Abstract
Neuropsychiatric disorders such as anxiety and depression have formidable economic and societal impacts. A dysregulation of the hypothalamo-pituitary-adrenal (HPA) axis leading to elevated endogenous glucocorticoid levels is often associated with such disorders. Chronically high glucocorticoid levels may act upon the central nucleus of the amygdala (CeA) to alter normally adaptive responses into those that are maladaptive and detrimental. In addition to glucocorticoids, other steroid hormones such as estradiol and androgens can also modify hormonal and behavioral responses to threatening stimuli. In particular, estrogen receptor beta (ERβ) agonists have been shown to be anxiolytic. Consequently, these experiments addressed the hypothesis that the selective stimulation of glucocorticoid receptor (GR) in the CeA would increase anxiety-like behaviors and HPA axis reactivity to stress, and further, that an ERβ agonist could modulate these effects. Young adult female Sprague-Dawley rats were ovariectomized and bilaterally implanted via stereotaxic surgery with a wax pellet containing the selective GR agonist RU28362 or a blank pellet, to a region just dorsal to the CeA. Four days later, animals were administered the ERβ agonist S-DPN or vehicle (with four daily sc injections). Anxiety-type behaviors were measured using the elevated plus maze (EPM). Central RU28362 implants caused significantly higher anxiety-type behaviors in the EPM and greater plasma CORT levels than controls given a blank central implant. Moreover, S-DPN treated animals, regardless of type of central implant, displayed significantly lower anxiety-type behaviors and post-EPM plasma CORT levels than vehicle treated controls or vehicle treated animals implanted with RU28362. These results indicate that selective activation of GR within the CeA is anxiogenic, and peripheral administration of an ERβ agonist can overcome this effect. These data suggest that estradiol signaling via ERβ prevents glucocorticoid-dependent effects of the CeA on behavior and neuroendocrine function.
Keywords: Glucocorticoids, Amygdala, Estrogen receptor, Stereotaxic, Anxiety
1. Introduction
Anxiety is one of the most prevalent mental disorders and has an immense impact on society. Approximately 25% of adults will suffer from one or more forms of anxiety disorder in their lifetime, and the yearly economic cost is estimated to be at least $40 billion per year in the United States and Europe (Balak and Elmaci, 2007; Gordon and Hen, 2004; Kessler et al., 2009). In addition to emotional symptoms, patients with anxiety disorder exhibit impaired hypothalamic-pituitary-adrenal (HPA) axis function (Ehlert et al., 2001; McEwen, 2005) as evidenced by the inability to respond appropriately to stress. For example, patients with late-life generalized anxiety disorder (GAD) exhibit higher salivary cortisol levels as compared to normal healthy individuals, and the level of cortisol positively correlates with severity of GAD symptoms (Mantella et al., 2008). Furthermore, anxiety disorders are more prevalent in females than males (Bekker and van Mens-Verhulst, 2007), suggesting a potential role for estradiol in the etiology of anxiety. Interestingly, estradiol has been shown to be both anxiolytic and anxiogenic in rodents (Koss et al., 2004; Leret et al., 1994; Mora et al., 1996; Palermo-Neto and Dorce, 1990). However, selective activation of estrogen receptor alpha (ERα) has been shown to increase anxiety-like behaviors, whereas selective activation of estrogen receptor beta (ERβ) has been shown to decrease these behaviors (Lund et al., 2005; Walf and Frye, 2005; Walf et al., 2008). Furthermore, different stressors can activate distinct neural circuits, each of which may have a different inherent sensitivity to either ERα- or ERβ-dependent signaling (Dayas et al., 2001). This suggests that estrogens can have vastly different effects on hormonal and behavioral responses to stress depending on the predominant ER involved in signaling.
One brain region underlying anxiety behaviors in humans and anxiety-like behaviors in rodents is the amygdala (Chapman et al., 1954; Davis, 1997; Kopchia et al., 1992). This brain region also potentiates HPA axis reactivity (Bhatnagar and Dallman, 1998; Prewitt and Herman, 1994), and neurons within this region contain high amounts of androgen and estrogen receptors (Gray et al., 1993; Laflamme et al., 1998; Shughrue and Merchenthaler, 2001). Consequently, it is possible that the amygdala may be a brain site that is important for the sex-specific or hormone dependent modulation of neurobiological changes underlying anxiety disorders. The amygdala coordinates behavioral, autonomic, and endocrine responses to perceived threats or danger to an animal (Davis, 1992; Davis, 1997; LeDoux, 1998). The central nucleus of the amygdala (CeA) in particular, controls major efferent pathways from the amygdalar complex (for review see (Sah et al., 2003)). The CeA has descending projections to regions involved in autonomic, endocrine, and behavioral responses to emotional stimuli (Gray et al., 1989; Marcilhac and Siaud, 1997; Veening et al., 1984). Accordingly, CeA lesions lead to decreased anxiety and decreased neuroendocrine responses to a spectrum of stressors (Beaulieu et al., 1986; Feldman et al., 1994; Kopchia et al., 1992).
