Abstract
BACKGROUND
We investigated whether combination therapy with a statin plus a fibrate, as compared with statin monotherapy, would reduce the risk of cardiovascular disease in patients with type 2 diabetes mellitus who were at high risk for cardiovascular disease.
METHODS
We randomly assigned 5518 patients with type 2 diabetes who were being treated with open-label simvastatin to receive either masked fenofibrate or placebo. The primary outcome was the first occurrence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. The mean follow-up was 4.7 years.
RESULTS
The annual rate of the primary outcome was 2.2% in the fenofibrate group and 2.4% in the placebo group (hazard ratio in the fenofibrate group, 0.92; 95% confidence interval [CI], 0.79 to 1.08; P = 0.32). There were also no significant differences between the two study groups with respect to any secondary outcome. Annual rates of death were 1.5% in the fenofibrate group and 1.6% in the placebo group (hazard ratio, 0.91; 95% CI, 0.75 to 1.10; P = 0.33). Prespecified subgroup analyses suggested heterogeneity in treatment effect according to sex, with a benefit for men and possible harm for women (P = 0.01 for interaction), and a possible interaction according to lipid subgroup, with a possible benefit for patients with both a high baseline triglyceride level and a low baseline level of high-density lipoprotein cholesterol (P = 0.057 for interaction).
CONCLUSIONS
The combination of fenofibrate and simvastatin did not reduce the rate of fatal cardiovascular events, nonfatal myocardial infarction, or nonfatal stroke, as compared with simvastatin alone. These results do not support the routine use of combination therapy with fenofibrate and simvastatin to reduce cardiovascular risk in the majority of high-risk patients with type 2 diabetes. (ClinicalTrials.gov number, NCT00000620.)
Patients with type 2 diabetes mellitus have an increased incidence of atherosclerotic cardiovascular disease.1–4 This increase is attributable, in part, to associated risk factors, including hypertension and dyslipidemia. The latter is characterized by elevated plasma triglyceride levels, low levels of high-density lipoprotein (HDL) cholesterol, and small, dense low-density lipoprotein (LDL) particles.5,6 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study was designed to test the effect of intensive treatment of blood glucose and either blood pressure or plasma lipids on cardiovascular outcomes in 10,251 patients with type 2 diabetes who were at high risk for cardiovascular disease. Here we present the findings of the ACCORD lipid trial (ACCORD Lipid).
Although statins are efficacious in patients with type 2 diabetes, rates of cardiovascular events remain elevated in such patients even after statin treatment.7–9 Fibrate therapy in patients with type 2 diabetes reduced the rate of coronary heart disease events in the Veterans Affairs HDL Intervention Trial (VA-HIT; ClinicalTrials.gov number, NCT00035711)10 but not in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial (Current Controlled Trials number, ISRCTN64783481).11 However, a post hoc analysis of data from the FIELD study suggested a benefit for patients with both elevated triglyceride levels and low HDL cholesterol levels.12 Previous fibrate studies in subjects with diabetes10,11 or in those without diabetes13–15 did not address the role of such drugs in patients receiving statin therapy. The hypothesis that we tested in ACCORD Lipid was that in high-risk patients with type 2 diabetes, combination treatment with a fibrate (both to raise HDL cholesterol levels and to lower triglyceride levels) and a statin (to reduce LDL cholesterol levels) would reduce the rate of cardiovascular events, as compared with treatment with a statin alone.
METHODS
STUDY DESIGN
The rationale and designs for the various components of ACCORD have been reported previously.16–20 The ACCORD study was a randomized trial conducted at 77 clinical sites organized into seven networks in the United States and Canada. (For a full list of participating institutions and investigators, see Section 20 in Supplementary Appendix 1, available with the full text of this article at NEJM.org The trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI), and the protocol was approved by a review panel at the NHLBI, as well as by the institutional review board or ethics committee at each center.
In the ACCORD study, all patients were randomly assigned to receive either intensive glycemic control (targeting a glycated hemoglobin level below 6.0%) or standard therapy (targeting a glycated hemoglobin level of 7.0 to 7.9%). The results of this comparison have been reported previously.20 A subgroup of patients in the ACCORD study were also enrolled in the ACCORD Lipid trial and underwent randomization, in a 2-by-2 factorial design, to receive simvastatin plus either fenofibrate or placebo. Randomization occurred between January 11, 2001, and October 29, 2005. End-of-study visits were scheduled between March and June 2009. Additional details regarding the trial protocol and amendments are provided in Supplementary Appendix 2, also available with the full text of this article at NEJM.org.
