholar]
- 27.Franklin PJ, Turner SW, Mutch RC, Stick SM. Comparison of single-breath and tidal breathing exhaled nitric oxide levels in infants. Eur Respir J. 2004;23:369–72. doi: 10.1183/09031936.04.00084604. [DOI] [PubMed] [Google Scholar]
- 28.Nordvall SL, Janson C, Kalm-Stephens P, Foucard T, Torén K, Alving K. Exhaled nitric oxide in a population-based study of asthma and allergy in schoolchildren. Allergy. 2005;60(4):469–75. doi: 10.1111/j.1398-9995.2005.00735.x. [DOI] [PubMed] [Google Scholar]
- 29.Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, Young DA, Spahn JD. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003;142(5):469–75. doi: 10.1067/mpd.2003.187. [DOI] [PubMed] [Google Scholar]
- 30.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2007. Vital Health Stat. 2009;239:1–80. [PubMed] [Google Scholar]
- 31.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 32.Beigelman A, Mauger DT, Phillips BR, Zeiger RS, Taussig LM, Strunk RC, Bacharier LB. Effect of elevated exhaled nitric oxide levels on the risk of respiratory tract illness in preschool-aged children with moderate-to-severe intermittent wheezing. Ann Allergy Asthma Immunol. 2009;103(2):108–13. doi: 10.1016/S1081-1206(10)60162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964-1999. J Allergy Clin Immunol. 2002;109(2):189–94. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 35.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg SB, Faerber EN, Aspinall CL, Adams RC. High-dose chloral hydrate sedation for children undergoing MR imaging: safety and efficacy in relation to age. Am J Roentgenol. 1993;161:639–41. doi: 10.2214/ajr.161.3.8352124. [DOI] [PubMed] [Google Scholar]
- 37.Borrego LM, Stocks J, Leiria-Pinto P, Peralta I, Romeira AM, Neuparth N, Rosado-Pinto JE, Hoo AF. Lung function and clinical risk factors for asthma in infants and young children with recurrent wheeze. Thorax. 2009;64(3):203–9. doi: 10.1136/thx.2008.099903. [DOI] [PubMed] [Google Scholar]
- 38.Lum S, Gustafsson P, Ljungberg H, Hülskamp G, Bush A, Carr SB, Castle R, Hoo AF, Price J, Ranganathan S, Stroobant J, Wade A, Wallis C, Wyatt H, Stocks J. Early detection of cystic fibrosis lung disease: multiple-breath washout versus raised volume tests. Thorax. 2007;62(4):341–7. doi: 10.1136/thx.2006.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Code of Federal Regulations. Title 45 Public Welfare, DHHS NIH Office for Protection from Research Risks, Part 46 Protection of Human Subjects, Section 46.406. http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html. [PubMed]
- 40.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 41.ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 42.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005;115:1130–6. doi: 10.1016/j.jaci.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999;159(1):69–73. doi: 10.1164/ajrccm.159.1.9804134. [DOI] [PubMed] [Google Scholar]
- 44.Kissoon N, Duckworth LJ, Blake KV, Murphy SP, Taylor CL, DeNicola LR, Silkoff PE. Exhaled nitric oxide concentrations: online versus offline values in healthy children. Pediatr Pulmonol. 2002;33(4):283–92. doi: 10.1002/ppul.10023. [DOI] [PubMed] [Google Scholar]
- 45.Shin HW, George SC. Microscopic modeling of NO and S-nitrosoglutathione kinetics and transport in human airways. J Appl Physiol. 2001;90:777–788. doi: 10.1152/jappl.2001.90.3.777. [DOI] [PubMed] [Google Scholar]


