Abstract
Background
The Chernobyl accident caused an unprecedented increase in papillary thyroid carcinoma (PTC) incidence with a surprisingly short latency and unusual morphology. We have investigated whether unexpected features of the PTC incidence after Chernobyl were radiation specific or influenced by iodine deficiency.
Methods
PTCs from children from Belarus, Ukraine, and the Russian Federation exposed to fallout from Chernobyl were compared with PTCs from children not exposed to radiation from the same countries, from England and Wales (E&W) and from Japan. The degree and type of differentiation, fibrosis, and invasion were quantified.
Results
There were no significant differences between PTCs from radiation-exposed children from Belarus, Ukraine, and the Russian Federation and PTCs from children from the same countries who were not exposed to radiation. Childhood PTCs from Japan were much more highly differentiated (p < 0.001), showed more papillary differentiation (p < 0.001) and were less invasive (p < 0.01) than “Chernobyl” tumors, while tumors from E&W generally showed intermediate levels of degree and type of differentiation and invasion. There was a marked difference between the sex ratios of children with PTCs who were radiation exposed and those who were not exposed (F:M exposed vs. unexposed 1.5:1 vs. 4.2:1; χ2 = 7.90, p ≤ 0.01005).
Conclusions
The aggressiveness and morphological features of Chernobyl childhood PTCs are not associated with radiation exposure. The differences found between tumors from the Chernobyl area, E&W, and Japan could be influenced by many factors. We speculate that dietary iodine levels may have wide implications in radiation-induced thyroid carcinogenesis, and that iodine deficiency could increase incidence, reduce latency, and influence tumor morphology and aggressiveness.
Introduction
A thyroid cancer endemic has occurred among those exposed to high levels of fallout from the Chernobyl nuclear power plant accident in 1986 (1–4). The tumors are dominantly papillary thyroid carcinomas (PTCs), and many early cases were aggressive and showed an unusual solid phenotype (5,6), uncommon in unexposed populations except young children (7). The great increase in papillary rather than follicular carcinoma, and the morphologic features have been linked to the molecular findings (8,9). The most common oncogenes involved in PTCs generally are rearrangements of tyrosine kinase (tk) genes, usually RET, less often TRK, and point mutations in BRAF (10–14). Radiation is the likely cause of the rearrangements; it preferentially induces double-strand DNA breaks and is less effective than other mutagens in inducing point mutations (15). PTCs with a solid (immature) morphology more often involve RET-PTC3 than tumors with a papillary (more differentiated) phenotype (8,9). A short latent period is linked to PTCs with a dominantly solid phenotype (16), and to a higher frequency of RET-PTC3 rearrangements (11). Mutations in BRAF are less frequent in childhood PTCs, exposed or unexposed, than in adult PTCs (17–19); it is not currently possible to separate the roles of age, latency, or mutagen. BRAF activation by rearrangement has occurred in short latency Chernobyl-related PTCs (20), supporting the relevance of radiation-induced double-strand breaks.
Early reports on Chernobyl-related thyroid carcinomas suggested that the solid morphology, aggressiveness, and high frequency of RET-PTC3 rearrangements were features of radiation-induced tumors (5,6,8). These features change with increasing latency and age (11,16,21). We set out to determine whether radiation-induced thyroid carcinomas differ in morphology and aggressiveness from non-radiation-induced tumors.
Materials and Methods
Three groups of PTCs from unexposed children under 15 were studied: U1, all 23 available childhood PTCs from Belarus, Ukraine, and the Russian Federation, born more than 6 months after the accident; U2, 18 cases of childhood PTCs from England and Wales (E&W), selected from a national survey for the age distribution found in the exposed group; and U3, 27 unselected cases of childhood PTCs from Japan, representing all available cases from three hospitals. Two groups of exposed cases were selected from a previous study (14): Ec, all 84 exposed cases within the same age range as the unexposed cases studied, and Es, 23 exposed cases age and sex matched to unexposed cases from the same geographical region (U1). (Table 1) Appropriate informed consent was obtained for all tissues studied, and the study was approved by the relevant institutional review boards. The statistical analyses were carried out by S.L.V.
Table 1.
