Abstract
We demonstrate that autosomal-dominant STAT3-deficient hyper-IgE syndrome confers late-onset susceptibility to molds, especially Aspergillus, despite preserved myeloid functions, in association with previous parenchymal lung damage (bronchiectasis/pneumatoceles). This suggests a critical role for non-hematopoietic processes in innate antifungal immunity in AD-HIES.
Keywords: autosomal dominant hyper-IgE syndrome, STAT3, fungus, aspergillus, aspergillosis
To the Editor:
Autosomal-dominant hyper-IgE (Job’s) syndrome (AD-HIES), due to heterozygous dominant-negative mutations in STAT3, is characterized by staphylococcal skin abscesses, eczematous dermatitis, connective tissue defects and elevated serum IgE[1, 2]. Recurrent bacterial pneumonias, attributed to dysfunctional STAT3, frequently lead to bronchiectasis and formation of pneumatoceles. Fungal pneumonias, particularly aspergillosis, have also been reported in patients diagnosed with AD-HIES, albeit by clinical scoring system, and are a cause of mortality[3]. However, the few reports of invasive mycoses in patients with HIES pre-dated the ability to genotypically confirm the patients’ diagnoses, potentially admixing heterogeneous phenocopies and precluding comprehensive understanding of fungal disease in AD-HIES. We sought to define the epidemiology of invasive mycoses in patients with confirmed STAT3-mutant AD-HIES. Further, since STAT3 is implicated in multiple hematopoietic and non-hematopoietic processes, and the former are critical to antifungal resistance in other at-risk groups, we evaluated myeloid function related to fungal susceptibility.
Medical records of all patients with the diagnosis of AD-HIES enrolled on protocols of the National Institute of Allergy and Infectious Diseases, NIH and confirmed by STAT3 sequencing[2] were reviewed. Patients with clinical, computed tomography (CT), and laboratory evidence of “proven” and “probable” invasive fungal disease, based on EORTC/MSG consensus definitions[4], were identified.
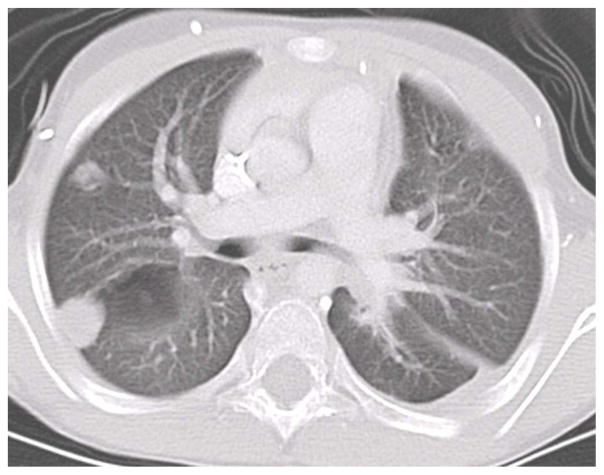
Sixty-four STAT3 AD-HIES patients were identified: 23 (36%) had no CT evidence of lung bronchiectasis/cysts/cavities/pneumatoceles, while 41 (64%) did. Of those without lung damage, none had concurrent or previous invasive mycoses. Among the 41 patients with parenchymal lung defects, 18 (44%) had at least 1 episode of invasive mold disease, typically involving the respiratory tract. Thus overall, 28.1% of all patients developed an invasive mycosis. The isolated molds were Aspergillus spp. (n=16; 83.3%), Scedosporium apiospermum (n=2), and Histoplasma capsulatum (n=2). One patient had aspergillosis and subsequently S. apiospermum; another had laryngeal histoplasmosis followed by aspergillosis. The other case of histoplasmosis manifested as a tongue ulcer with right middle lobe lung involvement necessitating lobectomy. Mold disease did not solely manifest as “fungus balls” restricted to lung cavities but also as consolidations without halo sign (Figure 1); 5 patients had disseminated disease. Two cases due to the yeast, Cryptococcus sp., presented as an esophageal mass[6] and meningitis, respectively; both occurred in patients with abnormal lung.
Figure 1.
CT of invasive fungal pneumonia in AD-HIES.
Stratification of patients based on location of STAT3 mutation (DNA-binding domain vs. SH2 domain) was performed to identify any genotype-phenotype correlation in regards to the development of fungal infections (Table). The occurrence of structural lung disease, fungal disease, median age of onset of first fungal pneumonia, and mortality due to fungal infection were not statistically different between domains (Fisher’s exact test). Treatment was variable (systemic antifungal therapy, with/without intracavitary instillation of antifungals or surgical resection). Overall, mortality rate among those who developed fungal disease was 16.7%.
Table.
