Abstract
Previous studies demonstrate impaired nitric oxide (NO) signaling in children and animal models with congenital heart defects and increased pulmonary blood flow. However, the molecular mechanisms underlying these alterations remain incompletely understood. The purpose of this study was to determine if early changes in arginine metabolic pathways could play a role in the reduced NO signaling demonstrated in our lamb model of congenital heart disease with increased pulmonary blood flow (Shunt lambs). The activities of the arginine recycling enzymes, argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) were both decreased in lung tissues of Shunt lambs while arginase activity was increased. Associated with these alterations, lung L-arginine levels were decreased. These changes correlated with an increase in NO synthase-derived reactive oxygen species (ROS) generation. This study provides further insights into the molecular mechanisms leading to decreased NO signaling in Shunt lambs and suggests that altered arginine metabolism may play a role in the development of the endothelial dysfunction associated with pulmonary hypertension secondary to increased pulmonary blood flow.
Keywords: Pulmonary hypertension, Argininosuccinate lyase, Argininosuccinate synthetase, Cationic amino acid transporter-1, Arginase
1. Introduction
Children with congenital heart disease and increased pulmonary blood flow suffer significant morbidity and mortality due to altered pulmonary vascular reactivity and remodeling. Endothelial dysfunction may contribute to the pathogenesis of pulmonary hypertension through impaired production and bioavailability of, and responsiveness to, NO. Utilizing in utero placement of an aorto-pulmonary vascular graft, we have previously generated a lamb model (Reddy et al., 1995) that mimics a congenital heart defect with increased pulmonary blood flow. In these Shunt lambs, we have previously identified the development of progressive endothelial dysfunction (Steinhorn et al., 2001), decreased NO signaling (Sud et al., 2007), increased oxidative stress (Sud et al., 2007), and loss of mitochondrial function (Sud et al., 2007). However, the molecular mechanisms of impaired NO signaling in pulmonary hypertension secondary to increased pulmonary blood flow remain unclear.
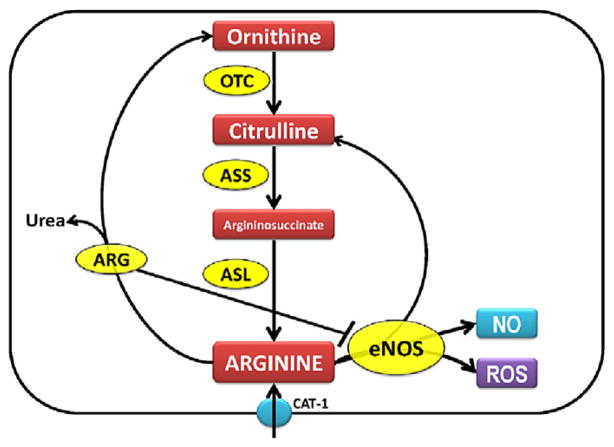
One key regulator of NO generation is arginine bioavailability. L-arginine in the endothelial cells can be metabolized by arginase to form urea and ornithine or bind to eNOS to form NO and citrulline (Fig. 1). Ornithine and citrulline can be recycled to synthesize L-arginine by the enzymes ornithine transcarbamylase, ASS, and ASL (Fig. 1). However, an early, detailed evaluation of the balance of arginine and its catabolic byproducts, its mechanisms of alterations, and its potential association with decreased NO signaling, has not been performed. Thus, in this study we tested the overall hypothesis that early changes in arginine metabolic pathways are involved in the reduced NO signaling observed in Shunt lambs. Therefore, in the present study we investigated whether there were alterations in the arginine recycling and metabolic pathways, that were associated with altered NO signaling in our Shunt model of increased pulmonary blood flow.
Fig. 1.
The arginine–nitric oxide pathway showing the role of enzymes involved in the arginine metabolism and NO signaling. ASS: argininosuccinate synthetase; ASL: argininosuccinate lyase; CAT-1: cationic amino acid transporter; ARG: arginase; ROS: reactive oxygen species; OTC: ornithine transcarbamylase; eNOS: endothelial nitric oxide synthase; NO: nitric oxide.
