Abstract
Chronic inflammation is associated with aging and plays a causative role in several age-related diseases such as cancer, atherosclerosis and osteoarthritis. The source of this chronic inflammation is often attributed to the progressive activation of immune cells over time. However, recent studies have shown that the process of cellular senescence, a tumor suppressive stress response that is also associated with aging, entails a striking increase in the secretion of pro-inflammatory proteins and might be an important additional contributor to chronic inflammation. Here, we list the secreted factors that make up the pro-inflammatory phenotype of senescent cells and describe the impact of these factors on tissue homeostasis. We also summarize the cellular pathways/processes that are known to regulate this phenotype – namely, the DNA damage response, microRNAs, key transcription factors and kinases and chromatin remodeling.
Keywords: aging, cancer, inflammation, cytokines, interleukins, tumor suppression
Acute Inflammation
External signs of inflammation – pain, redness, heat and swelling – were known long before biologists began to investigate their molecular and cellular mechanisms. We now know that the external signs of inflammation are caused by the dilation of blood vessels and action of phagocytes at the site of injury. Phagocytes, in turn, produce pro-inflammatory factors such as cytokines and chemokines, which attract leukocytes to deal with the presence of foreign organisms or particles. Normally, the inflammatory response ceases within hours or days, once the foreign objects have been removed, and damaged tissue then begins to heal. This type of inflammation is known as acute inflammation.
Chronic inflammation in aging and age-related diseases
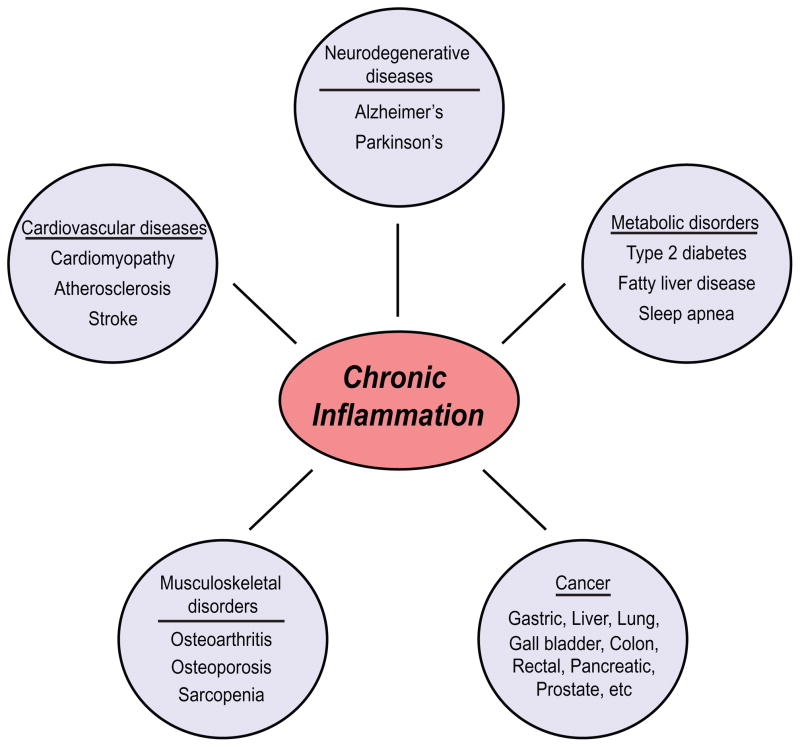
Chronic inflammation, by contrast, is the continued presence (sometimes over many years) of pro-inflammatory factors at levels higher than baseline, but many fold lower than those found in acute inflammation. Chronically inflamed tissues are characterized by the presence of infiltrating lymphocytes and macrophages, abundant blood vessels, fibrosis, and often, tissue necrosis [1, 2]. Chronic inflammation, as measured by the serum levels of pro-inflammatory mediators near sites of pathology, is associated with many age-related pathophysiologic processes and diseases, including Alzheimer’s disease, diabetes, atherosclerosis, osteoarthritis and cancer, among others [3, 4] (Figure 1). Chronic inflammation is also associated with normal aging. For example, on average, there is a 2–4 fold increase in serum levels of pro-inflammatory mediators (e.g. interleukin (IL)-6 and tumor necrosis factor (TNF)α) in aged individuals (>50 years of age), compared to younger individuals [5, 6]. Moreover, individuals who experience unusually healthy aging – for example, healthy centenarians – typically have a lower inflammatory profile than frail centenarians [7] (or individuals that display obvious signs of aging and age-related disease). The inflammatory status of a tissue or plasma profile is determined by a balance between pro- and anti-inflammatory factors. For example, although both frail and healthy centenarians often have plasma levels of pro-inflammatory mediators that are higher than young individuals, healthy centenarians often also have increased levels of anti-inflammatory mediators (for example, cortisol and IL-10) and, overall, reduced chronic inflammation [7].
Figure 1. Chronic inflammation is associated with most age-related diseases.
There is an extensive range of conditions and diseases that are associated with chronic inflammation or that have an inflammatory component. Chronic inflammation lies at the root of heart disease, cancer, osteoporosis, Alzheimer’s disease, diabetes and many other age-related diseases.
