Abstract
Purpose
Studies have shown that women who survive breast cancer have an increased risk of a future primary lung cancer, though many are based only on data recorded in tumor registries and none have conducted pathological confirmation. Previous studies and future use of large registries may be affected by misdiagnosis.
Methods
Pathological analysis was conducted on tumors from 110 women with breast cancer followed by lung cancer using morphology, Estrogen Receptor-alpha (ER), and Thyroid Transcription Factor-1 (TTF1). We developed an algorithm to classify lung tumors as unlikely lung cancer (score=1) to likely lung cancer (score=5).
Results
Mean time to diagnosis of lung cancer after breast cancer was 13 years. 76% of breast tumors and 20% of lung tumors were positive for ER and 51% of lung tumors were positive for TTF-1. 86% of the lung tumors were probable primaries, 7% were probable metastases from the breast, and 7% were of undetermined status. 70% of probable metastases had a latency of longer than 10 years.
Conclusion
Prior studies identifying the association of breast cancer and breast cancer treatments with lung cancer are likely to reflect true associations not confounded by misdiagnosis, as evidenced by the low rate of misclassification detected in this study. Analysis of the years of diagnosis suggests that latency may not be an accurate criterion for assignment of primary status, which could be significant in a clinical setting. These data may also benefit future retrospective studies using large registries.
Keywords: lung neoplasms, breast neoplasms, neoplasms, second primary
Through earlier detection and improved treatments, the number of women surviving breast cancer is increasing, as is survival time [1, 2]. Mortality rates among women with breast cancer have declined over the last decades and 5-year relative survival has risen from 75% to 90% [3]. This has led to an increasing population of women at risk for a second cancer [4–7]. As women live longer, there is an increased risk of developing a new primary cancer related to therapy, shared risk factors, genetic predisposition, or a combination of these [8, 9]. Many studies have shown that women who have had breast cancer are subsequently at increased risk for a new primary lung cancer, with risks estimates ranging from 1.2–4.5 [10–17]. This risk is increased in women who have been treated with radiotherapy and there is an interaction for increased risk from smoking and radiotherapy [18–21]. Using the Swedish Cancer Registry, established in 1958, our group has previously reported that the standardized incidence ratio (SIR) overall for developing lung cancer following breast cancer is 1.27 within 10 years, 1.66 for 10–14 years, 2.0 for 15–19 years, and 2.53 for greater than 20 years after breast cancer diagnosis. Further, concordance of breast and lung cancer on the same side (ipsilateral; inferring a radiotherapy-related secondary lung cancer) had a relative risk of 2.0, while the SIR for contralateral cancers was 0.8 [16].
Most studies of breast and lung cancer within the same woman are conducted using tumor registries and these preclude pathological confirmation of the cancers. None to date have examined pathological tissue for confirmation. Development of lung metastases has been reported to occur in 10–20% of female breast cancer patients, making confirmation of primary lung cancer status important in order to provide appropriate treatment for the specific tumor. Several studies have included a review of medical records but not actual tumors [12, 22]. Even with access to current complete medical records, there were still cases that could not be definitively categorized, emphasizing the challenge of verifying second primary lung tumors in registry studies. If the lung tumors were misclassified as primary lung cancer, rather than metastatic breast cancer, then an overestimation of secondary lung cancer risk could result.
As part of a study investigating biomarkers of susceptibility to lung cancer in women with breast cancer, we assessed the pathologic incidence for secondary lung cancers by conducting an in-depth, joint review with pathologists from Georgetown University (GU) and the Karolinska Institutet (KI) in Stockholm, Sweden. This review used patient records, morphological review, and immunohistochemistry (IHC). Use of morphology and patient information alone may not correctly assign tumors, but the use of IHC improves classification [23–28]. Comparisons of breast and lung cancer IHC staining also are helpful as primary tumors and their metastases share similar marker profiles [23, 29–32]. We conducted a review using tumor tissue and TTF-1 and ER immunohistochemistry stains to determine whether there is misclassification of lung tumors following breast cancer in a population of Swedish women.
METHODS
Study Design
Cases from Stockholm were identified from the Swedish Cancer Registry (SCR), where a unique identification number assigned to all residents of Sweden identifies individuals in national registries. The SCR began in 1958 and records the mandatory reports of cancer from pathologists and physicians, so it includes 96% of all cancers in Sweden [16]. Data in this registry includes diagnosis, date of diagnosis, mode of detection of tumor, department and hospital where the tumor was diagnosed, carcinoma in situ, date of death, and underlying cause of death. Cases were women with an initial diagnosis of breast cancer of any histological type (including DCIS) and a later primary lung cancer. Also, cases had to have sufficient archived tumor tissue for analysis. Lung cancer diagnosis had to be at least one year after the breast cancer diagnosis. Cases were excluded if they were diagnosed only by autopsy or x-ray or if they had been previously diagnosed with any cancer. Of the 180 cases identified through the Swedish Cancer Registry, 70 were excluded for lack of tissue, resulting in a total of 110 cases analyzed. There were three cases included in the study with tumor tissue but missing clinical data.