ELIGIBILITY
All patients in the ACCORD study had type 2 diabetes and a glycated hemoglobin level of 7.5% or more. If patients had evidence of clinical cardiovascular disease, the age range was limited to 40 to 79 years; if they had evidence of subclinical cardiovascular disease or at least two additional cardiovascular risk factors, the age range was compressed to 55 to 79 years. Patients were specifically eligible to participate in the lipid trial if they also had the following: an LDL cholesterol level of 60 to 180 mg per deciliter (1.55 to 4.65 mmol per liter), an HDL cholesterol level below 55 mg per deciliter (1.42 mmol per liter) for women and blacks or below 50 mg per deciliter (1.29 mmol per liter) for all other groups, and a triglyceride level below 750 mg per deciliter (8.5 mmol per liter) if they were not receiving lipid therapy or below 400 mg per deciliter (4.5 mmol per liter) if they were receiving lipid therapy. All patients provided written informed consent. Additional details regarding eligibility and the protocol for the enrollment of patients are available in Section 3 in Supplementary Appendix 1.
STUDY PROCEDURES
Randomization was performed centrally on the trial’s Web site with the use of permuted blocks to maintain concealment of study-group assignments. Open-label simvastatin therapy began at the randomization visit, and the masked administration of either fenofibrate or placebo began 1 month later. The initial dose of simvastatin complied with national lipid guidelines at the time the study began.21 The dose of simvastatin was modified over time in response to changing guidelines (see Section 6 in Supplementary Appendix 1).18
At the start of the trial, the dose of fenofibrate was 160 mg per day. Because of a rise in serum creatinine levels in some patients while receiving this dose of fenofibrate,22 starting in 2004, the dose of fenofibrate was adjusted according to the estimated glomerular filtration rate (GFR) with the use of the abbreviated Modification of Diet in Renal Disease (MDRD) equation (see Section 7 in Supplementary Appendix 1).23
A fasting plasma lipid profile was measured at the ACCORD central laboratory at 4, 8, and 12 months after randomization, annually thereafter, and at the end of the study. Safety profiles, including liver-function tests and measurements of creatine kinase levels, were determined at 1, 4, 8, and 12 months after randomization and annually thereafter. If symptoms or signs suggestive of drug-induced toxic effects developed, tests of liver function (including measurement of alanine aminotransferase), creatine kinase, or both were obtained. If liver-function values were elevated, lipid medications were temporarily discontinued; if creatine kinase values were elevated, lipid medications were permanently discontinued.
PRESPECIFIED OUTCOMES
The prespecified primary outcome was the first occurrence of a major cardiovascular event, including nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Secondary outcomes included the combination of the primary outcome plus revascularization or hospitalization for congestive heart failure (termed the “expanded macrovascular outcome”); a combination of a fatal coronary event, nonfatal myocardial infarction, or unstable angina (termed “major coronary disease events”); nonfatal myocardial infarction; fatal or nonfatal stroke; nonfatal stroke; death from any cause; death from cardiovascular causes; and hospitalization or death due to heart failure. Definitions of each prespecified outcome and methods of ascertainment are detailed in Section 8 in Supplementary Appendix 1.
STUDY OVERSIGHT
Fenofibrate and matching placebo were donated by Abbott Laboratories; simvastatin was donated by Merck. The drug manufacturers had no role in the design of the study, in the accrual or analysis of the data, or in the preparation of the manuscript. All authors vouch for the accuracy and completeness of the reported data.
STATISTICAL ANALYSIS
The study was designed to recruit 5800 patients, with a power of 87% to detect a 20% reduction in the rate of the primary outcome for patients in the fenofibrate group, as compared with placebo, assuming a two-sided alpha level of 0.05, a primary outcome rate of 2.4% per year in the placebo group, and an average follow-up of approximately 5.6 years for patients who did not have an event. All statistical analyses were conducted at the coordinating center with the use of S-Plus software, version 8.0 (Insightful) or SAS software, version 9.1 (SAS Institute). Baseline characteristics were compared between study groups with the use of the chi-square test, Fisher’s exact test, Wilcoxon rank-sum test, and two-sample t-tests. The incidence of key safety outcomes was compared with the use of Fisher’s exact test.
Analyses of primary and secondary outcomes were performed with the use of time-to-event methods, according to the intention-to-treat principle, and occurrences of outcomes were compared with the use of hazard ratios and 95% confidence intervals. Two-sided P values were obtained from likelihood ratio tests from Cox proportional-hazards regression analyses. The Cox models contained a term representing study-group assignment plus terms for the following prespecified variables: assignment to the intensive glycemic intervention, the seven clinical-center networks, and the presence or absence of a previous cardiovascular event. Between-group differences were also examined in prespecified subgroups on 10 baseline characteristics (see Section 9 in Supplementary Appendix 1). Event rates are expressed as the percentage of events per years of follow-up, taking into account the censoring of follow-up data. Kaplan–Meier estimates were used to obtain the proportion of patients who had an event during follow-up.
The primary outcome and total rates of death were monitored by the data and safety monitoring board, using O’Brien–Fleming boundaries determined by the Lan–DeMets approach. For the primary outcome and rates of death, P values have been adjusted to account for the number, timing, and results of interim analyses. Further details regarding the analytic methods are available in Section 11 in Supplementary Appendix 1.
