Abstract
Photodynamic inactivation (PDI) is a rapidly developing antimicrobial technology which combines a non-toxic photoactivatable dye or photosensitizer (PS) in combination with harmless visible light of the correct wavelength to excite the dye to its reactive triplet state that will then generate reactive oxygen species (ROS) that are highly toxic to cells. Buckminsterfullerenes are closed-cage molecules entirely composed of sp2 hybridized carbon atoms and although their main absorption is in the UV, they also absorb visible light and have a long-lived triplet state. When C60 fullerene is derivatized with cationic functional groups it forms molecules that are more water-soluble and can mediate PDT efficiently upon illumination, and moreover cationic fullerenes can selectively bind to microbial cells. In this report we describe the synthesis and characterization of several new cationic fullerenes. Their relative effectiveness as broad-spectrum antimicrobial photosensitizers against Gram-positive, Gram-negative bacteria, and a fungal yeast was determined by quantitative structure function relationships.
Keywords: Photodynamic therapy, functionalized fullerenes, antimicrobial photoinactivation, quantitative structure function relationships, broad-spectrum antimicrobials
Introduction
There has been a relentless rise in antibiotic resistance that has taken place over many years in most regions of the world and has been observed in many different classes of microbial cells (1). In recent times the phenomenon has become even more worrying, with concerns that hitherto fairly trivial infections could again become untreatable as in the days before antibiotics were discovered (2). In fact the present time has been referred to as the “end of the antibiotic era” (3). The rise in multidrug resistance amongst microbial pathogens has motivated an international search for alternative antimicrobial strategies, particularly those which could be applied to infections in wounds and burns.
Photodynamic therapy (PDT) employs a nontoxic dye termed a photosensitizer (PS) and low-intensity visible light, which in the presence of oxygen produce reactive oxygen species (ROS), such as singlet oxygen, superoxide and hydroxyl radical (4). PDT has the advantage of dual selectivity in that the PS can be targeted to its destination cell or tissue, and in addition the illumination can be spatially directed to the lesion. PDT was originally discovered over a hundred years ago by its light-mediated killing effect on microorganisms (5), but since then has been principally developed as a treatment for cancer (6) and age-related macular degeneration (7). PDT has recently attracted attention as a possible alternative treatment for localized infections (8-10). It is proposed that for antimicrobial PDT the PS would be topically or locally applied to the infected tissue and, after a relatively short time interval, light would be delivered to the area and depending on the effectiveness of the antimicrobial PS, up to three logs of bacterial or fungal cells would be killed without causing unacceptable damage to the host tissue. A three-log reduction is accepted as an effective antimicrobial intervention equivalent to disinfectants (11). It has been reported that PDI is equally effective (12) (or even more effective (13)) against multi-drug resistant bacteria as it was against naïve species, and moreover, the PDI treatment itself is considered to be unlikely to cause bacterial resistance to damage by ROS to arise (14). This is because the PDI insult is relatively brief (many logs killed over minutes) compared to antibiotics that are typically present for many days or weeks, and because the type of damage to bio-molecules done by PDT is relatively non-speciific compared to antibiotics that in general specifically inhibit a target enzyme.
It is known that Gram-negative bacteria are resistant to PDI with many commonly used PS that will readily lead to phototoxicity for Gram-positive species (15) and that PS bearing a cationic charge (16) or the use of agents that increase the permeability of the outer membrane will increase the efficacy of killing of gram-negative organisms (17). The ideal PS for killing bacteria should possess an overall cationic charge and preferably multiple cationic charges (18).
Buckminsterfullerenes are closed-cage molecules entirely composed of sp2 hybridized carbon atoms. The presence of many conjugated double bonds gives these molecules a high optical absorption in visible wavelengths and a high triplet yield from the excited singlet state (19). The triplet state of the fullerene molecule can interact with molecular oxygen via two reaction pathways (Figure 1). Firstly energy transfer can take place between the fullerene triplet and ground state triplet oxygen giving the reactive species singlet oxygen (Type II pathway) and the ground state fullerene. Secondly, either the triplet fullerene (or possible the excited singlet fullerene) can accept an electron from a biological reducing agent such as nicotinamide adenine dinucleotide reduced form (NAD(P)H) to give the fullerene radical anion. This species can then transfer an electron to molecular oxygen to form superoxide radical anion and restoring the fullerene ground state. Superoxide anion can subsequently give rise to hydrogen peroxide and the highly reactive hydroxyl radical (20). Although pristine C60 in the form of nanoaggregates (21) or cyclodextrin complexes (22) is able to produce ROS upon illumination, the process is more efficient in biological media when the fullerenes have been chemically derivatized to make them water soluble. We have previously shown that fullerenes derivatized with one or more cationic charges via quaternary ammonium groups efficiently bind and penetrate all classes of microbial cells, and therefore act as broad-spectrum light-activated antimicrobials (23). In this report we describe the preparation and testing of new functionalized fullerene-derivatives with cationic charges as broad-spectrum antimicrobial PS against a panel of human pathogens.
Figure 1.
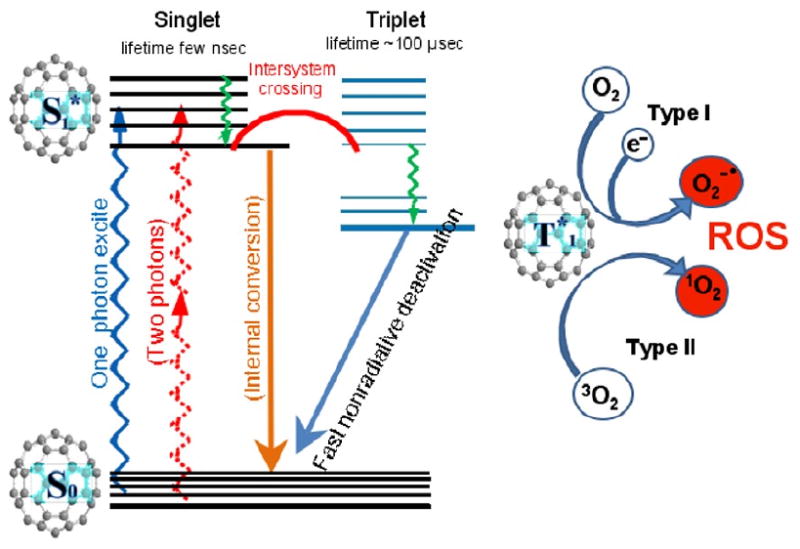
Schematic illustration of fullerene-mediated photodynamic therapy including the Jablonski diagram and the formation of Type I and Type II reactive oxygen species.