Tissue processing
Researchers in Stockholm collected tumor tissue blocks and tissue sections were placed on Silane-prep slides treated with DEPC in thicknesses of 5, 20, or 50 microns. Slides were baked at 60°C for two hours. The slides were then sent to GU by express shipping. Hematoxylin and eosin (H&E) slides were prepared either at KI before shipping or by the histopathology shared resource facility at GU.
Immunohistochemistry
IHC was done to detect ER protein expression in breast and lung tumors and TTF-1 in lung tumors. Five micron slides were deparaffinized with xylene and ethanol. Antigen retrieval was achieved by a Citra-Plus Buffer microwave protocol and slides were treated with peroxide and protein block (Biogenex; San Ramon, CA). The ER antibody (Clone F-10, Santa Cruz Biotechnology; Santa Cruz, CA) was used for 60 minutes at room temperature. The slides were then treated with a biotinylated second antibody link, enzyme-conjugated label, and Chromagen DAB (Biogenex). Slides were analyzed according to the Allred system, where intensity of staining is recorded as a score of 0–3 (0 is no staining, 1 is weak, 2 is moderate, and 3 is strong) and proportion of cells stained is recorded as a score of 0–5 (0 is no staining, 1 is 20%, 2 is 40%, 3 is 60%, 4 is 80%, and 5 is 100%) [33]. These scores are added together for a total of 0 or 2–8; scores of 2 or higher were considered positive for ER expression. Immunohistochemistry with the TTF-1 antibody (Clone 8G7G3/1, Invitrogen; Carlsbad, CA) was conducted using a similar protocol. Tissues were positive for TTF-1 if 10% or more of the cells were stained.
Pathology Review
Two pathologists collaborated to confirm histology, one with expertise in breast cancer (BS) and one in lung cancer (AH). First, the initial diagnosis of breast cancer was confirmed morphologically and by ER staining. An algorithm was established to classify lung tumors based on morphology, ER and TTF-1 lung tumor staining. A scoring system was used that ranged from 1–5 for the lung tumors, indicating a definite diagnosis of breast cancer metastasis (score of 1) to a definite diagnosis of a primary lung cancer (score of 5) (Table 1). Positive TTF-1 staining was considered indicative of primary lung tumor status. A case with an ER positive, TTF-1 negative lung tumor, an ER positive matched breast tumor, and a finding of no significant morphologic differences was considered a probable metastasis. Different ER staining for the breast and lung tumors from an individual was considered indicative of a probable lung primary. All tumors were initially reviewed independently. When the initial diagnoses differed, the pathologists reviewed these cases together, following repeat immunohistochemical staining using newly sectioned tumor blocks.
Table 1.
Description of the five score categories used to classify cases with breast and lung cancer in the joint pathology review.
| Criteria for Pathology Review Scores | |
|---|---|
| 1 | Definitely metastatic: significantly similar morphology, TTF-1 negative. |
| 2 | Probably metastatic: similar morphology, TTF-1 negative |
| 3 | Undecided: unclear morphology comparison, poorly preserved tissue. |
| 4 | Probably primary: different morphology or a weak TTF-1 stain with similar morphology. |
| 5 | Definitely primary: significantly different morphology and/or positiveTTF-1 stain. |
RESULTS
Clinical Characteristics
Cases (n=110) were women who had breast cancer and developed lung cancer at least one year after breast cancer diagnosis and whose original breast tumor was diagnosed between 1958 and 2000. Their clinical characteristics are shown in Table 2. Mean age at breast cancer diagnosis for all cases was 57 (+/− 13) and 68 (+/− 11) for lung cancer. Availability of information on menopausal status in medical records was inconsistent, so age greater or less than 50 was used as a surrogate for menopausal status; 35% of cases were premenopausal at the time of breast cancer diagnosis. Mean time to diagnosis of lung cancer after breast cancer for all cases was 13 years (range 1–35). About 48% of lung cancers were ipsilateral (occurring on the same side in the lung as the breast cancer), 38% were contralateral (occurring on the opposite side from the breast cancer), and 14% were of unrecorded location.
Table 2.