RESULTS
STUDY PATIENTS
A total of 5518 patients were enrolled in the ACCORD Lipid study, with 2765 assigned to receive fenofibrate plus simvastatin and 2753 assigned to receive placebo plus simvastatin. Baseline characteristics were similar between the two groups (Table 1). The mean age was 62 years, and 31% of the patients were female. Thirty-seven percent had a history of a cardiovascular event, and about 60% were taking a statin before enrollment.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | All Patients (N = 5518) |
Fenofibrate (N = 2765) |
Placebo (N = 2753) |
P Value |
|---|---|---|---|---|
| Age — yr | 62.3±6.8 | 62.2±6.7 | 62.3±6.9 | 0.69 |
| Female sex — no. (%) | 1694 (30.7) | 851 (30.8) | 843 (30.6) | 0.90 |
| Race or ethnic group — no. (%)† | ||||
| White | 3774 (68.4) | 1909 (69.0) | 1865 (67.7) | 0.30 |
| Black | 834 (15.1) | 392 (14.2) | 442 (16.1) | 0.05 |
| Hispanic | 407 (7.4) | 213 (7.7) | 194 (7.0) | 0.35 |
| Education — no. (%) | 0.19 | |||
| Less than high school | 750 (13.6) | 394 (14.2) | 356 (12.9) | |
| High-school graduate or GED | 1433 (26.0) | 712 (25.8) | 721 (26.2) | |
| Some college | 1827 (33.1) | 885 (32.0) | 942 (34.2) | |
| College degree or higher | 1505 (27.3) | 772 (27.9) | 733 (26.6) | |
| Missing data | 3 (<0.1) | 2 (0.1) | 1 (<0.1) | |
| Previous cardiovascular event — no. (%) | 2016 (36.5) | 1008 (36.5) | 1008 (36.6) | 0.90 |
| Previous congestive heart failure — no. (%) | 291 (5.3) | 151 (5.5) | 140 (5.1) | 0.54 |
| Cigarette-smoking status — no. (%) | 0.42 | |||
| Current | 803 (14.6) | 410 (14.8) | 393 (14.3) | |
| Former | 2546 (46.2) | 1292 (46.7) | 1254 (45.6) | |
| Never | 2161 (39.2) | 1059 (38.3) | 1102 (40.0) | |
| Missing data | 8 (0.1) | 4 (0.1) | 4 (0.1) | |
| Weight — kg | 94.8±18.7 | 94.5±18.5 | 95.2±18.8 | 0.21 |
| Body-mass index‡ | 32.3±5.4 | 32.2±5.4 | 32.4±5.4 | 0.32 |
| Blood pressure — mm Hg | ||||
| Systolic | 133.9±17.8 | 133.8±17.7 | 134.0±17.9 | 0.79 |
| Diastolic | 74.0±10.8 | 73.9±10.7 | 74.0±10.9 | 0.58 |
| Medications — no. (%) | ||||
| Insulin | 1836 (33.3) | 919 (33.2) | 917 (33.3) | 0.95 |
| Metformin | 3420 (62.0) | 1712 (61.9) | 1708 (62.0) | 0.92 |
| Any sulfonylurea | 2892 (52.4) | 1440 (52.1) | 1452 (52.7) | 0.62 |
| Any thiazolidinedione | 973 (17.6) | 480 (17.4) | 493 (17.9) | 0.59 |
| Angiotensin-converting–enzyme inhibitor | 2967 (53.8) | 1473 (53.3) | 1494 (54.3) | 0.46 |
| Angiotensin-receptor blocker | 838 (15.2) | 405 (14.6) | 433 (15.7) | 0.26 |
| Aspirin | 3106 (56.3) | 1583 (57.3) | 1523 (55.3) | 0.15 |
| Beta-blocker | 1798 (32.6) | 912 (33.0) | 886 (32.2) | 0.53 |
| Any thiazide diuretic | 1473 (26.7) | 740 (26.8) | 733 (26.6) | 0.91 |
| Statin | 3299 (59.8) | 1641 (59.3) | 1658 (60.2) | 0.51 |
| Any lipid-lowering agent | 3558 (64.5) | 1773 (64.1) | 1785 (64.8) | 0.58 |
| Duration of diabetes — yr | ||||
| Median | 9 | 10 | 9 | 0.83 |
| Interquartile range | 5–15 | 5–15 | 5–15 | |
| Glycated hemoglobin — % | ||||
| Mean | 8.3±1.0 | 8.3±1.0 | 8.3±1.0 | 0.52 |
| Median | 8.1 | 8.1 | 8.1 | |
| Interquartile range | 7.6–8.8 | 7.6–8.8 | 7.5–8.8 | |
| Fasting plasma glucose — mg/dl | 175.8±54.9 | 176.5±54.5 | 175.1±55.3 | 0.38 |
| Amputation due to diabetes — no. (%) | 110 (2.0) | 59 (2.1) | 51 (1.9) | 0.45 |
| Potassium — mg/dl | 4.5±0.4 | 4.5±0.4 | 4.5±0.4 | 0.31 |
| Serum creatinine — mg/dl | 0.9±0.2 | 0.9±0.2 | 0.9±0.2 | 0.96 |
| Estimated glomerular filtration rate — no. (%) | ||||
| 30–49 ml/min/1.73 m2 | 141 (2.6) | 71 (2.6) | 70 (2.5) | 0.89 |
| >50 ml/min/1.73 m2 | 5347 (97.4) | 2668 (97.4) | 2679 (97.5) | |
| Plasma cholesterol — mg/dl | ||||
| Total | 175.2±37.3 | 174.7±36.8 | 175.7±37.9 | 0.36 |
| Low-density lipoprotein | 100.6±30.7 | 100.0±30.3 | 101.1±31.0 | 0.15 |