Although the correlation between inflammation and aging is well established, it is difficult to demonstrate a causal connection. This difficulty stems from both the systemic, diffuse nature of chronic inflammation, and the lengthy times that are required for definitive studies. Nonetheless, it is now clear that chronic inflammation plays an important role in the initiation and/or progression of several age-related diseases, including atherosclerosis, Alzheimer’s disease, osteoarthritis, and cancer [8–10]. Important outstanding questions remain, though. What is the relationship between chronic inflammation and normal (disease-free) aging? Does aging drive chronic inflammation, or does something else cause chronic inflammation, which in turn drives aging? Are aging and chronic inflammation too intricately intertwined to be neatly separated? There are, as yet, no definitive answers to these questions, but here we consider working hypotheses regarding the relationship between aging and chronic inflammation. First, we consider how chronic inflammation might contribute to the general aging process. Next, we consider how chronic inflammation might arise during aging. We discuss a chronically active immune system, which has been termed “inflammaging”, as a source of age-related inflammation. Then, we discuss the pro-inflammatory phenotype of senescent cells as a possible additional source. We describe the known effects of the senescence-associated secretory phenotype (SASP), or senescence-messaging secretome (SMS) [11], and the known pathways that regulate the SASP.
Chronic inflammation might propel basic aging processes
Chronic inflammation might contribute to general aging in several ways. First, the continual presence of circulating pro-inflammatory factors may keep the immune system in a state of chronic low-level activation. Eventually, this chronic immune activation will cause immunosenesence, commonly defined as the functional decline of the adaptive immune system with age. Immunosenescence is caused primarily by an exhaustion of the pool of naïve T cells, clonal expansion among T and B cells, and the consequent shrinkage of “immunological space”; together, these phenomena reduce the body’s ability to respond to new antigens [12, 13]. In addition to causing immunosenescence, some pro-inflammatory factors may degrade tissue microenvironments [14], for example, the matrix metalloproteinase (MMP)-3 (stromelysin) produced by senescent cells disrupts normal branching morphogenesis by mammary epithelial cells [15]. Other cytokines produced by senescent cells, such as IL-6 and IL-8, are potent attractors and activators of innate immune cells, which can destroy tissue environments by virtue of the oxidizing molecules they release (designed to kill pathogens) [16].
In addition, chronic inflammation can disrupt stem cell function. This disruption can be direct, as inflammatory mediators can drive stem cell differentiation [17–21]. It can also be indirect because proteases and the destructive activities of immune cells can destroy stem cell niches, for example, by thickening the basal lamina around muscle satellite cells by extracellular matrix deposition, impeding satellite cell function [17]. These effects may well be tissue and cell context-specific. For example, breast cancer stem cells are maintained by a positive feedback loop of which IL-6 is a critical component [22].
Age-related chronic inflammation is often attributed to the immune system [3, 10, 12]. As we age, we accumulate an “antigenic burden,” the sum of all the antigenic stresses (both internal and external) that we unavoidably encounter throughout life, which causes the progressive activation of macrophages and other immune-cell types. This low-level chronic activation leads to the continuous production of inflammatory factors such as cytokines and chemokines, which raises basal levels of these factors throughout the body. This process, termed “inflammaging”, has been thoroughly described elsewhere and is supported by a substantial body of data [3, 12].
Senescent cells as a source of chronic inflammation
Although the immune system plays a major role in modulating the levels of pro- and anti-inflammatory factors, it is not the only source of these factors. Recent evidence in fibroblasts and epithelial cells has shown that cellular senescence is accompanied by a striking increase in the secretion of 40–80 factors that participate in intercellular signaling [23–25]. Secretion of this set of factors has been termed the “senescence-associated secretory phenotype”, or SASP [23] (Table 1). SASP proteins are generally induced at the level of mRNA [23] and include a wide range of growth factors, proteases, chemokines and cytokines. Proteins that are known to stimulate inflammation, including IL-6, IL-8, IL-1, granulocyte macrophage colony-stimulating factor (GM-CSF), growth regulated oncogene (GRO)α, monocyte chemotactic protein (MCP)-2, MCP-3, MMP-1, MMP-3, and many of the Insulin-like growth factor (IGF)-binding proteins [23, 26, 27], are among the most robustly induced and secreted of these factors (Table 1).
Table 1. SASP factors.
The SASP is a complex, plastic phenotype that varies with cell type and mode of senescence induction. The SASP factors are categorized according to the fold change in secreted protein level over presenescent controls. The categories are approximate by necessity, as each cell type has different fold changes of each factor. The “senescence inducers” increase the expression of a given factor. “+” indicates well-documented pro-inflammatory proteins; “−” indicates well-documented anti-inflammatory proteins. Factors lacking + or − might also have pro- or anti-inflammatory activity, but these activities are either not well documented or highly context-dependent. Abbreviations: OIS, oncogene-induced senescence; DDIS, DNA-damage-induced senescence; REP, replicative senescence; RAS, oncogenic RAS overexpression; MEK, oncogenic MEK overexpression; XRA, high dose X-irradiation; BLEO, bleomycin treatment; ETOP, etoposide treatment. Cell types: HCA2, BJ – human foreskin fibroblasts; Wi-38, IMR-90 – human embryonic lung fibroblasts; PrECs – normal human prostate epithelial cells; BPH1, RWPE1, PC3 –transformed human prostate epithelial cells; PSC27, PSC31, PSC32 – human prostate fibroblasts. The last names in the reference column refer to the following papers: Acosta: [51]; Bavik: [75]; Coppe: [23] and [76]; Rodier: [56]; Wajapeyee: [52]; Liu: [38]; Krizhanovksy: [44]; Parrinello: [15]; West: [77].