Clinical characteristics of cases with breast and lung cancer. Data is shown for all cases and for individual pathology review score groups. Complete clinical data was missing for three cases, which led to only 107 cases for some categories.
| Clinical Characteristics | All cases2 | Cases with scores 4,53 | Cases with score 1,2,34 | |||
|---|---|---|---|---|---|---|
| Mean age at breast cancer diagnosis | 57 | 55 | 60 | |||
| Age at breast cancer diagnosis1 | n=110 | % | n=94 | % | n=16 | % |
| 50 or younger | 38 | 35 | 33 | 35 | 5 | 31 |
| 51 or older | 69 | 62 | 59 | 63 | 10 | 63 |
| No data | 3 | 3 | 2 | 2 | 1 | 6 |
| Year of breast cancer diagnosis | n=110 | % | n=94 | % | n=16 | % |
| 1958–1969 | 37 | 34 | 30 | 32 | 7 | 44 |
| 1970–1979 | 35 | 32 | 28 | 30 | 7 | 44 |
| 1980–1989 | 28 | 25 | 27 | 29 | 1 | 6 |
| 1990–2000 | 7 | 6 | 7 | 7 | 0 | 0 |
| No Data | 3 | 3 | 2 | 2 | 1 | 6 |
| Mean age at lung cancer diagnosis | 68 | 67 | 71 | |||
| Mean latency | 13 | 13 | 11 | |||
| Latency | n=110 | % | n=94 | % | n=16 | % |
| >3 | 7 | 6 | 7 | 7 | 0 | 0 |
| 3–5 years | 14 | 13 | 12 | 13 | 2 | 13 |
| 6–10 years | 29 | 26 | 26 | 28 | 3 | 19 |
| 11–20 years | 35 | 32 | 26 | 28 | 9 | 56 |
| 21–30 years | 18 | 16 | 17 | 18 | 1 | 6 |
| >30 years | 4 | 4 | 4 | 4 | 0 | 0 |
| No Data | 3 | 3 | 2 | 2 | 1 | 6 |
| Lung cancer location | n=110 | % | n=94 | % | n=16 | % |
| Ipsilateral | 53 | 48 | 48 | 52 | 5 | 31 |
| Contralateral | 42 | 38 | 34 | 37 | 8 | 49 |
| Unknown | 15 | 14 | 12 | 11 | 3 | 20 |
Age 50 used as a surrogate for menopause due to lack of information in medical records.
All cases with tissue available identified in the Swedish Cancer Registry.
Only cases with scores of 4 and 5 in the pathology review, classified as primary.
Only cases with a score of 1,2, or 3 in the pathology review, classified as undetermined or metastatic.
Pathology verification of cancer diagnosis
Upon the initial morphology review, 61 cases were given concordant diagnoses by the two pathologists. For cases where the two pathologists initially disagreed on the diagnosis, an algorithm was applied for establishing a final diagnosis. The first step in the algorithm was to examine morphology and TTF-1 staining in the 49 lung tumors that had discordant diagnoses. There were 7 squamous cell cancers in the review, which were subsequently considered primary lung cancers based solely on the squamous histology (score = 5). For the non-squamous cell cancers, there were 25 adenocarcinomas and 17 tumors with mixed histology or other non-small cell lung cancer. For these, 50% of the adenocarcinomas were positive for TTF-1 and so were considered to be primary lung cancers, as were 44% of the other non-squamous tumors. For ER staining, 29% of the adenocarcinomas from the lung were positive, compared to 11% of the other non-squamous tumors from the lung. Examining the matched breast and lung tumors, 17% of pairs stained positively for ER in the breast and lung, indicating that the tumors from the lung were probably metastatic, while 50% of non-squamous cell lung cancers were discordant for ER staining, indicating a probable new lung primary.
21% of the cases with initial discordant pathological diagnoses were classified as possible metastases or undetermined. This accounted for 14% of the tumors in the entire study. 94 (86%) of the original study set of 110 cases were considered to have primary lung tumors, namely scores with 4 or 5 (Table 3). Among these confirmed cases, 29% of lung tumors were adenocarcinoma, 24% squamous cell carcinoma, 17% small cell carcinoma, 13% adenocarcinoma/squamous cell carcinoma, 10% bronchioloalveolar carcinoma, 3% mucinous adenocarcinoma, 3% large cell carcinoma, and 1% squamous cell carcinoma with sarcomatoid features. Of the probable or likely metastatic breast tumors (n=8), 87.5% were adenocarcinoma, and 12.5% mucinous adenocarcinoma. Of the undetermined tumors (n=8), 63% were adenocarcinoma and 37% were mixed adenocarcinoma and squamous cell carcinoma. All squamous cell carcinomas and small cell carcinomas were considered primary lung tumors, while 71% of adenocarcinomas were diagnosed as primary.
Table 3.