2. Materials and methods
2.1. Surgical preparations and care
Six mixed-breed Western pregnant ewes (137–141 days gestation, term=145 days) were operated on as previously described in detail (Reddy et al., 1995). The twin gestation lamb served as controls; they were exposed to hysterotomy but did not undergo sham thoracotomy. Thus, for each study a total of twelve lambs were analyzed: 6 control and 6 shunts. We have previously demonstrated that these sham-operated twin control lambs do not differ from sham-operated controls (Gutierrez et al., 2001). Two weeks after spontaneous delivery just prior to sacrifice, the lambs were instrumented to measure vascular pressure and pulmonary blood flow, and the patency of the vascular graft was confirmed by inspection of a thrill, and an increase in oxygen saturation between the right ventricle and the distal pulmonary artery. Four peripheral lung biopsies (~300 mg) were then collected and blood was obtained from the femoral artery. At the end of the protocol, all lambs were killed with a lethal injection of sodium pentobarbital followed by bilateral thoracotomy as described in the NIH Guidelines for the Care and Use of Laboratory Animals. All animal surgery was performed at UCSF. All tissue analysis was performed at MCG. All protocols and procedures were approved by the Committees on Animal Research at UCSF and MCG.
2.2. Hemodynamic measurements
Pulmonary arterial, and right and left atrial pressures were measured using Sorenson Neonatal Transducers (Abbott Critical Care Systems). Mean pressures were obtained by electrical integration. Heart rate was measured by a cardiotachometer triggered from the phasic systemic arterial pressure pulse wave. Left pulmonary blood flow was measured on an ultrasonic flow meter (Transonic Systems). All hemodynamic variables were measured continuously utilizing the Gould Ponemah Physiology Platform (Version 4.2) and Acquisition Interface (Model ACG-16, Gould Inc). Shunt fraction (Qp/Qs) was determined utilizing the Fick principle.
2.3. Generation of ASS and ASL-specific antibodies
As there are no commercially available antibodies for ASS and ASL, polyclonal antisera for each protein were prepared by injecting rabbits with protein fragments corresponding to NH2-GCEDFEEARKKALKL-GAKKV-COOH for ASS and NH2-GCNDEDIHTANERRLKELI-COOH for ASL. These sequences were determined based on sequence alignments of all available mammalian species for ASS and ASL. Rabbits were bled at 6-, 8-and 10-weeks, and the plasma from the 8-week bleed was immuno-purified (BioSynthesis Inc.) and used for the Western blot analyses.
2.4. Western blot analysis
Lung protein extracts were prepared as previously described (Sud et al., 2007; Sharma et al., 2008) and used for Western blot analyses. Protein extracts (25 μg) were separated on 4–20% Tris–SDS–Hepes gels and electrophoretically transferred to Immuno-Blot™ PVDF membrane (Bio-Rad Laboratories), then blocked with 5% nonfat dry milk in Tris-buffered saline. These membranes were probed with antibodies to arginase 1 and 2 (Santa Cruz Biotechnology, Inc.), eNOS (Transduction Laboratories), ASS, ASL (rabbit polyclonal antibody, Biosynthesis Inc) and the CAT-1 transporter (rabbit polyclonal antibody raised against intracellular domain 6 of mouse CAT-1). Reactive bands were visualized using chemiluminescence (Pierce Laboratories) on a Kodak 440CF image station. Band intensity was quantified using Kodak 1D image processing software. Protein expression was normalized by re-probing with a mouse anti β-actin with the exception of the mitochondrial assays evaluating eNOS translocation which were normalized by re-probing with voltage-dependent anion channel (anti-VDAC, 1:1000, Cell Signaling) antibody. Western blot analyses using antiserum raised against either ASS or ASL protein on whole cell lysates from COS-7 cells transfected with either a control plasmid (containing a cDNA for GFP) or plasmids containing the complete coding sequences for ASS or ASL was also carried out to verify specificity.
