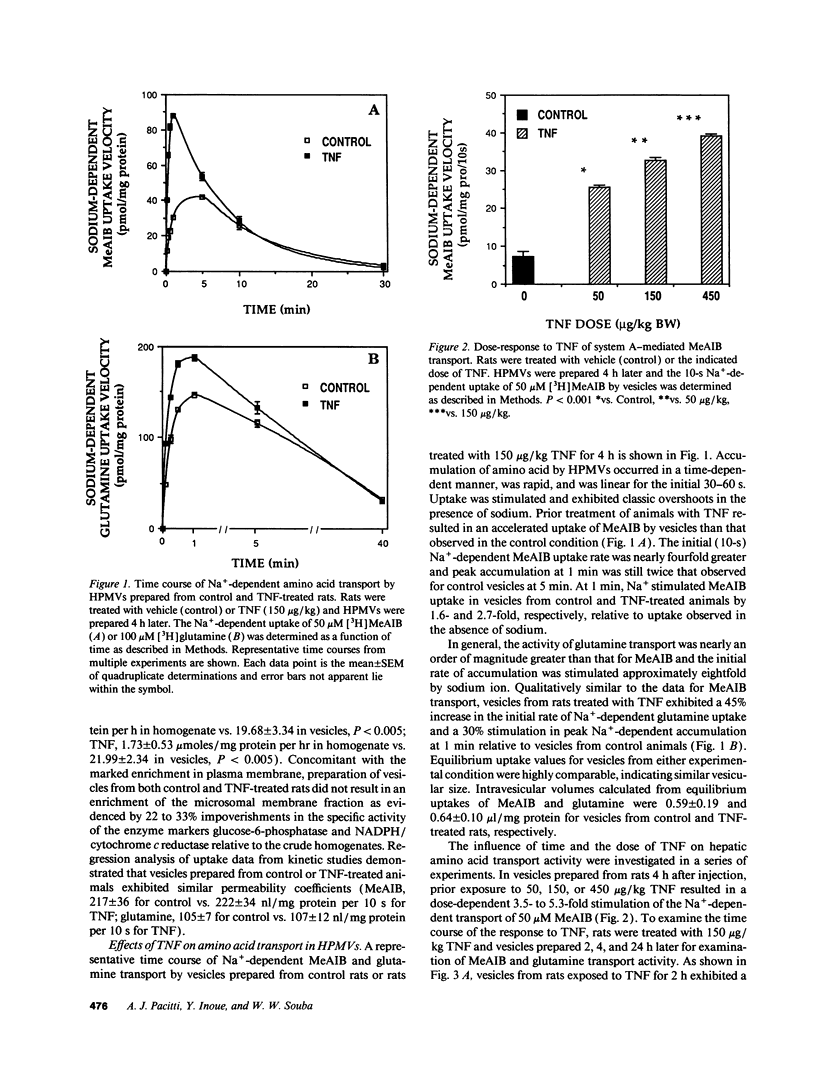
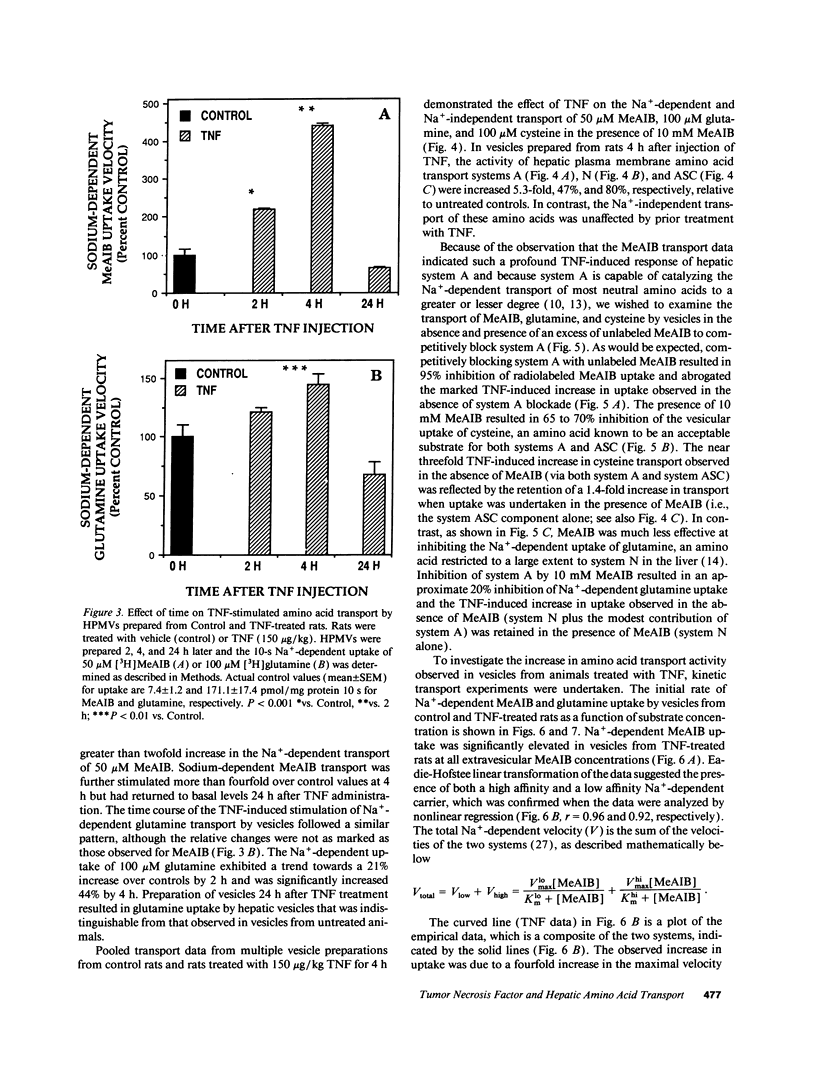
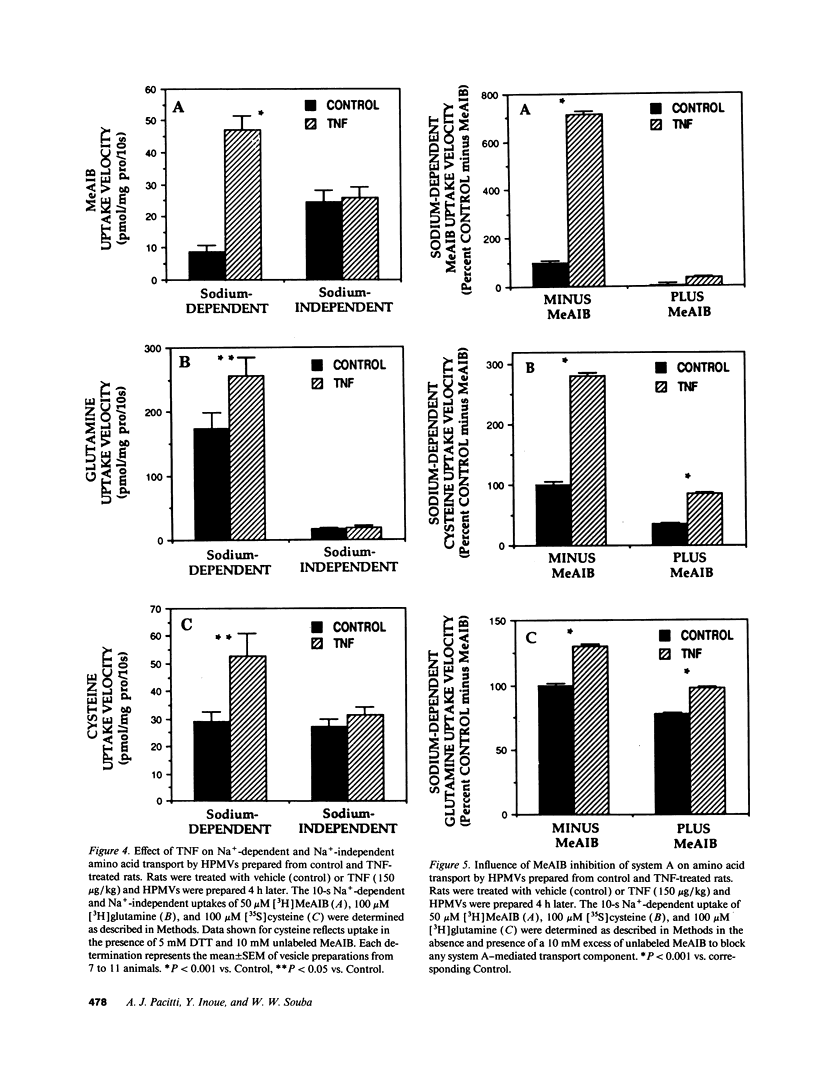
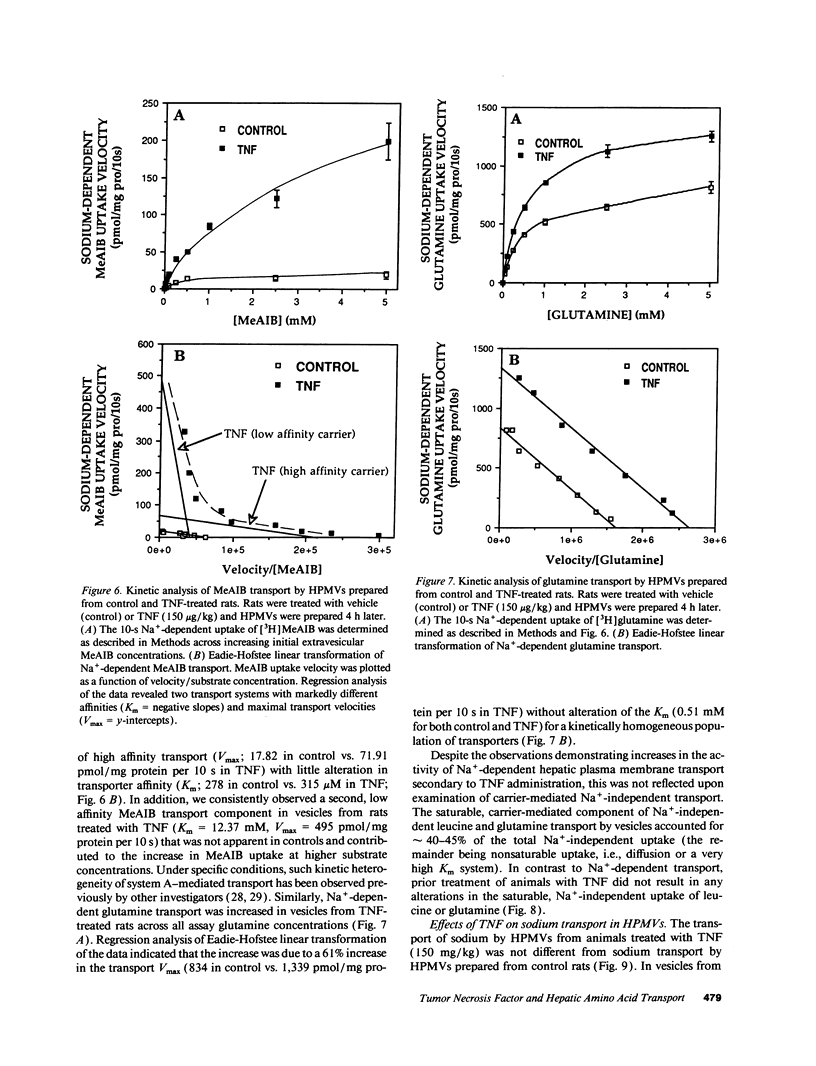
Abstract
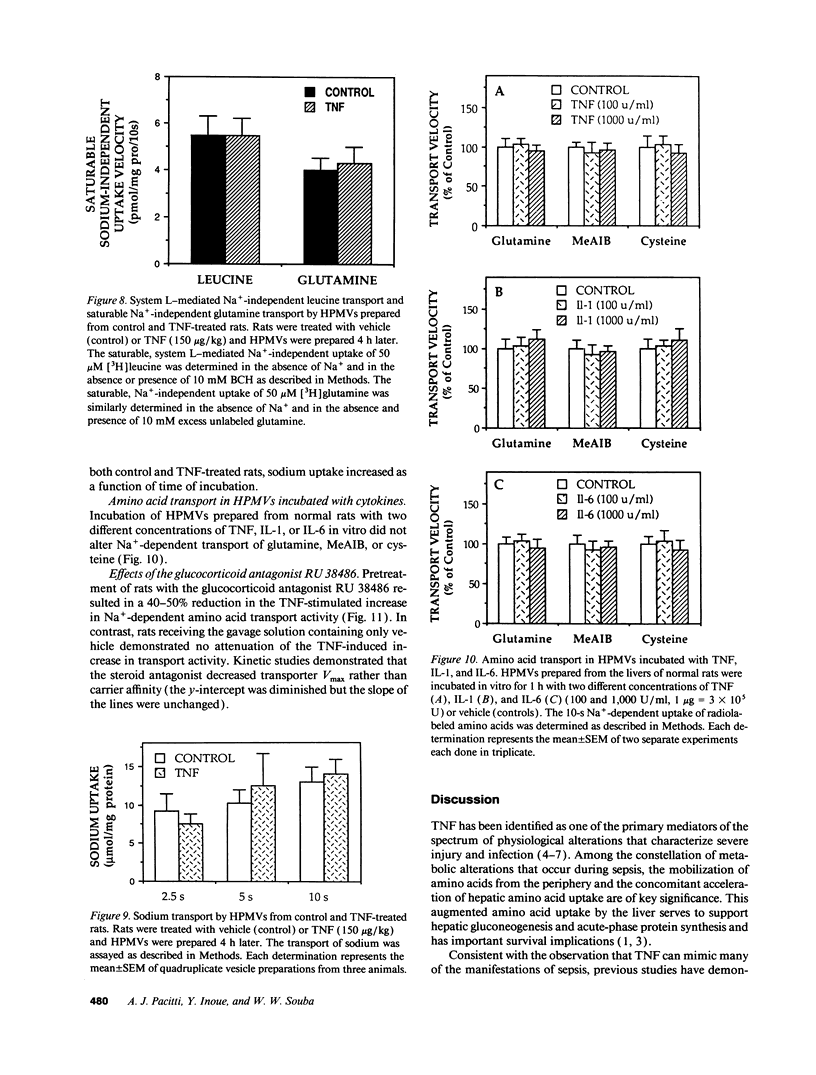
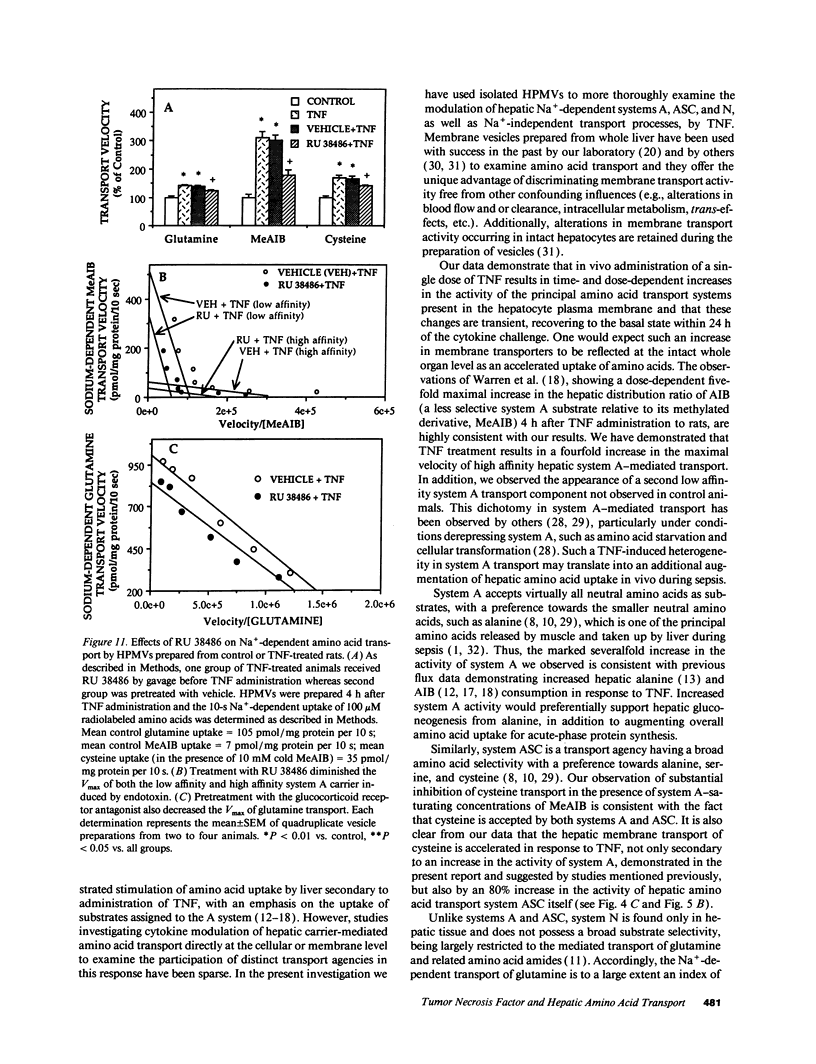
Severe infection is characterized by a translocation of amino acids from the periphery to the liver, an event that is mediated in part by cytokines such as tumor necrosis factor-alpha (TNF). We investigated the activities of Na(+)-dependent transport systems A, ASC, and N in hepatic plasma membrane vesicles (HPMVs) prepared from rats treated with TNF in vivo. TNF did not alter sodium uptake but resulted in time- and dose-dependent fivefold and 50% maximal increases in system A and system N activity, respectively, in HPMVs secondary to an increase in the transport Vmax. Maximal increases in transport were observed 4 h after exposure to TNF and had returned to basal levels within 24 h. Similarly, system ASC activity was stimulated 80% in HPMVs from rats treated with TNF. Incubation of HPMVs from normal rats in vitro with TNF did not alter transport activity. Pretreatment of animals with the glucocorticoid receptor antagonist RU 38486 attenuated the TNF-induced enhancement in transport activity by 50%. The marked increase in Na(+)-dependent amino acid transport activity by TNF is mediated in part by the glucocorticoid hormones and represents an important mechanism underlying the accelerated hepatic amino acid uptake that occurs during critical illness.