Distribution of lung tumor histologies within pathology review score groups.
| Primary (Scores 4,5) | ||
|---|---|---|
| n | % | |
| Adenocarcinoma | 27 | 29 |
| Squamous Cell Carcinoma | 23 | 24 |
| Adeno/Squamous Cell Carcinoma | 12 | 13 |
| Bronchioloalveolar Carcinoma | 9 | 10 |
| Squamous Cell Carcinoma/Sarcomatoid | 1 | 1 |
| Mucinous Adenocarcinoma | 3 | 3 |
| Large Cell | 3 | 3 |
| Small Cell | 16 | 17 |
| Total | 94 | |
| Metastatic or Undetermined (Score 1,2,3) | ||
| n | % | |
| Adenocarcinoma | 12 | 75 |
| Squamous Cell Carcinoma | 0 | 0 |
| Adeno/Squamous Cell Carcinoma | 3 | 19 |
| Bronchioloalveolar Carcinoma | 0 | 0 |
| Squamous Cell Carcinoma/Sarcomatoid | 0 | 0 |
| Mucinous Adenocarcinoma | 1 | 6 |
| Large Cell | 0 | 0 |
| Small Cell | 0 | 0 |
| Total | 16 | |
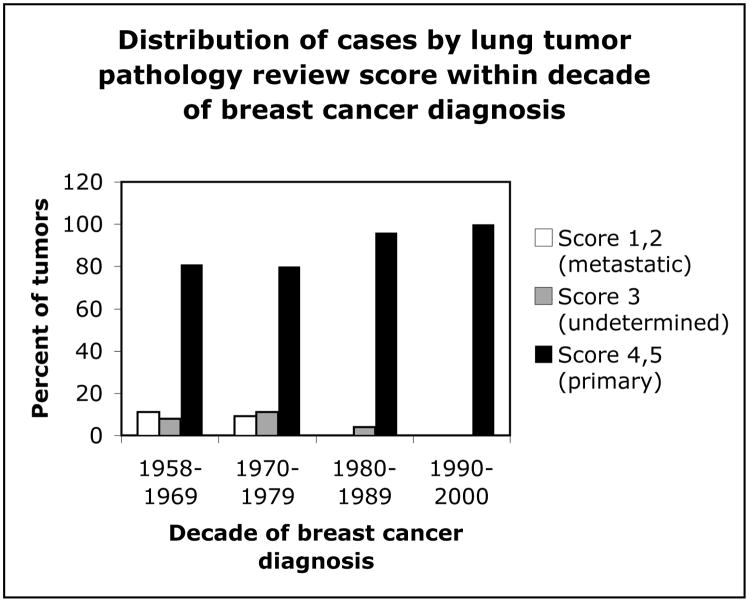
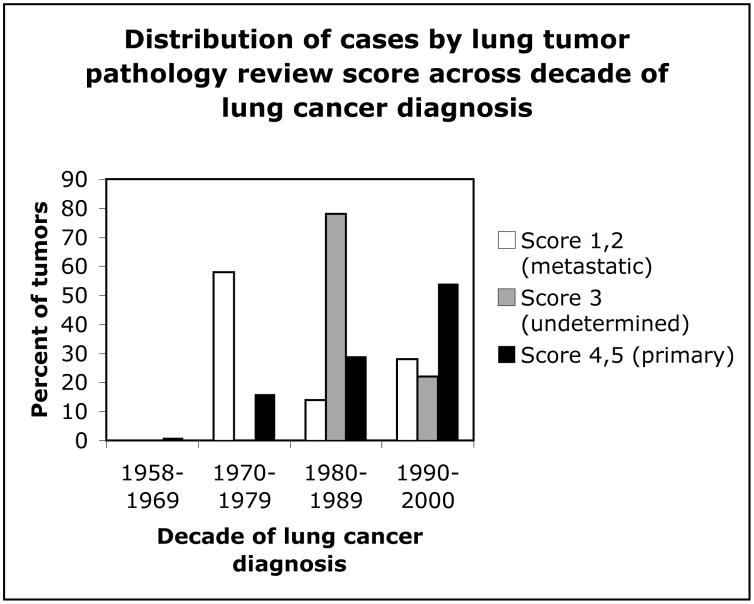
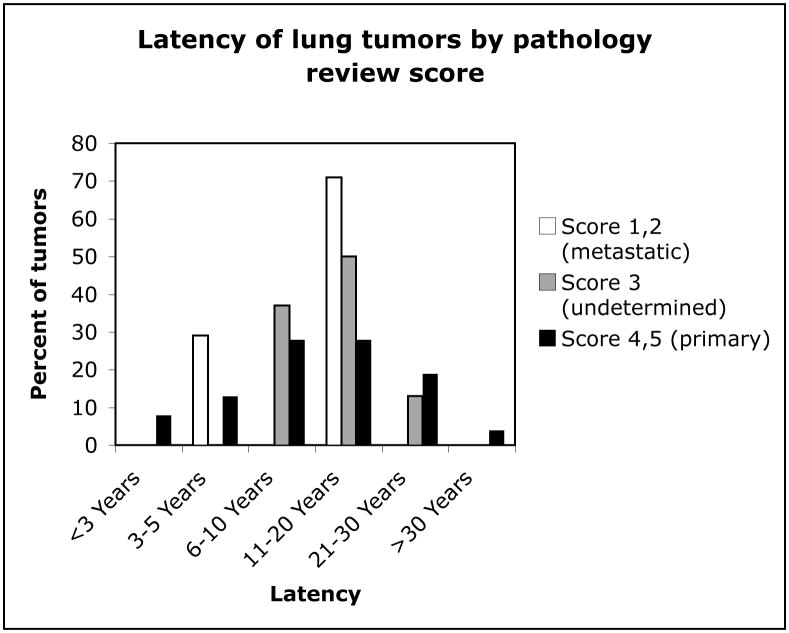
Figure 1 shows the distribution of cases by lung tumor pathology review score within decades of breast cancer diagnosis. The two earliest decades each contained about 20% of lung tumors that were classified as metastases or undetermined, while the later two decades had less than 5% combined. Figure 2 shows the distribution of cases by review score across decades of lung tumor diagnosis. Accurate diagnosis of primary lung tumors increased with progressive decades, with most misdiagnoses occurring between 1970–1989. Figure 3 shows the distribution of latency (time between diagnosis of breast and lung cancers) in cases for each of the review scores. Latency periods were not used as criteria for classification of tumors in the pathology review. About 50% of the undetermined tumors, about 70% of probable metastases, and about 50% of primary tumors had a latency of longer than 10 years.
Figure 1.
Distribution of lung tumors by pathology review score group within decades of matched breast tumor diagnoses from 1958–2000.
Figure 2.
Distribution of lung tumors by pathology review score group across decades of lung tumor diagnoses from 1958–2000.
Figure 3.
Latency of lung tumors after breast cancer for each category of pathology review score group.
DISCUSSION
The risk of secondary lung cancer following radiotherapy is a well described, albeit rare, complication [34–36]. Of fundamental importance to any study examining second cancers is the establishment that the cancer is actually a new lung cancer and not a metastasis from another site. Most studies of second cancers rely only on registry information and/or pathology reports. Lung tumors after breast cancer are especially challenging because lung adenocarcinomas can have similar morphology