2.5. Quantification of arginase 1 and 2 mRNA by real-time RT-PCR
Quantitative RT-PCR by SYBR green I dye for specific detection of double-stranded DNA was employed to determine arginase 1 and arginase 2 mRNA levels by our previously described method (Kumar et al., 2008). The sequences were arginase 1 Forward, 5′-CACACGGAC ATCAACACTCC-3′, Reverse, 5′-AGGGAGCCACCCAGTAGAAT-3′; arginase 2 Forward, 5′-AGACCTTGGTGTGATCTGGG-3′, Reverse, 5′-AGGAAAATC CTGGGAGCTGT-3′; β-actin Forward, 5′-CTC TTC CAG CCT TCC TTC CT-3′, Reverse, 5′-GGG CAG TGA TCT CTT TCT GC-3′. Each sample was normalized to β-actin mRNA levels.
2.6. Measurement of arginine and citrulline levels
L-arginine and L-citrulline levels were analyzed by high-performance liquid chromatography (HPLC) as we have previously described (Sud et al., 2008). L-arginine and L-citrulline concentrations were calculated using standards and homoarginine as an internal standard.
2.7. Measurement of ornithine and lysine levels
Ornithine and lysine levels were measured in the 2-week old control and Shunt lung homogenates and plasma using methods described by Erbas et al (2004) and Hsieh et al (1995) respectively. Tissue ornithine and lysine levels were expressed as nmols/gww and plasma levels were expressed as μmols/L.
2.8. Measurement of activities of arginine metabolizing enzymes
The ASS and ASL enzyme activities were measured in lung homogenates as described by Dhanakoti et al (1992) except, for the measurement of argininosuccinate and arginine levels, a sensitive fluorimetric HPLC method was used (Wu et al., 1994).
2.9. Measurement of arginase activity
Arginase activity was measured by the conversion of L-arginine to urea, as previously described (Liu et al., 2005). The amount of urea formed was determined spectrophotometrically at 540 nm and the final results expressed as microgram urea/milligram protein.
2.10. Measurement of NOS activity
NO and its metabolites were determined in the peripheral lung tissue from Shunt and control lambs as we have previously described (Black et al., 1999). In addition, total NOS activity (Vmax) was determined using the conversion of 3H–L-arginine to 3H–L-citrulline (Black et al., 1999).
2.11. Measurement of superoxide levels in peripheral lung tissue
EPR measurements were performed as described previously (Sud et al., 2007; Sharma et al., 2008). NOS-derived superoxide levels were determined by incubating samples with the NOS-inhibitor, ethylisothiourea (ETU, 100 μM).
2.12. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4.01 for Windows (GraphPad Software). The mean±SEM were calculated for all samples and significance was determined either by the unpaired t-test (for 2 groups) or ANOVA (for ≥ 3 groups) with Newman–Keuls post-hoc testing. A value of p<0.05 was considered significant.
3. Results
3.1. Hemodynamics
The hemodynamic data for the 2-week old control and Shunt lambs are shown in Table 1. Pulmonary arterial pressure (PAP), left atrial pressure (LAP) and left pulmonary blood flow (LPF) were significantly greater in Shunt lambs than twin controls (Table 1). In addition, there was a significant difference in pulmonary to systemic blood flow ratio in Shunt lambs (Table 1). There were no significant differences in the mean systemic blood pressure, heart rate and right atrial pressure (Table 1).
Table 1.
Hemodynamic measurements.
| 2-week Shunt | 2-week control | |
|---|---|---|
| PAP (mean, mmHg) | 21.6±4.3* | 15.4±3.2 |
| LAP (mean, mmHg) | 6.5±4.1* | 3.1±1.9 |
| RAP (mean, mm Hg) | 4.9±3.2 | 2.5±1.4 |
| Mean BP (mmHg) | 75.8±31.5 | 65.0±7.2 |
| Left pulmonary flow (ml/min/kg) | 166.0±15.7* | 55.5±15.9 |
| Heart rate (beats/min) | 162.7±30.4 | 177.4±26.7 |
| Qp:Qs | 3.4±1.2* | 1.1±0.1 |
PAP, pulmonary arterial pressure; LAP, left atrial pressure; RAP, right atrial pressure; BP, systemic blood pressure; Qp:Qs, pulmonary to systemic blood flow ratio. N=6 for each group.