| High-density lipoprotein | 38.1±7.8 | 38.0±7.8 | 38.2±7.8 | 0.25 |
| Plasma triglyceride — mg/dl | ||||
| Median | 162 | 164 | 160 | 0.15 |
| Interquartile range | 113–229 | 114–232 | 112–227 | |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. To convert the values for glucose to millimoles per liter, multiply by 0.055551. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for potassium to millimoles per liter, multiply by 0.2558. To convert the values for creatinine to micromoles per liter, multiply by 88.4. GED denotes general equivalency diploma.
Race or ethnic group was self-reported, and patients could check multiple categories.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The mean duration of follow-up was 4.7 years for the primary outcome and 5.0 years for total rates of death. At the final study visit, 77.3% of the patients in the fenofibrate group and 81.3% of those in the placebo group were taking their assigned medication. At the end of the study, approximately 80% of patients were still taking simvastatin in each group, and an additional 6% were taking an alternative study-approved agent for lowering LDL cholesterol. Additional details related to adherence are in presented in Section 12 in Supplementary Appendix 1. The average daily dose of simvastatin during the follow-up period was 22.3 mg in the fenofibrate group and 22.4 mg in the placebo group.
SAFETY
Elevations of creatine kinase of more than 10 times the upper limit of the normal range at any time during the trial occurred in 10 patients (0.4%) in the fenofibrate group and 9 (0.3%) in the placebo group (for details, see Section 13 in Supplementary Appendix 1). An elevation in alanine aminotransferase of more than three times the upper limit of the normal range occurred in 52 patients (1.9%) in the fenofibrate group and 40 (1.5%) in the placebo group.
As noted in other fenofibrate trials,11,22 mean serum creatinine levels increased from 0.93 to 1.10 mg per deciliter (82 to 97 µmol per liter) in the fenofibrate group within the first year and remained relatively stable thereafter. In the placebo group, mean serum creatinine levels increased from 0.93 to 1.04 mg per deciliter (82 to 92 µmol per liter) during the course of the trial (see Section 15 in Supplementary Appendix 1). The study drug was discontinued by 66 patients (2.4%) in the fenofibrate group and 30 (1.1%) in the placebo group because of a decrease in the estimated GFR. At the last clinic visit, 440 patients (15.9%) in the fenofibrate group and 194 (7.0%) in the placebo group were receiving a reduced dose of either fibrate or placebo because of a decreased estimated GFR. There was no significant between-group difference in the incidence of both hemodialysis and end-stage renal disease (75 patients in the fenofibrate group vs. 77 in the placebo group). There was a lower incidence of both microalbuminuria and macroalbuminuria in the fenofibrate group than in the placebo group (see Section 13 in Supplementary Appendix 1).
PLASMA LIPIDS
By the end of the study, the mean LDL cholesterol level fell from 100.0 to 81.1 mg per deciliter (2.59 to 2.10 mmol per liter) in the fenofibrate group and from 101.1 to 80.0 mg per deciliter (2.61 to 2.07 mmol per liter) in the placebo group (Fig. 1, and Section 16 in Supplementary Appendix 1). Mean HDL cholesterol levels increased from 38.0 to 41.2 mg per deciliter (0.98 to 1.07 mmol per liter) in the fenofibrate group and from 38.2 to 40.5 mg per deciliter (0.99 to 1.05 mmol per liter) in the placebo group. Median plasma triglyceride levels decreased from 164 to 122 mg per deciliter (1.85 to 1.38 mmol per liter) in the fenofibrate group and from 160 to 144 mg per deciliter (1.81 to 1.63 mmol per liter) in the placebo group.
Figure 1. Lipid Values.
