Abstract
The primary cilium is a solitary, immotile cilium that is present in almost every mammalian cell type. Primary cilia are thought to function as chemosensors, mechanosensors, or both, depending on cell type, and have been linked to several developmental signaling pathways. Primary cilium malfunction has been implicated in several human diseases, the symptoms of which include vision and hearing loss, polydactyly, and polycystic kidneys. Recently, primary cilia have also been implicated in the development and homeostasis of the skeleton. In this review, we discuss the structure and formation of the primary cilium and some of the mechanical and chemical signals to which it could be sensitive, with a focus on skeletal biology. We also raise several unanswered questions regarding the role of primary cilia as mechanosensors and chemosensors and identify potential research avenues to address these questions.
Keywords: primary cilium, kidneys, cilia
The primary cilium is a solitary, immotile cilium that is present in almost every mammalian cell type (Wheatley et al., 1996). Multiple developmental signaling pathways have been linked to primary cilia (reviewed in Christensen et al., 2007), and they are thought to function as chemosensors, mechanosensors, or both, depending on cell type. Primary cilium malfunction has been implicated in several human diseases, the symptoms of which include vision and hearing loss, polydactyly, and polycystic kidneys (reviewed in Tobin and Beales 2007; Yoder 2007). Recently, primary cilia have also been implicated in the development (Zhang et al., 2003; Xiao et al., 2006; Haycraft et al., 2007; Koyama et al., 2007; McGlashan et al., 2007) and homeostasis (Malone et al., 2007) of the skeleton. In this review, we discuss the structure and formation of the primary cilium and some of the mechanical and chemical signals to which it could be sensitive, with a focus on skeletal biology. We also raise several unanswered questions regarding the role of primary cilia as mechanosensors and chemosensors and identify potential research avenues to address these questions.
PRIMARY CILIUM STRUCTURE
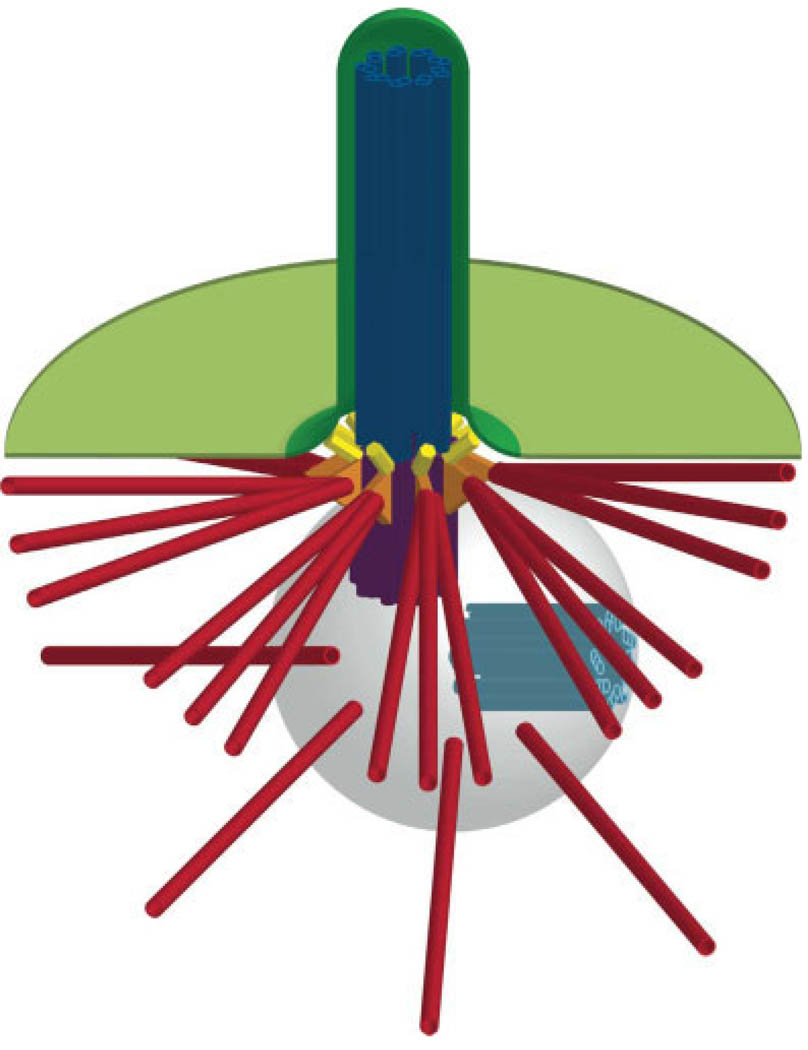
The structural core of the primary cilium is an axoneme of nine doublet microtubules that extends from a basal body (also known as a mother centriole; Fig. 1). Unlike the motile cilia that exist in cells lining the airway and other tissues, primary cilia lack a central pair of microtubules and are thus called 9+0 cilia. The primary cilium is enclosed in a specialized membrane (Vieira et al., 2006) to which receptors for Sonic hedgehog (Shh), platelet-derived growth factor (PDGF), serotonin, and somatostatin localize (Handel et al., 1999; Brailov et al., 2000; Schneider et al., 2005; Rohatgi et al., 2007). The ciliary membrane forms a compartment that is separated from the cytoplasm by transition fibers (Fig. 1) (Sorokin, 1962; Deane et al., 2001), which connect the distal end of the basal body/mother centriole to the plasma membrane. The basal body/mother centriole also possesses subdistal appendages on its sides that directly contact cytoplasmic microtubules (Sorokin 1962), linking the primary cilium to the microtubule cytoskeleton. The basal body/mother centriole is part of the centrosome, which organizes the interphase microtubule cytoskeleton in many cell types and exists at the poles of mitotic spindles (reviewed in Bettencourt-Dias and Glover 2007). Centrosomes also contain a daughter centriole that lacks a cilium, and the two centrioles are surrounded by pericentriolar material from which microtubules are nucleated (Fig. 1).