Anxiety disorders are often accompanied by HPA axis dysfunction that results in chronically elevated glucocorticoids. Glucocorticoids typically act through high affinity mineralocorticoid receptors (MR) or low affinity glucocorticoid receptors (GR) (Reul and de Kloet, 1985). During the basal state, circulating levels of glucocorticoids are sufficient to occupy primarily MR, whereas following a stressor, elevated glucocortiocoid levels can occupy both MR and GR. The CeA has a robust expression of GR, and accordingly can respond to acute or chronically elevated CORT (Morimoto et al., 1996; Reul and de Kloet, 1985). In contrast to the paraventricular nucleus of the hypothalamus (PVN), where elevated CORT inhibits CRH expression, similar levels of CORT increase CRH mRNA in the CeA. Correspondingly, adrenalectomy increases CRH expression in the PVN whereas it decreases CRH mRNA within the CeA (Makino et al., 1994; Palkovits et al., 1998; Swanson and Simmons, 1989; Viau et al., 2001; Watts and Sanchez-Watts, 1995) and direct administration of a corticosterone pellet just dorsal to the CeA increases CRH mRNA in the CeA (Shepard et al., 2000). Recent studies also show that CORT delivery to the CeA causes increased anxiety-type behaviors, and plasma CORT following exposure to the elevated plus maze (Myers et al., 2005; Shepard et al., 2000; Shepard et al., 2003). Such results, when taken together indicate that the CeA likely plays an important role in integrating the response to acutely or chronically elevated glucocorticoids. Alterations in this response by glucocorticoid hormones could precipitate the maladaptive behaviors associated with anxiety.
The gonadal steroid hormone, estradiol, has also been shown to augment HPA axis activity and alter anxiety-type behaviors (Burgess and Handa, 1992; Handa et al., 1994; Lund et al., 2004; Lund et al., 2005). Estradiol can act via two receptors, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) (Green et al., 1986; Kuiper et al., 1996). Treatment of ovariectomized female rats with the ERβ agonist diarylpropionitrile (DPN) results in decreased anxiety-type behaviors and plasma CORT following exposure to the elevated plus maze, whereas ERα activation appears to have the opposite effect (Lund et al., 2005). Such data suggest that it is the ERα activating component of estradiol that may augment hormonal and behavioral responses to stress, an effect that is opposite of any ERβ activating components.
The CeA is integral in the behavioral responses of fear and anxiety. It also plays a major role in regulating the stress responsiveness of the HPA axis, and expresses low levels of ERβ (Laflamme et al., 1998; Osterlund et al., 1998; Shughrue and Merchenthaler, 2001), and ERα (Laflamme et al., 1998; Shughrue et al., 1997). Therefore, in these studies we sought to determine if local administration of a GR agonist to the CeA can induce anxiety-type behaviors which can be counteracted by peripheral administration of an ERβ agonist.
2. Results
Bilateral implantation of the GR agonist RU28362 results in nuclear localization of GR within the central nucleus of the amygdala
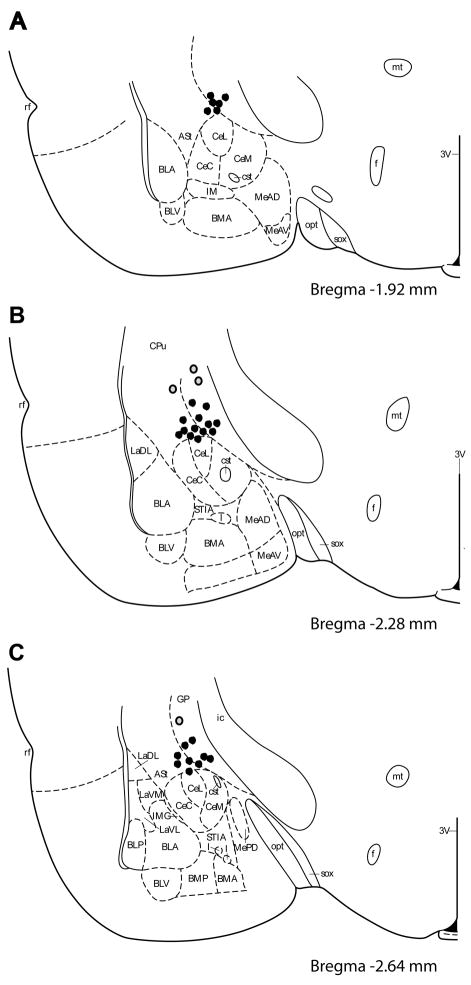
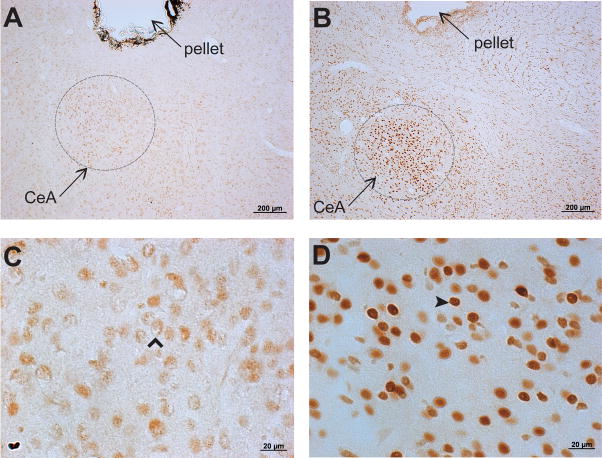
We utilized the translocation of GR immunoreactivity (GR-IR) to the cell nucleus to verify the spread of RU28362 from the wax pellet. GR-IR was examined seven days following bilateral implantation of a beeswax pellet containing either RU28362 or nothing (blank) to a region dorsal to the CeA (Figure 1). RU28362 implants resulted in nuclear GR-IR within the CeA (dark brown staining within cell body) (Figure 2B and 2D). Blank implants resulted in largely cytoplasmic GR-IR within the CeA (light brown staining in cytoplasm) (Figure 2A and 2C). Nuclear GR-IR was primarily contained within the area of the CeA, and observed no further than 1.0mm from the implanted pellet (including basolateral amygdala, paraventricular nucleus of the hypothalamus, and hippocampus). Based on these observations we excluded animals from analysis in which both implants were greater than 1.0mm from the CeA (4/32 or 12.5% of animals, represented by grey dots in Figure 1). There were three animals in which one of the two implants was beyond 1.0mm of the CeA, however the second implant was within 1.0mm of the CeA. The dependent measures obtained from these animals were similar to those that received two correct bilateral implants, and therefore were included in the statistical analysis.