3.2. Lung and plasma amino acid levels
Our data indicate that although plasma L-arginine concentrations trended lower they did not reach statistical significance, whereas, tissue L-arginine concentrations were significantly lower in Shunt lambs (Table 2). Lung L-citrulline levels were also significantly decreased while plasma levels were significantly higher in the Shunt lambs (Table 2). We also found significantly increased ornithine and lysine levels in lung tissue in Shunt lambs, whereas, in plasma, only ornithine levels were significantly increased (Table 2). We also determined the biomarkers of arginine bioavailability (arginine/ornithine; arginine/[ornithine+lysine]) in plasma and peripheral lung. These ratios were significantly lower in peripheral lung tissue of the Shunt lambs but were not altered in the plasma (Table 2).
Table 2.
Measurement of amino acid levels associated with arginine–nitric oxide pathway.
| Amino acid | Lung tissues (nmols/gww) |
Plasma (μmol/L) |
||
|---|---|---|---|---|
| Control | Shunt | Control | Shunt | |
| Arginine | 510.9±109.66 | 347.5±54.66* | 113.6±63.30 | 82.7±29.71 |
| Ornithine | 686.2±157.413 | 876.2±54.48* | 108.2±6.60 | 125.8±13.81* |
| Lysine | 376.9±39.96 | 439.4±22.51* | 117.6±15.22 | 114.9±21.78 |
| Citrulline | 135.3±41.89 | 75.49±16.38* | 51.70±16.48 | 75.24±8.87* |
| Arginine/ornithine | 0.65±0.13 | 0.40±0.05* | 1.02±0.35 | 0.73±0.24 |
| Arginine/(Orn+Lys) | 0.43±0.08 | 0.27±0.03* | 0.49±0.19 | 0.38±0.09 |
(mean±SD;
p<0.05 vs control, N=6 for each group).
3.3. Expression and activity of arginine biosynthetic enzymes
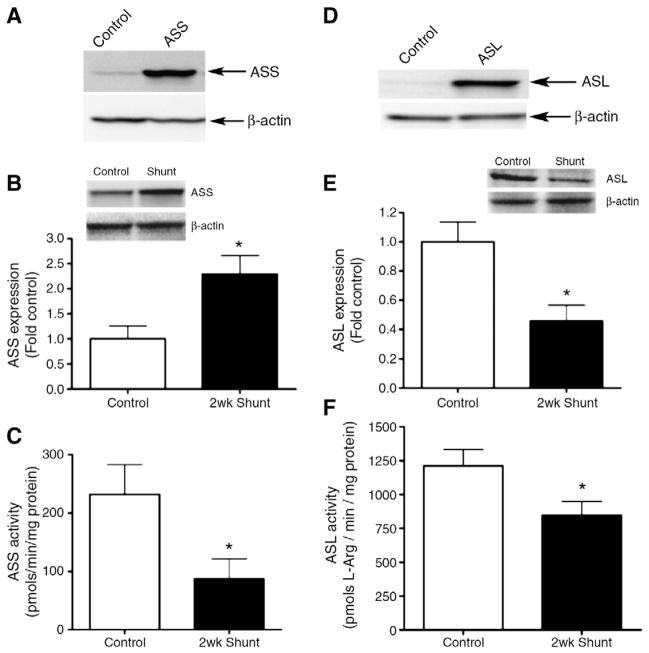
We next examined the expression and activity of the arginine recycling enzymes, argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL). We initially verified our ASS (Fig. 2A) and ASL (Fig. 2D) antibodies by over-expressing each protein in COS-7 cells. Our results indicated that ASS expression was significantly increased in Shunt lambs (Fig. 2B). However, although ASS expression was increased there was a significant decrease in ASS activity in Shunt lambs (Fig. 2C). We found that ASL was significantly decreased in Shunt lambs (Fig. 2E) and this was associated with a significant decrease in ASL activity (Fig. 2F).