to breast adenocarcinomas that metastasize to the lung. The lung is a common site of metastasis for breast cancer, with 10–20% of patients hospitalized with breast cancer developing a metastasis in the lung [37]. In an examination of isolated pulmonary nodules in women with a history of breast cancer, 44% of the patients were presumed to have metastatic breast cancer and a tissue diagnosis was not pursued [37]. In this study, evaluation of factors often used as indicators of the histology of a pulmonary nodule after breast cancer, such as age, size of breast tumor, number of nodules, or axillary lymph node involvement, did not reliably exclude primary lung tumors. In our study, after a pathological review of 110 cases, we found that 7% were confirmed misclassified as primary lung cancer, with an additional 7% possibly misclassified. This misclassification was related to decade of diagnosis and latency from breast to lung cancer. Thus, while previously identified associations for breast cancer and lung cancer are not substantially confounded by the misdiagnosis of lung cancer, there may be a slight overestimation of risk based on incorrect lung cancer diagnoses.
Algorithms developed to classify tumors of unknown origin facilitate correct clinical diagnosis, but in a retrospective study, when tissue or medical records are unavailable, researchers must rely on recorded diagnoses [23]. These registries may be subject to diagnostic bias, diagnoses based on outdated technology or knowledge, or data entry errors. In this study, unlike previous studies of secondary lung cancer, we retrieved tumor blocks and conducted pathological diagnoses. An algorithm was established based on combined evaluation of morphology and IHC. TTF-1 stains 65–95% of lung adenocarcinomas and is highly specific for lung and thyroid tissue. It has been suggested as a useful clinical marker to differentiate between primary and metastatic tumors in the lung [23–26]. A positive TTF-1 stain in our study was sufficient for classification of the lung tumor as a primary cancer. ER status alone did not classify lung tumors as primary or metastatic, however, breast metastases are more likely to maintain the ER status of the primary tumor. An ER positive lung tumor from a patient with an ER positive breast tumor would support the diagnosis of the lung tumor as a probable breast metastasis or as undecided in the absence of clear morphologic differences [22, 29–32]. Use of different ER antibodies may lead to different rates of ER positivity in lung tumors, which may affect the comparison of our study with previous studies of ER status in lung tumors from women.