Materials and Methods
Fullerene synthesis and characterization
The compounds were characterized by 1H and 13C NMR, 500 MHz (Innova500 spectrometer), FTIR (ATR), and mass spectrometry (MALDI-TOF).
BF1: C60 (100 mg, 0.14 mmol) was dissolved in 150 mL toluene. Sarcosine (30.82 mg, 0.35 mmol) and 4-pyridinecarboxaldehyde (46 μL, 0.59 mmol) were added and the reaction mixture was refluxed for 48 h. The toluene was removed and the product dried overnight in vacuo. The product was purified on a silica gel column using a solvent ratio of 1000:1:1 of toluene, triethylamine, and ethyl acetate, respectively. MALDI TOF-MS calculated for C68H10N2 854.8, found 853.2 [M – H]-. The brown powder was treated with a large excess of iodomethane at ambient temperature for 48 h. The material was collected by filtration and washed with methanol.
ESI-MS calculated for C69H13N2 869.4 [M]+, found 869.1
1H NMR (DMSO-d6, TMS ref.) δ = 2.759 (3H, s), 4.352 (3H, s), 4.437 (1H, d), 5.161 (1H, d), 5.553 (1H, s), 8.593 (2H, d), 9.047 (2H, d)
13C NMR (DMSO-d6, TMS ref.) δ = 48.48, 69.09, 69.93, 76.53, 79.84, 126.0, 128.2, 128.9, 129.6, 136.0, 136.2, 137.0, 138.0, 139.6, 140.1, 140.3, 140.4, 141.8, 141.9, 143.3, 144.5, 144.7, 145.0, 145.3, 145.5, 145.6, 145.7, 145.8, 145.9, 146.1, 146.3, 146.5, 146.8, 147.4, 147.5, 151.8, 152.7, 154.3, 156.2, 156.6.
FT-IR ν (cm-1): 2919, 1454, 1210, 1006, 739, 520
Log KOW = -1.331
BF2 (mixture of bis and tris adduct): C60 (100 mg, 0.14 mmol) was dissolved in 50 mL chlorobenzene and the solution degassed with N2 for 30 minutes. Piperazine-2-carboxylic acid dihydrochloride (84.6 mg, 0.42 mmol) in methanol (3 mL) and triethylamine (300 μL) was added followed by 4-pyridinecarboxaldehyde (52.4 μL, 0.56 mmol) and the reaction was refluxed for 48 h under N2. The solvent was removed in vacuo, then the solid was dissolved in 10 mL methanol and 20 mL chloroform and loaded onto a silica gel column. The column was eluted with chloroform followed by chloroform:methanol:triethylamine (94:3:3) to remove the mono adducts. The remaining column material was then extracted with methanol to obtain the pure product precursors. The material was dissolved in 10 mL chloroform and treated with 3.0 mL iodomethane for 1 h followed by the addition of 10 mg K2CO3 and stirring at ambient temperature for 24 h. After filtration, the product was washed with chloroform and dried in vacuo to give a brown solid.
FT-IR ν (cm-1): 2922, 1454, 1210, 1008, 737
Log Kow = -3.02
Synthesis of N-hexylglycine: N-hexylamine (2 mL, 15.2 mmol) was dissolved in ethanol (1.43 mL) and water (686 μL) and stirred in an ice bath. Iodoacetic acid (649 mg, 3.49 mmol) was added slowly and the reaction mixture was allowed to stand overnight at room temperature. The reaction mixture was added to acetone (50 mL) and placed in the fridge. The resulting white precipitate was recrystallized using methanol/acetone. The resulting white crystals were dried overnight at 40 °C under vacuum.
Synthesis of BF3, BF4, and BF5: C60 (264.6 mg, 0.367 mmol) was dissolved in 130 mL toluene. N-hexylglycine (117 mg, 0.735 mmol) and paraformaldehyde (55 mg, 1.84 mmol) were added and the solution was refluxed for 2 h. After solvent removal, product was re-dispersed in minimal toluene and loaded onto a silica gel column. Elution with toluene gave unreacted C60 followed by monoadduct and then two bands corresponding to bisadduct regioisomers of increasing polarity. The fractions were methylated at room temp for 4 days with a large excess of methyl iodide. BF3 31.6 mg, BF4 29.9 mg, BF5 39.1 mg. ESI MS calculated for C69H20N 862.8 [M]+, found 862.2 for BF3, calculated. for C78H40N2 502.4 [M]2+, found 502.2 for BF4 and BF5 (regioisomers).
BF3 1H NMR (TMS ref.) δ = 0.95 (t, 3H) 1.20-1.59 (bm, 8H), 2.19 (bm, 2H), 4.14 (s, 3H), 4.22 (b, 2H), 5.68 (d, 2H), 5.82 (d, 2H)
13C NMR (TMS ref.) δ = 14.63, 22.59, 23.72, 26.24, 31.39, 48.50, 66.17, 69.21, 72.30, 136.2, 140.1, 140.2, 141.8, 142.4, 142.9, 143.0, 144.6, 144.7, 145.5, 145.7, 145.9, 146.3, 146.6, 147.6, 152.5, 153.3
FT-IR ν (cm-1): 2920, 1245, 741, 572
Log Kow = 0.542
BF4 and BF5 1H NMR (TMS ref.) δ = 0.95 (bm), 1.15-1.61 (bm), 2.15 (bm), 3.8-4.2 (m) 5.45-5.95 (m)
FT-IR ν (cm-1): 2925, 1454, 1213, 1010, 738
BF4: Log Kow = 0.184
BF5: Log Kow = -0.440
LogP values
The measured logKow values were determined by dissolving a few milligrams of each compounds in 100 μL dimethlysulfoxide followed by dilution in 10 mL deionized water. The solution was shaken vigorously for > 1 h with 10 mL of n-octanol (equilibrium was established by extended shaking times). After allowing the phases to separate, each was analyzed by absorption spectroscopy at 405 nm and a ratio of the concentration in each phase determined. The calculated logP values were found using ACD/LogP DB software version 12.0 (ACD/Labs, Toronto, Ontario, Canada).