Fig. 2.
Activity and expression of the arginine biosynthetic enzymes. The specificity of the ASS and ASL antibodies were confirmed by transfecting COS-7 cells with expression plasmids containing coding sequences for ASS (A) or ASL (D). ASS (B) or ASL (E) protein levels were measured using Western blot analysis. Protein loading was normalized using β-actin. There was a significant increase in normalized densitometric values for ASS (B) but a significant decrease in ASL expression (E) in Shunt lambs. There is a significant decrease in both ASS (C) and ASL (F) activities in Shunt lambs. Values are mean±SEM. N=6 for each group. *p<0.05 vs. control lambs.
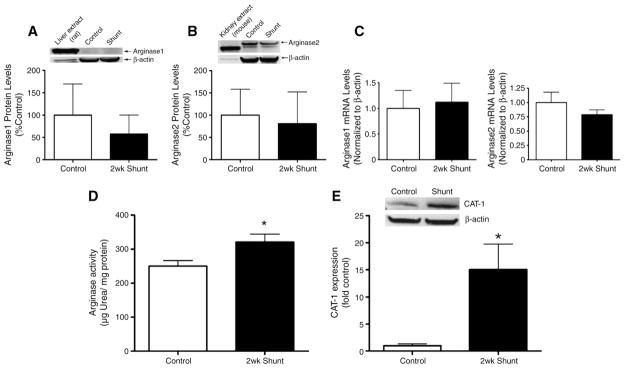
3.4. Arginase expression and activity
Using Western blot analysis we found no significant changes in protein levels of arginase 1 (Fig. 3A) or arginase 2 (Fig. 3B) nor were the mRNA levels of each isoform altered (Fig. 3C). However, there was a significant increase in arginase activity (Fig. 3D) and CAT-1 transporter protein (Fig. 3E) in Shunt lambs.
Fig. 3.
Arginase expression and activity. Arginase 1 and 2 protein levels were measured using Western blot analysis with liver (A) and kidney (B) extracts as positive controls, respectively. In addition, arginase 1 and arginase 2 mRNA levels were quantified by real-time RT-PCR analysis. There were no significant changes in the protein or mRNA levels of either arginase 1 or 2 (A–C). However, a significant increase in arginase enzyme activity is observed in Shunt lambs (D). CAT-1 expression in peripheral lung tissue of control and Shunt lambs was also determined using Western blot analysis (E). There was a significant increase in normalized (using β-actin) densitometric values for CAT-1 in Shunt lambs. Values are mean±SEM, N=6 for each group. *p<0.05 vs. control lambs.
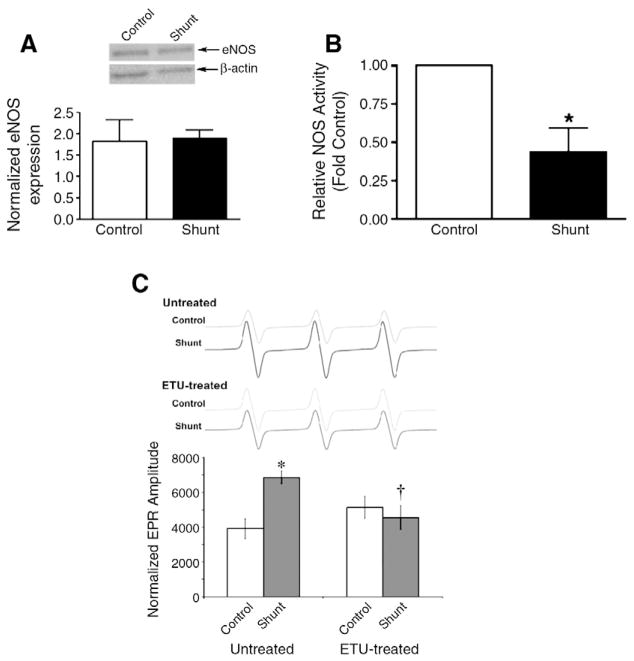
3.5. NO signaling
Finally, we determined if the alterations in arginine metabolism altered NO signaling in the Shunt lambs. Although we found no change in total eNOS protein levels (Fig. 4A), relative NOS activity (determined by tissue NOx levels as a fraction of calcium-dependent [3H]–L-arginine to [3H]–L-citrulline conversion) was significantly decreased in Shunt lambs (Fig. 4B). Further this decrease in relative NOS activity correlated with a significant increase in NOS-dependent superoxide generation, indicative of eNOS uncoupling (Fig. 4C).