Details of Patients in the Five Groups
| Group | Ec | Es | U1 | U2 | U3 |
|---|---|---|---|---|---|
| Number | 84 | 23 | 23 | 18 | 27 |
| F:M | 1.5:1 | 4.75:1 | 4.75:1 | 3.5:1 | 4.4:1 |
| Age at operation, mean | 11.2 | 9.6 | 9.8 | 11.1 | 11.9 |
| Age at operation, median | 12.7 | 8.4 | 9.0 | 11.5 | 12.0 |
| Age at exposure, median | 1.49 | 1.48 | −2.64 |
Note that the Es group was selected from among exposed patients to match the unexposed group from the same area (U1) for age and gender.
Ec: exposed, control; Es: exposed, selected.
The extent and type of tumor differentiation and of invasion were quantitatively assessed using previously reported techniques (16). Eight pathologists with a thyroid carcinoma interest assessed sections from each case using an agreed protocol. Four differentiation patterns—two less mature (solid and trabecular), and two more mature (papillary and follicular), scored as absent, minor (<10%), moderate (11–50%), or dominant (51–100%)—were analyzed using the midrange score for each component, corrected to bring the total to 100%. Mean scores for all participants were used for each tumor. Intratumor and peritumor (capsular) fibrosis and intrathyroid invasion were each scored as absent [0], moderate [1], or marked [2]; extrathyroid invasion was recorded as absent, connective tissue invasion, muscle invasion, or not assessable. For each variable, groups were compared using the Kruskal–Wallis test. Where this was significant the Mann–Whitney U was used in post hoc tests. For extrathyroid invasion the modal class for each tumor was determined, that is, the category listed by the largest number of observers.
We aimed to achieve a consensus estimate of morphological features requiring a subjective assessment, not a comparison of the reproducibility of individual assessors results. However, multirater kappa coefficients showed that all values (except for the trabecular component present in very small amounts) lay between 0.40 and 0.56, a moderate level of agreement.
Results
Group characteristics
Unexposed 1 (U1). PTCs from 23 unexposed children from the area around Chernobyl, born more than 6 months after the accident: 19 females and 4 males, mean age at operation 9.86 years (median 9.0; F:M, 4.75:1).
Exposed, selected (Es). PTCs from 23 exposed children, 19 females and 4 males chosen to match U1 for age and sex, mean age at operation 9.68 years (median 8.4; F:M, 4.75:1).
Exposed, control (Ec). PTCs from 84 exposed children with the same age range as unexposed cases, mean age at operation 11.24 years (median 12.7; F:M, 1.5:1).
U2. PTCs from 18 children from E&W, mean age 11.1 years (median 11.5; F:M, 3.5:1).
U3. PTCs from 27 children from Japan, mean age 11.9 years (median 12.0; F:M, 4.4:1).
The sex ratios of the three unexposed groups were similar; the sex ratio for all unexposed cases combined was significantly different from the exposed group (Ec) (4.2:1 vs. 1.5:1; χ2 = 7.90, p ≤ 0.01005).
Level of differentiation
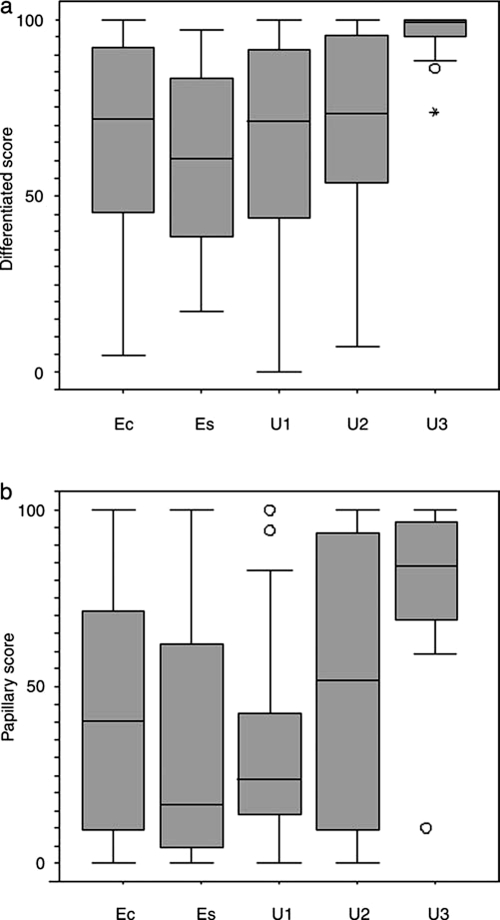
Tumors from unexposed children from countries around Chernobyl showed very similar quantitative results to both the age- and sex-matched exposed group and to all 84 cases from exposed children. Less mature areas (solid and trabecular combined) formed a mean of 36.9% (median 28.96) of the tumor area in group U1, compared to 34.12% (median 28.3) in group Ec and 39.44 (median 39.3) in group Es. Unexposed cases from E&W showed a slight reduction in the amount of less mature tumor (mean 27.3%, median 26.6), while the unexposed tumors from Japan showed a significant reduction (mean 3.98%, median 0.8; U3 vs. Ec, U = 254, p < 0.0001); almost all Japanese cases were dominated by well-differentiated tumor (Figs. 1a & 2).
