Abstract
Background
Ranolazine is an antianginal drug that inhibits the cardiac late Na+ current (INa). The selectivity of ranolazine to block late INa relative to peak INa at rapid heart rates has not been determined, but is potentially important to drug efficacy and safety.
Objective
To quantify use-dependent block (UDB) of cardiac peak and late INa by ranolazine.
Methods
Wild-type (WT) and LQT3 mutation R1623Q channels were expressed in HEK293 cells and studied using whole-cell patch-clamp technique. Ranolazine (1–300 μM) caused tonic (0.1 Hz) and UDB (1, 2 and 5 Hz) of WT and R1623Q peak INa. The IC50 values for block WT and R1623Q peak INa at 0.1, 1, 2 and 5 Hz were 430, 260, 160 and 150μM, and 95, 78, 37 and 25μM, respectively. The IC50 values for block of R1623Q late INa at 0.1, 1, 2 and 5 Hz were 7.5, 7.3, 2.2 and 1.9 μM, respectively. Ranolazine (10 μM) caused a hyperpolarizing shift of WT and R1623Q peak INa steady-state inactivation without affecting steady-state activation, suggesting that ranolazine interacts with inactivated states of the channels. Ranolazine (30 μM) significantly slowed the recovery from inactivation of peak INa of both WT and R1623Q and late INa of R1623Q.
Conclusion
Ranolazine slowed recovery of late INa from inactivation and thus caused UDB of late INa. These data suggest that the effect of ranolazine to block late INa may be increased, and the selectivity to block late INa relative to peak INa may be retained, during tachycardia.
Keywords: Angina, sodium channel, late sodium, ranolazine, tachycardia
Introduction
Local anesthetic and class I antiarrhythmic drugs inhibit peak Na+ current (INa) by binding to voltage-gated Na+ channels. Inhibition of peak INa produced by these drugs is often enhanced by rapid, repetitive stimulation. The resultant block is called use-dependent block (UDB) or frequency-dependent block. Recently, we and others1, 2 have shown that the antianginal drug ranolazine causes UDB of skeletal (Nav1.4), cardiac (Nav1.5) and peripheral (Nav1.7 and Nav1.8) peak INa., but UDB of late INa has not been characterized.
Many excitable tissues have been shown to have a component of INa that is resistant to inactivation. The existence of inactivation-resistant (persistent or late) INa was first identified in cardiac Purkinje fibers of dogs and rabbits.3 Recently, Maltsev et al4 demonstrated the presence of late INa in human mid-myocardial myocytes isolated from normal and failing hearts. Similar to peak INa, late INa has been shown to be blocked by local anesthetics5–7 and by ranolazine.8 The block of late INa by ranolazine has been shown to occur at significantly lower concentrations than the block of peak INa. The values of IC50 for ranolazine to block late and peak INa in canine ventricular myocytes were reported as 5.98 and 294 μM9, respectively.
Because late INa may play an important mechanistic role to induce tachyarrhythmias10, 11, it is important to understand whether the effect of drugs to inhibit late INa is increased or decreased at rapid heart rates. Therefore, in this study, our goal was to determine if the effect of ranolazine to inhibit late INa is use-dependent and if the selectivity of ranolazine to inhibit late INa relative to peak INa is maintained at high stimulating frequencies. Because the amplitude of endogenous late INa is normally very small4, 12, we utilized cells expressing Na+ channels with a long QT3 (LQT3) mutation, R1623Q, a missense mutation13 in the voltage-sensing region of the Na+ channel that leads to an increase of late INa. The endogenous late INa in R1623Q was sufficiently large to allow characterization of the UDB by ranolazine of peak and late INa using the whole-cell patch clamp technique.
Methods
Heterologous Expression of SCN5A wild-type and R1623Q
Human embryonic kidney (HEK293) cells stably expressing the human heart Na+ channel (hH1a; Nav1.5) clone of SCN5A gene (α-subunit alone) were purchased from Cytomyx, Cambridge, UK. The LQT3 mutation, R1623Q was generated by site-directed mutagenesis of WT SCN5A cDNA using overlap extension PCR strategy.13, 14 HEK293 cells were transiently transfected using PolyFect (Qiagen, Valencia, CA). After 48 hours following transfection, green fluorescence protein-positive cells were selected for recording of INa. Cells were grown in minimum essential medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% non-essential amino acids, and 400 μg/mL G418 (Invitrogen, Carlsbad, CA) and incubated at 37°C in an atmosphere of 5% CO2 in air.