Fig. 4.
Altered NO signaling in Shunt lambs. eNOS protein levels were measured using Western blot analysis and protein loading was normalized using β-actin. There was no significant change in eNOS protein levels (A). However, relative NOS activity (calculated from tissue NOx levels as a fraction of calcium-dependent [3H]–L-arginine to [3H]–L-citrulline conversion) is significantly decreased in Shunt lambs (B) while NOS-derived superoxide levels are increased (C). Protein loading was normalized using the mitochondrial specific marker, VDAC. Values are mean±SEM. N=6 for each group. *p<0.05 compared to control lambs, †p<0.05 compared to untreated (no ETU).
4. Discussion
In this study we investigated the role of the arginine metabolism in the progressive loss of NO signaling and the development of endothelial dysfunction and pulmonary hypertension in 2-week old Shunt lambs. The important findings of this study are as follows: 1) Shunt lambs have decreased activities of the arginine recycling enzymes (ASS, ASL) while arginase activity is increased. 2) L-arginine levels are significantly decreased whereas, there is increased cationic amino acid transporter (CAT-1) expression. 3) These changes correlate with an increase in NOS-derived superoxide and a decrease in relative NO signaling.
Alterations in arginine bioavailability have been implicated in a variety of vascular disorders. It can be impacted by increased consumption by arginase and/or decreased recycling by ASS and ASL. Alterations in the normal balance of arginine and other amino acids involved in arginine–NO pathway (citrulline, ornithine, and lysine) can also significantly affect the cellular arginine uptake and its bioavailability. In this study we identified a decrease in biomarkers of arginine bioavailability in the lungs, but not the plasma of Shunt lambs. This discrepancy may be due to the fact that plasma levels reflect levels from sources in addition to the lung. Indeed, our findings are consistent with a recent study in sickle cell disease patients where lower arginine bioavailability (arginine–ornithine ratio) secondary to increased arginase activity was associated with greater severity of pulmonary hypertension and mortality (Morris et al., 2005). Similarly, arginine levels are decreased in children with congenital heart disease and increased pulmonary blood flow (Gorenflo et al., 2001), and abnormalities in arginine metabolism are associated with increased mortality in patients with cardiogenic shock after acute myocardial infarction (Nicholls et al., 2007). Arginine bioavailability can be impacted by increased consumption by arginase and we found that arginase activity was increased in the lung tissues of Shunt lambs. However, this increase in activity was independent of changes in either arginase-1 or -2 protein levels suggesting that a yet unresolved post-translational modification is involved. One possibility is that arginase activity can be regulated by its sub-cellular localization with the redistribution of arginase from the cytoskeleton to the cytosol associated with increased activity (Ryoo et al., 2006). However, further studies will be required to test this possibility. It is possible that the increased arginase activity we have identified is involved in the development of pulmonary hypertension in this model by shifting arginine metabolism towards ornithine. Indeed, increased arginase-2 has been shown to be involved in reducing NO synthesis in PAH (Xu et al., 2004). While arginase activity is elevated in the serum of sickle cell patients who had associated secondary pulmonary hypertension (Morris et al., 2003). In addition, a recent study has shown decreased plasma arginine levels in sickle cell patients (Morris et al., 2005). Further, although no significant differences in plasma arginine levels was observed within the sickle cell patients with or without PAH, there were alterations in “arginine bioavailability” between sickle cell patients with severe PAH vs. those with mild or no PAH, when ornithine and citrulline levels were taken into consideration. Another study reported decreased plasma L-arginine levels in human infants with pulmonary hypertension (Pearson et al., 2001). In addition, although we found only a trend towards a decrease in plasma L-arginine levels in this study using 2-week old lambs, our previous studies have found that plasma levels of L-arginine are significantly decreased at one month of age in the Shunt lamb (Reddy et al., 1996) suggesting L-arginine levels in the plasma may decrease with advancing disease. Further developmental studies will be required to test this possibility.