Fig. 1.
Schematic of primary cilium structure. The primary cilium is enclosed in a specialized membrane (dark green) that is continuous with the plasma membrane (light green). Membranes are shown in cutaway view. The axoneme of the primary cilium (dark blue) extends from the mother centriole/basal body (purple), which possesses subdistal appendages (orange) that are connected to a subset of cytoplasmic microtubules (red). The mother centriole/basal body is part of the centrosome, which includes a daughter centriole (light blue) and pericentriolar material (light gray) from which other cytoplasmic microtubules (red) are nucleated). The mother centriole/basal body is connected to the plasma membrane via transition fibers (yellow) that separate the ciliary compartment from the cytoplasm. Cytoplasmic microtubules are reduced in number and shortened for simplicity; they actually extend far beyond the boundaries of the image. Image not to scale.
PRIMARY CILIUM GROWTH
Primary cilium growth begins when the distal end of the mother centriole docks with a ~300 nm diameter vesicle of unknown origin (Sorokin 1962; Alieva and Vorobjev 2004). This docking event precedes the extension of nine doublet microtubules from the nine triplet microtubules of the centriole. Further axoneme growth is accomplished by a process called intraflagellar transport (IFT), wherein cargo is transported up and down the axoneme via complexes of IFT adaptor proteins by the motor proteins kinesin-2 and cytoplasmic dynein 1b, respectively (reviewed in Scholey and Anderson 2006). The processes by which cargo is acquired and released and the regulation of motor attachment to the axonemal microtubules are unclear, as is the mechanism that determines the final length of the axoneme, which varies from a few µm to over 100 µm, depending on cell type (unpublished observations). The membrane surrounding the axoneme then fuses with the plasma membrane, allowing the cilium to protrude into the extracellular space. In cycling cells, primary cilia appear early in G1 (Alieva and Vorobjev 2004) and are disassembled prior to mitosis by an Aurora A-dependent mechanism (Pugacheva et al., 2007).
PRIMARY CILIA AS MECHANOSENSORS IN BONE
The skeleton is primarily a mechanical organ whose function is to support the body against gravitational force and aid in locomotion. The skeleton adapts to mechanical stimuli: increased loading results in greater bone formation, whereas reduced loading results in bone loss. The ability of bone to sense and respond to mechanical stimuli depends on bone cells, including osteocytes, osteoblasts, and osteoclasts. Osteocytes have been proposed as candidate mechanosensors because of their location within the bone matrix. Osteocytes are housed in small pockets, or lacunae, which are connected by a network of channels, or canaliculi (Lanyon 1993). Osteocytes extend projections throughout this canalicular network and communicate with one another and with preosteoblasts on bone surfaces through gap junctions on these projections.
Osteocytes respond to mechanical stimuli both in vivo (Robling et al., 2006) and in vitro (Klein-Nulend et al., 1995). Osteoblasts and mesenchymal stem cells (MSCs), the main source of osteoblast progenitors, have also been shown to respond to mechanical stimuli and appear to play an important role in bone mechanotransduction (Reich and Frangos 1993; Meinel, Karageorgiou et al. 2004). However, the relative importance of different types of local, mechanical stimuli to which bones cells respond is unclear. One such mechanical stimulus is oscillatory fluid flow. During dynamic loading of the skeleton, bending moments are created, causing bone matrix deformation and intramedullary pressure gradients. These pressure gradients result in flow through the canalicular network from regions of high pressure to regions of low pressure (Knothe Tate et al., 1998). Flow-induced osteocyte surface shear stress is estimated to be in the range of 8–30 dynes/cm2 under normal loading conditions (Weinbaum et al., 1994). Fluid flow may also cause drag force on cell processes resulting in a hoop strain of >0.5% (Han et al., 2004); however, this model assumes that osteocyte processes are tethered to the lacunar wall, which has not been clearly established. Osteocytes may also experience cytoskeletal strain or deformation as the lacunar spaces in which they reside are deformed.