Electrophysiology
Whole-cell INa was recorded using an Axopatch 700B amplifier (Axon Instruments, Sunnyvale, CA, USA). Patch pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL, USA) with a DMZ Universal puller (Dagan Corporation, Minneapolis, MN) Pipette resistance was 1–1.5 MΩ when filled with a pipette (internal) solution containing (mM): 20 CsCl, 120 CsF, 2 EGTA and 5 HEPES (pH adjusted to 7.4 with CsOH). Cells were superfused with a bath (external) solution containing (mM): 140 NaCl, 4.0 KCl, 1.8 CaCl2, 0.75 MgCl2, and 5 HEPES (pH adjusted to 7.4 with NaOH). In all recordings, 75–80% of the series resistance compensation was achieved, thus yielding a maximum voltage error of ~5 mV. In all experiments, the temperature of experimental solutions was maintained at 20±1°C using a CL-100 bipolar temperature controller (Warner Instruments, Hamden, CT). Research grade ranolazine (racemic mixture) was synthesized by the Department of Bio-Organic Chemistry at CV Therapeutics, Inc (Palo Alto, CA) and dissolved in 0.1 N HCl to give a stock solution of 10 mM. Further dilutions were freshly made in Tyrode solution on the day of each experiment.
Data Analysis
pCLAMP 10.0 software (Axon Instruments, Sunnyvale, CA) was used to generate voltage clamp protocols and acquire data. Data were analyzed using Clampfit and Microcal Origin (MicroCal, Northampton, MA) software. Results are expressed as mean±S.E.M. and n refers to number of cells with p<0.05 indicating statistical significance (Student t-test). Concentration-response relationships were fitted using the Hill equation, Idrug/Icontrol=1/[1+(D/IC50)nH], where Idrug/Icontrol is fractional block, D is drug concentration, IC50 is the drug concentration that causes 50% block and nH is the Hill coefficient.
Recovery from inactivation was measured using a standard two-pulse protocol of 24-(for peak INa) or 50-msec (for late INa) duration with an incremental time delay of 1 msec to 8 sec between the two pulses (holding potential = −140 mV; test potential = −20 mV). The peak INa or the mean value of INa between 46 and 48 msec (for late INa) elicited by the second pulse (I) was normalized relative to the current elicited by the first pulse (I0). The duration of every cycle of the double pulse protocol was 20 sec. I/I0 was plotted against the time delay between the two pulses and fit to a double exponential function,
where t = recovery time interval, τF and τS = fast and slow time constants, and AF and AS = relative amplitudes of the fast and slow recovery components.
The voltage dependence of activation was determined using 50-msec depolarizing pulses from a holding potential of −140 mV to test potentials ranging from −120 to +40 mV in 5 mV increments. To determine the voltage dependence of channel activation, Na+ conductance (GNa) was calculated from the peak current (INa), using the equation:
where V is the test pulse potential and Vrev is the calculated reversal potential. Normalized Na+ conductance was plotted against test pulse potential and fit to a Boltzmann equation:
where G is the measured conductance, Gmax is the maximal conductance, V½ is the membrane potential at which the half-maximal channel open probability occurs and k is the slope of the curve. For assessing the voltage dependence of steady-state inactivation, prepulses ranging from −140 to 0 mV were applied for a period of 1 sec, followed by a 24-msec depolarizing step to 0 mV. The peak current (I) was normalized relative to the maximal value (Imax) obtained at a holding potential (Vh) of −140 mV and plotted against the conditioning pulse potential. Data were fit to a Boltzmann equation:
where V is the membrane potential during the pre-pulse, V½ the potential at which the half-maximal channel inactivation occurs and k is the slope factor.