| High increase (4+ fold) | ||||
|---|---|---|---|---|
| Factor | Senescence inducer | Cell type | Reference | |
| GM-CSF | OIS (RAS, MEK), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Acosta, Coppe |
| GROα | OIS (RAS, MEK), DDIS (XRA, BLEO), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3, prostate broblasts (PSC27, PSC31, and PSC32) | Acosta, Coppe, Bavik |
| GROα,β,γ | OIS (RAS, MEK), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Acosta, Rodier, Coppe |
| IGFBP-7 | OIS (BRAF) | + | melanocytes | Wajapeyee |
| IL-1α | OIS (RAS, MEK), DDIS (XRA, BLEO) | + | IMR-90, HCA2, PrECs, BPH1, RWPE1, PC3 | Acosta, Coppe, Liu |
| IL-6 | OIS (RAS, MEK), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Acosta, Rodier, Coppe |
| IL-7 | OIS (RAS), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ | Coppe |
| IL-8 | OIS (RAS, MEK), DDIS (XRA, BLEO), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3, prostate broblasts (PSC27, PSC31, and PSC32) | Acosta, Rodier, Coppe, Bavik |
| MCP-1 | OIS (RAS, MEK), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Acosta, Rodier, Coppe, Liu |
| MCP-2 | OIS (RAS), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ | Coppe |
| MIP-1α | OIS (RAS), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe |
| MMP-1 | OIS (RAS), DDIS (XRA, BLEO), REP | IMR-90, HCA2, WI-38, BJ | Coppe, Liu | |
| MMP-10 | OIS (RAS), DDIS (XRA, BLEO, ETOP), REP | IMR-90, HC A2, WI-38, ti c myo BJ, hepa broblasts | Coppe, Krizhanovsky | |
| MMP-3 | OIS (RAS), DDIS (XRA, BLEO), REP | IMR-90, HCA2, WI-38, BJ | Coppe, Liu, Parrinello | |
| Intermediate increase (2–4 fold) | ||||
| Factor | Senescence inducer | Cell type | Reference | |
| Amphiregulin | OIS (RAS), DDIS (XRA, BLEO) | PrECs, BPH1, RWPE1, PC3, prostate broblasts (PSC27, PSC31, and PSC32) | Coppe, Bavik | |
| ENA-78 | OIS (RAS, MEK), DDIS (XRA) | + | IMR-90, PrECs, BPH1, PC3 | Acosta, Coppe |
| Eotaxin-3 | OIS (RAS), DDIS (XRA), REP | + | IMR-90, HCA2 | Coppe |
| GCP-2 | OIS (RAS), DDIS (XRA) | + | HCA2, PrECs, RWPE1, PC3 | Coppe |
| GITR | OIS (RAS), DDIS (XRA) | HCA2, PrECs, BPH1, RWPE1, PC3 | Coppe | |
| HGF | OIS (RAS), DDIS (XRA, BLEO), REP | IMR-90, HCA2, WI-38, BJ, prostate broblasts (PSC27, PSC31, and PSC32) | Coppe, Bavik, Liu | |
| ICAM-1 | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Rodier, Coppe | |
| IGFBP-2 | DDIS (XRA, BLEO), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3, prostate broblasts (PSC27, PSC31, and PSC32) | Rodier, Coppe, Bavik |
| IGFBP-4 | OIS (RAS), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ | Coppe |
| IGFBP-5 | DDIS (BLEO), REP | + | prostate broblasts (PSC27, PSC31, and PSC32) | Bavik |
| IGFBP-6 | OIS (RAS), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe |
| IL-13 | OIS (RAS), DDIS (XRA), REP | + | IMR-90, WI-38 | Coppe |
| IL-1β | OIS (RAS), DDIS (XRA, BLEO), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe, Liu |
| MCP-4 | OIS (RAS, MEK), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Acosta, Coppe |
| MIF | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe | |
| MIP-3α | OIS (RAS, MEK), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Acosta, Coppe |
| MMP-12 | DDIS (XRA, ETOP) | IMR-90, HCA2, WI-38, BJ, hepatic myo broblasts | Coppe, Krizhanovsky | |
| MMP-13 | DDIS (XRA, ETOP) | IMR-90, HCA2, WI-38, BJ, hepati c myo broblasts | Coppe, Krizhanovsky | |
| MMP-14 | DDIS (XRA) | IMR-90, HCA2, WI-38, BJ | Coppe | |
| NAP2 | OIS (MEK) | IMR-90 | Acosta | |
| Oncostati n M | OIS (MEK), DDIS (XRA) | IMR-90, WI-38 | Acosta, Coppe | |
| Osteoprotegerin | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Rodier, Coppe | |
| PIGF | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe | |
| RANTES | DDIS (BLEO) | HCA2 | Liu | |
| sgp130 | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Rodier, Coppe | |
| TIMP-2 | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Rodier, Coppe | |
| TRAIL-R3 | DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ | Rodier, Coppe | |
| Small increase (below 2 fold) | ||||
| Factor | Senescence inducer | Cell type | Reference | |
| Acrp30 | DDIS (XRA) | PrECs | Coppe | |
| Angiogenin | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe | |
| Axl | DDIS (XRA), REP | WI-38 | Coppe | |