The involvement of the arginine homeostasis pathways in pulmonary hypertension is likely very complex and may be altered in several ways. One of the main sources of the L-arginine utilized by eNOS is the cationic amino acid (CAT-1) transporter which is responsible for 60–80% of total carrier mediated arginine transport into endothelial cells (McDonald et al., 1997). Thus, the increase in CAT-1 expression we have identified in Shunt lambs may be a mechanism to maintain normal levels of L-arginine in the face of increased L-arginine degradation. In addition, there is another arginine metabolic pathway termed the citrulline–NO cycle, involved in regulating NO signaling. Citrulline produced in the conversion of arginine to NO is efficiently recycled to arginine by two urea cycle enzymes: ASS and ASL. In this study we have observed a significant decrease in the activity of both ASS and ASL. However, the mechanism by which this occurs appears to be complex. Although decreases in ASL protein levels correlated with decreased activity, ASS protein levels were significantly increased in Shunt lambs but activity decreased. Again, suggesting that a post-translational mechanism can inhibit ASS activity. However, this mechanism is unknown and will require further studies to unravel. This failure of the Shunt lambs to adequately recycle L-citrulline back to L-arginine is also of interest for ongoing clinical studies that are evaluating the therapeutic potential of L-citrulline to decrease the development of pulmonary hypertension after surgical repair of various CHD (Smith et al., 2006; Barr et al., 2007). Indeed, studies have shown the cardio-pulmonary bypass during the surgical correction of both ventricular septal defects and atrioventricular septal defects leads to a decrease in urea cycle repair intermediates including arginine (Smith et al., 2006) suggesting that there may be alterations in enzyme activity that compromises the ability of arginine recycling and it is possible this could limit the efficacy of L-citrulline therapy.
In conclusion our data indicate that lambs with increased pulmonary blood flow have alterations in the enzyme activities of the arginine biosynthetic and metabolic pathways within the pulmonary system. Further, these alterations lead to the disruption of the arginine pool utilized by the eNOS reaction. Thus, we speculate that the arginine metabolic pathway could be a potential therapeutic target to maintain NO signaling and endothelial function.
Acknowledgments
This research was supported in part by grants HL60190 (to SMB), HL67841 (to SMB), HL72123 (to SMB), HL70061 (to SMB), HL084739 (to SMB), R21HD057406 (to SMB), and HL61284 (to JRF) all from the National Institutes of Health, by a Transatlantic Network Development Grant from the Fondation Leducq (to SMB, JRF & SF), and by 09BGIA2310050 from the Southeast Affiliates of the American Heart Association (to SS). This work was also supported by a Programmatic Development award (to SMB) and Seed Awards (to SS and SJ) from the Cardiovascular Discovery Institute of the Medical College of Georgia. Neetu Sud was supported in part by an AHA postdoctoral fellowship from the AHA Southeast affiliates and by 1K99HL097153 from the National Institutes of Health.