Results
Use-dependent Block of WT and R1623Q Na+ Channels by Ranolazine
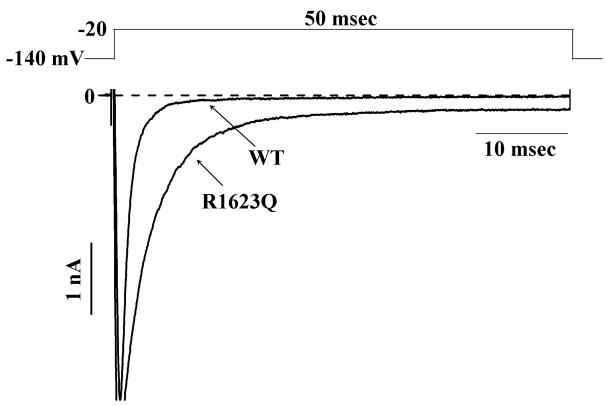
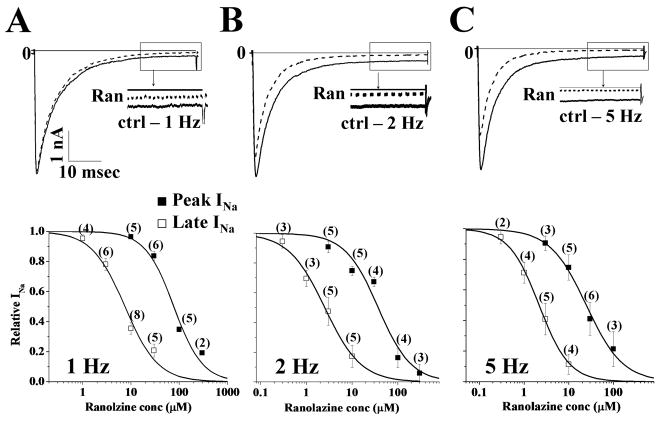
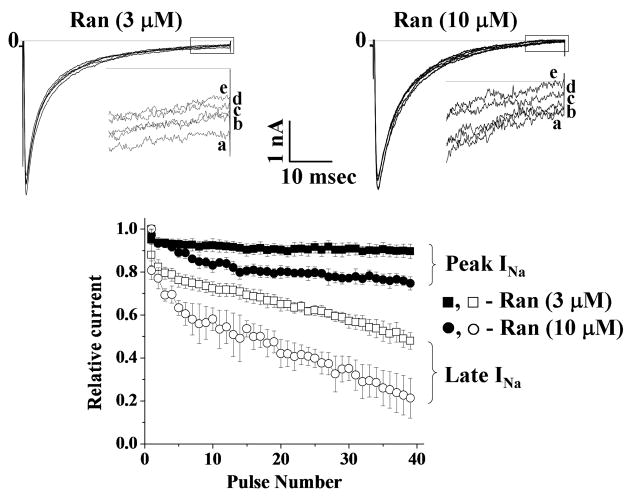
The late component of INa in HEK293 cells transiently expressing R1623Q mutation was greater than the late component of INa in HEK293 cells stably expressing WT channels (Fig. 1), as previously shown.13 To study the UDB by ranolazine of peak (WT and R1623Q) and late (R1623Q) INa, a series of 40 pulses (50-msec in duration) to −20 mV from a holding potential of −140 mV were applied at rates of 1, 2 and 5 Hz. Late INa for R1623Q was measured as the mean value of INa between 46 and 48 msec following the step to −20 mV. For WT channels stimulated at rates of 1, 2 and 5 Hz, the amplitudes of peak INa of the 1st and 40th pulses were similar (data not shown). For R1623Q channels stimulated at 1, 2 and 5 Hz, frequency-dependent reductions in both peak and late INa amplitude were observed. The amplitudes of peak and late INa at 1, 2 and 5 Hz relative to 0.1 Hz were 100.0±0.1%, 95.3±1.6%, 88.2±5.6% and 99.3±1.9%, 88.1±7.4%, 83.1±3.1%, respectively. Ranolazine reduced peak INa in WT channels in a concentration-and frequency-dependent manner; the values of IC50 for ranolazine reduce WT peak INa at 4 different tested frequencies are shown in Table 1. Ranolazine also blocked R1623Q peak and late INa. Original traces recorded from HEK293 cells transiently expressing R1623Q channels and stimulated at frequencies of 1, 2 and 5 Hz are shown in Fig. 2 (top panels A–C, respectively), and indicate that block of peak and late INa was use-dependent. Summary data for concentration- and frequency-dependence (amplitude of the 40th pulse relative to the first pulse) of block of R1623Q peak and late INa are shown in Fig. 2 (lower panels). The IC50 values derived from fits of the data in Figure 2 are summarized in Table 1. The accumulation of block of late INa from pulse 1 to pulse 40 in the presence of ranolazine (3 and 10 μM) was greater than the accumulation of block of peak INa (Fig. 3). Thus the late component of R1623Q INa was more sensitive to UDB by ranolazine than the peak component of INa.
Figure 1.