Figure 1.
Location of beeswax pellet implants. One side of the bilateral implant is depicted for clarity. Each dot represents the ventral border of the wax pellet. Grey dots correspond to implants in animals in which placement was beyond 1.0mm of the central nu of the amygdala in both hemispheres (excluded from analysis). Illustration adapted from Paxinos and Watson (Paxinos and Watson, 2007). 3V, third ventricle; ASt, amygdalostriatal transition; BLA, anterior basolateral amygdaloid nu; BLP, posterior basolateral amygdaloid nu; BLV, ventral basolateral amygdaloid nu; BMA, anterior basomedial amygdaloid nu; BMP, posterior basomedial amygdaloid nu; CeC, central amygdaloid nu, capsular part; CeL, central amygdaloid nu, lateral div; CeM, central amygdaloid nu, medial div; CPu, caudate putamen; cst, commissural stria terminalis; f, fornix; I, intercalated nuclei of the amygdala; ic, internal capsule; IM, intercalated amygdaloid nu, main part; IMG, amygdaloid intramedullary gray; LaDL, lateral amygdaloid nu, dorsolateral part; LaVL, lateral amygdaloid nu, ventrolateral part; LaVM, lateral amygdaloid nu, ventromedial part; MeAD, medial amygdaloid nucleus, ant dorsal; MeAV, medial amygdaloid nucleus, anteroventral part; MePD, medial amygdaloid nucleus, posterodorsal part; MePV, medial amygdaloid nucleus, posteroventral part; mt, mammillotegmental tract; MTu, medial tuberal nu; opt, optic tract; rf, rhinal fissure; sox, supraoptic decussation; STIA, bed nucleus of the stria terminalis, intraamygdaloid division.
Figure 2.
Glucocorticoid receptor immunoreactivity (GR-IR) in the central nucleus of the amygdala (CeA) following bilateral implantation of RU28362 pellets. Representative photomicrographs (A) and (C) of GR-immunoreactivity (GR-IR) in an animal with bilateral implants of blank pellets to a region dorsal to the CeA show largely cytoplasmic GR-IR within the CeA (open arrowhead in (C), light brown staining in cytoplasm). Representative photomicrographs (B) and (D) of GR-IR in an animal with bilateral implants of the GR agonist RU28362 show nuclear GR-IR within the CeA (closed arrowhead in (D), dark brown staining within cell body). Accumulation of nuclear GR-IR was observed no further than 1.0mm from pellet.
The GR agonist RU28362 administered to the central nucleus of the amygdala decreases anxiolytic behavior and ERβ activation blocks this effect
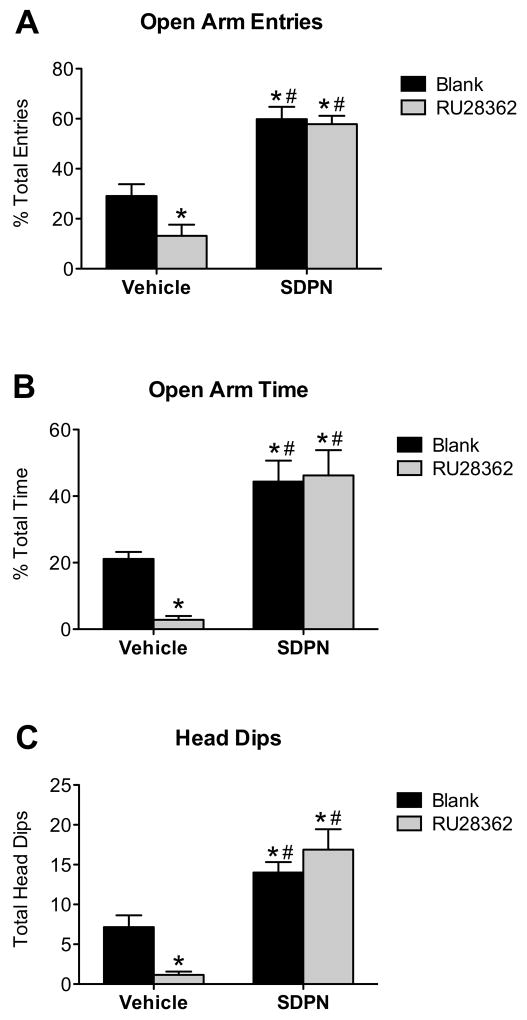
We next examined whether GR activation in the CeA alters anxiety-like behaviors on the elevated plus maze (EPM), and the possibility that systemic treatment with an ERβ agonist (S-DPN) could alter these effects. We measured the open arm entries and open arm time as a percentage of total entries and total time, respectively, to normalize for non-specific treatment effects on and/or individual variations in locomotor activity. Two-way ANOVA analysis showed a trend for systemic treatment-by-central treatment interaction effect for percentage of open arm entries (F(1,26) = 2.52; P = 0.126) and percentage of open arm time (F(1,26) = 3.07; P = 0.093), but did reveal a significant systemic treatment-by-central treatment interaction effect in total head dips (F(1,26) = 5.86; P = 0.024). Post hoc pairwise comparison revealed that RU28362 caused a significant decrease in the percentage of open arm entries (Figure 3A) (F(3,26) = 30.85; P < 0.05), percentage open arm time (Figure 3B) (F(3,26) = 14.44; P < 0.05), and number of head dips (Figure 3C) (F(3,26) = 14.90; P < 0.05) as compared to animals with blank (beeswax) implants. There was no significant effect of GR activation on total entries, rearing, grooming, or number of fecal boli (data not shown).