Abbreviations
- ASL
Argininosuccinate lyase
- ASS
Argininosuccinate synthetase
- CAT-1
Cationic amino acid transporter-1
References
- Barr F, Tirona R, Taylor M, Rice G, Arnold J, Cunningham G, Smith H, Campbell A, Canter JA, Christian K, Drinkwater D, Scholl F, Kavanaugh-McHugh A, Summar M. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery: potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;134:319–326. doi: 10.1016/j.jtcvs.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Black SM, Heidersbach RS, McMullan DM, Bekker JM, Johengen MJ, Fineman JR. Inhaled nitric oxide inhibits NOS activity in lambs: a potential mechanism for rebound pulmonary hypertension. Am J Physiol. 1999;277:H1849–H1856. doi: 10.1152/ajpheart.1999.277.5.H1849. [DOI] [PubMed] [Google Scholar]
- Dhanakoti SN, Brosnan ME, Herzberg GR, Brosnan JT. Cellular and subcellular localization of enzymes of arginine metabolism in rat kidney. Biochem J. 1992;282 (Pt 2):369–375. doi: 10.1042/bj2820369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbas H, Aydogdu N, Kaymak K. Effects of N-acetylcysteine on arginase, ornithine and nitric oxide in renal ischemia-reperfusion injury. Pharmacol Res. 2004;50:523–527. doi: 10.1016/j.phrs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gorenflo M, Zheng C, Pöge A, Bettendorf M, Werle E, Fiehn W, Ulmer H. Metabolites of the L-arginine-NO pathway in patients with left-to-right shunt. Clin Lab. 2001;47:441–447. [PubMed] [Google Scholar]
- Gutierrez JA, Parry AJ, McMullan DM, Chapin CJ, Fineman JR. Decreased surfactant proteins in lambs with pulmonary hypertension secondary to increased blood flow. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1264–1270. doi: 10.1152/ajplung.2001.281.5.L1264. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Hsiung KP, Su JC. Determination of lysine with ninhydrin-ferric reagent. Anal Biochem. 1995;224:187–189. doi: 10.1006/abio.1995.1027. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sun X, Wedgwood S, Black SM. Hydrogen peroxide decreases endothelial nitric oxide synthase promoter activity through the inhibition of AP-1 activity. Am J Physiol Lung Cell Mol Physiol. 2008;295:L370–377. doi: 10.1152/ajplung.90205.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Drew P, Gaugler AC, Cheng Y, Visner GA. Pirfenidone inhibits lung allograft fibrosis through L-arginine-arginase pathway. Am J Transplant. 2005;5:1256–1263. doi: 10.1111/j.1600-6143.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, Machado L, Kuypers FA, Vichinsky EP. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls SJ, Wang Z, Koeth R, Levison B, DelFraino B, Dzavik V, Griffith OW, Hathaway D, Panza JA, Nissen SE, Hochman JS, Hazen SL. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation. 2007;116:2315–2324. doi: 10.1161/CIRCULATIONAHA.107.693986. [DOI] [PubMed] [Google Scholar]
- Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, Scott N, Summar ML. Neonatal pulmonary hypertension–urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344:1832–1838. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts: a model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92:1–8. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- Reddy VM, Wong J, Liddicoat JR, Johengen M, Chang R, Fineman JR. Altered endothelium-dependent responses in lambs with pulmonary hypertension and increased pulmonary blood flow. Am J Physiol. 1996;271:H562–570. doi: 10.1152/ajpheart.1996.271.2.H562. [DOI] [PubMed] [Google Scholar]
- Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, Fineman JR, Black SM. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L46–56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Canter J, Christian K, Drinkwater D, Scholl F, Christman B, Rice G, Barr F, Summar M. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Steinhorn R, Russell J, Lakshminrusimha S, Gugino S, Black S, Fineman J. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H311–H317. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1444–1453. doi: 10.1152/ajplung.00175.2007. [DOI] [PubMed] [Google Scholar]
- Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Knabe DA, Flynn NE. Synthesis of citrulline from glutamine in pig enterocytes. Biochem J. 1994;299 (Pt 1):115–121. doi: 10.1042/bj2990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. Faseb J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]