Representative sodium current (INa) traces recorded from HEK293 cells stably expressing WT Na+ channels, or transiently expressing R1623Q Na+ channels. Peak Na+ currents have been truncated to enable better visualization of the late currents.
Table 1.
Potencies of ranolazine block of SCN5A R1623Q (peak and late INa) and WT (peak INa).
| Stimulating Frequency | IC50 values (μM) |
||
|---|---|---|---|
| R1623Q |
WT |
||
| Peak INa (■) | Late INa (□) | Peak INa | |
| 0.1 Hz | 95.32±2.25 [1.01±0.02] | 7.45±0.11 [1.41±0.02] | 427.98±35.21 [1.61±0.02] |
| 1 Hz | 77.08±9.77 [1.46±0.24] | 7.28±1.03 [1.24±0.20] | 259.32±2.71 [0.93±0.01] |
| 2 Hz | 37.05±7.16 [1.20±0.25] | 2.17±0.19 [1.09±0.09] | 157.18±3.19 [1.15±0.03] |
| 5 Hz | 24.60±2.46 [1.06±0.13] | 1.94±0.01 [1.39±0.09] | 154.01±17.81 [0.78±0.14] |
Data were recorded using voltage-clamp protocols described in Fig. 1 and were fitted using Hill equation. The potencies (IC50 values) for inhibition of peak and late INa by ranolazine arc given in μM and Hill coefficients are listed in brackets.
Figure 2.
Concentration-dependence of block by ranolazine of R1623Q peak and late INa in cells stimulated at frequencies of 1 (panel A), 2 (panel B) and 5 (panel C) Hz. Representative INa traces (upper panels) recorded from R1623Q Na+ channels in the absence (solid line) and presence (dashed line) of 10 μM ranolazine. Insets: Expanded traces show the last 10 msec (following the depolarizing pulse) of late INa in the absence (solid line) and presence of 10 μM ranolazine (Ran, dashed line). Concentration-response relationships (lower panels) for the use-dependent block (UDB) by ranolazine of peak (■) and late (□) INa in cells stimulated at 1, 2 and 5 Hz. The amplitude of current evoked by the 40th pulse was normalized to that of the current evoked by the first pulse and plotted as a function of ranolazine concentration. Data represent mean±SEM; the number of experiments is indicated in parentheses. Values of IC50 and Hill coefficient are given in Table 1.
Figure 3.
Time courses of the development of UDB by ranolazine of R1623Q peak and late INa during stimulation of HEK293 cells at a rate of 2 Hz. Original traces recorded from HEK293 cells expressing R1623Q channels stimulated at 2 Hz in the presence of 3 and 10 μM ranolazine are shown in Fig. 2 (top panels). Insets: Expanded traces show the last 10 msec (following the depolarizing pulse) of late INa in the presence of 3 μM (left panel) and 10 μM (right panel) ranolazine at pulse number 1 (a), 10 (b), 20 (c), 30 (d) and 40 (e), respectively. The amplitudes of peak (■ 3 μM; ● 10 μM) and late (□ 3 μM; ○ 10 μM) Na+ currents elicited by each pulse were normalized to the respective amplitudes of currents elicited by the first pulse, and plotted against the pulse number are shown in the lower panel.
Kinetics of Activation and Inactivation of WT and R1623Q Peak INa in the absence and presence of Ranolazine
Drugs that bind preferentially to the inactivated state of Na+ channels shift steady-state inactivation (voltage-dependent inactivation) curves toward more negative potentials.15, 16 Therefore the effect of 10 μM ranolazine on voltage-dependent activation and voltage-dependent inactivation of WT and R1623Q INa were measured. Compared to control, ranolazine (10 μM) did not cause a significant change in either the midpoint (V½) or the slope factor (k; in mV/e-fold change in current) of activation of either WT or R1623Q INa (Fig. 4A, 4B; Table 2). The values of V½ of the voltage dependence of steady-state inactivation of WT and R1623Q INa (Fig. 4A, 4B) were also not significantly different from each other; however, the slope (k) factors of the current-voltage relationships for WT and R1623Q inactivation were significantly different (see Table 3). These data are similar to those published previously.13, 14 Ranolazine (10 μM) caused a significant (p<0.05) negative (hyperpolarized) shift in the midpoints of inactivation of both WT and R1623Q INa, respectively (Fig. 4A and B and Table 2), without affecting the slope factor. This finding is consistent with the interpretation that ranolazine blocked the inactivated states of both WT and R1623Q Na+ channels.