Figure 3.
Behavior on the elevated plus maze. The GR agonist RU28362 delivered bilaterally to the central nucleus of the amygdala (CeA) decreases anxiolytic behaviors on the elevated plus maze (#). Peripheral treatment with the ERβ agonist S-DPN increases anxiolytic behaviors and blocks the anxiogenic effect of RU28362 administered to the CeA (*). Panel A: Open arm entries expressed as percentage of total arm entries (closed + open). Panel B: Open arm time expressed as percentage of total time on maze. Panel C: Number of head dips over edge of open arm. Data are represented as mean ± SEM; n = 6–8 animals per treatment group. *, indicates significant difference (P < 0.05) compared with animals implanted with blank pellets and vehicle treated (controls); #, indicates significant effect of peripheral treatment in groups with same central treatment.
Two-way ANOVA indicated an overall systemic treatment effect, whereby S-DPN treatment significantly increased the percentage of open arm entries (Figure 3A) (F (1,26) = 74.49; P < 0.0001), percentage open arm time (Figure 3B) (F (1,26) = 33.63; P < 0.0001), and head dips (Figure 3C) (F (1,26) = 37.78; P < 0.0001) as compared to animals with vehicle treatment regardless of implant type. There was no effect of S-DPN on total entries (F (1,26) = 0.32; P = 0.7025). Therefore, peripheral administration of S-DPN effectively blocked the anxiety-like behaviors seen in RU28362 implanted animals, and further increased exploratory behavior on the EPM.
The GR agonist RU28362 administered to the central nucleus of the amygdala increases plasma corticosterone, and peripheral administration of the ERβ agonist S-DPN prevents this effect
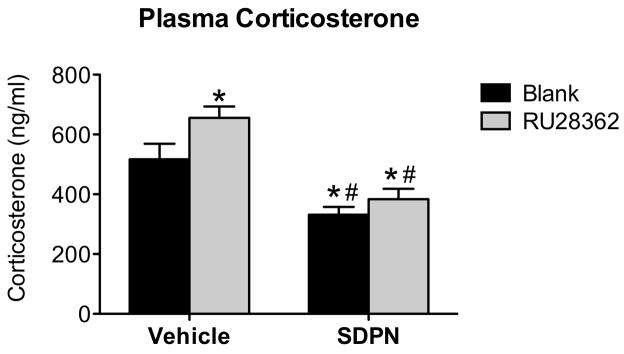
To examine the corresponding hormonal response caused by exposure to the EPM, corticosterone (CORT) levels were measured in plasma obtained from trunk blood collected 20 minutes after initial exposure to the elevated plus maze. Two-way ANOVA revealed an overall systemic treatment effect where peripheral S-DPN treatment significantly decreased plasma CORT following the EPM regardless of implant type (F (1,23) = 34.73; P < 0.0001). There was no systemic treatment-by-central treatment interaction effect for plasma corticosterone (F (1,23) = 1.25; P = 0.276). Post hoc pairwise comparisons indicate that animals with RU28362 implants had significantly higher plasma CORT following the EPM than animals with blank implants (Figure 4; F (3,23) = 13.77; P < 0.05). Therefore, peripheral administration of S-DPN effectively blocked the increase in stress-induced plasma CORT seen with RU28362 implanted animals given vehicle injections, and further reduced CORT levels as compared to controls.
Figure 4.
Plasma corticosterone levels 20 minutes after initial maze exposure. The GR agonist RU28362 delivered bilaterally to the central nucleus of the amygdala (CeA) increases plasma corticosterone 20 minutes following the initiation of elevated plus maze (#). Peripheral treatment with the ERβ agonist S-DPN decreases plasma corticosterone following the elevated plus maze and blocks the augmentation of plasma corticosterone seen with RU28362 administration to the CeA (*). Data are represented as mean ± SEM; n = 6–7 animals per treatment group. *, indicates significant difference (P < 0.05) compared with animals implanted with blank pellets and vehicle treated (controls); #, indicates significant effect of peripheral treatment in groups with same central treatment.
3. Discussion
In these studies we examined the ability of selective glucocorticoid receptor activation in the CeA to augment anxiety-like behaviors and determined whether the concurrent activation of estrogen receptor beta could counteract glucocorticoid effects. Our result show that chronic exposure of a GR agonist, RU28362, directly to the CeA results in elevated anxiety-type behaviors and plasma CORT in response to a psychological stressor (elevated plus maze, EPM). Interestingly, concomitant systemic treatment with a selective ERβ agonist, S-DPN, completely abolished the behavioral and hormonal effect of local application of RU28362 to the CeA.
The CeA is well poised to integrate glucocorticoid and gonadal steroid hormone signaling during time of perceived or actual danger. The presence of steroid hormone receptors within the CeA allow this brain region to respond to fearful situations in a manner dependent upon the internal hormone milieu (Bingaman et al., 1994; Laflamme et al., 1998; Morimoto et al., 1996; Osterlund et al., 1998; Reul and de Kloet, 1985; Shughrue and Merchenthaler, 2001). The CeA contains the highest density of GR expression in the amygdala, and is responsive to elevated levels of glucocortiocoids (Makino et al., 1994; Morimoto et al., 1996; Swanson and Simmons, 1989; Watts and Sanchez-Watts, 1995). Therefore, in these studies, we delivered the GR agonist RU28362 bilaterally to an area dorsal to the CeA via stereotaxic implantation. This results in chronic activation of GR within the CeA independent of exogenous CORT levels. The placement of the pellet insures sustained GR occupancy without mechanical damage to the CeA.