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argilés J. M., López-Soriano F. J. The effects of tumour necrosis factor-alpha (cachectin) and tumour growth on hepatic amino acid utilization in the rat. Biochem J. 1990 Feb 15;266(1):123–126. doi: 10.1042/bj2660123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilés J. M., López-Soriano F. J., Wiggins D., Williamson D. H. Comparative effects of tumour necrosis factor-alpha (cachectin), interleukin-1-beta and tumour growth on amino acid metabolism in the rat in vivo. Absorption and tissue uptake of alpha-amino[1-14C]isobutyrate. Biochem J. 1989 Jul 15;261(2):357–362. doi: 10.1042/bj2610357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgen T. R., Chen M. K., Flynn T. C., Souba W. W. The effects of endotoxin on the splanchnic metabolism of glutamine and related substrates. J Trauma. 1991 Jun;31(6):742–752. doi: 10.1097/00005373-199106000-00003. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bereta J., Kurdowska A., Koj A., Hirano T., Kishimoto T., Content J., Fiers W., Van Damme J., Gauldie J. Different preparations of natural and recombinant human interleukin-6 (IFN-beta 2, BSF-2) similarly stimulate acute phase protein synthesis and uptake of alpha-aminoisobutyric acid by cultured rat hepatocytes. Int J Biochem. 