Microbial strains and culture conditions
The following microbial strains were used: Staphylococcus aureus 8325-4, Escherichia coli K12 and Pseudomonas aeruginosa (ATCC 19660), as well as the DAY286 reference strain (24) of Candida albicans (a gift from Aaron Mitchell, Department of Microbiology, Columbia University, New York, NY). Planktonic bacterial cells were cultured in brain-heart infusion (BHI) with aeration at 37°C in mid-log growth phase (unless otherwise stated). Cell numbers were estimated by measuring the OD at 600-nm (OD of 0.5 = 108 cells/mL). Yeasts were cultured in yeast peptone dextrose (YPD) with aeration at 30°C and cell number was assessed with a hematocytometer (25).
Photodynamic inactivation studies and CFU determination
Cells were grown overnight at 37°C (30°C for Candida) and refreshed for 2-4 hours before being collected through centrifugation and suspended in phosphate-buffered saline (PBS). A cell suspension consisting of 108 cells/mL for bacteria (107 cells/mL for Candida (26) was incubated with various concentrations of the BF compounds for 30 min at room temperature in the dark. 1 mL aliquots were transferred to a 24 well plate and illuminated at room temperature with a broad-band white light source (400-700-nm band pass filter, Lumacare, Newport Beach, CA) to deliver 10 J/cm2 at an irradiance of 100 mW/cm2 as measured with a power meter (Coherent). Cells treated with BF in the dark were incubated covered with aluminum foil for the same time as the PDT groups (30 min) At the completion of illumination (or dark incubation) aliquots (100 μl) were taken from each well to determine CFU. The aliquots were serially diluted 10-fold in PBS to give dilutions of 10−1 to 10−5 times in addition to the original concentration and 10 μl aliquots of each of the dilutions were streaked horizontally on square BHI or YPD (Candida) plates by the method of Jett and colleagues (27). Plates were streaked in triplicate and incubated for 12-36 h at 30°C or 37°C in the dark to allow colony formation.
A control group of cells treated with light alone (no BF added) showed the same number of CFU as absolute control (data not shown). Survival fractions were routinely expressed as ratios of CFU of microbial cells treated with light and BF (or BF in the absence of light) to CFU of microbes treated with neither.
Results
Synthesis and characterization of fullerenes
The newly reported fullerene derivatives whose structures are shown in Figure 2 were designed to be amphiphilic in nature. In previous work, we (23) and others (28) have shown that the presence of one or more cationic charges greatly increases the antimicrobial photoinactivation efficacy of fullerene based PS. The charge increases the association of PS with negatively charged pathogen membranes while the hydrophobic character increases association with and/or penetration into the lipid components of the membrane. The compounds were designed to increase either the lipophilic or the cationic character of previously synthesized compounds for comparison. Compounds BF1-2 were designed to increase cationic charge per addend, while compounds BF3-5 were designed to increase overall lipophilicity of the PS. The syntheses were based on the fulleropyrrolidine construct, first developed by Prato, et. al. (29). By column chromatography, the monosubstituted addend was collected as the first eluting brown band. However, by simple chromatography, the bis- and tris- mixture always co-eluted under all tested conditions. Thus, BF2, is a mixture of bis- and tris- substitution ratios. Based on multiple consistent mass spec analyses, it is estimated that the mixture is ∼3:1 bis:tris. Monoadduct BF3 eluted first on the column followed by two bands of regioisomeric bis- product in order of increasing polarity, BB4-5.
Figure 2.
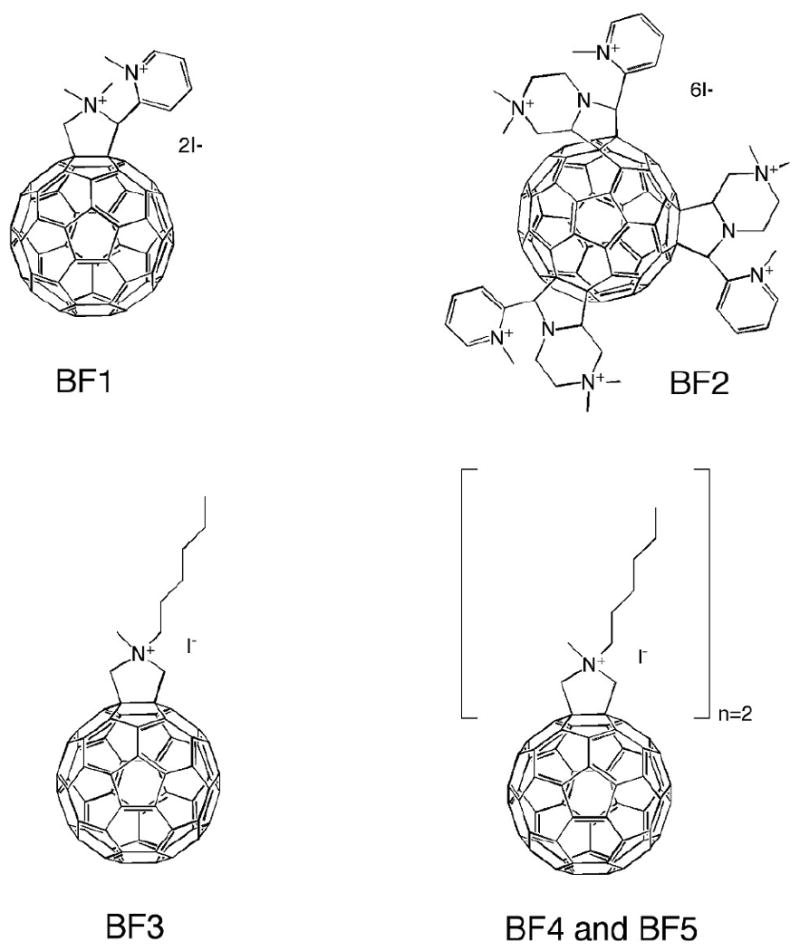
Structural formulas of the fullerenes studied in this report. Note that BF2 is a mixture of bis- (not shown) and tris- (shown) substituted fullerenes.