There are no prior studies with pathologic confirmation of secondary lung cancer. In the two published studies of lung cancer following breast cancer that did some evaluation of the status of lung tumors, both relied on review of medical records and not pathology samples for confirmation [22, 38]. A study by Ford et al. found 4% of lung cancers after breast cancer were possible metastases and that 8% were misclassified in the registry as the wrong tissue [38]. By examining actual tumor samples, our study found 14% of potential cases could not be unequivocally classified as having primary lung tumors. In our previously reported study, the risk for secondary lung cancer was 1.27 within 10 years of breast cancer diagnosis (95% CI 1.07–1.49), 1.66 for 10–14 years (95% CI 1.37–1.99), 2.0 for 15–19 years (95% CI 1.61–2.47), and 2.53 (95% CI 2.10–3.04) for 20 or more years [16]. In studies using the Connecticut Tumor Registry, a risk estimate of 2.7 (95% CI 6.9–154) was observed with exposure to radiotherapy and smoking [19]. Data from the SEER registry reported a relative risk of 2.06 (95% CI 1.53–2.78) and 2.09 (95% CI, 1.50–2.90) for ipsilateral lung carcinoma at 10–14 years and 15+ years after postmastectomy radiotherapy [[19, 21]. The National Surgical Adjuvant Breast and Bowel Project reported an increase in the rate of lung cancer among breast cancer patients who had received postmastectomy radiotherapy [22]. Given the estimated 14% misclassification rate in our report, these studies would still likely be positive.
The range of latency times for primary lung tumors in this study suggests that latency is not a reliable criterion for primary or metastatic status. This is an important clinical finding, as some pathologists may still rely on latency to determine the metastatic or primary status of a tumor. Our data also indicate that misclassification of tumors occurs somewhat by decade of diagnosis. After the pathology review, 8 tumors were classified as undetermined and 8 as probable metastases. Most patients with lung tumors misclassified as primary had their breast cancers diagnosed between 1958 and 1979 and most lung tumors that were misclassified were diagnosed between 1970 and 1979. Most confirmed primary lung tumors were diagnosed between 1980 and 2000. This may be due to changes in diagnostic criteria or antibody availability since 1958, resulting in more metastatic tumors misidentified as primary in early decades. In large studies of secondary lung cancer after breast cancer that do not include pathology review, identifying the years of tumor diagnoses may help to identify possible misclassification bias.
While this study examined misclassification of primary lung cancer, we did not examine the misclassification rate for women erroneously diagnosed with metastatic breast cancer, for example, by presumption of metastasis without biopsy by treating clinicians. Given the low rate of secondary lung cancer, it is not feasible to examine cases for this type of misclassification, which would bias the results toward the null. If there were substantial misclassification, for example, in early years after a breast cancer diagnosis, then the risks of secondary lung cancer might be underestimated. The status of 7% of lung tumors in this study was undetermined after thorough review. This reinforces the limitation of any study reviewing tumors regarding metastatic or primary status, as some tumors are very difficult to confidently classify. If all 7% of undetermined tumors were misclassified metastases, the misclassification rate for this study would double.
This data validates that in general, diagnoses of primary lung tumors after breast cancer are accurate. It is clinically reassuring that the problem of misdiagnosis seems to be decreasing with calendar period. Also clinically important is the suggestion that latency is not a reliable criterion for assignment of metastatic or primary status of lung tumors. This study was strengthened by access to medical records and tissue blocks through the Swedish Cancer Registry, effectively giving us the ability to review the tissues as the original diagnosing physicians did. Additionally, the participation of two pathologists, specializing in breast or lung cancer, strengthened the final pathological diagnoses. We were able to review lung tumors and matched breast tumors from cases and to confirm the primary status of 86% of the lung tumors. The algorithm proposed could be useful in clinical settings when initial pathologic findings do not clearly indicate a diagnosis of primary or metastasis status. While the underestimation of risk by misclassifying lung tumors as metastatic breast tumors remains unknown, this study indicates that current risks of lung cancer after breast cancer are only mildly overestimated because of misclassification due to incorrectly diagnosed lung tumors.
Acknowledgments
This study was supported by DOD DAMD17-03-1-0300 and NIH R01CA092705.