Figure 4.
Top panel: Original steady-state inactivation traces recorded from HEK293 cells expressing WT (A) or R1623Q (B) channels in the absence and presence 10 μM ranolazine are shown. Inset: voltage-clamp protocol. Bottom panel: The effects of ranolazine on the voltage dependence of steady-state activation and inactivation of WT (A) and R1623Q (B) SCN5A Na+ channels. INa was measured in the absence (filled symbols, control) and presence of 10 μM ranolazine (open symbols), normalized to maximum in each experiment, and plotted as a function of either the potential of conditioning pulse that preceded a test pulse to 0 mV (for inactivation), or the potential of the test pulse from a holding potential of −140 mV (for activation). Symbols indicate the mean and SEM of values from 5 cells. The average midpoint (50% reduction) and slope factor of each relationship were determined by fitting the data to the Boltzmann function, and these values are summarized in Table 2.
Table 2.
Comparative activation and inactivation parameters of WT and R1623Q in the absence (control) and presence of ranolazine (10 μM).
| SCN5A – WT |
SCN5A – R1623Q |
||||
|---|---|---|---|---|---|
| V½ (mV) | k (mV/e-fold) | V½ (mV) | k (mV/e-fold) | ||
| Activation | Control (●) | −43.01±2.29 | 5.61±0.22 | −38.12±1.34 | 6.44±0.16 |
| Ranolazine (○) | −46.30±1.22 | 5.93±0.18 | −42.84±1.42 | 6.08±0.22 | |
| Inactivation | Control (■) | −83.4±2.4 | 5.6±0.5 | −84.3±2.6 | 10.3±0.6† |
| Ranolazine (□) | −94.9±4.4* | 5.4±0.3 | −96.9±4.8* | 11.1±0.5† | |
Data were recorded using voltage-clamp protocol described in Fig. 3 and fitted with Boltzmann Equation.
p<0.05 versus control:
p<0.05 versus WT.
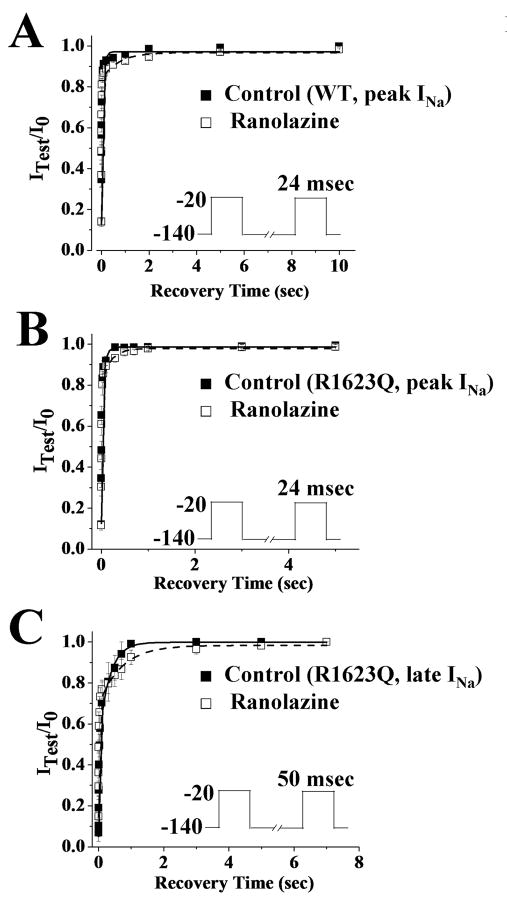
Table 3.
Recovery inactivation parameters of peak and late INa recorded from cells expressing WT or R1623Q in the absence (control) and presence of ranolazine (30 μM).
| Control | Ranolazine | ||
|---|---|---|---|
| WT Peak INa | AF | 0.83±0.04 | 0.74±0.02* |
| AS | 0.16±0.01 | 0.17±0.02 | |
| τF | 2.57±0.34 | 3.44±1.44 | |
| τS | 55.43±7.06 | 537.44±185.46* | |
| R1623Q Peak INa | AF | 0.74±0.03 | 0.69±0.02 |
| AS | 0.25±0.04 | 0.31±0.01 | |
| τF | 5.06±0.51 | 5.14±1.12 | |
| τS | 61.31±15.53 | 568.79±172.98* | |
| R1623Q Late INa | AF | 0.49±0.02 | 0.28±0.04* |
| AS | 0.44±0.02 | 0.59±0.10* | |
| τF | 6.78±1.05 | 8.81±3.48 | |
| τS | 306.41±38.43 | 835.89±254.14* | |
Data were recorded using voltage-clamp protocol described in Fig. 5A and 5C and fitted with double exponential equation.
p<0.05.