FIG. 1.
Tumor differentiation. (a) Extent of tumor differentiation (papillary and follicular combined), expressed as % of tumor area, showing the similarity between the two exposed groups (exposed, control [Ec], and exposed, selected [Es]), and the unexposed group from the same geographical area (U1), and the considerable increase in the % of differentiation in tumors of group U3 from Japan (U3 vs. Ec; p < 0.0001). (b) Type of differentiation, expressed as % of differentiated tumor (papillary and follicular patterns combined); papillary differentiation is shown. There is a close similarity between the exposed and unexposed groups from the same area, but a considerable increase in the % of the papillary component in the tumors of group U3 from Japan (U3 vs. Ec; p < 0.0001). In both (a) and (b), the England and Wales findings were intermediate between the Chernobyl and the Japanese cases. The whiskers are lines that extend from the box to the highest and lowest values, excluding outliers. A line across the box indicates the median. Circles represent outliers (cases between 1.5 and 3 box lengths from the upper or lower end of the box). Stars represent extremes (cases that are beyond 3 box lengths from the upper or lower end of the box).
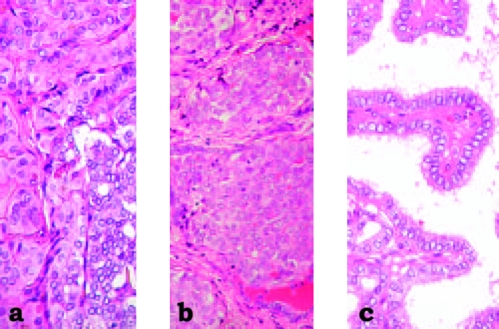
FIG. 2.
Tumor morphology of papillary thyroid carcinomas. (a) and (b) show representative tumors from the Chernobyl area (a) exposed and (b) unexposed to radiation. (c) shows a typical childhood tumor from Japan, almost entirely composed of well-differentiated papillae, contrasting with the more solid less mature tumors found in children from the areas around Chernobyl.
Type of differentiation
This is expressed as the proportion that each differentiated component forms of the sum of the two differentiated components, follicular and papillary. Tumors from areas around Chernobyl were broadly similar whether exposed to radiation or not, with a mean of 41.7% papillary differentiation (median 40.3) in all exposed children together, 52.3% in the matched exposed group (median 16.5), and 55.7% in the unexposed children from the same area (median 23.9). The other unexposed groups showed more papillary differentiation, 70.9% (median 51.6) in children from E&W (U2 vs. Ec, U = 601, p = 0.21), and considerably more, 84.1% (median 84.2), in Japanese children (U3 vs. Ec, U = 353.5, p < 0.0001) (Figs. 1b & 2).
Fibrotic response
Intratumor fibrosis was less marked in Japanese tumors (median score 0.75) than in tumors from E&W (0.94), both were less than any of the groups from the Chernobyl area, unexposed (U1, 1.13) or exposed (Ec, 1.13, Es, 1.13); there was no evidence that the differences reached significance. There was no significant difference in the extent of peritumor (capsular) fibrosis between any of the unexposed groups (χ2kw = 2.95, df = 2, p = 0.23) (Fig. 3).
FIG. 3.