Shown are mean plasma levels of total cholesterol (Panel A), low-density lipoprotein (LDL) cholesterol (Panel B), and high-density lipoprotein (HDL) cholesterol (Panel C) and median levels of triglycerides (Panel D) at baseline, 4 months, 8 months, 1 year, and annually thereafter. Nominal P values for differences between the study groups at 4 months and at the end of the study were, respectively: total cholesterol, P<0.001 and P = 0.02; LDL cholesterol, P = 0.11 and P = 0.16; HDL cholesterol, P<0.001 and P=0.01; and triglycerides, P<0.001 for both comparisons with the use of nonparametric tests. End-of-study visits were those that occurred in early 2009 and included follow-up at years 4, 5, 6, and 7. The I bars represent 95% confidence intervals. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
CLINICAL OUTCOMES
The annual rate of the primary outcome was 2.2% in the fenofibrate group, as compared with 2.4% in the placebo group (hazard ratio in the fenofibrate group, 0.92; 95% confidence interval [CI], 0.79 to 1.08; P = 0.32 after adjustment for monitoring) (Table 2 and Fig. 2). Hazard ratios for the secondary outcomes, including the individual components of the primary outcome, ranged from 0.82 to 1.17 (P≥0.10 for all comparisons) (Table 2). Annual rates of death from all causes were 1.5% in the fenofibrate group and 1.6% in the placebo group (hazard ratio, 0.91; 95% CI, 0.75 to 1.10; P = 0.33 for the adjusted comparison). Specific causes of death and enlarged versions of the Figure 2 insets are presented in Sections 17 and 18 in Supplementary Appendix 1.
Table 2.
Prespecified Primary and Secondary Outcomes
| Outcome | Fenofibrate (N = 2765) |
Placebo (N = 2753) |
Hazard Ratio (95% CI) |
P Value | ||
|---|---|---|---|---|---|---|
| no. of events | rate/yr | no. of events | rate/yr | |||
| Primary outcome (major fatal or nonfatal cardiovascular event) | 291 | 2.24 | 310 | 2.41 | 0.92 (0.79–1.08) | 0.32* |
| Secondary outcomes | ||||||
| Primary outcome plus revascularization or hospitalization for congestive heart failure |
641 | 5.35 | 667 | 5.64 | 0.94 (0.85–1.05) | 0.30 |
| Major coronary disease event† | 332 | 2.58 | 353 | 2.79 | 0.92 (0.79–1.07) | 0.26 |
| Nonfatal myocardial infarction | 173 | 1.32 | 186 | 1.44 | 0.91 (0.74–1.12) | 0.39 |
| Stroke | ||||||
| Any | 51 | 0.38 | 48 | 0.36 | 1.05 (0.71–1.56) | 0.80 |
| Nonfatal | 47 | 0.35 | 40 | 0.30 | 1.17 (0.76–1.78) | 0.48 |
| Death | ||||||
| From any cause | 203 | 1.47 | 221 | 1.61 | 0.91 (0.75–1.10) | 0.33* |
| From cardiovascular cause | 99 | 0.72 | 114 | 0.83 | 0.86 (0.66–1.12) | 0.26 |
| Fatal or nonfatal congestive heart failure | 120 | 0.90 | 143 | 1.09 | 0.82 (0.65–1.05) | 0.10 |
P values were adjusted for interim monitoring.
A major coronary disease event was defined as a fatal coronary event, nonfatal myocardial infarction, or unstable angina.
Figure 2. Kaplan-Meier Analyses of the Primary Outcome, Expanded Macrovascular Outcome, and Death.
Shown are the cumulative incidence of the primary outcome (nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) (Panel A), the expanded macrovascular outcome (a combination of the primary outcome plus revascularization or hospitalization for congestive heart failure) (Panel B), and death from any cause (Panel C) or from cardiovascular causes (Panel D) during follow-up. The insets show close-up versions of the graphs in each panel.
