Abstract
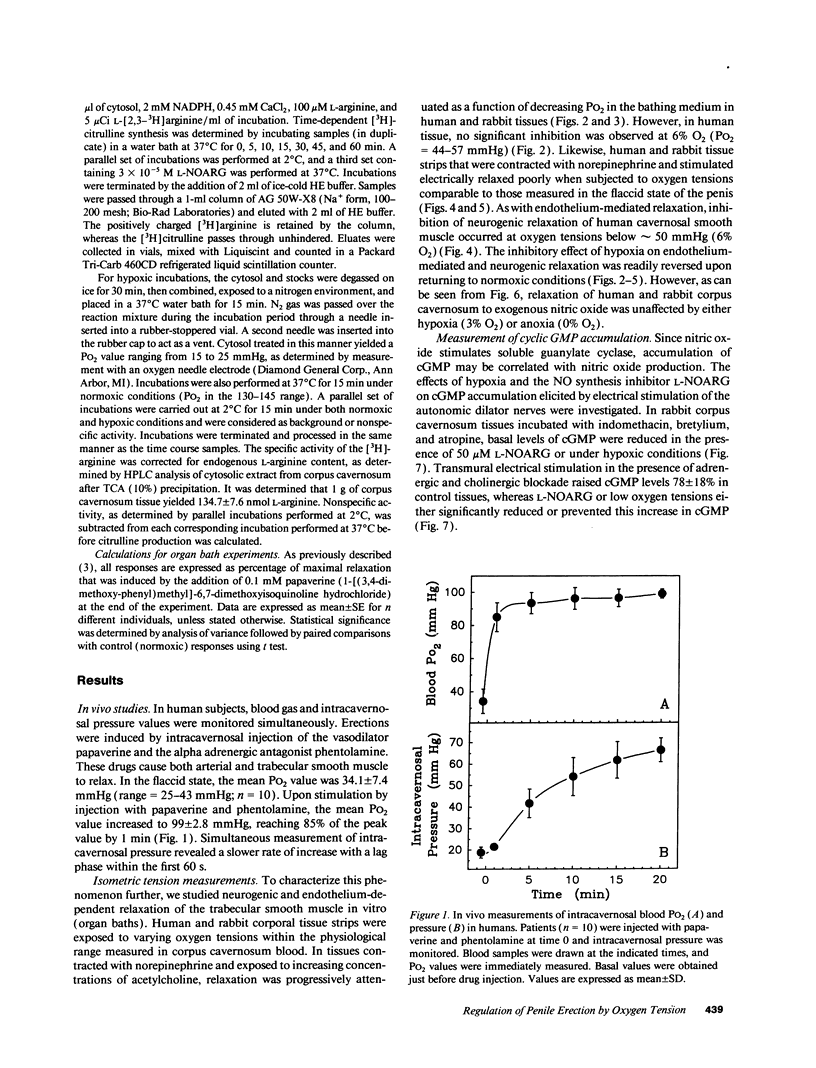
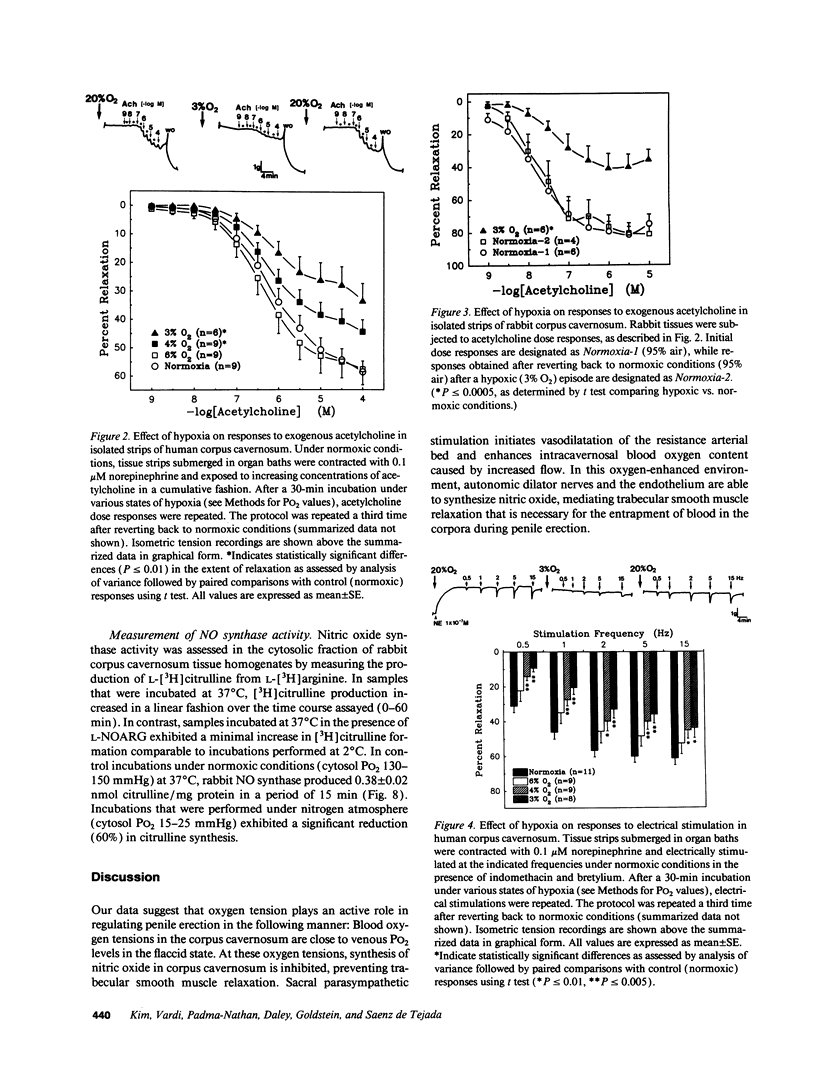
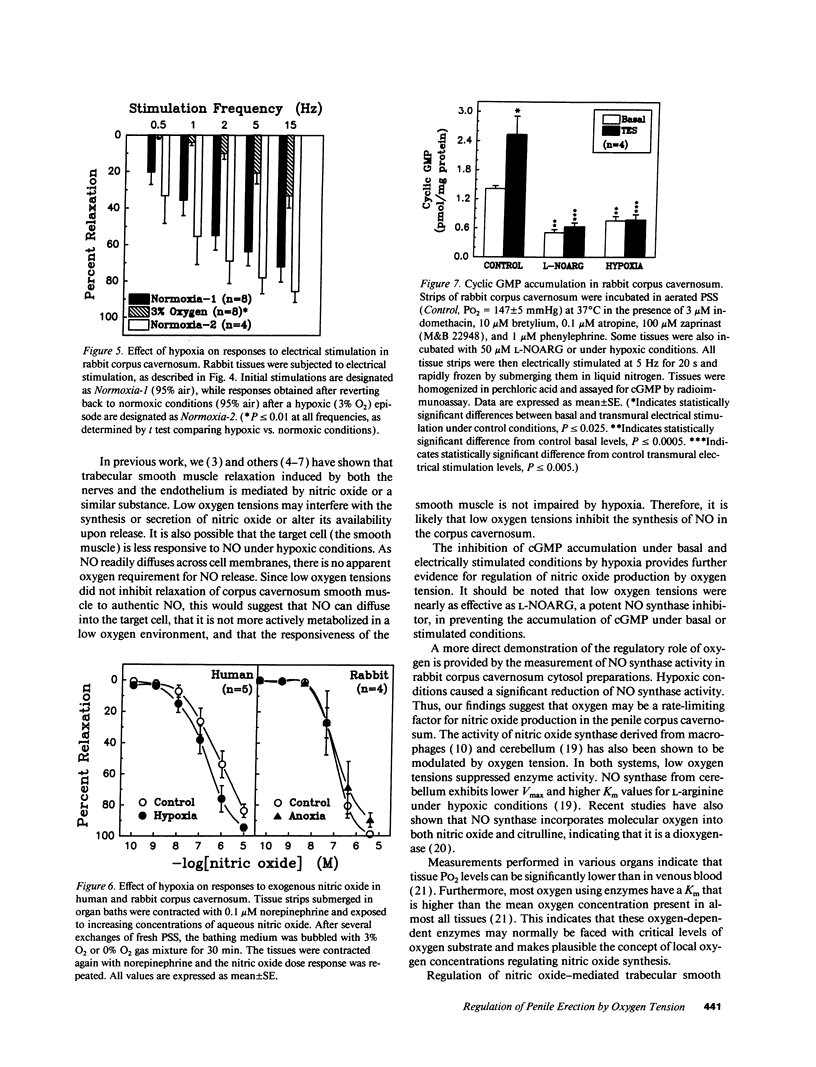
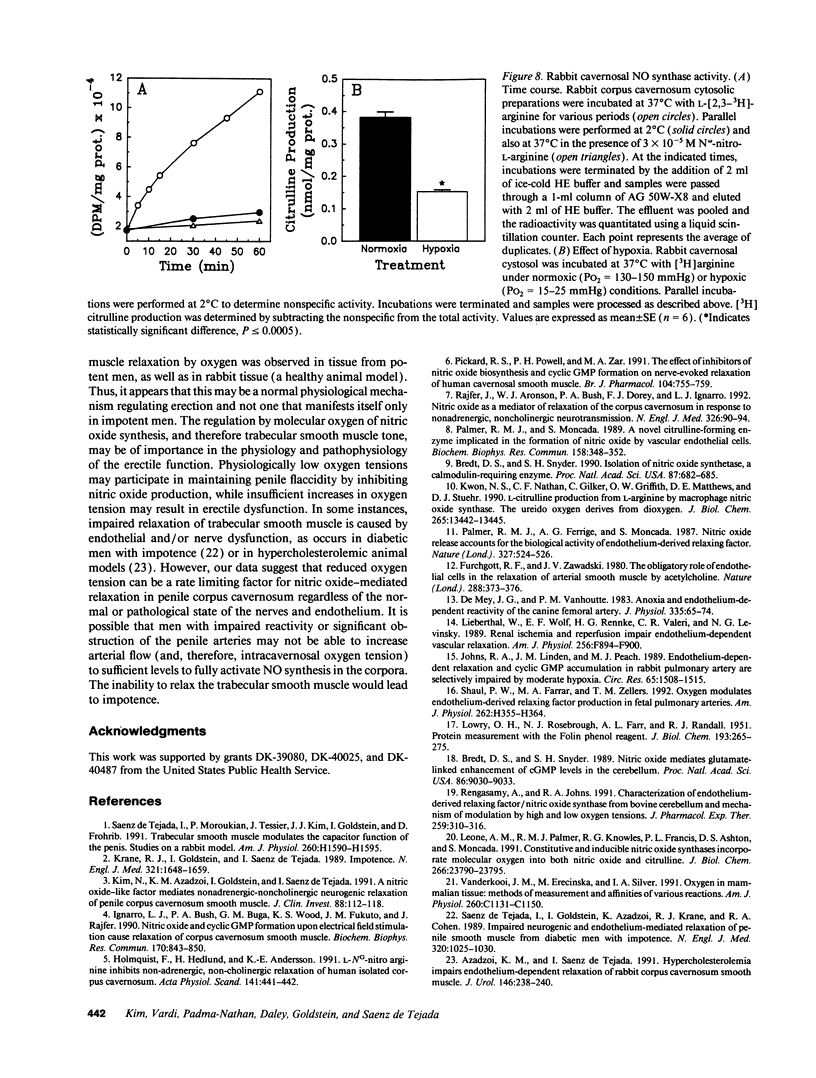
Relaxation of the trabecular smooth muscle of the corpus cavernosum (the erectile tissue) of the penis is mediated by nitric oxide released by the nerves and endothelium. We have investigated the physiological role of oxygen tension in the regulation of trabecular smooth muscle tone. In human subjects, measurement of intracavernosal PO2 in blood drawn from corpus cavernosum in the flaccid state was comparable to that of venous blood (25-43 mmHg). Vasodilatation of the resistance arteries and trabecular smooth muscle relaxation by intracavernosal injection of papaverine and phentolamine caused oxygen tension to rise rapidly to arterial levels (PO2 approximately 100 mmHg). Isolated human and rabbit corpus cavernosum tissue strips in organ baths, exposed to arterial-like PO2 relaxed to the endothelium-dependent dilator acetylcholine and to electrical stimulation of the autonomic dilator nerves. These nitric oxide-mediated responses were progressively inhibited as a function of decreasing PO2 to levels measured in the flaccid penis. Reverting to normoxic conditions readily restored