| bFGF | OIS (RAS), DDIS (XRA), REP | IMR-90, HC A2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe | |
| BLC | OIS (RAS) | + | HCA2, BJ | Coppe |
| BTC | DDIS (XRA) | PrECs | Coppe | |
| CTACK | DDIS (XRA) | + | RWPE1, PC3 | Coppe |
| EGF-R | DDIS (XRA) | PrECs, BPH1, PC3 | Coppe | |
| Fas | DDIS (XRA), REP | WI-38 | Coppe | |
| FGF-7 | DDIS (XRA, BLEO), REP | HCA2, WI-38, BJ, prostate broblasts (PSC27, PSC31, and PSC32) | Coppe, Bavik | |
| G-CSF | OIS (RAS) | + | HCA2, BJ | Coppe |
| GDNF | DDIS (XRA) | PrECs | Coppe | |
| HCC-4 | OIS (RAS), DDIS (XRA), REP | + | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Coppe |
| I-309 | OIS(RAS), DDIS (XRA) | + | HCA2 | Coppe |
| IFN-γ | OIS (RAS) | + | HCA2, BJ | Coppe |
| IGFBP-1 | DDIS (XRA), REP | + | HCA2 | Coppe |
| IGFBP-3 | DDIS (XRA, BLEO), REP | + | HCA2, prostate broblasts (PSC27, PSC31, and PSC32) | Rodier, Bavik |
| IL-1 R1 | DDIS (XRA) | HCA2 | Rodier | |
| IL-11 | DDIS (XRA), REP | − | HCA2, BJ | Coppe |
| IL-15 | DDIS (XRA), REP | IMR-90, HCA2, BJ | Coppe | |
| IL-2R-α | DDIS (XRA) | PrECs, PC3 | Coppe | |
| IL-6 R | OIS (RAS), DDIS (XRA) | PrECs, BPH1, RWPE1, PC3 | Coppe | |
| I-TAC | OIS (RAS), DDIS (XRA) | PrECs, BPH1, RWPE1, PC3 | Coppe | |
| Lepti n | DDIS (XRA), REP | IMR-90, HCA2, BJ | Coppe | |
| LIF | OIS (MEK) | IMR-90 | Acosta | |
| MMP-2 | DDIS (BLEO), REP | prostate broblasts (PSC27, PSC31, and PSC32) | Bavik | |
| MSP-a | DDIS (XRA) | RWPE1, PC3 | Coppe | |
| PAI-1 | REP | IMR-90, BJ, JAS-3, HUVEC | West | |
| PAI-2 | REP | IMR-90, BJ, JAS-3 | West | |
| PDGF-BB | DDIS (XRA) | BPH1, RWPE1, PC3 | Coppe | |
| SCF | DDIS (XRA), REP | IMR-90, HCA2, BJ | Coppe | |
| SDF-1 | OIS (RAS), DDIS (XRA) | + | prostate broblasts (PSC27, PSC31, and PSC32) | Bavik |
| sTNF RI | OIS (RAS), DDIS (XRA), REP | − | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Rodier, Coppe |
| sTNF RII | DDIS (XRA), REP | − | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC | 3 Coppe |
| Thrombopoietin | DDIS (XRA) | BPH1, RWPE1, PC3 | Coppe | |
| TIMP-1 | OIS (RAS), DDIS (XRA, BLEO) | HCA2, PrECs, BPH1, PC3, prostate broblasts (PSC27, PSC31, and PSC32) | Coppe, Bavik | |
| tPA | DDIS (BLEO), REP | HCA2, IMR-90, BJ, JAS-3 | West, Liu | |
| uPA | DDIS (BLEO), REP | HCA2, IMR-90, BJ, JAS-3 | West, Liu | |
| uPAR | OIS (RAS), DDIS (XRA), REP | IMR-90, HCA2, WI-38, BJ, PrECs, BPH1, RWPE1, PC3 | Rodier, Coppe | |
| VEGF | OIS (RAS), DDIS (XRA) | PrECs, BPH1, RWPE1, PC3 | Coppe | |
Cellular senescence is a state of essentially irreversible proliferative arrest caused by stresses that are potentially oncogenic. Cellular senescence is now recognized as an important tumor suppressive mechanism that prevents cancer progression in vivo; the senescence growth arrest is, by definition, limited to mitotic tissues [28–30]. Whether post-mitotic cells such as mature muscle cells and neurons develop a secretory phenotype when damaged is unknown. Much like apoptosis, senescence can be induced by a wide range of stimuli, including severe DNA damage, the expression of certain oncogenes, oxidative stress and strong mitogenic signals [14, 31–34]. However, unlike apoptotic cells, senescent cells remain metabolically active and undergo widespread gene expression changes, of which the SASP is part [35]. Thus, senescent cells can alter their microenvironment for as long as they persist.
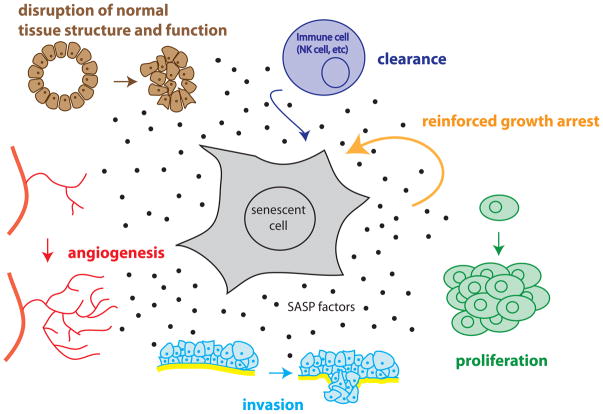
The SASP has many paracrine effects – some beneficial, but some deleterious if left unchecked, as expected for pro-inflammatory molecules. Senescent cells can disrupt normal tissue structure and function in mammary gland culture models [15], accelerate the invasion of transformed cells in a Boyden chamber assay via an epithelial to mesenchymal transition [23], stimulate both endothelial cell invasion in a Boyden chamber assay and angiogenesis in a xenograft model [36], and promote the proliferation of premalignant or malignant epithelial cells in culture and in vivo [37][38] (Figure 2). Further, senescent endothelial cells and fibroblasts are sometimes found adjacent to malignant tumors in humans [39, 40], and tumor cells themselves can senesce in vivo in human patients treated with DNA-damaging chemotherapy agents or in mice forced to express the potent tumor suppressor protein p53 [23, 41].