LogP values
The correlation between measured (mLogP) and calculated (cLog P) values is shown in Figure 3A. The R value is 0.9825 which was surprisingly high considering that cLogP is approximately eight orders of magnitude higher than mLogP. It is known that highly hydrophobic compounds (such as fullerenes) tend to aggregate in polar solvents, and this process of individual molecules aggregating in water makes actual logP measurements unreliable (30) and explains why there was such a large difference between mLogP and cLogP. Aggregates and clusters of the hydrophobic compounds are solid-state particles and the logP definition loses its validity because the system contains both individual molecules and aggregates. The linear correlation between mLogP (aggregates, individual molecules) and cLogP (assuming that there are only individual molecules) implies that the effect of aggregation on logP depends on the difference in polarity of the two solvents and not on the relative hydrophobicities of the molecules. There was also a good linear correlation of 0.92 between cLogP and the number of cationic charges (Figure 3B) as might be expected considering the rest of the fullerene molecules (and the hydrophobicity attributed to the side-chain functionality) remains relatively similar.
Figure 3.
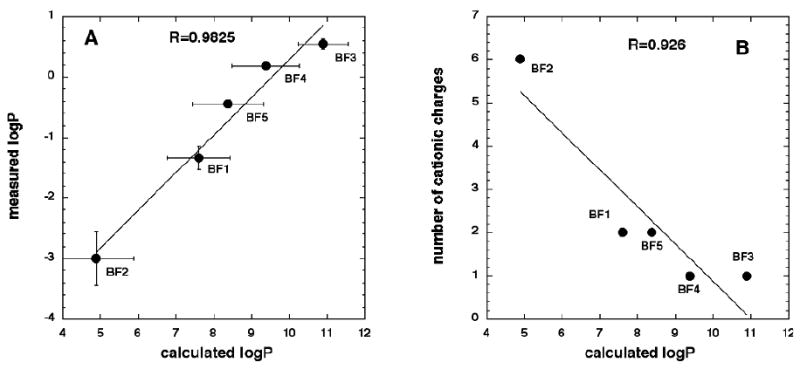
(A) Linear correlation between measured and calculated logP values. (B) Linear correlation between calculated logP values and number of cationic charges.
Antimicrobial photoinactivation
The best way to compare PDT efficiencies of different fullerene molecules is to vary the fullerene concentration while keeping the light fluence constant either as dark (0 J/cm2) or as 10 J/cm2. We studied the PDT effects on Gram-positive bacteria (S. aureus), two different Gram-negative bacteria (E. coli and P. aeruginosa) and a fungal yeast C. albicans.
Figure 4 shows the killing curves for BF1 against the four microbial species. S. aureus (Figure 4A) was most susceptible with 1 μM BF1 giving complete eradication and only modest (1.5 log) dark toxicity. E coli (Figure 4B) was next most susceptible with over 5 logs of killing at 10 μM BF1 and three logs of dark toxicity. P aeruginosa (Figure 4C) and C. albicans (Figure 4D) were least susceptible with 4 and 5 logs of killing at 100 μM respectively and considerable dark toxicity (2 and 4 logs respectively).
Figure 4.
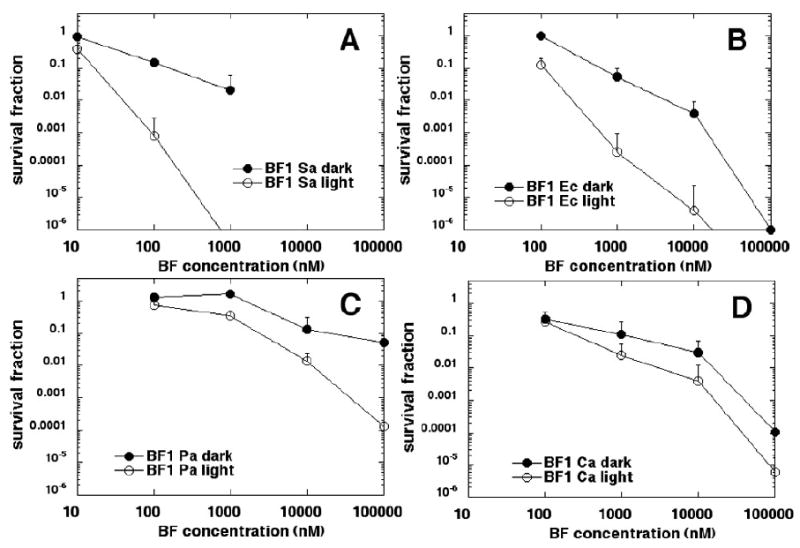
Killing curves for microbial cells with increasing concentrations of BF1 in dark and after illumination with 10 J/cm2 of 400-700-nm white light. (A) S. aureus; (B) E. coli; (C) P. aeruginosa; (D) C. albicans. Plots shown are representative of three repetitions.
Figure 5 shows that BF2 was overall the most powerful of the five fullerenes tested. More than 3 logs of S. aureus (Figure 5A) was killed at the surprisingly low concentration of 100 nM with no dark toxicity, and cells were eliminated at 1 μM. E. coli (Figure 5B) was eliminated at 10 μM with three logs of dark toxicity. In the case of BF2, P. aeruginosa (Figure 5C) was more susceptible to PDT than E. coli with over 4 logs of killing at 1 μM with no dark toxicity and elimination at 10 μM. C. albicans (Figure 5D) had over 5 logs of killing at 10 μM and was eliminated at 100 μM with considerable (over 5 logs) dark toxicity.