A and τ arc the amplitude and the time constants, respectively, of the fast (F) and slow (S) components of recovery of inactivation of peak and late INa.
The time course of recovery of peak INa from inactivation of WT and R1623Q channels in the absence of drug had fast and slow components (Fig. 5A and 5B, Table 3). Ranolazine (30 μM) caused a significant slowing of the recovery of peak INa (WT and R1623Q) and late INa (R1623Q) from inactivation (Fig. 5, Table 3). The values of the slow time constants (τS) of recovery from inactivation of WT and R1623Q peak INa and R1623Q late INa in the absence and presence of ranolazine were 55.43±7.06, 61.31±15.53, 306.41±38.43 msec and 537.44±185.46 (p<0.05), 568.79±172.98 (p<0.05), 835.89±254.14 msec (p<0.05), respectively. In addition, ranolazine (30 μM, n=4 cells) caused significant (p<0.05) changes in the fractions of fast (from 0.49 to 0.28) and slow (from 0.44 to 0.59) components of the recovery of late INa from inactivation. Thus, in the presence of ranolazine, there were a significant greater number of channels in the slow inactivated state, and a significantly prolonged recovery of channels from this state than in the absence of ranolazine. In addition, R1623Q late INa was more sensitive to ranolazine than either WT or R1623Q peak INa.
Figure 5.
Time-course of recovery from inactivation of WT peak (panel A), R1623Q peak (panel B), and R1623Q late INa (panel C) in the absence (filled squares, control) and presence of 30 μM ranolazine (open squares). The pulse protocols are shown as insets and described in Methods. Symbols indicate the mean±SEM of values from 5 cells. The data were fit with a double exponential equation and the parameters of the fit and numbers of experiments are reported in Table 3.
Discussion
The major new finding of this study was that ranolazine caused a UDB of late INa, in addition to UDB of peak INa. The potencies for ranolazine to cause tonic (0.1 Hz) and UDB (at 5 Hz) of R1623Q peak INa were 95.3 and 24.6 μM, respectively; for R1623Q late INa, the ranolazine potency values for tonic and UDB were 7.45 and 1.94 μM, respectively. Thus, the potencies of ranolazine to cause block of peak and late INa were similarly increased ~3 fold with increased stimulating frequency, and the selectivity of ranolazine for block of late relative to peak INa was maintained at both low and high frequencies. Because the range of therapeutic concentrations of ranolazine as an anti-ischemic agent is ~2–9 μM, these data suggest that an increase in stimulating frequency (i.e. heart rate) will significantly augment the effect of ranolazine to block late but not peak INa in patients with tachycardia.
Ranolazine slowed the recovery from inactivation of peak INa in WT and R1623Q and late INa in R1623Q channels (Fig. 5). This finding suggests that the mechanism for the UDB of both peak and late INa is incomplete recovery from block between pulses. In the presence of ranolazine (30 μM), the time constant (τs) and the amplitude (As) of the component of R1623Q late INa that recovered slowly from inactivation were both significantly (p<0.05; Table 3) increased. However, only the time constant but not the amplitude of the slow component of peak INa (WT and R1623Q) was significantly (p<0.05; Table 3) increased by 30 μM ranolazine. These results suggest that the UDB of late INa would be greater than that of peak INa in the presence of ranolazine, because fewer channels at any particular recovery time would have recovered from the UDB of late INa than from the UDB of peak INa. In support of this, UDB of R1623Q late INa by 3 and 10 μM ranolazine was greater than UDB of peak INa (Fig. 3).