Figure 2. Effects of the SASP on tissue homeostasis.
The response of cells to the SASP depends on cell type and cell context. The SASP affects the original senescent cell by stimulating clearance by NK cells and reinforcing the senescence growth arrest. The SASP affects surrounding non-immune cells as well; it increases the proliferation of nearby epithelial and stromal cells, promotes invasion of any nearby preneoplastic or neoplastic cells via an epithelial to mesenchymal transition, stimulates angiogenesis by stimulating endothelial cell migration and invasion, and disrupts normal tissue structures and function.
In addition to these tumor-promoting effects, there is correlative in vivo evidence that senescent cells are present near, and are thus presumed to contribute to, age-related pathologies that are unrelated to cancer. First, cells that express senescence markers accumulate with age in a variety of vertebrates, including zebrafish, rodents, non-human primates and humans, especially in renewable tissues such as the stroma, hematopoietic system and epithelial organs [42–44]. Second, senescent cells such as chondrocytes and endothelial cells are found at sites of age-related pathologies. These pathologies include degenerative conditions such as atherosclerosis, osteoarthritis and eroded vertebral discs [45–48]. They also include hyperproliferative diseases associated with aging, such as benign prostatic hyperplasia [49, 50] and melanotic nevi [28]. Although the cell type has not been identified in all cases, there is strong evidence that senescent cells, and in some cases the accompanying SASP, increase with age and in many age-related pathologies.
Although the age-related results are only correlative, they suggest that, similar to chronic activation of the immune system, the senescence response, and in particular the SASP, may reduce fitness by promoting both the generalized inflammation associated with aging as well as the development of specific age-related diseases. At first glance, this conclusion seems paradoxical, considering that the SASP originates from a fitness-promoting tumor-suppressive response. However, the paradox is consistent with the evolutionary theory of antagonistic pleiotropy, which states that because of the high level of extrinsic mortality in most natural populations, there is little selective pressure for any trait that promotes fitness past the age when an organism will probably die from external causes [35]. Therefore, as long as a trait has a beneficial function in early life, it can have a neutral or even deleterious function in late life without being negatively selected.
Multiple roles of the SASP
The senescence response, with the potentially long-term deleterious consequences of the SASP, might persist because its tumor-suppressing function keeps young organisms cancer-free. However, this explanation implies that the SASP itself has a beneficial role in early life. If the SASP were strictly deleterious, or even neutral (given the energy cost of maintaining high secretory activity), selective pressure should remove the SASP from the senescence response, leaving only the growth arrest phenotype.
Indeed, recent evidence suggests the SASP has at least two beneficial roles. First, certain key SASP factors such as IL-6, IL-8, GROα and IGFBP-7 act in an autocrine feedback loop to reinforce the senescence growth arrest [51–54] (Figure 2). These factors cooperate with the p53 and pRb tumor suppressor pathways to reduce the risk of oncogenic transformation in a cell-autonomous manner. Second, the SASP might signal to the immune system to clear senescent cells (Figure 2). In a mouse model of liver carcinoma, reactivation of p53 in tumor cells induces a senescence response in vivo; this response is followed by increases in expression of several inflammatory cytokines, which stimulate an infiltrating immune response to clear senescent tumor cells [41]. In a mouse model of liver fibrosis, liver damage produces fibrosis and a senescence response in hepatic stellate cells; the cytokines and extracellular matrix-degrading enzymes produced by the senescent cells promote both the clearance of the senescent cells by natural killer (NK) cells and degradation of the fibrotic mass [55].
These findings suggest that the SASP is important, especially early after senescence induction, for ensuring efficient growth arrest and, eventually, for stimulating the immune system to clear senescent cells. Despite the ability of the innate immune system to remove them, senescent cells accumulate with age in vivo [14, 47]. Thus, either immune clearance is not 100% efficient or the rate at which senescent cells are produced outpaces the rate of clearance. Consequently, the deleterious chronic-inflammatory effects of the SASP might only become apparent with time.
The SASP may contribute to aging by disrupting tissue structure and function directly, or indirectly, by attracting the immune system. Given that senescence is also important for tumor suppression, it is important to determine how the senescence growth arrest and the SASP are regulated and whether the potentially deleterious effects of the SASP can be mitigated without interfering with the beneficial effects of growth arrest.
Molecular mechanisms that control the SASP
Transcriptional regulation
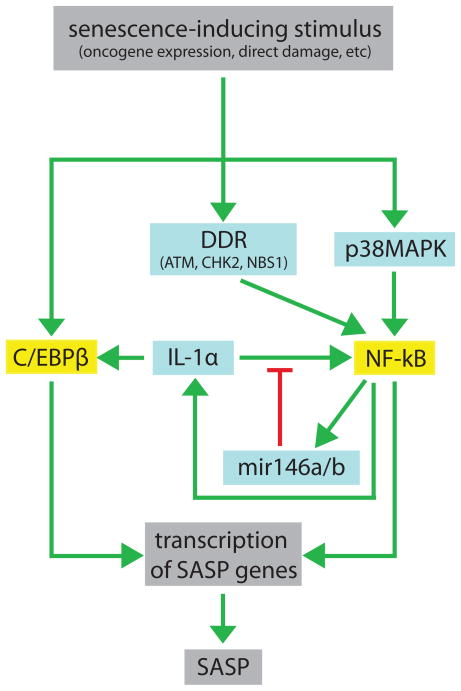
Although our understanding of how the SASP is controlled remains incomplete, several key features of SASP regulation have been elucidated (Figure 3). Most components of the SASP are upregulated at the level of mRNA abundance [23]. Moreover, the increase in mRNA levels of many factors depends on the transcription factors NF-κB and C/EBPβ, which have increased activity in senescent cells [51, 54]. Depletion of C/EBPβ substantially diminishes the expression of both IL-6 and IL-8, which are among the most strongly upregulated SASP cytokines [54], and inhibition of NF-κB significantly decreases the levels of ENA-78, NAP-2, MCP-1, MCP-4, MIP-3a, and the GRO family members [51].