In order to test the spread of ligand from the implanted pellet, we examined nuclear localization of GR using immunocytochemistry. The rationale for this approach is based upon the ability of ligand binding to translocate GR to the cell nucleus whereas it is largely cytoplasmic in the absence of ligand (Madan and DeFranco, 1993; Munck and Holbrook, 1984; Raaka and Samuels, 1983; Sackey et al., 1996). Therefore nuclear GR immunoreactivity (GR-IR) provides a good marker to determine the extent of agonist spread. We observed nuclear GR-IR within a radius of approximately 1.0mm from the RU28362 pellet, which included the CeA. This is consistent with what was shown previously for other hormones using the same procedure for estradiol (Lund et al., 2006) and for androgens (Bingham et al., 2009). Predominantly cytoplasmic GR-IR was observed around blank pellets. Previous studies where CORT pellets (30 μg) are placed in this same region resulted in a CORT diffusion radius of 0.5 to 1.0 mm, which included the CeA, but excludes other glucocorticoid sensitive regions such as the BNST, PVN, and hippocampus (Shepard et al., 2003).
Our results demonstrate that administration of a selective GR agonist to the CeA results in increased anxiety-type behaviors in the elevated plus maze (EPM) and increased plasma CORT following maze exposure. Generally, treatments that reduce open arm exploration (time spent on open arm and open arm entries) and increase head dipping and rearing (explorative behaviors) are considered anxiogenic (Handley and McBlane, 1993; Pellow et al., 1985). These results implicate GR activation in the CeA alone as responsible for the generation of anxiety-type behaviors in the rat. Our data are in accordance with previous studies where CORT was delivered to the CeA. CORT exposure was shown to be anxiogenic and augment CeA CRH mRNA expression and plasma CORT secretion (Myers et al., 2005; Shepard et al., 2000; Shepard et al., 2003). Furthermore, this effect of CORT on anxiogenesis can be blocked via peripheral administration of a CRHR1 antagonist, suggesting that the anxiogenic effect of CORT delivered to the CeA is mediated through CRH signaling (Myers et al., 2005). It is important to note that these studies, including the one presented here, examine the consequences of a relatively high dose and long-term (days) exposure to glucocorticoids within the CeA. While this does not entirely match a physiological phenomenon, it may be representative of what occurs during chronic glucocorticoid hypersecretion as is seen with chronic stress and in certain psychological disorders. However, it is necessary to keep in mind that endogenous glucocorticoids are secreted in a diurnal circadian pattern consisting of frequent pulses that are necessary for normal physiology (Lightman et al., 2008; Stavreva et al., 2009).
Since CORT has affinity for both MR and GR and preferentially binds MR, it could not be determined from the studies of Shephard et al. (2000 Shephard et al. (2003) if the effect observed was truly GR mediated. Indeed, elevated glucocorticoid levels allow binding to GR and this increases CRH levels in the CeA, an effect that is opposite that of glucocorticoids in the PVN. Upregulation of CeA CRH may in turn result in increased CRH neurotransmission to other brain regions integral in the behavioral and endocrine responses to stressors. Furthermore, the CeA does have direct stimulatory projections to the medial parvocellular region of the PVN (Gray et al., 1989; Marcilhac and Siaud, 1997), a brain region that contains the CRH and AVP neurons that comprise the main effector arm of the HPA axis. Alternatively, the CeA could access the PVN through an indirect pathway involving the lateral BNST. The CeA sends CRH-positive axons to the lateral BNST, which in turn has direct stimulatory projections to the medial parvocellular PVN (Gray, 1993; Herman et al., 1994; Herman et al., 2003). Correspondingly, stimulation of the lateral BNST increases CRH mRNA expression in the medial parvocellular PVN and plasma CORT levels (Dunn, 1987; Herman et al., 1994). Interestingly, the hippocampus also accesses the PVN via an indirect path through the BNST (Herman et al., 1998). In this regard, it is possible that the CeA may counteract the inhibitory influence of the hippocampus on HPA axis output, especially during times of chronically elevated glucocorticoids. Studies examining the effect of selective activation of ERβ within the CeA, BNST, or PVN on CRH gene expression is certainly warranted given the results described in this study.
Gonadal steroids play an integral role in modulating mood and HPA axis function (Fink et al., 1998; Handa et al., 1994; McCormick et al., 2002; Shors and Leuner, 2003; Weiser et al., 2008). Estrogen in particular has been reported to have opposing effects on both mood and HPA axis activity. This could be explained, in part, by the existence of two main receptors for estrogen, ERα and ERβ (Green et al., 1986; Kuiper et al., 1996). Animal studies have implicated estrogen signaling via ERα in generating anxiety-type behaviors and in augmentation of HPA axis activity (Lund et al., 2005). Alternatively, studies have shown an opposite role for estrogen signaling through ERβ (Imwalle et al., 2005; Krezel et al., 2001; Lund et al., 2005; Walf and Frye, 2006). Specifically, treatment of OVX females with the selective ERβ agonist, S-DPN, decreases anxiety-type behaviors in the EPM, despair in the forced swim test, and plasma CORT secretion in response to restraint (Weiser et al., 2009). However, the mechanism of ERβ-dependent effects on anxiety-like behavior and HPA axis function remain to be determined. Therefore, in this study, we addressed the hypothesis that systemic treatment of S-DPN could alter glucocorticoid receptor specific actions on the CeA. Our results indicate that peripheral administration of S-DPN blocks the anxiogenic effect of a locally administered GR agonist to the CeA. In fact, S-DPN treatment proved to be anxiolytic to the same extent regardless of GR activation in the CeA. Therefore, glucocorticoid-sensitive modulation of anxiety-type behaviors through the CeA can be inhibited or overcome by activation of ERβ.