Footnotes
Conflict of Interest Statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilkins KL, Woodgate RL. Preventing second cancers in cancer survivors. Oncol Nurs Forum. 2008;35:E12–22. doi: 10.1188/08.ONF.E12-E22. [DOI] [PubMed] [Google Scholar]
- 2.Desandes E, Berger C, Tron I, Demeocq F, Bellec S, Blouin P, Casagranda L, De Lumley L, Freycon F, Goubin A, Le Gall E, Sommelet D, Lacour B, Clavel J. Childhood cancer survival in France, 1990–1999. Eur J Cancer. 2008;44:205–215. doi: 10.1016/j.ejca.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Zhou CX, Gao Y. Frequent genetic alterations and reduced expression of the Axin1 gene in oral squamous cell carcinoma: involvement in tumor progression and metastasis. Oncol Rep. 2007;17:73–79. [PubMed] [Google Scholar]
- 4.Matesich SM, Shapiro CL. Second cancers after breast cancer treatment. Semin Oncol. 2003;30:740–748. doi: 10.1053/j.seminoncol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, Aldape KD, Yu TK, Hortobagyi GN, Gonzalez-Angulo AM. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008 doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 6.Kirova YM, Gambotti L, De Rycke Y, Vilcoq JR, Asselain B, Fourquet A. Risk of second malignancies after adjuvant radiotherapy for breast cancer: a large-scale, single-institution review. Int J Radiat Oncol Biol Phys. 2007;68:359–363. doi: 10.1016/j.ijrobp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki K, Zhang H, Sundquist J, Lorenzo Bermejo J. Modification of risk for subsequent cancer after female breast cancer by a family history of breast cancer. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9759-5. [DOI] [PubMed] [Google Scholar]
- 8.Park SM, Lim MK, Jung KW, Shin SA, Yoo KY, Yun YH, Huh BY. Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National Health Insurance Corporation Study. J Clin Oncol. 2007;25:4835–4843. doi: 10.1200/JCO.2006.10.3416. [DOI] [PubMed] [Google Scholar]
- 9.Soliman H, Agresta SV. Current issues in adolescent and young adult cancer survivorship. Cancer Control. 2008;15:55–62. doi: 10.1177/107327480801500107. [DOI] [PubMed] [Google Scholar]
- 10.Evans HS, Lewis CM, Robinson D, Bell CM, Moller H, Hodgson SV. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84:435–440. doi: 10.1054/bjoc.2000.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levi F, Te VC, Randimbison L, La Vecchia C. Cancer risk in women with previous breast cancer. Ann Oncol. 2003;14:71–73. doi: 10.1093/annonc/mdg028. [DOI] [PubMed] [Google Scholar]
- 12.Volk N, Pompe-Kirn V. Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control. 1997;8:764–770. doi: 10.1023/a:1018487506546. [DOI] [PubMed] [Google Scholar]
- 13.Soerjomataram I, Louwman WJ, de Vries E, Lemmens VE, Klokman WJ, Coebergh JW. Primary malignancy after primary female breast cancer in the South of the Netherlands, 1972–2001. Breast Cancer Res Treat. 2005;93:91–95. doi: 10.1007/s10549-005-4016-2. [DOI] [PubMed] [Google Scholar]
- 14.Mellemkjaer L, Friis S, Olsen JH, Scelo G, Hemminki K, Tracey E, Andersen A, Brewster DH, Pukkala E, McBride ML, Kliewer EV, Tonita JM, Kee-Seng C, Pompe-Kirn V, Martos C, Jonasson JG, Boffetta P, Brennan P. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118:2285–2292. doi: 10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Tsukuma H, Koyama H, Kinoshita Y, Kinoshita N, Oshima A. Second primary cancers following breast cancer in the Japanese female population. Jpn J Cancer Res. 2001;92:1–8. doi: 10.1111/j.1349-7006.2001.tb01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prochazka M, Granath F, Ekbom A, Shields PG, Hall P. Lung cancer risks in women with previous breast cancer. Eur J Cancer. 2002;38:1520–1525. doi: 10.1016/s0959-8049(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman EL, Jacobson JS, Hershman DL, Desai M, Neugut AI. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol. 2008;26:392–398. doi: 10.1200/JCO.2007.13.3033. [DOI] [PubMed] [Google Scholar]
- 18.Prochazka M, Hall P, Gagliardi G, Granath F, Nilsson BN, Shields PG, Tennis M, Czene K. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol. 2005;23:7467–7474. doi: 10.1200/JCO.2005.01.7335. [DOI] [PubMed] [Google Scholar]
- 19.Neugut AI, Murray T, Santos J, Amols H, Hayes MK, Flannery JT, Robinson E. Increased risk of lung cancer after breast cancer radiation therapy in cigarette smokers. Cancer. 1994;73:1615–1620. doi: 10.1002/1097-0142(19940315)73:6<1615::aid-cncr2820730612>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Inskip PD, Stovall M, Flannery JT. Lung cancer risk and radiation dose among women treated for breast cancer. J Natl Cancer Inst. 1994;86:983–988. doi: 10.1093/jnci/86.13.983. [DOI] [PubMed] [Google Scholar]