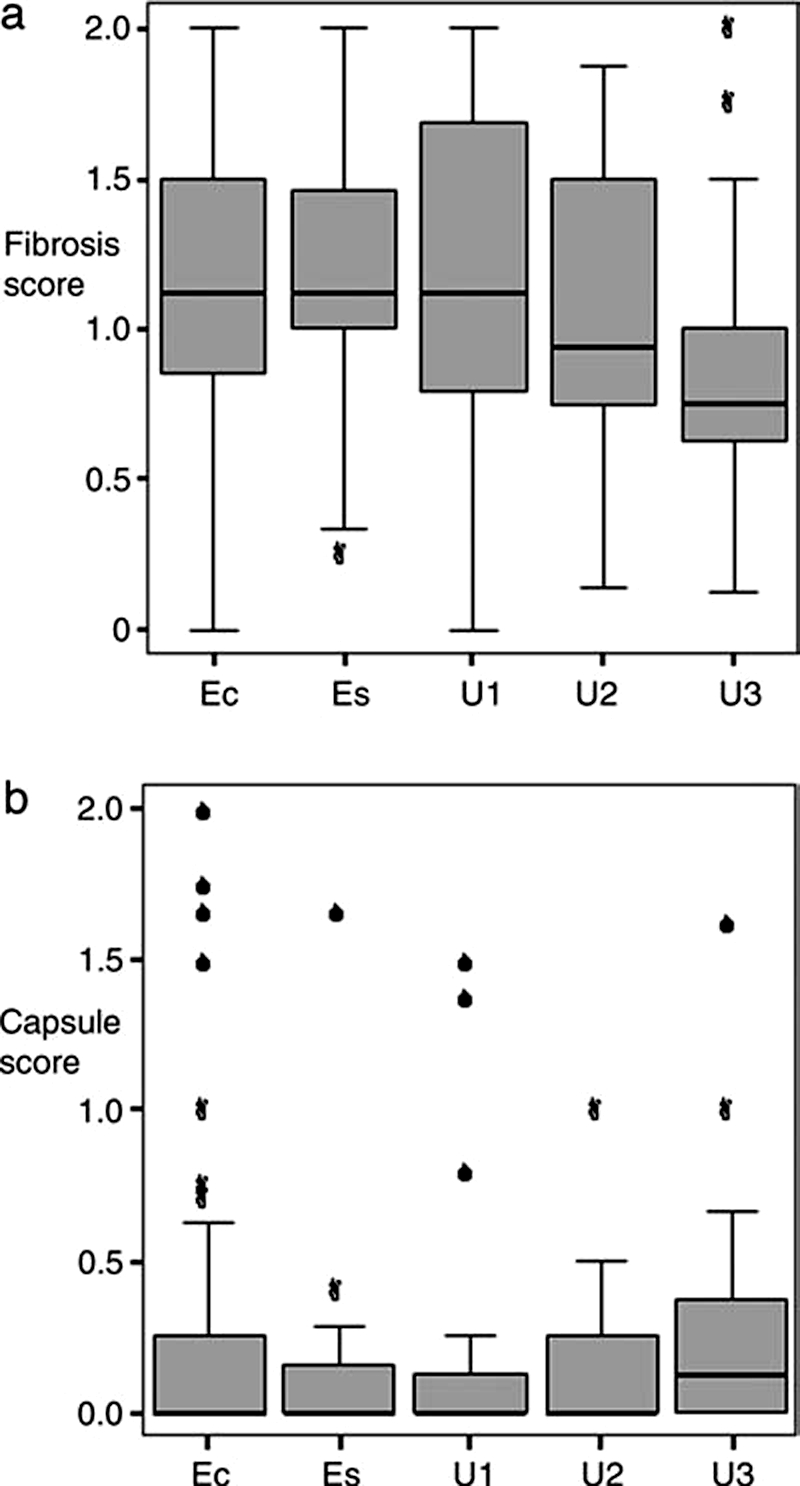
Tumor fibrosis. (a) Extent of intratumor fibrosis and (b) extent of peritumor (capsular) fibrosis. While the change in the extent of intratumor or capsular fibrosis in the tumors from Japan as compared to the tumors from the fallout areas did not reach significance, the trend in both was that expected to be associated with a reduction in aggressiveness. In both (a) and (b), the England and Wales findings were intermediate between the Chernobyl and the Japanese cases. The whiskers are lines that extend from the box to the highest and lowest values, excluding outliers. A line across the box indicates the median. Filled circles represent extremes (cases that are beyond 3 box lengths from the upper or lower end of the box).