In this study S-DPN, the bioactive enantiomer of the ERβ agonist, diarypropionitrile (Weiser et al., 2009), was administered peripherally, and consequently several different modes of action are possible. DPN has been shown to pass the blood-brain barrier, and thus likely exerts its effect centrally (Lund et al., 2005). The CeA does express ERβ, albeit at low levels (Laflamme et al., 1998; Osterlund et al., 1998; Shughrue and Merchenthaler, 2001), indicating a potential direct effect of ERβ on the activity of CeA neurons. However, it is not known if ERβ and GR co-exist within the same neurons in the CeA. Aside from a direct action on CeA projecting neurons, an alternative possibility is that ERβ is expressed in GABAergic interneurons of the CeA and upon ligand-activation acts to inhibit nearby CRH neurons. Interestingly, infusion into the CeA of the benzodiazepine midazolam, an allosteric modulator of the GABAA receptor, produces anxiolytic effects that can be blocked by injection of the benzodiazepine receptor antagonist, flumazenil, into the CeA (Pesold and Treit, 1995). Furthermore, local application of the GABA agonist, muscimol, to the CeA results in decreased anxiety in the elevated plus maze (Moreira et al., 2007). This highlights the importance of GABA signaling for CeA-specific modulation of behaviors and raises the possibility that GABA mediates ERβ’s actions. Future studies could examine the extent of ERβ, GR and GABA colocalization in the CeA, and to what extent ERβ activation has an effect on GABAergic signaling and/or CRH expression in the CeA.
Another potential mechanism that might explain the anxiolytic effects of ERβ on CeA function could be through ERβ-containing sites that project to the CeA or impinge on projection areas from the CeA. For example, S-DPN may act by activation of ERβ-expressing areas that project to the CeA such as the medial amygdala, BNST, PVN, arcuate nucleus, hippocampus, and dorsal raphe nucleus (Canteras and Swanson, 1992; Dong and Swanson, 2004; Dong and Swanson, 2006; Ottersen, 1980; Ottersen, 1981). Alternatively, there could be activation of ERβ containing neurons in brain regions that have direct and indirect projections to the parvocellular PVN (e.g. BNST, hippocampus, arcuate nucleus) which may act to override the CeA-mediated augmentation of HPA axis drive. Although, ERβ is expressed in a small percentage of CRH-expressing neurons of the PVN (Laflamme et al., 1998; Suzuki and Handa, 2005), suggesting a potential direct effect on HPA axis output, the majority of ERβ-expressing neurons in the PVN are brainstem- and spinal cord- projecting neurons and not neurosecretory neurons (Bingham et al., 2006). Selective activation of ERβ within these discrete brain regions in combination with or without GR activation in the CeA would provide clues into potential sites of action and neural pathways that may be involved.
In summary, the data presented here indicate that GR activation in the CeA can induce anxiety-type behaviors and an elevated hormonal (CORT) response to a psychological stressor. Additionally, treatment with an ERβ agonist can override the effect of GR in the CeA and further attenuates anxiety-type behaviors and dampens CORT secretion following a stressor. These data suggest a role for estradiol signaling via ERβ in modulating glucocorticoid-dependent effects of the CeA on behavior and neuroendocrine function.
4. Materials and Methods
Animals
Young adult female Sprague Dawley rats (~225g) were obtained from Harlan Laboratories (San Diego, CA), housed individually, and maintained on a 12h:12h light schedule (lights on at 0600h) in temperature and humidity controlled rooms at the Laboratory Animal Research Facility at Colorado State University. We chose female rats due to a few factors: one of their primary gonadal hormones is estrogen, there is a significant sex difference in anxiety-type behaviors in female rats as compared to males, and there is a sex difference in anxiety and depression in humans where women suffer greater from either in comparison to men. Animals had ad libitum access to a soy free diet (modified AIN-93G, DYET#101591 from DYETS, Inc., Allentown, PA, with corn oil substituted for soy oil) to minimize activation of ERβ by phytoestrogenic compounds. One week after arrival, animals were ovariectomized under isofluorane anesthesia. Following ovariectomy, animals were handled daily (5 minutes each animal) by the same experimenter. All animal protocols were previously approved by the Animal Care and Use Committee at Colorado State University.
Stereotaxic implantation
One week following ovariectomy animals were fitted bilaterally with two 22 gauge stainless-steel cannulae (Small Parts, Miami Lakes, FL) with the aid of a small animal stereotaxic instrument (David Kopf Instruments, Tujunga, CA). The tips of the cannulae were previously packed with the glucocorticoid receptor agonist RU28362 (Roussel-Uclaf, Romainville, France), which was dissolved into warmed beeswax (Sigma-Aldrich, St. Louis, MO) to a final concentration of 0.5 uM, and packed to a height of 0.5 mm within the end of the cannulae. We chose this concentration of RU28362 since a similar concentration of estradiol diffuses approximately 0.5mm from a beeswax pellet when examined sven days after implantation in the brain (Lund et al., 2006). Controls received cannulae packed with beeswax alone. Stereotaxic coordinates to allow placement of the cannula tip to the region just above the central nucleus of the amygdala were: 2.3 mm posterior and 3.8 mm lateral to bregma, and 6.3 mm below the skull surface. A 28 gauge stainless steel wire cut to extend 1.0 mm past the length of the cannulae was inserted into the cannulae to expell the pellet. Following animal sacrifice, location of pellet implants was confirmed in cresyl violet-stained sections. Animals in which both implants were more than 1.0mm from the central nucleus of the amygdala (4 out of 32 animals, 12.5%) were excluded from analysis based on the observed spread of RU28362 from the beeswax pellet via glucocorticoid receptor immunocytochemistry.