Clinical characteristics of invasive fungal disease in AD-HIES patients based on location of STAT3 mutation
| Mutation in DNA-binding domain | Mutation in SH2-binding domain | |
|---|---|---|
| Number of patients | 34 | 30 |
| Frequency of patients with structural lung disease | 23 (67.6%) | 18 (60%) |
| Frequency of patients with invasive fungal disease | 10 (29.4%)* | 8 (26.7%)* |
| Median age of onset of first fungal pneumonia (years) | 32 | 30 |
| Mortality due to invasive fungal disease | 3 | 0 |
All cases occurred in patients with structural lung disease
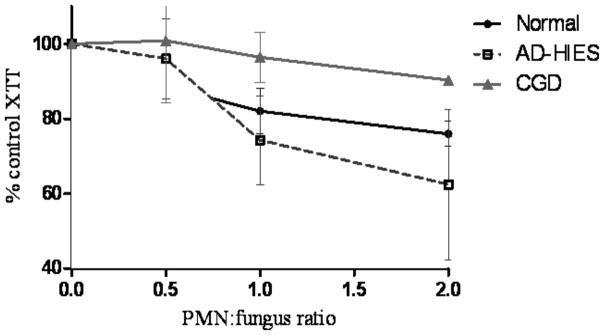
The integrity of myeloid functions associated with susceptibility to invasive mycoses was investigated. Neutrophils, peripheral blood mononuclear cells (PBMC), and plasma were isolated from patients (3 AD-HIES; 3 CGD) and healthy donors (n=4), and Aspergillus fumigatus B-5233, a pulmonary isolate from a leukemia patient, was prepared, as previously described[5]. Patients’ neutrophils were previously investigated for reactive oxygen species production via nitroblue tetrazolium histochemical staining, cytochrome c reduction, and dihydrorhodamine-123 flow cytometry; no deficiency was detected in the AD-HIES patients. The capacities of neutrophils or PBMC to inhibit growth of Aspergillus swollen conidia (the earliest metabolically-active spore form) and of germlings (the earliest hyphal form) in autologous plasma were assessed by modified XTT method at leukocyte:fungus ratios of 0, 0.5, 1 and 2[5]. This method has previously been used to demonstrate that neutrophils from patients with chronic granulomatous disease (CGD) have impaired ability to control A. fumigatus germlings[5], recapitulating the in vivo phenomenon. Data analysis was performed using GraphPad Prism software (v.5).
Swollen conidia were inhibited equally well by neutrophils from controls and patients (data not shown). Neutrophils from AD-HIES patients inhibited the growth of germlings as effectively as normal donors, whereas neutrophils from CGD patients were significantly less effective (Figure 2). PBMC from controls and patients equally suppressed swollen conidia but mediated no significant inhibition of germlings at ratios tested (data not shown).
Figure 2.
Growth inhibition of A. fumigatus hyphae by neutrophils from AD-HIES patients compared to normal donors and CGD patients.
Among primary immunodeficiencies, only 2 demonstrate consistent susceptibility to invasive aspergillosis: CGD and AD-HIES. The former results from mutations in the phagocyte NADPH oxidase complex, impairing their ability to generate reactive oxygen species. Consequently, patients with CGD demonstrate high frequency of invasive aspergillosis (25–35%), whereby fungal disease typically occurs within the first 2 decades of life in otherwise normal lung. AD-HIES patients are also at risk for invasive mold infections (28% of this cohort), most commonly Aspergillus. In contrast to CGD, invasive fungal disease typically occurred in the fourth decade of life and only when there were concurrent anatomical lung defects from previous bacterial pneumonias. Despite therapy, mortality from invasive fungal disease was significant (~17%).
Late-onset invasive fungal disease in the setting of anatomical lung defects suggests that susceptibility to molds in AD-HIES is distinct from that in CGD. In agreement with this hypothesis, leukocytes from AD-HIES patients performed as well as those from healthy donors in in vitro killing of Aspergillus conidia and hyphae and markedly better than CGD, suggesting that an intrinsic myeloid defect is not the prime reason these patients are susceptible to molds. Furthermore, chemotaxis of AD-HIES neutrophils towards A. fumigatus was not different from controls (Supplemental videos). Therefore, STAT3 mutations do not appear to impair the capacity of neutrophils or PBMC to inhibit Aspergillus in vitro. However, STAT3 may be operational in the myeloid control of intracellular fungi (e.g. Histoplasma).
STAT3 enables the epithelium to respond to Th17-based cytokines by upregulating antimicrobial peptides; defects in this pathway likely account for susceptibility to bacterial infections[7]. STAT3 also has numerous roles in lung processes relevant to epithelial integrity following injury[8, 9], such as bacterial infection; defects in these healing processes likely create a nidus for subsequent susceptibility to fungal pneumonias, similar to cystic fibrosis or post-tuberculosis cavities.
AD-HIES confers a significant late-onset risk of invasive fungal disease despite preserved myeloid functions. This temporally-dynamic susceptibility is associated with bronchiectasis/pneumatoceles, likely related to STAT3’s role in lung epithelial homeostasis and defense.
Supplementary Material
Acknowledgments
Sources of financial support: Canadian Institutes of Health Research (CIHR); Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH
Footnotes
Potential conflicts of interest: none to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 2.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 Mutations in the Hyper-IgE Syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 3.Freeman AF, Kleiner D, Nadiminti H, et al. Causes of death in hyper-IgE syndrome. J Allergy Clin Immunol. 2007;119:1234–40. doi: 10.1016/j.jaci.2006.12.666. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin infect dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI. Human Polymorphonuclear Leukocytes Inhibit Aspergillus fumigatus Conidial Growth by Lactoferrin-Mediated Iron Depletion. J Immunol. 2007;178:6367–6373. doi: 10.4049/jimmunol.178.10.6367. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs DH, Macher AM, Handler R, Bennett JE, Collen MJ, Gallin JI. Esophageal cryptococcosis in a patient with the hyperimmunoglobulin E-recurrent infection (Job’s) syndrome. Gastroenterology. 1984;87:201–3. [PubMed] [Google Scholar]
- 7.Minegishi Y, Saito M, Nagasawa M, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuzaki Y, Xu Y, Ikegami M, et al. Stat3 Is Required for Cytoprotection of the Respiratory Epithelium during Adenoviral Infection. J Immunol. 2006;177:527–537. doi: 10.4049/jimmunol.177.1.527. [DOI] [PubMed] [Google Scholar]
- 9.Kida H, Mucenski ML, Thitoff AR, et al. GP130-STAT3 Regulates Epithelial Cell Migration and Is Required for Repair of the Bronchiolar Epithelium. Am J Pathol. 2008;172:1542–1554. doi: 10.2353/ajpath.2008.071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.