endothelium-dependent and neurogenic relaxation. Relaxation to exogenous nitric oxide was not impaired in low PO2. In rabbit corpus cavernosum, low PO2 reduced basal levels of cGMP and prevented cGMP accumulation induced by stimulation of dilator nerves. Furthermore, low PO2 inhibited nitric oxide synthase activity in corpus cavernosum cytosol. It is concluded that physiological concentrations of oxygen modulate penile erection by regulating nitric oxide synthesis in corpus cavernosum tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azadzoi K. M., Saenz de Tejada I. Hypercholesterolemia impairs endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J Urol. 1991 Jul;146(1):238–240. doi: 10.1016/s0022-5347(17)37759-5. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983 Feb;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Holmquist F., Hedlund H., Andersson K. E. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxation of human isolated corpus cavernosum. Acta Physiol Scand. 1991 Mar;141(3):441–442. doi: 10.1111/j.1748-1716.1991.tb09103.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Bush P. A., Buga G. M., Wood K. S., Fukuto J. M., Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990 Jul 31;170(2):843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Johns R. A., Linden J. M., Peach M. J. Endothelium-dependent relaxation and cyclic GMP accumulation in rabbit pulmonary artery are selectively impaired by moderate hypoxia. Circ Res. 1989 Dec;65(6):1508–1515. doi: 10.1161/01.res.65.6.1508. [DOI] [PubMed] [Google Scholar]
- Kim N., Azadzoi K. M., Goldstein I., Saenz de Tejada I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J Clin Invest. 1991 Jul;88(1):112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane R. J., Goldstein I., Saenz de Tejada I. Impotence. N Engl J Med. 1989 Dec 14;321(24):1648–1659. doi: 10.1056/NEJM198912143212406. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Gilker C., Griffith O. W., Matthews D. E., Stuehr D. J. L-citrulline production from L-arginine by macrophage nitric oxide synthase. The ureido oxygen derives from dioxygen. J Biol Chem. 1990 Aug 15;265(23):13442–13445. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leone A. M., Palmer R. M., Knowles R. G., Francis P. L., Ashton D. S., Moncada S. Constitutive and inducible nitric oxide synthases incorporate molecular oxygen into both nitric oxide and citrulline. J Biol Chem. 1991 Dec 15;266(35):23790–23795. [PubMed] [Google Scholar]