Drug treatments
Beginning four days following stereotaxic implant, animals were given a single daily subcutaneous injection of either vehicle (hydroxypropyl betacyclodextran; 27% w/w in saline; CTD Inc., High Springs, FL), or the ERβ agonist, S-DPN (1.0 mg/kg) in a total volume of 0.2 ml. This dose of DPN has been previously shown to be anxiolytic in female rats (Lund et al., 2005). Injections occurred at 0800 h for 4 days. DPN was synthesized de novo as previously described (Lund et al., 2005) and separated into S- and R- enantiomers by HPLC (Weiser et al., 2009). Because we have previously demonstrated that the S-enantiomer contains all of the ERβ activity of DPN (Weiser et al., 2009), only the S-enantiomer was tested in these studies. Three hours after the injection on day 4, animals underwent behavioral testing on the elevated plus maze (EPM).
Elevated plus maze
Animals were transported in their home cage to the behavioral testing room and maze performance was evaluated for 5 minutes on the fourth day of treatment (seventh day post-implantation) as previously described (Handley and McBlane, 1993). Behaviors measured included number of entries into open and closed arms (where both forelimbs enter maze arm), total time spent in open and closed arms, rearing (picking up both forelimbs from the surface of the maze in an effort to explore), head dips (placing head over the edge of the maze in an effort explore area below and around the maze), total time spent grooming, and the number of fecal boli. Animals that fell off the maze during testing (two total) were excluded from analysis.
Plasma corticosterone analysis
Animals were returned to their home cage following EPM testing and 15 min later (20 min following the start of EPM), animals were rapidly decapitated and trunk blood collected into ice-chilled tubes containing 0.5M EDTA and aprotinin (4 mg/ml, Sigma, St. Louis, MO). Plasma levels of corticosterone were determined by radioimmunoassay as previously described (Lund et al., 2005). Intra-assay and Inter-assay coefficient of variance (CV) were 3.9% and 5.1% respectively.
Immunocytochemistry
To examine the extent of RU28362 spread from implanted pellets, two animals from each central treatment group (four total, blank or RU28362) were intracardially perfused with saline followed by 4% buffered paraformaldehyde 90 minutes after maze exposure. Brains were postfixed in 4% paraformaldehyde, cryoprotected in 30% sucrose, sectioned at 35uM on a Leitz cryostat, and stored in cryopreservative at −20 C until processed for immunocytochemistry. After standard washes, the free floating sections were incubated in 1.5% NGS in 0.01 M PBS with 0.03% TX to block nonspecific binding, then incubated for 24 hours at 4 C with the GR antibody (1:400) in 0.01 M PBS with TX in the presence of 1.5% NGS. The rabbit antiserum used (PA-511, Affinity Bioreagents, lot# 019-147) was prepared against a synthetic peptide corresponding to residues 346–367 from human GR. This antibody detects a single band via western blot representing the 97 kDa GR protein, and has been shown to produce cytoplasmic staining in the absence of ligand, and nuclear staining after glucocorticoid administration (Cidlowski et al., 1990; Francis et al., 2006). Following primary antibody incubation, the tissue was washed three times for 10 minutes each in PBS with TX and incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA; 1:400) in PBS with TX in the presence of 1.5% NGS for 2 hours at room temperature. Sections are subsequently washed and processed according to the avidin-biotin-peroxidase procedure (Vector Laboratories; 1:400). After standard washes, the tissue was rinsed in 0.1 M Tris-buffered saline (TBS, pH 8.0) for 15 minutes and then developed with 3,3′-diaminobenzidine (DAB; 0.5 mg/ml; Sigma, St. Louis, MO) in 0.1 M Tris-buffered saline) and 0.01% hydrogen peroxide in 0.1M TBS for 3–5 minutes to produce a brown reaction product. The reaction is stopped by several washes in 0.01 M PBS. Control sections, where primary antibody was omitted, were run in parallel. Staining for the corresponding antigen was absent in these sections.
Statistics
For behavioral measures, 27 out of 32 total animals were included in the statistical analysis and exclude four animals in which pellet placement was incorrect and two animals that fell off the maze (including one animal that had incorrect pellet placement and fell off the maze). For corticosterone, 24 out of 32 total animals were included in the statistical analysis and exclude four animals perfused for ICC (one of which fell off the maze) and four animals in which pellet placement was incorrect (including one animal that had incorrect pellet placement and fell off the maze). Two-way analysis of variance was performed on data from each experiment using Prism analysis software (v. 5.0, GraphPad Software Inc., San Diego CA). Newman-Keuls multiple comparison test was used post hoc for pairwise comparisons. Differences were considered significant when p < 0.05. Data are expressed as group means ± SEM.