Invasion
There was no significant difference in intrathyroid invasion between any unexposed group (U1, 1.43; U2, 1.38; U3, 1.43), or between these and exposed children (Ec 1.50, Es, 1.57).
Extrathyroid invasion was present in 57% of all exposed children (Ec), 62% in group Es, age matched to U1, and 65% of group U1. The frequency in children from E&W (67%) was similar, but extrathyroid invasion was much less frequent in Japanese children (30%, U3 vs. Ec, χ2 = 7.66, p ≤ 0.01006). Direct invasion of muscle was present in 8% of group Ec, 14% of Es, 13% of U1, 11% of U2, and 0% of U3.
Discussion
The great increase in thyroid carcinoma incidence in areas exposed to high levels of Chernobyl fallout is most marked in those who were young children when exposed. Their thyroid dose is higher than adults, due to increased dietary intake from milk and increased thyroid uptake. Children are known to be more susceptible to the carcinogenic effect of thyroid X-radiation (22). To determine whether there are specific morphologic and behavioral characteristics of radiation-induced thyroid tumors, we have compared the findings in unexposed children from Belarus, Ukraine, and the Russian Federation with those in an age- and sex-matched subgroup of exposed children, and with a larger exposed group. The exposed and unexposed tumors from the same geographical area are essentially identical morphologically and in their degree of aggressiveness. There were, however, striking differences between tumors from around Chernobyl and tumors from E&W or Japan. Tumors from Japanese children were very different from tumors from countries close to Chernobyl, whether exposed or unexposed, showing significantly more overall differentiation, more papillary differentiation, and less extrathyroid invasion, while tumors from E&W were generally intermediate. The changes in intratumor fibrosis and capsule formation did not reach significance, but showed the same trends as other features.
We considered the possibility that children from the Chernobyl region born more than 6 months after the accident were in fact exposed to a carcinogenic level of radiation. The fetal thyroid gland begins to concentrate iodine at about 12–14 weeks, the two unexposed cases in utero at the time of the accident were both less than 8 weeks gestational age. Iodine-131, generally accepted as the cause of the increased thyroid carcinoma incidence (3,4,21), rapidly disappeared from the environment (half-life 8.1 days). Cesium (Cs) isotopes have a much longer half-life, and some contamination with radioactive Cs persists. However, the Cs activity released was more than an order of magnitude less than radioiodine, and Cs is not concentrated in the thyroid. The incidence of thyroid carcinoma fell very rapidly in those born after the accident, reaching levels close to normal, and the geographical distribution of unexposed cases does not show the association with fallout levels seen in exposed cases. The sex ratio in unexposed children with thyroid cancer from Belarus, Ukraine, and the Russian Federation is similar to that of other unexposed cases, and differs from that of exposed children. We conclude that radiation due to fallout from Chernobyl is not relevant to the findings in children born after the accident.
We have shown that radiation-induced childhood PTCs from countries around Chernobyl are morphologically indistinguishable from non-radiation-induced PTCs from the same areas. However, compared to two other countries these tumors are the least differentiated, show the least papillary differentiation, and are the most aggressive, while tumors from Japan are the most differentiated, show the most papillary differentiation, and are the least aggressive; tumors from E&W generally show intermediate features. The areas around Chernobyl are iodine deficient; the urinary iodide excretion in Gomel, the most heavily exposed oblast in Belarus, was found to be 41 μG/L (23). In contrast, Japan is one of the most iodine-rich countries (urinary iodide up to >1000 μG/L (22), while E&W have an intermediate intake (urinary iodide 100–200 μG/day) (23–25). Our findings, therefore, are compatible with the possibility that papillary carcinomas developing in children in iodine-deficient areas are more aggressive and less differentiated than those from iodide-rich areas. This could be mediated through thyroid-stimulating hormone (e.g., an effect on promoters of rearranged genes), or be a direct effect of iodide. We cannot exclude an influence of ethnic or other factors, but the possibility that changes in dietary iodide are responsible is strengthened by evidence that dietary iodide levels also influence the incidence of Chernobyl-related PTCs (26,27). The higher proportion of less mature, more aggressive PTCs occurring in the iodine-deficient areas around Chernobyl could well be relevant to the unexpectedly short latency seen after Chernobyl. Tumors with this phenotype in unexposed children occurred predominantly in younger cases, again suggesting a link to short latency. The possible role of iodine intake could be further explored by comparing the morphology and molecular pathology of papillary carcinomas occurring before and after dietary iodination in iodine-deficient countries. Material for molecular analysis was unfortunately not available from the tumors in the present study, so we were unable to ascertain whether the changes in morphologic type frequency reflected changes in oncogene frequency.