1989;21(4):361–366. doi: 10.1016/0020-711x(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Bereta J., Szuba K., Fiers W., Gauldie J., Koj A. Transforming growth factor-beta and epidermal growth factor modulate basal and interleukin-6-induced amino acid uptake and acute phase protein synthesis in cultured rat hepatocytes. FEBS Lett. 1990 Jun 18;266(1-2):48–50. doi: 10.1016/0014-5793(90)81503-g. [DOI] [PubMed] [Google Scholar]
- Bode B., Tamarappoo B. K., Mailliard M., Kilberg M. S. Characteristics and regulation of hepatic glutamine transport. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):51S–55S. doi: 10.1177/014860719001400404. [DOI] [PubMed] [Google Scholar]
- Boerner P., Saier M. H., Jr Adaptive regulatory control of System A transport activity in a kidney epithelial cell line (MDCK) and in a transformed variant (MDCK-T1). J Cell Physiol. 1985 Feb;122(2):308–315. doi: 10.1002/jcp.1041220221. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Hirsch E., George B. C., Bigatello L. M., Mazuski J. E., Villee C. A., Jr Survival from sepsis. The significance of altered protein metabolism regulated by proteolysis inducing factor, the circulating cleavage product of interleukin-1. Ann Surg. 1985 Oct;202(4):446–458. doi: 10.1097/00000658-198510000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collarini E. J., Oxender D. L. Mechanisms of transport of amino acids across membranes. Annu Rev Nutr. 1987;7:75–90. doi: 10.1146/annurev.nu.07.070187.000451. [DOI] [PubMed] [Google Scholar]