Acknowledgments
Support: USPHS: NIH/NINDS R01 NS039951
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balak N, Elmaci I. Costs of disorders of the brain in Europe. Eur J Neurol. 2007;14:e9. doi: 10.1111/j.1468-1331.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–54. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4(Suppl B):S178–93. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Baeckman LM, Yracheta JM, Handa RJ, Gray TS. Localization of androgen receptor within peptidergic neurons of the rat forebrain. Brain Res Bull. 1994;35:379–82. doi: 10.1016/0361-9230(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-beta distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J Comp Neurol. 2006;499:911–23. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- Bingham BI, Gray M, Innala L, Viau V. Cellular responses of the paraventricular nucleus of the hypothalamus to testosterone and hydroxyflutamide implants in the medial amygdala. Society for Neuroscience; Chicago, IL: 2009. [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–9. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–94. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Chapman WP, Schroeder HR, Geyer G, Brazier MA, Fager C, Poppen JL, Solomon HC, Yakovlev PI. Physiological evidence concerning importance of the amygdaloid nuclear region in the integration of circulatory function and emotion in man. Science. 1954;120:949–50. doi: 10.1126/science.120.3127.949. [DOI] [PubMed] [Google Scholar]
- Cidlowski JA, Bellingham DL, Powell-Oliver FE, Lubahn DB, Sar M. Novel antipeptide antibodies to the human glucocorticoid receptor: recognition of multiple receptor forms in vitro and distinct localization of cytoplasmic and nuclear receptors. Mol Endocrinol. 1990;4:1427–37. doi: 10.1210/mend-4-10-1427. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–98. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–78. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407:327–31. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol. 2001;57:141–52. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658:21–6. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25:764–75. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Francis AB, Pace TW, Ginsberg AB, Rubin BA, Spencer RL. Limited brain diffusion of the glucocorticoid receptor agonist RU28362 following i.c.v. administration: implications for i.c.v. drug delivery and glucocorticoid negative feedback in the hypothalamic-pituitary-adrenal axis. Neuroscience. 2006;141:1503–15. doi: 10.1016/j.neuroscience.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Hen R. Genetic approaches to the study of anxiety. Annu Rev Neurosci. 2004;27:193–222. doi: 10.1146/annurev.neuro.27.070203.144212. [DOI] [PubMed] [Google Scholar]
- Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–46. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–24. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–9. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 1993;29:129–38. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6:433–42. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–59. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–63. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustun TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchia KL, Altman HJ, Commissaris RL. Effects of lesions of the central nucleus of the amygdala on anxiety-like behaviors in the rat. Pharmacol Biochem Behav. 1992;43:453–61. doi: 10.1016/0091-3057(92)90176-g. [DOI] [PubMed] [Google Scholar]
- Koss WA, Gehlert DR, Shekhar A. Different effects of subchronic doses of 17-beta estradiol in two ethologically based models of anxiety utilizing female rats. Horm Behav. 2004;46:158–64. doi: 10.1016/j.yhbeh.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–82. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–78. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–38. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Leret ML, Molina-Holgado F, Gonzalez MI. The effect of perinatal exposure to estrogens on the sexually dimorphic response to novelty. Physiol Behav. 1994;55:371–3. doi: 10.1016/0031-9384(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583:255–62. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Lund T, Hinds L, Handa R. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–56. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16:272–8. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Madan AP, DeFranco DB. Bidirectional transport of glucocorticoid receptors across the nuclear envelope. Proc Natl Acad Sci U S A. 1993;90:3588–92. doi: 10.1073/pnas.90.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–12. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–81. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilhac A, Siaud P. Identification of projections from the central nucleus of the amygdala to the paraventricular nucleus of the hypothalamus which are immunoreactive for corticotrophin-releasing hormone in the rat. Exp Physiol. 1997;82:273–81. doi: 10.1113/expphysiol.1997.sp004022. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–47. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–3. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–20. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Moreira CM, Masson S, Carvalho MC, Brandao ML. Exploratory behaviour of rats in the elevated plus-maze is differentially sensitive to inactivation of the basolateral and central amygdaloid nuclei. Brain Res Bull. 2007;71:466–74. doi: 10.1016/j.brainresbull.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–69. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Munck A, Holbrook NJ. Glucocorticoid-receptor complexes in rat thymus cells. Rapid kinetic behavior and a cyclic model. J Biol Chem. 1984;259:820–31. [PubMed] [Google Scholar]
- Myers DA, Gibson M, Schulkin J, Greenwood Van-Meerveld B. Corticosterone implants to the amygdala and type 1 CRH receptor regulation: effects on behavior and colonic sensitivity. Behav Brain Res. 2005;161:39–44. doi: 10.1016/j.bbr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–80. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol. 1980;194:267–89. doi: 10.1002/cne.901940113. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–56. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, Dorce VA. Influences of estrogen and/or progesterone on some dopamine related behavior in rats. Gen Pharmacol. 1990;21:83–7. doi: 10.1016/0306-3623(90)90600-q. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Young WS, 3rd, Kovacs K, Toth Z, Makara GB. Alterations in corticotropin-releasing hormone gene expression of central amygdaloid neurons following long-term paraventricular lesions and adrenalectomy. Neuroscience. 1998;85:135–47. doi: 10.1016/s0306-4522(97)00621-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CS. The Rat Brain in Stereotaxic Coordinates. Academic Press; London: 2007. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pesold C, Treit D. The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res. 1995;671:213–21. doi: 10.1016/0006-8993(94)01318-c. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Lesion of the central nucleus of the amygdala decreases basal CRH mRNA expression and stress-induced ACTH release. Ann N Y Acad Sci. 1994;746:438–40. doi: 10.1111/j.1749-6632.1994.tb39279.x. [DOI] [PubMed] [Google Scholar]
- Raaka BM, Samuels HH. The glucocorticoid receptor in GH1 cells. Evidence from dense amino acid labeling and whole cell studies for an equilibrium model explaining the influence of hormone on the intracellular distribution of receptor. J Biol Chem. 1983;258:417–25. [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Sackey FN, Hache RJ, Reich T, Kwast-Welfeld J, Lefebvre YA. Determinants of subcellular distribution of the glucocorticoid receptor. Mol Endocrinol. 1996;10:1191–205. doi: 10.1210/mend.10.10.9121487. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–95. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res. 2003;963:203–13. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord. 2003;74:85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Stavreva D, Wiench M, John S, Conway-Campbell B, McKenna M, Pooley J, Johnson T, Voss T, Lightman S, Hager G. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009 doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–35. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–57. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13:442–52. doi: 10.1046/j.1365-2826.2001.00653.x. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2006 doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–81. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. J Physiol. 1995;484(Pt 3):721–36. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57:309–20. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–25. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]