In a nonirradiated population dietary iodine deficiency is associated with an increase of the relative incidence of follicular carcinoma. The dominance of PTCs in the exposed population since the Chernobyl accident probably relates to the ability of radiation to produce double-strand DNA breaks, necessary precursors of the rearrangements found in many PTCs. Although BRAF point mutations are uncommon in childhood thyroid carcinoma, rearranged BRAF has been found in 3 of 28 short latency thyroid cancers in children exposed to Chernobyl (20). The PAX8-PPARγ rearrangement found in some follicular adenomas and carcinomas (28) could also be radiation induced, although unlike RET rearrangements it is probably not an early event in tumorogenesis. External radiation causes both papillary and follicular tumors of the thyroid; follicular adenomas show a particularly long mean latent period (29). An increase in follicular carcinomas may yet occur in the exposed population, and BRAF point mutations may occur more frequently in papillary carcinomas as the cohort of those irradiated in infancy ages and the latent period increases.
Previous studies of radiation-induced thyroid carcinomas have drawn widely differing conclusions about gender-associated risks. We find that the F:M ratio in exposed children with PTC is 1.5:1, while in unexposed children it is 4.2:1. Further analysis shows that the ratio for exposed children changes with latency (<10 years vs. >10, 1.3:1 vs. 1.9:1), similar to the findings (1.5:1 vs. 1.9:1) in a study of 191 exposed cases (11). The changes were not accounted for by changes in the type of RET rearrangement. The ratio also changes with age at operation; comparing exposed to unexposed cases, we find the F:M ratio for children under 10 is 1.3:1 vs. 2.4:1, and for over 10 is 1.6:1 vs. 5.2:1. Latency and age are not independent variables. These findings could represent a much greater relative risk for males than females, as discussed by Jacob et al. (30). They are also consistent with an approximately equal excess absolute risk for the sexes, with the proportion of sporadic cases increasing with age. A shorter latent period in males is another possible relevant factor. It is clearly important to continue to follow the effects of the Chernobyl accident for many reasons, including studies of sex ratio changes with increasing latency.
Exposure to fallout from the Chernobyl accident has so far induced specifically papillary carcinomas of the thyroid (21). The present study shows that the morphology and aggressiveness of PTCs in children exposed to fallout from the Chernobyl accident are indistinguishable from PTCs in the unexposed population from the same area. There are significant differences between both these groups and unexposed tumors from other countries, particularly Japan. While ethnic and other factors cannot be excluded, the large difference in dietary iodine intake between these populations is a possible cause. The link between iodine deficiency and less mature, more aggressive PTCs could be a major factor in the unexpectedly short latent period after Chernobyl, as these tumors formed the majority of the cases with a short latency. Dietary iodine status is important in thyroid cancer susceptibility after fallout exposure; these findings reinforce the need to prevent iodine deficiency generally.
Acknowledgments
We are grateful to the European Commission, the United States National Cancer Institute, the World Health Organisation, and the Sasakawa Memorial Health Foundation for their financial support to the Chernobyl Tumour Bank, which provided the infrastructure on which this work depended, and to the people and Governments of Belarus, the Russian Federation, and Ukraine. We thank all those who have helped in the collection of the material, particularly Kuma Hospital (Kobe, Japan) and Ito Hospital (Tokyo, Japan) for providing the Japanese cases. We are also grateful to Professor Nick Day for advice and to the Rockefeller Foundation for their support of this study.