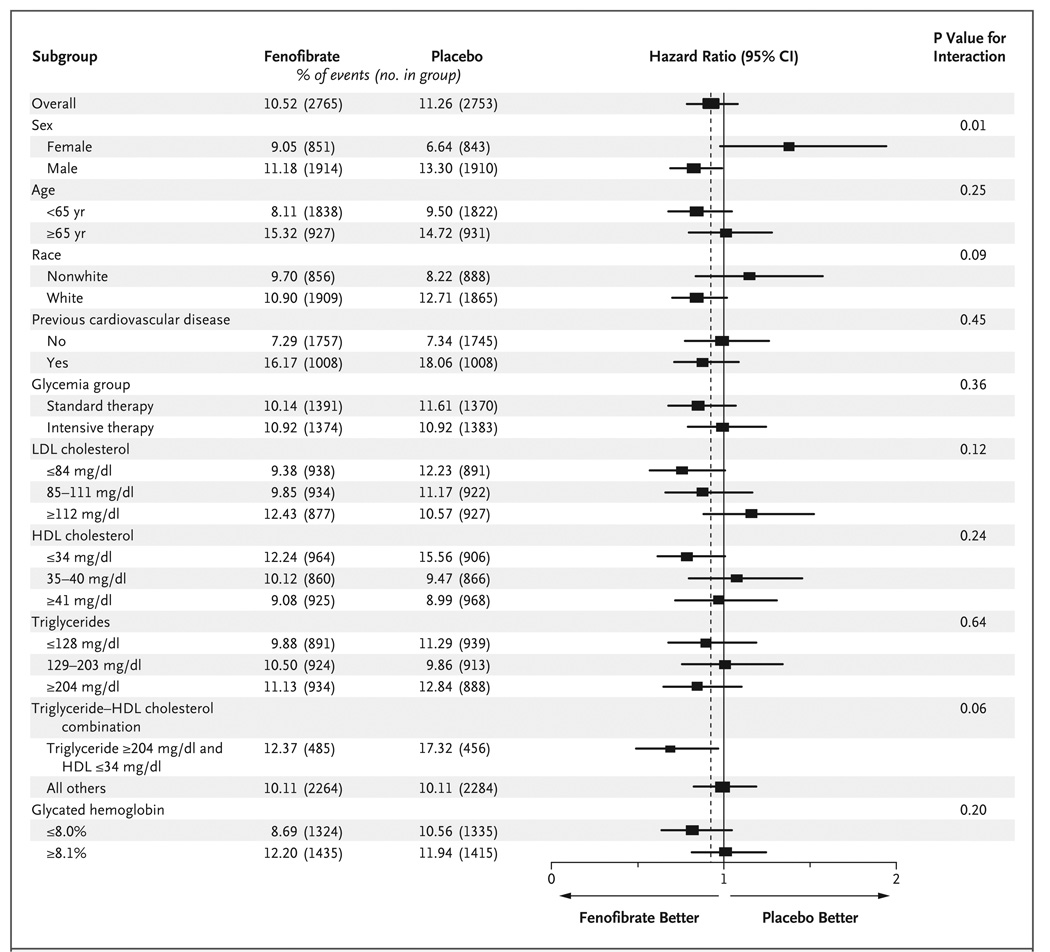
Study-group effects on the primary outcome across prespecified baseline subgroups are shown in Figure 3. Only sex showed evidence of an interaction according to study group: the primary outcome for men was 11.2% in the fenofibrate group versus 13.3% in the placebo group, whereas the rate for women was 9.1% in the fenofibrate group versus 6.6% in the placebo group (P = 0.01 for interaction). There was also a nonsignificant suggestion of heterogeneity when patients who had a triglyceride level in the highest third (≥204 mg per deciliter [≥2.30 mmol per liter]) and an HDL cholesterol level in the lowest third (≤34 mg per deciliter [≤0.88 mmol per liter]) were compared with all the other patients (P = 0.057 for interaction). In this subgroup of patients with high triglyceride levels and low HDL cholesterol levels, the primary outcome rate was 12.4% in the fenofibrate group, versus 17.3% in the placebo group, whereas such rates were 10.1% in both study groups for all other patients.
Figure 3. Hazard Ratios for the Primary Outcome in Prespecified Subgroups.
The horizontal bars represent 95% confidence intervals, and the vertical dashed line indicates the overall hazard ratio. The size of each square is proportional to the number of patients. P values are for tests for interaction. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
DISCUSSION
In this trial, we tested the hypothesis that the use of fenofibrate to increase plasma HDL cholesterol levels and to reduce plasma triglyceride levels in patients with type 2 diabetes who were already receiving simvastatin therapy would result in an additional cardiovascular benefit, as compared with simvastatin therapy alone. However, the rates of the primary outcome did not differ significantly between the fenofibrate group and the placebo group during 4.7 years of treatment and follow-up.
When a study does not support the central hypothesis, it is critical to examine potential reasons for this outcome. One possibility is that the addition of fenofibrate to statin therapy benefited only certain subgroups of patients and that other subgroups that did not benefit diluted the overall effect. Our study was part of a factorial design to simultaneously test the effects of intensive glycemic control17,20 and combination lipid therapy on cardiovascular outcomes. To allow for efficient enrollment of the entire cohort of 10,000 patients while including a group for whom the results of the lipid trial could be widely extrapolated, we used broader inclusion criteria for plasma lipid levels than might have been used if the lipid trial had been an independent study.
A second possibility is that the trial might have had fewer events than anticipated. However, the annual rate of 2.4% in the placebo group was the rate used in the power calculations. Another possibility is poor adherence to the experimental protocol. However, adherence at the end of the study was approximately 80% in both the fenofibrate and placebo groups and 80% for simvastatin. Furthermore, unlike the FIELD study, in which there was a disproportionate drop-in to statin therapy in the placebo group,11 the prevalence of statin therapy in our study was similar in the fenofibrate and placebo groups. A fourth possibility is that fenofibrate is not as effective as gemfibrozil, which showed benefit in the Helsinki Heart Study (HHS) and VA-HIT,13,15 studies in which there was no background statin therapy.