- 21.Inskip PD. Second cancers following radiotherapy. In: Neugut AIMAT, editor. Multiple Primary Cancers. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 91–135. [Google Scholar]
- 22.Deutsch M, Land SR, Begovic M, Wieand HS, Wolmark N, Fisher B. The incidence of lung carcinoma after surgery for breast carcinoma with and without postoperative radiotherapy. Results of National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials B-04 and B-06. Cancer. 2003;98:1362–1368. doi: 10.1002/cncr.11655. [DOI] [PubMed] [Google Scholar]
- 23.Dennis JL, Hvidsten TR, Wit EC, Komorowski J, Bell AK, Downie I, Mooney J, Verbeke C, Bellamy C, Keith WN, Oien KA. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 24.Moldvay J, Jackel M, Bogos K, Soltesz I, Agocs L, Kovacs G, Schaff Z. The role of TTF-1 in differentiating primary and metastatic lung adenocarcinomas. Pathol Oncol Res. 2004;10:85–88. doi: 10.1007/BF02893461. [DOI] [PubMed] [Google Scholar]
- 25.Chang YL, Lee YC, Liao WY, Wu CT. The utility and limitation of thyroid transcription factor-1 protein in primary and metastatic pulmonary neoplasms. Lung Cancer. 2004;44:149–157. doi: 10.1016/j.lungcan.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Zamecnik J, Kodet R. Value of thyroid transcription factor-1 and surfactant apoprotein A in the differential diagnosis of pulmonary carcinomas: a study of 109 cases. Virchows Arch. 2002;440:353–361. doi: 10.1007/s00428-001-0552-2. [DOI] [PubMed] [Google Scholar]
- 27.Jan IS, Chung PF, Weng MH, Huang MS, Lee YT, Kuo SH. Utility of thyroid transcription factor-1 expression in the differential diagnosis of metastatic adenocarcinoma of serous effusion specimens prepared using the cell transfer technique. J Formos Med Assoc. 2006;105:695–700. doi: 10.1016/S0929-6646(09)60196-0. [DOI] [PubMed] [Google Scholar]
- 28.Su YC, Hsu YC, Chai CY. Role of TTF-1, CK20, and CK7 immunohistochemistry for diagnosis of primary and secondary lung adenocarcinoma. Kaohsiung J Med Sci. 2006;22:14–19. doi: 10.1016/S1607-551X(09)70214-1. [DOI] [PubMed] [Google Scholar]
- 29.Kayser K, Biechele U, Kayser G, Dienemann H, Andre S, Bovin NV, Gabius HJ. Pulmonary metastases of breast carcinomas: ligandohistochemical, nuclear, and structural analysis of primary and metastatic tumors with emphasis on period of occurrence of metastases and survival. J Surg Oncol. 1998;69:137–146. doi: 10.1002/(sici)1096-9098(199811)69:3<137::aid-jso4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Umekita Y, Sagara Y, Yoshida H. Estrogen receptor mutations and changes in estrogen receptor and progesterone receptor protein expression in metastatic or recurrent breast cancer. Jpn J Cancer Res. 1998;89:27–32. doi: 10.1111/j.1349-7006.1998.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lower EE, Glass EL, Bradley DA, Blau R, Heffelfinger S. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat. 2005;90:65–70. doi: 10.1007/s10549-004-2756-z. [DOI] [PubMed] [Google Scholar]
- 32.Koda M, Sulkowski S, Kanczuga-Koda L, Surmacz E, Sulkowska M. Expression of ERalpha, ERbeta and Ki-67 in primary tumors and lymph node metastases in breast cancer. Oncol Rep. 2004;11:753–759. [PubMed] [Google Scholar]
- 33.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 34.Brenner DJ, Hall EJ, Curtis RE, Ron E. Prostate radiotherapy is associated with second cancers in many organs, not just the colorectum. Gastroenterology. 2005;129:773–774. doi: 10.1016/j.gastro.2005.06.045. author reply 774–775. [DOI] [PubMed] [Google Scholar]
- 35.Bassal M, Mertens AC, Taylor L, Neglia JP, Greffe BS, Hammond S, Ronckers CM, Friedman DL, Stovall M, Yasui YY, Robison LL, Meadows AT, Kadan-Lottick NS. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 36.Tucker MA, Murray N, Shaw EG, Ettinger DS, Mabry M, Huber MH, Feld R, Shepherd FA, Johnson DH, Grant SC, Aisner J, Johnson BE. Second primary cancers related to smoking and treatment of small-cell lung cancer. Lung Cancer Working Cadre. J Natl Cancer Inst. 1997;89:1782–1788. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 37.Chang EY, Johnson W, Karamlou K, Khaki A, Komanapalli C, Walts D, Mahin D, Johnson N. The evaluation and treatment implications of isolated pulmonary nodules in patients with a recent history of breast cancer. Am J Surg. 2006;191:641–645. doi: 10.1016/j.amjsurg.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Ford MB, Sigurdson AJ, Petrulis ES, Ng CS, Kemp B, Cooksley C, McNeese M, Selwyn BJ, Spitz MR, Bondy ML. Effects of smoking and radiotherapy on lung carcinoma in breast carcinoma survivors. Cancer. 2003;98:1457–1464. doi: 10.1002/cncr.11669. [DOI] [PubMed] [Google Scholar]