Figure 5.
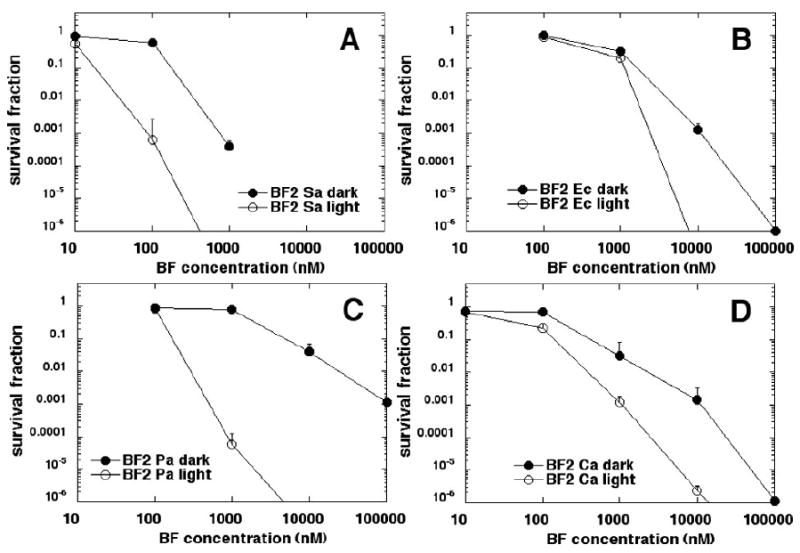
Killing curves for microbial cells with increasing concentrations of BF2 in dark and after illumination with 10 J/cm2 of 400-700-nm white light. (A) S. aureus; (B) E. coli; (C) P. aeruginosa; (D) C. albicans. Plots shown are representative of three repetitions.
BF3 (Figure 6) was the least effective PS of those fullerenes tested. Three logs of S. aureus was killed at 1 μM (Figure 6A) and 3 logs of E. coli was killed at 100 μM (Figure 6B) without dark toxicity, while no P. aeruginosa (Figure 6C) or C. albicans (Figure 6D) was killed at the concentrations tested.
Figure 6.
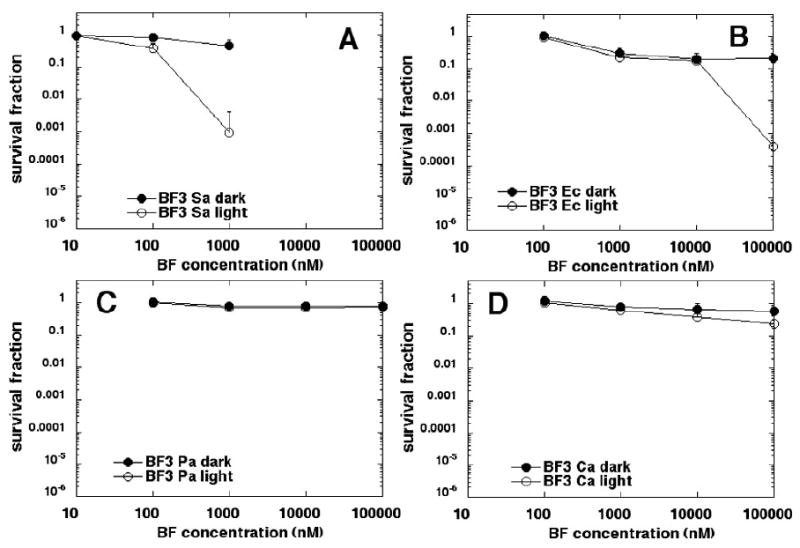
Killing curves for microbial cells with increasing concentrations of BF3 in dark and after illumination with 10 J/cm2 of 400-700-nm white light. (A) S. aureus; (B) E. coli; (C) P. aeruginosa; (D) C. albicans. Plots shown are representative of three repetitions.
BF4 (Figure 7) was somewhat more effective than BF3. More than 3 logs of S. aureus (Figure 7A) was killed at 1 μM and E. coli (Figure 7B) was eliminated at 100 μM. There was modest killing of P aeruginosa (Figure 7C) at 100 μM and in all three cases dark toxicity was moderate. No C. albicans was killed with either BF4 or BF5 at concentrations up to 100 μM (data not shown).
Figure 7.
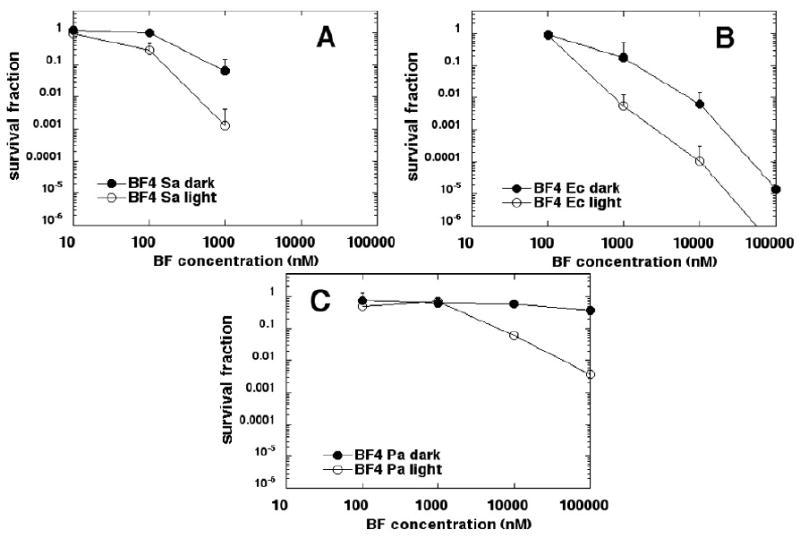
Killing curves for microbial cells with increasing concentrations of BF4 in dark and after illumination with 10 J/cm2 of 400-700-nm white light. (A) S. aureus; (B) E. coli; (C) P. aeruginosa. Plots shown are representative of three repetitions.