In examined subgroups, only sex had a significant interaction with treatment: men seemed to benefit from fenofibrate therapy, whereas there was a trend toward harm among women. This is in contrast to the results of the FIELD study, in which there was no significant interaction effect between treatment and sex on outcome.11
There was also a suggestion of heterogeneity according to baseline lipid levels: patients who had both a triglyceride level in the highest third and an HDL cholesterol level in the lowest third (which we termed the subgroup with dyslipidemia) appeared to benefit from fenofibrate, whereas all other patients receiving fenofibrate did not. The mean baseline HDL cholesterol level in the subgroup with dyslipidemia was 29.5 mg per deciliter (0.76 mmol per liter), and the median triglyceride level was 284 mg per deciliter (3.21 mmol per liter), in contrast to the rest of the patients, in whom the mean HDL cholesterol level was 39.9 mg per deciliter (1.03 mmol per liter) and the median triglyceride level was 144 mg per deciliter (1.63 mmol per liter). From baseline to 4 months in the fenofibrate group, the HDL cholesterol level rose 12.9% and the triglyceride level fell 35.0% among patients in the subgroup with dyslipidemia, as compared with a 7.3% rise in the HDL cholesterol level and a 24.1% decrease in the triglyceride level among all other patients receiving fenofibrate. The treatment interaction according to sex for the entire ACCORD Lipid cohort was not observed in the subgroup with dyslipidemia (data not shown).
The results for patients in the subgroup with dyslipidemia are similar to those in post hoc subgroup analyses performed in three of four major fibrate trials, including HHS,24 the Bezafibrate Infarction Prevention (BIP) trial,14 and the FIELD trial12 (see Section 19 in Supplementary Appendix 1 for details). Our subgroup results and those of these previous trials support the view that the addition of fenofibrate to a statin may benefit patients with type 2 diabetes who have substantial dyslipidemia. The use of combination fibrate–statin therapy in such patients is consistent with current guidelines that recommend treatment for patients with hypertriglyceridemia and low HDL cholesterol levels that persist despite statin therapy.25
Previous studies11,22 have raised concern about increases in serum creatinine levels during fenofibrate treatment. Serum creatinine levels increased in the fenofibrate group soon after randomization but thereafter remained constant, as compared with those in the placebo group. In the FIELD study, there was a return of serum creatinine to baseline levels by 8 weeks after the end of the trial.11 In our study, there was no significant difference in the incidence of end-stage renal disease or need for dialysis between the fenofibrate group and the placebo group. There was a reduction in both microalbuminuria and macroalbuminuria in the fenofibrate group. There has also been longstanding concern regarding an increased risk of myositis or rhabdomyolysis when fibrates are added to statins.26,27 No evidence for such a risk was noted in our study, a finding that was compatible with evidence that fenofibrate, in contrast to gemfibrozil, does not increase plasma concentrations of statins.28
In conclusion, we found that combination therapy with the use of fenofibrate and simvastatin (at a daily dose of 40 mg or less) did not reduce rates of cardiovascular disease, as compared with simvastatin alone. Our findings do not support the use of combination fibrate–statin therapy, rather than statin therapy alone, to reduce cardiovascular risk in the majority of patients with type 2 diabetes who are at high risk for cardiovascular disease.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAAY1-HC-9035, and IAAY1-HC-1010), the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, the National Eye Institute, the Centers for Disease Control and Prevention, and General Clinical Research Centers at many sites. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca Pharmaceuticals, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline Pharmaceuticals, King Pharmaceuticals, Merck, Novartis Pharmaceuticals, Novo Nordisk, Omron Healthcare, Sanofi-Aventis, and Takeda Pharmaceuticals.