References
- 1.Kazakov VS. Demidchik EP. Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 2.Baverstock K. Egloff B. Pinchera A. Williams D. Thyroid cancer after Chernobyl. Nature. 1992;359:21–22. doi: 10.1038/359021b0. [DOI] [PubMed] [Google Scholar]
- 3.Williams ED. International Atomic Energy Authority; Vienna: 1996. Effects on the Thyroid in Populations Exposed to Radiation as a Result of the Chernobyl Accident., In One Decade after Chernobyl; pp. 207–230. [Google Scholar]
- 4.Annex J. UNSCEAR Report. United Nations. Vol. 2. New York and Geneva: 2000. [Google Scholar]
- 5.Furmanchuk AW. Averkin JI. Egloff B. Ruchti C. Abelin T. Schappi W. Korotkevich EA. Pathomorphological findings in thyroid cancers of children from the Republic of Belarus. Histopathology. 1992;21:401–408. doi: 10.1111/j.1365-2559.1992.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 6.Nikiforov Y. Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster. Cancer. 1994;74:748–766. doi: 10.1002/1097-0142(19940715)74:2<748::aid-cncr2820740231>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Harach HR. Williams ED. Childhood thyroid cancer in England and Wales. Br J Cancer. 1995;72:777–783. doi: 10.1038/bjc.1995.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikiforov YE. Rowland JM. Bore KE. Montfort-Mungo H. Fagin JA. Distinct pattern of RET oncogene rearrangements in morphological variants of radiation induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 9.Thomas GA. Bunnell H. Cook HA. Williams ED. Nerovnya A. Cherstvoy ED. Bogdanova TI. Chiappetta G. Viglietto G. Pentimalli F. Salvatore G. Fusco A. Vecchio G. High prevalence of RET PTC rearrangements in Ukrainian and Belarussian post Chernobyl thyroid papillary carcinomas: a strong correlation between RET-PTC 3 and the solid follicular variant. J Clin Endocrinol Metab. 1999;84:4232–4238. doi: 10.1210/jcem.84.11.6129. [DOI] [PubMed] [Google Scholar]
- 10.Santoro M. Carlomagno F. Hay ID. Herrman NA. Grieco M. Melillo R. Pierotti MA. Bongarzone I. DellaPorta G. Berger N. Peix JL. Fabien N. Vecchio G. Jenkins RB. Fusco A. RET oncogene activation in human thyroid neoplasms is restricted to the papillary carcinoma subtype. J Clin Invest. 1992;89:1517–1522. doi: 10.1172/JCI115743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabes HM. Demidchik EP. Siderow JD. Lengfelder E. Beimfohr C. Hoelzel D. Klugbauer S. Pattern of radiation induced RET and NTRK1 rearrangements in 191 post Chernobyl papillary carcinomas: biologic, phenotypic and clinical implications. Clin Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 12.Kimura ET. Nikiforova MN. Zhu Z. Knauf JA. Nikiforov YE. Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 13.Soares P. Trovisco V. Rocha AS. Lima J. Castro P. Preto A. Máximo V. Botelho T. Seruca R. Sobrinho-Simões M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 14.Cohen Y. Xing M. Mambo E. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 15.Sankaranarayanan K. Ionizing radiation and genetic risks. Mutat Res. 1991;258:75–97. doi: 10.1016/0165-1110(91)90029-u. [DOI] [PubMed] [Google Scholar]