BF5 (Figure 8) was more effective than either BF3 or BF4. S. aureus (Figure 8A) was killed 3.5 logs at 1 μM; E. coli (Figure 8B) was killed 5 logs at 100 μM and P. aeruginosa (Figure 8C) was killed 3 logs at 100 μM, with dark toxicity only apparent in the case of E. coli.
Figure 8.
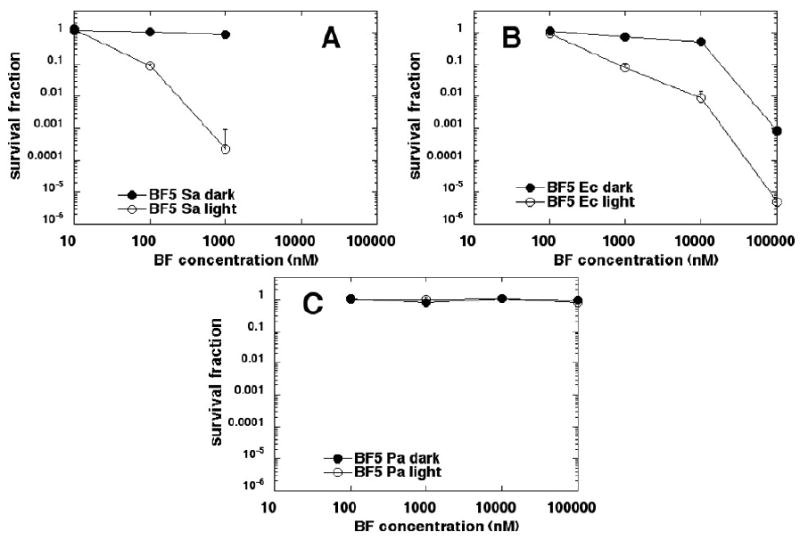
Killing curves for microbial cells with increasing concentrations of BF5 in dark and after illumination with 10 J/cm2 of 400-700-nm white light. (A) S. aureus; (B) E. coli; (C) P. aeruginosa. Plots shown are representative of three repetitions.
Quantitative structure function relationship (QSAR) correlations
We plotted the concentration of fullerenes necessary to kill 2 logs (LD99) of microbial cells (after illumination with 10 J/cm2 of white light), against the calculated values of logP (Figure 9). Although all three microbial species gave extremely good exponential correlations, the correlation for S. aureus (Figure 9A) was the least good with R = 0.862. The correlation for E. coli (Figure 9B) gave a R value of 0.976, and the correlation for P. aeruginosa (Figure 9C) was almost perfect at R = 0.999. Therefore for these fullerenes their effectiveness as antimicrobial PS against both Gram-positive and Gram-negative bacteria negatively correlates with their calculated logP values.
Figure 9.
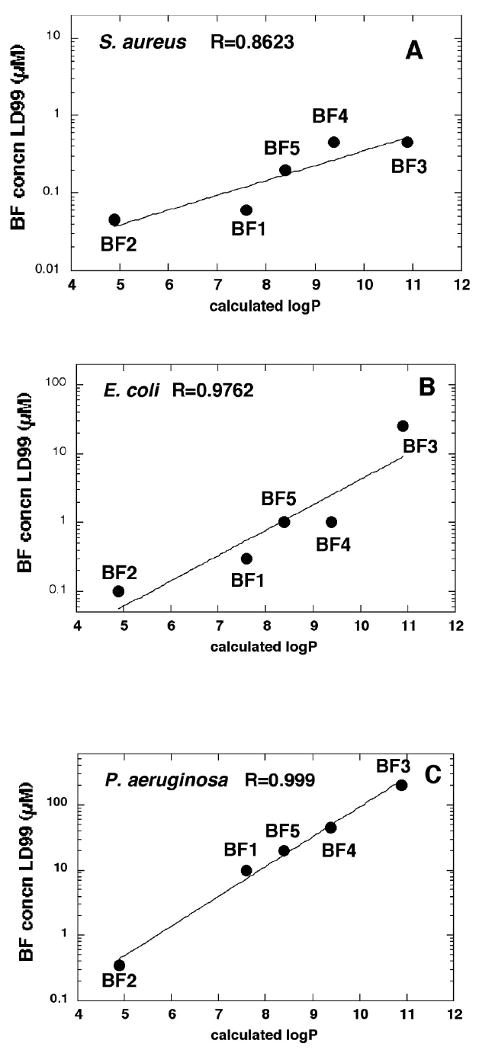
Linear correlation plots between the concentration of fullerene need to kill 99% of cells after illumination (LD99) and calculated logP values. (A) S. aureus; (B) E. coli; (C) P. aeruginosa.
Discussion
Functionalized fullerenes have several attractive features as antimicrobial PS (19). They have a high degree of photostability compared to tetrapyrrole-based PS, they tend to produce more Type I ROS such as hydroxyl radical and superoxide compared to alternative PS structures (20), and they show selectivity for microbial cells over mammalian cells (31). Fullerenes have been an important feature of the nanotechnology revolution (32). Although their molecular diameter is only of the order of 1 nm, they are generally accepted as nanoparticles, and their use as antimicrobial PS may be a real application of nanotechnology in medicine.
It is well known that cationic charges are a highly desirable feature in antimicrobial PS (33). It is usually considered that S. aureus as a typical Gram-positive bacterium is easily killed by PDT using PS of diverse molecular structures. The structure of the Gram-positive bacterial cell includes a relatively porous outer coat of peptidoglycan as well as polysaccharides and lipoteichoic acids that encircles a single cytoplasmic membrane composed of a lipid bilayer. Many dyes and PS of diverse structures and various overall molecular charges can bind and penetrate the Gram-positive cell wall. By contrast the Gram-negative bacterial structure is characterized by a double lipid bilayer encasing a thin layer of peptidoglycan, and it is accepted that this structure presents a much more effective permeability barrier to many classes of molecule than the Gram-positive bacterial cell wall structure.