Clinical Implications
The LQT3 mutation R1623Q used in this study may be relevant to both genetic and pathological (e.g. ischemia, heart failure, acidosis)17–20 conditions in which late INa is increased. These conditions wherein late INa is increased are associated with prolongation of the QT interval, and predispose patients to polymorphic ventricular tachycardia (i.e.,torsades de pointes). The results of this study suggest that block of late INa by ranolazine would be greater during tachycardia than at normal heart rates in disease situations in which that current is increased, and are consistent with the observation that ranolazine was found to reduce the incidence of tachycardia in the MERLIN-TIMI-36 clinical outcome trial.10 Furthermore, ranolazine has been shown to be effective to shorten the QTc interval in patients with the LQT3 mutation ΔKPQ. In these patients, 2 and 4 μM ranolazine were found to shorten the QTc interval by approximately 20 and 40 msec, respectively, without altering PR, QRS and RR intervals.21 These findings and those in the present study suggest that ranolazine may be effective in suppressing tachyarrhythmias whose origin or maintenance depends on enhanced late INa.
Limitations
Because the results in this study were obtained using R1623Q Na+ channels expressed in HEK293 cells studied at room temperature, the interpretation of these data should be extrapolated with caution to other pathophysiological conditions that increase the magnitude of late INa in the intact heart. In addition, ranolazine’s effects on other ion channel currents (e.g. HERG K+ current) and interactions among genetic and environmental factors that determine the response of individual patients to ranolazine were not considered in this study.
Acknowledgments
Financial support: All experiments were funded by and performed at Gilead Sciences, Inc (formerly CV Therapeutics, Inc) Palo Alto, CA.
Footnotes
Conflict of interest: SR, NB, JCS, and LB are employees of Gilead Sciences, Inc. (owner of ranolazine). JCM has no disclosure.
References
- 1.Rajamani S, Shryock JC, Belardinelli L. Block of tetrodotoxin-sensitive, Na(V)1.7 and tetrodotoxin-resistant, Na(V)1.8, Na(+) channels by ranolazine. Channels (Austin) 2008;2:449–460. doi: 10.4161/chan.2.6.7362. [DOI] [PubMed] [Google Scholar]
- 2.Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav1.4 and neuronal Nav1.7 voltage-gated Na+ channel isoforms by ranolazine. Mol Pharm. 2008;73:940–948. doi: 10.1124/mol.107.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudel J, Peper K, Rudel R, et al. Effect of tetrodotoxin on membrane currents in mammalian cardiac fibres. Nature. 1967;213:296–297. doi: 10.1038/213296a0. [DOI] [PubMed] [Google Scholar]
- 4.Maltsev VA, Sabbah HN, Higgins RS, et al. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 5.Nagatomo T, January CT, Makielski JC. Preferential block of late sodium current in the LQT3 DeltaKPQ mutant by the class I(C) antiarrhythmic flecainide. 2000;57:101–107. [PubMed] [Google Scholar]
- 6.Dumaine R, Kirsch GE. Mechanism of lidocaine block of late current in long Q-T mutant Na+ channels. Am J Physiol. 1998;274:H477–487. doi: 10.1152/ajpheart.1998.274.2.H477. [DOI] [PubMed] [Google Scholar]
- 7.Wang DW, Yazawa K, Makita N, et al. Pharmacological targeting of long QT mutant sodium channels. J Clin Invest. 1997;99:1714–1720. doi: 10.1172/JCI119335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Undrovinas AI, Belardinelli L, Undrovinas NA, et al. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17 (Suppl 1):S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 11.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac “late sodium current”. Pharmacol Ther. 2008;119:326–339. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Vassalle M, Bocchi L, Du F. A slowly inactivating sodium current (INa2) in the plateau range in canine cardiac Purkinje single cells. Exp Physiol. 2007;92:161–173. doi: 10.1113/expphysiol.2006.035279. [DOI] [PubMed] [Google Scholar]
- 13.Makita N, Shirai N, Nagashima M, et al. A de novo missense mutation of human cardiac Na+ channel exhibiting novel molecular mechanisms of long QT syndrome. FEBS Lett. 1998;423:5–9. doi: 10.1016/s0014-5793(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 14.Oginosawa Y, Nagatomo T, Abe H, et al. Intrinsic mechanism of the enhanced rate-dependent QT shortening in the R1623Q mutant of the LQT3 syndrome. Cardiovasc Res. 2005;65:138–147. doi: 10.1016/j.cardiores.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Hondeghem LM, Katzung BG. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- 16.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett PB, Yazawa K, Makita N, et al. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 19.Undrovinas AI, Belardinelli L, Undrovinas NA, et al. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17 (Suppl 1):S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valdivia CR, Chu WW, Pu J, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Moss AJ, Zareba W, Schwarz KQ, et al. Ranolazine Shortens Repolarization in Patients with Sustained Inward Sodium Current Due to Type-3 Long-QT Syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]