- Fong Y. M., Marano M. A., Moldawer L. L., Wei H., Calvano S. E., Kenney J. S., Allison A. C., Cerami A., Shires G. T., Lowry S. F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990 Jun;85(6):1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt R., Kleemann E. Hormonal regulation of amino acid transport system N in primary cultures of rat hepatocytes. Eur J Biochem. 1987 Jul 15;166(2):339–344. doi: 10.1111/j.1432-1033.1987.tb13520.x. [DOI] [PubMed] [Google Scholar]
- Hall-Angerås M., Angerås U., Zamir O., Hasselgren P. O., Fischer J. E. Effect of the glucocorticoid receptor antagonist RU 38486 on muscle protein breakdown in sepsis. Surgery. 1991 Apr;109(4):468–473. [PubMed] [Google Scholar]
- Jacob R., Rosenthal N., Barrett E. J. Characterization of glutamine transport by liver plasma membrane vesicles. Am J Physiol. 1986 Nov;251(5 Pt 1):E509–E514. doi: 10.1152/ajpendo.1986.251.5.E509. [DOI] [PubMed] [Google Scholar]
- Kalimi M. Y., Agarwal M. K. Interaction of antiglucocorticoid RU 486 with rat kidney glucocorticoid receptor. Biochem Biophys Res Commun. 1988 May 31;153(1):365–371. doi: 10.1016/s0006-291x(88)81232-4. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S. Amino acid transport in isolated rat hepatocytes. J Membr Biol. 1982;69(1):1–12. doi: 10.1007/BF01871236. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Christensen H. N. Electron-transferring enzymes in the plasma membrane of the Ehrlich ascites tumor cell. Biochemistry. 1979 Apr 17;18(8):1525–1530. doi: 10.1021/bi00575a021. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. 1980 May 10;255(9):4011–4019. [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of system ASC for transport of neutral amino acids in the isolated rat hepatocyte. J Biol Chem. 1981 Apr 10;256(7):3304–3312. [PubMed] [Google Scholar]
- Konagaya M., Bernard P. A., Max S. R. Blockade of glucocorticoid receptor binding and inhibition of dexamethasone-induced muscle atrophy in the rat by RU38486, a potent glucocorticoid antagonist. Endocrinology. 1986 Jul;119(1):375–380. doi: 10.1210/endo-119-1-375. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Pacitti A. J., Austgen T. R., Souba W. W. Adaptive regulation of alanine transport in hepatic plasma membrane vesicles from the endotoxin-treated rat. J Surg Res. 1991 Jul;51(1):46–53. doi: 10.1016/0022-4804(91)90068-w. [DOI] [PubMed] [Google Scholar]
- Prpić V., Green K. C., Blackmore P. F., Exton J. H. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol Chem. 1984 Feb 10;259(3):1382–1385. [PubMed] [Google Scholar]
- Rock C. S., Lowry S. F. Tumor necrosis factor-alpha. J Surg Res. 1991 Nov;51(5):434–445. doi: 10.1016/0022-4804(91)90146-d. [DOI] [PubMed] [Google Scholar]