Figure 3. Pathways that regulate the SASP.
A senescence-inducing stimulus causes genotoxic stress (e.g. DNA damage or chromatin unwinding), which activates the DDR and p38MAPK pathways. These pathways cooperate to activate NF-κB. C/EBPβ is activated through an unknown mechanism. NF-κB and C/EBPβ activate the transcription of SASP genes. IL-1α, which is regulated by NF-κB, further increases NF-κB activity via a positive feedback loop; this loop is dampened by mir146a/b, which are also induced by NF-κB. This complex regulatory network increases the expression of pro-inflammatory factors, which are then secreted. Green arrows indicate activation of the SASP; red lines indicate inhibition of the SASP; blue boxes are upstream signaling components; yellow boxes are transcription factors.
The activities of C/EBPβ and NF-κB are regulated by a plethora of pathways depending on the cellular context and the pathways that are particularly important for the senescence response are emerging. First, it appears that the SASP is caused primarily by genotoxic stress rather than by proliferative arrest per se: genotoxic senescence inducers, such as ionizing radiation, hyperproliferation caused by oncogene activity or dysfunctional telomeres, induce a SASP [23, 56]. However, the induction of senescence without genotoxic stress – for example, by overexpressing the pRb regulator p16INK4A – does not induce a SASP [25]. Further, p53, one of the central mediators of the senescence growth arrest, is not required for the SASP. In fact, p53 inactivation in senescent cells enhances the expression and secretion of many SASP factors, though the mechanism of that enhancement remains unknown [23].
In summary, NF-κB and C/EBPβ are induced by genotoxic stresses and are required for the transcription of many SASP factors. It is likely that there are other inflammation-associated transcription factors that contribute to the transcription of SASP-encoding genes, though they have yet to be identified. Because neither p16 nor p53 are required for the SASP, the pathways that regulate the SASP (eventually culminating in NF-κB and C/EBPβ activation) are distinct from the pathways that regulate the senescence growth arrest. We describe the known SASP regulatory pathways in the following sections.
DNA damage response
The DNA damage response (DDR) and several key DDR proteins are required for the expression of a subset of SASP factors, including IL-6 and IL-8 [56]. The DDR is a signal-amplification cascade that senses DNA damage, induces cell cycle arrest and initiates DNA damage repair. If the extent of DNA damage is severe, cells undergo either apoptosis or senescence, depending on the cell type and/or level of damage. In the case of senescence, cells arrest growth and maintain chronic low-level DDR signaling [57]. This persistent low-level DDR is necessary for a robust SASP; depletion of upstream components of the DDR cascade by RNA interference, specifically of ATM, NBS1, or CHK2, prevents the increased expression of SASP factors such as IL-6, IL-8, and GRO family members, among others [56]. It is probable that the DDR stimulates the SASP by activating NF-κB; NF-κB is a known target of ATM [58].
p38MAPK pathway
The DDR cannot be the sole regulator of the SASP because the DDR is activated immediately after damage, whereas the SASP, like other features of the senescence phenotype (for example, expression of senescence-associated β-galactosidase or formation of senescence-associated heterochromatin foci) develops slowly, generally over several days following the genotoxic stress [23, 56]. Additionally, a transient DDR, caused by low-level ionizing radiation that does not induce senescence, does not induce SASP factors [56]. Although the DDR is important, it is not sufficient to induce the SASP, suggesting that there must be other, slower events that cooperate with the DDR.
One such event may be p38MAPK activation. p38MAPK is a member of the mitogen-activated protein kinase (MAPK) family, which responds to a wide variety of extracellular and intracellular signals [59]. Inhibition of p38MAPK reduces the expression of IL-8 in MEK-induced senescence [51], and constitutive p38MAPK activation is sufficient to induce the senescence growth arrest by activation of both p53 and p16 [60–62]. It remains to be seen whether p38MAPK is required for SASP factors other than IL-8, and whether constitutive p38MAPK activation is sufficient to induce the SASP in addition to the senescence growth arrest.
IL-1α
Like many cytokine networks, the SASP also has an important positive feedback component. IL-1α is a cytokine that regulates its own synthesis through an autocrine, receptor-mediated positive feedback loop that entails activation of NF-κB; this has been observed in culture in human myeloid and pancreatic cancer cells [63, 64]. IL-1α is also a key positive regulator of IL-6 and IL-8 expression by senescent human cells in culture [65]. Loss of IL-1α signaling in senescent cells, whether by interfering with IL-1α expression or IL-1α receptor activity, markedly reduces the levels of IL-6 and IL-8, demonstrating that sustained IL-1R stimulation by surface-bound IL-1α is required to maintain senescence-associated IL-6 and IL-8 secretion [65]. Reduction of IL-1α signaling also decreases NF-κB and C/EBPβ transcriptional activities. IL-1α activates NF-κB activity via the Toll-like receptor pathway [65]. Thus, IL-1α triggers the formation of a complex between IL-1R and its coreceptor, activating a signaling cascade that ultimately permits nuclear translocation of NF-κB [66]. IL-1α may activate C/EBPβ activity indirectly via its regulation of IL-6 expression: depletion of IL-6 decreases C/EBPβ transcript levels [54]. These positive feedback loops sustain the SASP, reinforcing its expression and the senescence growth arrest (Figure 3).