The overall electrostatic charge borne by the outer surface of both classes of bacteria (Gram-positive and Gram-negative) is negative, and E. coli cells were reported to be the most negatively charged as measured by a zeta potential of − 16 mV, followed by S. aureus at − 10 mV and P. aeruginosa at − 7 mV (34). The other particular physical property of the different bacterial cells that is relevant to the binding of fullerene PS is the hydrophobicity. This is conveniently measured by the contact angle or wettability and S. aureus was reported to be the most hydrophobic at 72.2 deg, followed by P. aeruginosa at 43.3 deg and the most hydrophilic was E. coli at 33 deg (35).
The fact that S. aureus cells are most hydrophobic may explain why the negative correlation between fullerene logP value and PDT effectiveness for this species was the least good (Figure 9A). Because logP is negatively correlated with number of cationic charges in this series, and in general the number of cationic charges governs the binding of PS to bacteria, it would be expected that for hydrophilic bacterial cells like P. aeruginosa and E. coli the fullerene with the lowest logP value would be the best PS, but that hydrophobic S. aureus cells may bind fullerenes based on both cationic charge and on hydrophobicity, thus lessening the correlation. The QSAR studies showed a better correlation for LogP against P. aeruginosa than was found against E. coli (compare Figures 9B and 9C). The explanation for this is that cationic molecules are more suited to disrupting the outer-membrane lipolysaccharide in P. aeruginosa than in E. coli probably due to the greater role of divalent cations in the former case. It is thought that cationic molecules displace Ca++ and Mg++ and thereby weakening the outer structure (36).
Our previous study (23) on a series of three cationic fullerenes with 1, 2, and 3 cationic charges associated with dimethylpyrrolidinium groups demonstrated that increasing number of cationic charges correlated with increasing efficiency in light-mediated killing of Gram-positive bacteria (S. aureus) Gram-negative bacteria (E. coli and P. aeruginosa), and fungal yeast (C. albicans). The present study employed compounds with a wider range of different hydrophobicities, as well as an increased number of cationic charges (BF2). The finding that the effectiveness for antimicrobial PDT negatively correlates with hydrophobicity suggests that water solubility is important, and that drug-delivery vehicles that are necessary for hydrophobic more water insoluble compounds will not be needed if cationic fullerenes are used as clinical antimicrobial PS.
The extra permeability barrier characteristic of Gram-negative bacteria compared to Gram-positives is considered to be primarily against hydrophobic molecules (37). Small hydrophilic molecules tend to diffuse into bacteria via porin channels in Gram-negative species (38), but Yoshimura reported that even for hydrophilic molecules P. aeruginosa was 100 times less permeable than E. coli (39). P. aeruginosa is frequently considered to have high intrinsic antibiotic resistance due to its low outer membrane permeability (40). This intrinsic resistance is reflected in our finding that P. aeruginosa was the least susceptible of the three bacterial species tested to all the fullerenes except the polycationic BF2, where it was moderately susceptible.
The clear dependence of effectiveness of fullerene PS as anti-bacterial PS on the number of cationic charges may have two possible explanations. Firstly the amount of fullerene that binds to each negatively charged cell could be expected to depend on the number of positive charges on the molecule, and secondly the degree to which the outer membrane permeability barrier is breached by the self-promoted uptake pathway mediated by cationic molecules (41, 42) may be expected to depend on the number of cationic charges.
In contrast to bacteria, much less is known about structure function relationships amongst PS that are effective against fungal yeasts such as Candida. PS such as Photofrin that is an approved PS against cancer has been used to kill Candida (43), while in other studies cationic phenothiazinium salts such as methylene blue (44) and toluidine blue (45) have been employed. Candida cells also have an overall negative charge and may have varying hydrophobicity depending to some extent on growth conditions (46, 47). In the present study, only BF2 (and to a lesser extent BF1) was effective in killing Candida and the ratio between PDT toxicity and dark toxicity was somewhat less that that found for bacterial cells.
In conclusion, cationic fullerenes were found to be highly active broad spectrum antimicrobial PS and represent a potential contribution of nanotechnology to medicine. QSAR relationships were established that indicated that increasing the number of cationic charges and lowering the hydrophobicity tended to increase the PDT efficiency against both Gram-positive and Gram-negative bacteria. Although not specifically studied in this report, previous findings from our (23, 48) and other laboratories (49, 50) have indicated that at the short incubation times (10-30 min) typically employed in antimicrobial PDT experiments, there is a high level of selectivity for of antimicrobial PS for microbial cells over mammalian cells because compounds like cationic fullerenes are only slowly taken up by the latter cell types by the time-dependent process of endocytosis. We have undertaken a study of fullerene mediated PDT against a small animal model of bacterial wound infection that will be reported separately.
Acknowledgments
Funding sources
This work was supported by NIH (grants R44AI068400 and R44CA103177 to TW and R01AI050875 to MRH).
List of Abbreviations
- BF
buckminsterfullerene
- BHI
brain heart infusion
- CFU
colony forming units
- DMSO
dimethylsulfoxide
- ESI-MS
electro-spray ionization mass spectrometry
- FTIR
Fourier transform infra red (spectroscopy)
- MALDI TOF-MS
matrix assisted laser desorption ionization; time of flight; mass spectrometry
- NAD(P)H
nicotinamide adenine dinucleotide reduced form
- PBS
phosphate buffered saline
- PDI
photodynamic inactivation
- PDT
photodynamic therapy
- PS
photosensitizer
- ROS
reactive oxygen species
- YPD
yeast potato dextrose
Footnotes
Conflict of Interest Statement.