- Lieberthal W., Wolf E. F., Rennke H. G., Valeri C. R., Levinsky N. G. Renal ischemia and reperfusion impair endothelium-dependent vascular relaxation. Am J Physiol. 1989 May;256(5 Pt 2):F894–F900. doi: 10.1152/ajprenal.1989.256.5.F894. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Pickard R. S., Powell P. H., Zar M. A. The effect of inhibitors of nitric oxide biosynthesis and cyclic GMP formation on nerve-evoked relaxation of human cavernosal smooth muscle. Br J Pharmacol. 1991 Nov;104(3):755–759. doi: 10.1111/j.1476-5381.1991.tb12500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfer J., Aronson W. J., Bush P. A., Dorey F. J., Ignarro L. J. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992 Jan 9;326(2):90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- Rengasamy A., Johns R. A. Characterization of endothelium-derived relaxing factor/nitric oxide synthase from bovine cerebellum and mechanism of modulation by high and low oxygen tensions. J Pharmacol Exp Ther. 1991 Oct;259(1):310–316. [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Farrar M. A., Zellers T. M. Oxygen modulates endothelium-derived relaxing factor production in fetal pulmonary arteries. Am J Physiol. 1992 Feb;262(2 Pt 2):H355–H364. doi: 10.1152/ajpheart.1992.262.2.H355. [DOI] [PubMed] [Google Scholar]