microRNAs
MicroRNAs also play a role in SASP regulation. Thus far, two microRNAs, miR-146a and miR-146b (miR-146a/b), have been demonstrated to negatively regulate the senescence-associated secretion of IL-6 and IL-8 [67]. Senescent human fibroblasts with a strong SASP upregulate these microRNAs, which inhibit the production of inflammatory cytokines. These microRNAs target IRAK1, which is a positive regulator of NF-κB [68]. Indeed, overexpression of miR-146a/b in senescent human fibroblasts markedly reduces IRAK1 levels, along with reducing the secretion of IL-6 and IL-8. In addition, blockage of IL-1R signaling prevents the upregulation of miR-146a/b, consistent with these microRNAs being part of the NF-κB feedback loop (Figure 3; [68]). These data again highlight the important role NF-κB plays in both positive and negative regulation of the SASP.
Chromatin organization
Although the SASP is at least partly regulated by the activation of transcription factors, the global gene expression profile acquired at senescence probably entails large-scale changes in chromatin conformation, which is a feature of senescent cells [69–72]. Consistent with this idea, a number of genes that encode SASP proteins are physically clustered in the human and mouse genomes. Among these clusters are loci that contain MMP genes (MMP1, MMP3, MMP10 and MMP12) or CXCL and CCL cytokine family members. These loci are roughly the size of chromatin loops, which are an important unit of chromatin organization and transcriptional control [73]. Senescent cells also develop large heterochromatic structures termed senescence-associated heterochromatin foci (SAHFs) [69]. SAHFs physically contain, and most likely repress the expression of, several proliferation-promoting genes [74]. Virtually nothing is known about how senescence-causing stimuli bring about changes in chromatin organization, but it is likely that such changes are important for both the senescence-associated growth arrest and the SASP.
In summary, the data thus far suggest that the SASP is activated primarily at the transcriptional level by the transcription factors NF-κB and C/EBPβ. These transcription factors are activated by several pathways during senescence: the DNA damage response pathway, the p38MAPK pathway and an IL-1α positive feedback loop. However, these transcription factors are also subject to negative feedback during senescence: miR-146a/b act to inhibit NF-κB activity, decreasing the production of inflammatory cytokines. Lastly, senescent cells undergo global changes in chromatin organization, and it is likely that these changes have an effect on SASP gene regulation.
Concluding remarks
At the risk of over simplifying a complex network, the data summarized here are consistent with an “AND” logic gate regulating most of the SASP components. That is, there are a set of pathways that regulate most SASP factors (Figure 3), and it seems that all of these pathways must be active at senescence in order for the factors to be expressed. Molecularly, this may take the form of a large transcriptional complex involving NF-κB, C/EBPβ and a host of cofactors that are dependent on the individual pathways; without all the components, the complex cannot be fully active. Unlike a simple “AND” logic gate, disruption of the final complex can feed back onto the input signals, causing the collapse of the entire network. In this respect, the SASP is regulated in much the same way as the inflammatory response to other stresses (such as viral infection), and further clues about SASP regulation will almost certainly be found in analyses of the inflammatory response in other contexts.
It remains to be proven that senescent cells and the SASP are drivers of normal aging and age-related diseases. However, as researchers come closer to understanding the roles of senescence in vivo, it is possible to envision therapies aimed at mitigating the deleterious effects of the SASP. For example, the “AND” gate model suggests that the inhibition of any the SASP regulators may lead to successful therapeutic interventions. Of course, there may be potential complications. First, inhibiting SASP regulators may inadvertently compromise the beneficial effects of the SASP. For example, p38MAPK inhibition suppresses IL-8 production [51], but it also suppresses oncogene-induced senescence arrest by preventing p53 and p16 activation [60]. Likewise, disruption of the cytokine network during the early stages of senescence can prevent efficient growth arrest [51, 54, 62]. Additionally, it may be necessary to temporally separate the induction of localized senescence (e.g. by chemotherapy or radiation) from therapies designed to mitigate the SASP so that the clearance of senescent cells occurs unimpeded and only residual senescent cells are targeted by anti-SASP therapies. Lastly, because the SASP is regulated by many pathways that also regulate other important cellular processes, further research is needed to identify the pathways or combinations of pathways that are SASP-specific, to allow precise modulation of the SASP without compromising other systems. It may be possible to deliver SASP inhibitors locally – for example, directly into the joints of patients with osteoarthritis. Whether such complications will be difficult to overcome can only be answered by in vivo experiments, and the SASP pathways described in this review are a starting point to determine what effects the SASP has on an organism’s fitness, both in normal aging and after acute injuries such as radiation exposure.
Chronic inflammation, once established, often acquires momentum because of positive feedback loops in the immune system: cytokines activate leukocytes, which produce more cytokines, etc. Therefore, even a small pro-inflammatory stimulus, such a population of senescent cells scattered throughout organs and tissues, could seed a more systemic chronic inflammatory response. As we learn more about the role of senescent cells in vivo, we might find that the tradeoff between tumor suppression and longevity can be manipulated.