Ashlee Jahnke and Tim Wharton are employees of Lynntech Inc, a company with interests in fullerene synthesis. Tim Wharton and Michael R Hamblin are inventors on a patent for antimicrobial PDT with cationic fullerenes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoneyama H, Katsumata R. Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci Biotechnol Biochem. 2006;70:1060–1075. doi: 10.1271/bbb.70.1060. [DOI] [PubMed] [Google Scholar]
- 2.Dowell SF. Antimicrobial resistance: is it really that bad? Semin Pediatr Infect Dis. 2004;15:99–104. doi: 10.1053/j.spid.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J Am Geriatr Soc. 2002;50:S226–229. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- 4.Hamblin MR, Mroz P. Advances in photodynamic therapy: basic, translational and clinical. Norwood, MA: Artech House; 2008. [Google Scholar]
- 5.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–3600. [PubMed] [Google Scholar]
- 6.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 7.Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2007:CD002030. doi: 10.1002/14651858.CD002030.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jori G, Brown SB. Photosensitized inactivation of microorganisms. Photochem Photobiol Sci. 2004;3:403–405. doi: 10.1039/b311904c. [DOI] [PubMed] [Google Scholar]
- 10.Maisch T. Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci. 2007;22:83–91. doi: 10.1007/s10103-006-0409-7. [DOI] [PubMed] [Google Scholar]
- 11.Lilly HA, Lowbury EJ, Wilkins MD. Limits to progressive reduction of resident skin bacteria by disinfection. J Clin Pathol. 1979;32:382–385. doi: 10.1136/jcp.32.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soncin M, Fabris C, Busetti A, Dei D, Nistri D, Roncucci G, et al. Approaches to selectivity in the Zn(II)-phthalocyanine-photosensitized inactivation of wild-type and antibiotic-resistant Staphylococcus aureus. Photochem Photobiol Sci. 2002;1:815–819. doi: 10.1039/b206554a. [DOI] [PubMed] [Google Scholar]
- 13.Tang HM, Hamblin MR, Yow CM. A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens. J Infect Chemother. 2007;13:87–91. doi: 10.1007/s10156-006-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauro FM, Pretto P, Covolo L, Jori G, Bertoloni G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem Photobiol Sci. 2002;1:468–470. doi: 10.1039/b200977c. [DOI] [PubMed] [Google Scholar]
- 15.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Photobiol B. 1992;14:262–266. doi: 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- 16.Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 17.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 18.Alves E, Costa L, Carvalho CM, Tome JP, Faustino MA, Neves MG, et al. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009;9:70. doi: 10.1186/1471-2180-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photodynamic therapy with fullerenes. Photochem Photobiol Sci. 2007;6:1139–1149. doi: 10.1039/b711141j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, et al. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic Biol Med. 2007;43:711–719. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, et al. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2. J Am Chem Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- 22.Zhao B, He YY, Bilski PJ, Chignell CF. Pristine (C60) and hydroxylated [C60(OH)24] fullerene phototoxicity towards HaCaT keratinocytes: type I vs type II mechanisms. Chem Res Toxicol. 2008;21:1056–1063. doi: 10.1021/tx800056w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15:1150–1155. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 25.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 26.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 28.Spesia MB, Milanesio ME, Durantini EN. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C(60) derivatives. Eur J Med Chem. 2007 doi: 10.1016/j.ejmech.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Maggini M, Scorrano G, Prato M. Addition Of Azomethine Ylides To C-60 -Synthesis, Characterization, And Functionalization Of Fullerene Pyrrolidines. J Am Chem Soc. 1993;115:9798–9799. [Google Scholar]
- 30.Jafvert CT, Kulkarni PP. Buckminsterfullerene's (C60) octanol-water partition coefficient (Kow) and aqueous solubility. Environ Sci Technol. 2008;42:5945–5950. doi: 10.1021/es702809a. [DOI] [PubMed] [Google Scholar]
- 31.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, et al. Medicinal applications of fullerenes. Int J Nanomedicine. 2007;2:639–649. [PMC free article] [PubMed] [Google Scholar]
- 33.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottenbos B, van der Mei HC, Klatter F, Grijpma DW, Feijen J, Nieuwenhuis P, et al. Positively charged biomaterials exert antimicrobial effects on gram-negative bacilli in rats. Biomaterials. 2003;24:2707–2710. doi: 10.1016/s0142-9612(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 35.Mitik-Dineva N, Wang J, Truong VK, Stoddart P, Malherbe F, Crawford RJ, et al. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol. 2009;58:268–273. doi: 10.1007/s00284-008-9320-8. [DOI] [PubMed] [Google Scholar]
- 36.Fass RJ, Barnishan J. Effect of divalent cation concentrations on the antibiotic susceptibilities of nonfermenters other than Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1979;16:434–438. doi: 10.1128/aac.16.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hancock RE. The Pseudomonas aeruginosa outer membrane permeability barrier and how to overcome it. Antibiot Chemother. 1985;36:95–102. doi: 10.1159/000410475. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura F, Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982;152:636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hancock RE. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis. 1998;27 1:S93–99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 41.George S, Hamblin MR, Kishen A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem Photobiol Sci. 2009;8:788–795. doi: 10.1039/b809624d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merchat M, Spikes JD, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B. 1996;35:149–157. doi: 10.1016/s1011-1344(96)07321-6. [DOI] [PubMed] [Google Scholar]
- 43.Bliss JM, Bigelow CE, Foster TH, Haidaris CG. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother. 2004;48:2000–2006. doi: 10.1128/AAC.48.6.2000-2006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munin E, Giroldo LM, Alves LP, Costa MS. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT) J Photochem Photobiol B. 2007;88:16–20. doi: 10.1016/j.jphotobiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanco MT, Blanco J, Sanchez-Benito R, Perez-Giraldo C, Moran FJ, Hurtado C, et al. Incubation temperatures affect adherence to plastic of Candida albicans by changing the cellular surface hydrophobicity. Microbios. 1997;89:23–28. [PubMed] [Google Scholar]
- 47.Hazen KC, Brawner DL, Riesselman MH, Jutila MA, Cutler JE. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soukos NS, Wilson M, Burns T, Speight PM. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers Surg Med. 1996;18:253–259. doi: 10.1002/(SICI)1096-9101(1996)18:3<253::AID-LSM6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 50.Zeina B, Greenman J, Corry D, Purcell WM. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br J Dermatol. 2002;146:568–573. doi: 10.1046/j.1365-2133.2002.04623.x. [DOI] [PubMed] [Google Scholar]