- Roh M. S., Moldawer L. L., Ekman L. G., Dinarello C. A., Bistrian B. R., Jeevanandam M., Brennan M. F. Stimulatory effect of interleukin-1 upon hepatic metabolism. Metabolism. 1986 May;35(5):419–424. doi: 10.1016/0026-0495(86)90131-9. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S., Clowes G. H., Jr, George B. C., Hirsch E., Lindberg B. Exchange of amino acids by muscle and liver in sepsis. Arch Surg. 1983 Feb;118(2):167–175. doi: 10.1001/archsurg.1983.01390020023004. [DOI] [PubMed] [Google Scholar]
- Schenerman M. A., Kilberg M. S. Maintenance of glucagon-stimulated system A amino acid transport activity in rat liver plasma membrane vesicles. Biochim Biophys Acta. 1986 Apr 25;856(3):428–436. doi: 10.1016/0005-2736(86)90133-1. [DOI] [PubMed] [Google Scholar]
- Souba W. W. Glutamine: a key substrate for the splanchnic bed. Annu Rev Nutr. 1991;11:285–308. doi: 10.1146/annurev.nu.11.070191.001441. [DOI] [PubMed] [Google Scholar]
- Warren R. S., Donner D. B., Starnes H. F., Jr, Brennan M. F. Modulation of endogenous hormone action by recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8619–8622. doi: 10.1073/pnas.84.23.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Alcock N., Calvano S., Brennan M. F. Hormonal and metabolic response to recombinant human tumor necrosis factor in rat: in vitro and in vivo. Am J Physiol. 1988 Aug;255(2 Pt 1):E206–E212. doi: 10.1152/ajpendo.1988.255.2.E206. [DOI] [PubMed] [Google Scholar]