Acknowledgments
The authors are supported by the US National Institutes of Health research (AG09909, AG025901, AG017242) and center (AG025708, AG032117) grants, the Larry L. Hillblom Foundation (AVO), and a National Science Foundation Graduate Research Fellowship (AF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236(1):13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 3.Vasto S, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L, et al. A flame burning within. Aging Clin Exp Res. 2004;16(3):240–3. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- 5.Bruunsgaard H. The clinical impact of systemic low-level inflammation in elderly populations. With special reference to cardiovascular disease, dementia and mortality. Dan Med Bull. 2006;53(3):285–309. [PubMed] [Google Scholar]
- 6.Maggio M, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franceschi C, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Brennan FM, Maini RN, Feldmann M. Cytokine expression in chronic inflammatory disease. Br Med Bull. 1995;51(2):368–84. doi: 10.1093/oxfordjournals.bmb.a072967. [DOI] [PubMed] [Google Scholar]
- 9.Brod SA. Unregulated inflammation shortens human functional longevity. Inflamm Res. 2000;49(11):561–70. doi: 10.1007/s000110050632. [DOI] [PubMed] [Google Scholar]
- 10.Caruso C, et al. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 11.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 13.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21(4):418–24. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campisi J. Senescent cells, tumor suppression and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Parrinello S, et al. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118(Pt 3):485–96. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5(2):136–9. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7(4):590–8. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 18.Carlson ME, I, Conboy M. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6(3):371–82. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26(10):535–42. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16(10):1332–43. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 21.Seita J, et al. Interleukin-27 directly induces differentiation in hematopoietic stem cells. Blood. 2008;111(4):1903–12. doi: 10.1182/blood-2007-06-093328. [DOI] [PubMed] [Google Scholar]
- 22.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coppé, et al. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008;6(12):2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10(3):228–30. doi: 10.1038/embor.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppe JP, et al. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu Rev Pathol Mech Dis. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Millis AJ, Baglioni C. Expression of interleukin 1-inducible genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci U S A. 1992;89(10):4683–7. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, et al. Characterization of IGFBP-3, PAI-1 and SPARC mRNA expression in senescent fibroblasts. Mech Ageing Dev. 1996;92(2–3):121–32. doi: 10.1016/s0047-6374(96)01814-3. [DOI] [PubMed] [Google Scholar]
- 28.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human nevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 29.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 30.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10(1):51–7. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37(5):961–76. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res. 2006;66:2881–2884. doi: 10.1158/0008-5472.CAN-05-4006. [DOI] [PubMed] [Google Scholar]
- 34.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nature Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 35.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Rev Molec Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 36.Coppé JP, et al. Secretion of Vascular Endothelial Growth Factor by Primary Human Fibroblasts at Senescence. J Biological Chemistry. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 37.Krtolica A, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. PNAS. 2001;98:12071–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67(7):3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 39.Charalambous C, et al. Glioma-associated endothelial cells show evidence of replicative senescence. Exp Cell Res. 2007;313:1192–1202. doi: 10.1016/j.yexcr.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Studebaker AW, et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008;68:9087–9095. doi: 10.1158/0008-5472.CAN-08-0400. [DOI] [PubMed] [Google Scholar]
- 41.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeyapalan JC. Accumulation of senescent cells in mitotic tissue of aging primates. Mechanisms of Ageing and Development. 2006 doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price JS, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1(1):57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 46.Vasile E, et al. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. Faseb J. 2001;15(2):458–66. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 47.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40(8–9):634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Roberts S, et al. Senescence in human intervertebral discs. Eur Spine J. 2006;15:312–316. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro P, et al. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55:30–38. doi: 10.1002/pros.10204. [DOI] [PubMed] [Google Scholar]
- 50.Choi J, et al. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56:160–166. doi: 10.1016/s0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 51.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 52.Wajapeyee N, et al. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuilman T, et al. Oncogene-Induced Senescence Relayed by an Interleukin-Dependent Inflammatory Network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 55.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8(7):512–22. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 58.Elkon R, et al. Dissection of a DNA-damage-induced transcriptional network using a combination of microarrays, RNA interference and computational promoter analysis. Genome Biol. 2005;6(5):R43. doi: 10.1186/gb-2005-6-5-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773(8):1358–75. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 60.Kwong J, et al. p38alpha and p38gamma mediate oncogenic ras-induced senescence through differential mechanisms. J Biol Chem. 2009;284(17):11237–46. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8(2):131–44. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22(10):3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hiscott J, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13(10):6231–40. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niu J, et al. Identification of an autoregulatory feedback pathway involving interleukin-1alpha in induction of constitutive NF-kappaB activation in pancreatic cancer cells. J Biol Chem. 2004;279(16):16452–62. doi: 10.1074/jbc.M309789200. [DOI] [PubMed] [Google Scholar]
- 65.Orjalo AV, et al. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106(40):17031–6. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18(1):19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhaumik D, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1(4):402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taganov KD, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Funayama R, Ishikawa F. Cellular senescence and chromatin structure. Chromosoma. 2007;116(5):431–40. doi: 10.1007/s00412-007-0115-7. [DOI] [PubMed] [Google Scholar]
- 70.Mehta IS, et al. Alterations to nuclear architecture and genome behavior in senescent cells. Ann N Y Acad Sci. 2007;1100:250–63. doi: 10.1196/annals.1395.027. [DOI] [PubMed] [Google Scholar]
- 71.Adams PD. Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging. Gene. 2007;397(1–2):84–93. doi: 10.1016/j.gene.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narita M. Cellular senescence and chromatin organisation. Br J Cancer. 2007;96(5):686–91. doi: 10.1038/sj.bjc.6603636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horike S, et al. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat Genet. 2005;37(1):31–40. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- 74.Adams PD. Remodeling chromatin for senescence. Aging Cell. 2007;6(4):425–7. doi: 10.1111/j.1474-9726.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 75.Bavik C, et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 76.Coppe JP, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5(2):e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.West MD, et al. Altered expression of plasminogen activator and plasminogen activator inhibitor during cellular senescence. Exp Gerontol. 1996;31(1–2):175–93. doi: 10.1016/0531-5565(95)02013-6. [DOI] [PubMed] [Google Scholar]