- 16.Williams ED. Abrosimov A. Bogdanova T. Demidchik EP. Ito M. LiVolsi V. Lushnikov E. Rosai J. Sidorov Y. Tronko MD. Tsyb AF. Vowler SL. Thomas GA. Thyroid carcinoma after Chernobyl, latent period, morphology and aggressiveness. Br J Cancer. 2004;90:2219–2224. doi: 10.1038/sj.bjc.6601860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima J. Trovisco V. Soares P. Máximo V. Magalhães J. Salvatore G. Santoro M. Bogdanova T. Tronko M. Abrosimov A. Jeremiah S. Thomas G. Williams D. Sobrinho-Simões M. BRAF mutations are not a major event in post-Chernobyl childhood thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:4267–4271. doi: 10.1210/jc.2003-032224. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforova MN. Ciampi R. Salvatore G. Santoro M. Gandhi M. Knauf JA. Thomas GA. Jeremiah S. Bogdanova TI. Tronko MD. Fagin JA. Nikiforov YE. Low prevalence of BRAF mutations in radiation-induced thyroid tumors in contrast to sporadic papillary carcinomas. Cancer Lett. 2004;209:1–6. doi: 10.1016/j.canlet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Powell N. Jeremiah S. Morishita M. Dudley E. Bethel J. Bogdanova T. Tronko M. Thomas G. Frequency of BRAF T1796A mutation relates to age of patient at diagnosis and not to radiation exposure. J Pathol. 2005;205:558–564. doi: 10.1002/path.1736. [DOI] [PubMed] [Google Scholar]
- 20.Ciampi R. Knauf JA. Kerler R. Gandhi M. Zhu Z. Nikiforova MN. Rabes HM. Fagin JA. Nikiforov YE. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams ED. Cancer after nuclear fallout: lessons from the Chernobyl accident. Nat Rev Cancer. 2002;2:543–549. doi: 10.1038/nrc845. [DOI] [PubMed] [Google Scholar]
- 22.Ron E. Lubin JH. Shore RE. Mabuchi K. Modan B. Pottern LM. Schneider AB. Tucker MA. Boice JD., Jr Thyroid cancer after exposure to external radiation, a pooled analysis of 7 studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 23.Ishigaki K. Namba H. Takamura N. Saiwai H. Parshin V. Ohashi T. Kanematsu T. Yamashita S. Urinary iodine levels and thyroid disease in children; comparison between Nagasaki and Chernobyl. Endocr J. 2001;48:591–595. doi: 10.1507/endocrj.48.591. [DOI] [PubMed] [Google Scholar]
- 24.Lee SM. Lewis J. Buss DH. Holcombe GD. Lawrance PR. Iodine in British foods and diets. Br J Nutr. 1994;72:435–446. doi: 10.1079/bjn19940045. [DOI] [PubMed] [Google Scholar]
- 25.Vitti P. Delange F. Pinchera A. Zimmerman M. Dunn JT. Europe is iodine deficient. Lancet. 2003;361:1226. doi: 10.1016/S0140-6736(03)12935-2. [DOI] [PubMed] [Google Scholar]
- 26.Shakhtarin VV. Tsyb AF. Stepanenko VF. Orlov MY. Kopecky KJ. Davis S. Iodine deficiency, radiation dose, and the risk of thyroid cancer among children and adolescents in the Bryansk region of Russia following the Chernobyl power station accident. Int J Epidemiol. 2003;32:484–491. doi: 10.1093/ije/dyg205. [DOI] [PubMed] [Google Scholar]
- 27.Cardis E. Kesminiene A. Ivanov V. Malakhova I. Shibata Y. Khrouch V. Drozdovitch V. Maceika E. Zvonova I. Vlassov O. Bouville A. Goulko G. Hoshi M. Abrosimov A. Anoshko J. Astakhova L. Chekin S. Demidchik E. Galanti R. Ito M. Korobova E. Lushnikov E. Maksioutov M. Masyakin V. Nerovnia A. Parshin V. Parshkov E. Piliptsevich N. Pinchera A. Polyakov S. Shabeka N. Suonio E. Tenet V. Tsyb A. Yamashita S. Williams D. Risk of thyroid carcinoma after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 28.Cheung L. Messina M. Gill A. Detection of the PAX8-PPAR gamma fusion oncogene in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2003;88:354–357. doi: 10.1210/jc.2002-021020. [DOI] [PubMed] [Google Scholar]
- 29.Shore RE. Hildreth N. Dvoretsky P. Pasternack B. Andressa E. Benign thyroid adenomas among persons irradiated in infancy for enlarged thymus glands. Radiat Res. 1993;134:217–223. [PubMed] [Google Scholar]
- 30.Jacob P. Bogdanova TI. Buglova E. Chepurniy M. Demidchik Y. Gavrilin Y. Kenigsberg J. Meckbach R. Schotola C. Shinkarev S. Tronko MD. Ulanovsky A. Vavilov S. Walsh L. Thyroid cancer risk in areas of Ukraine and Belarus affected by the Chernobyl accident. Radiat Res. 2006;165:1–8. doi: 10.1667/rr3479.1. [DOI] [PubMed] [Google Scholar]