Dr. Ginsberg reports receiving consulting fees from Merck, Merck Schering-Plough, Bristol-Myers Squibb, AstraZeneca, Abbott, Roche, Isis/Genzyme, GlaxoSmithKline, Novartis, Pfizer, and Regeneron/Sanofi-Aventis and grant support from Merck, Isis/Genzyme, Roche, and AstraZeneca; Dr. Elam, receiving consulting fees from Pfizer, Abbott, and Merck Schering-Plough; Dr. Crouse, receiving consulting fees from the National Lipid Association, AstraZeneca, Merck, and Merck Schering-Plough and grant support from AstraZeneca; Dr. Leiter, receiving consulting fees from AstraZeneca, Merck, Pfizer, Roche, and Solvay and grant support from AstraZeneca, Merck, Pfizer, and Roche; Dr. Linz, having an equity interest in Pfizer, Novartis, and AstraZeneca; Dr. Buse, receiving consulting fees from Novo Nordisk, Amylin, Becton Dickinson, Eli Lilly, Hoffmann–La Roche, GlycoMark, Wyeth, Daiichi Sankyo, Bristol-Myers Squibb, Bayhill Therapeutics, LipoScience, MannKind, Veritas, MicroIslet, GlaxoSmithKline, Abbott, Exsulin, and GI Dynamics and grant support from Amylin, Novo Nordisk, Medtronic, Eli Lilly, Novartis, Tolerex, Osiris, Halozyme, Pfizer, Hoffmann–La Roche, InterKrin, Merck, Sanofi-Aventis, Dexcom, Johnson & Johnson, Bristol-Myers Squibb, and Fujisawa, having an equity interest in Insulet, and providing expert testimony for Novo Nordisk; Dr. Gerstein, receiving consulting fees from Sanofi-Aventis, GlaxoSmithKline, Eli Lilly, Novo Nordisk, AstraZeneca, Bristol-Myers Squibb, Roche, Medtronic, Merck, Bayer, Bioavail, and Janssen-Ortho, grant support from Sanofi-Aventis, GlaxoSmithKline, Novo Nordisk, Merck, Pronova, and Roche, and lecture fees from Sanofi-Aventis, GlaxoSmithKline, Solvay, Boehringer Ingelheim, Servier, Bayer, Eli Lilly, Novo Nordisk, and Takeda; Dr. Probstfield, receiving grant support from Sanofi-Aventis, Boehringer Ingelheim, and Abbott; Dr. Grimm, receiving consulting and lecture fees and grant support from Pfizer, Merck, and Novartis, consulting and lecture fees from Takeda, and lecture fees from AstraZeneca, Forest Laboratories, and Schering-Plough; Dr. Bigger, receiving consulting fees from Merck and Roche; Dr. Goff, receiving consulting fees from Takeda and grant support from Merck; and Dr. Cushman, receiving consulting fees from Novartis, Takeda, Sanofi-Aventis, Bristol-Myers Squibb, King, Daiichi-Sankyo, Gilead, Theravance, Pharmacopeia, and Sciele and grant support from Novartis, GlaxoSmithKline, and Merck. No other potential conflict of interest relevant to this article was reported.
APPENDIX
The affiliations of the members of the writing committee are as follows: the Department of Medicine, Columbia University College of Physicians and Surgeons, New York (H.N.G.); Memphis Veterans Affairs Medical Center, Memphis (M.B.E., W.C.C.); the Department of Public Health Sciences (L.C.L., D.C.G., R.P.B.) and Preventive Cardiology Program (J.R.C.), Wake Forest University School of Medicine, Winston-Salem, NC; University of Toronto, Toronto (L.A.L.); Naval Medical Center, San Diego, CA (P.L.); the Departments of Biostatistics and Epidemiology, Columbia University Mailman School of Public Health, New York (W.T.F.); the Division of Endocrinology, University of North Carolina School of Medicine, Chapel Hill (J.B.B.); the Department of Medicine and the Population Health Research Institute, McMaster University, Hamilton, ON, Canada (H.C.G.); the University of Washington, Seattle (J.P.); the Berman Center for Outcomes and Clinical Research, Minneapolis (R.H.G.), the Departments of Medicine and Physiology and Biophysics, Case Western Reserve University, Cleveland (F.I.-B.); the Division of Cardiology, Columbia University College of Physicians and Surgeons, New York (J.T.B.); and the National Heart, Lung, and Blood Institute, Bethesda, MD (D.G.S.-M.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen H, Lehto S, Salomaa V, et al. Impact of diabetes on mortality after the first myocardial infarction. Diabetes Care. 1998;21:69–75. doi: 10.2337/diacare.21.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35:491–510. doi: 10.1016/j.ecl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 9.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 10.Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT) Arch Intern Med. 2002;162:2597–2604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 11.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [Errata, Lancet 2006;368:1415, 1420.] [DOI] [PubMed] [Google Scholar]
- 12.Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia.Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 14.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 16.Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Goff DC, Jr, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:4i–20i. doi: 10.1016/j.amjcard.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg HN, Bonds DE, Lovato LC, et al. Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:56i–67i. doi: 10.1016/j.amjcard.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Cushman WC, Grimm RH, Jr, Cutler JA, et al. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:44i–55i. doi: 10.1016/j.amjcard.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 20.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Genest J, Frohlich J, Steiner G. Effect of fenofibrate-mediated increase in plasma homocysteine on the progression of coronary artery disease in type 2 diabetes mellitus. Am J Cardiol. 2004;93:848–853. doi: 10.1016/j.amjcard.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [Erratum, Circulation 2004;110:763.] [DOI] [PubMed] [Google Scholar]
- 26.Pasternak RC, Smith SC, Jr, BaireyMerz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024–1028. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 27.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120–122. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 28.Bergman AJ, Murphy G, Burke J, et al. Simvastatin does not have a clinically significant pharmacokinetic interaction with fenofibrate in humans. J Clin Pharmacol. 2004;44:1054–1062. doi: 10.1177/0091270004268044. [DOI] [PubMed] [Google Scholar]