The mechanism by which bone cells sense and respond to these mechanical stimuli is not known, but recent evidence (Malone et al., 2007) demonstrates that primary cilia are mechanically sensitive in bone cells. Primary cilia are known to act as flow sensors in renal epithelial cells (Liu et al., 2005), in which bending of the cilium results in an increase of intracellular calcium (Schwartz et al., 1997). They have also been observed in osteocytes and osteoblasts (Matthews and Martin, 1971; Tonna and Lampen, 1972; Xiao et al., 2006) and suggested as potential bone mechanical sensors (Whitfield, 2003; Xiao et al., 2006). Primary cilia projecting from osteocytes might experience fluid flow shear stress, mechanical deformation, or pressure gradients depending on their size and orientation. Mechanical stimuli that could affect primary cilia on preosteoblasts are less well defined, as these cells reside on bone surfaces rather than within the bone matrix. Determining the size, orientation and properties of primary cilia on different bone cell types in situ will be important for understanding their possible roles in vivo. Interestingly, chondrocytes in cartilage also have primary cilia, which possess integrins in their membranes and contact the extracellular matrix (Jensen et al., 2004; McGlashan et al., 2006). Since osteocytes likely experience fluid flow in the confined matrix-filled spaces between the cell surface and the lacunar wall, cilium-matrix interactions might also play a role in mechanosensation in bone.
PRIMARY CILIA AS CHEMOSENSORS IN BONE
Primary cilia may also function as specialized regions of the cell membrane that are equipped with receptors that transmit chemical or molecular signals from the environment into the cell. One of the best known of these molecular signaling pathways is mediated by secreted growth factors in the Hedgehog family. Hedgehog signaling is critical throughout embryonic development and adulthood: disruption in Hedgehog signaling has been associated with multiple birth defects and human cancers (reviewed in Helms et al., 2007). Much of our current knowledge of Hedgehog signaling comes from studies conducted in the fruit fly, Drosophila. Collectively, these studies indicate that the Hedgehog receptor complex is composed of two trans-membrane proteins, Smoothened and Patched. In the absence of Hedgehog, Patched represses pathway activity by inhibiting the function of Smoothened, but when Hedgehog is present and binds to Patched, Smoothened is relieved from this inhibition and activates target gene transcription. It is thought that a similar mechanism is responsible for Hedgehog signaling in mammalian cells, where Shh is one of the Hedgehog family ligands.
Recent evidence from a number of laboratories demonstrates that Shh signaling occurs at the primary cilium (Huangfu et al., 2003; Rohatgi et al., 2007). These groups showed that in the absence of Shh, Patched occupies the primary cilium, which may exclude Smoothened from this structure. Because Patched functions as a Shh receptor, its presence makes the primary cilium a receptacle for any secreted Shh in the vicinity. When Shh binds to its receptor, Patched leaves the cilium and Smoothened instead accumulates in the ciliary membrane (Rohatgi et al., 2007). Mutations that affect formation of the primary cilia thus lead to disruption in Shh signaling.
The connection between Shh signaling and primary cilia in bone was first hinted at by the finding that mice with mutations in IFT components have skeletal patterning defects that are similar to Shh mutants (Huangfu et al., 2003; Zhang et al., 2003). Null mutation of IFT components results in embryonic lethality (Marszalek et al., 1999; Murcia et al., 2000), precluding studies of complete skeletogenesis in these mutants, but tissue-specific mutation of these same IFT components causes a more restricted range of embryonic defects. For example, mice lacking the kinesin-2 subunit Kif3a in retinal photoreceptors display abnormal accumulation of opsin in the inner segment, causing photoreceptor death (Marszalek et al., 1999), and elimination of the IFT component Ift88 (also known as polaris) in the central nervous system of mice causes cerebellar disruptions (Chizhikov et al., 2007). Likewise, loss of Ift88/polaris or Kif3a in the developing skeleton leads to a plethora of skeletal malformations (Haycraft et al., 2007; Koyama et al., 2007) for reasons that are not yet clear. Analysis of additional tissue- and cell type-specific knockouts in mice and other experimental animals should provide insight into how primary cilia function as molecular detectors in bone. It will be important to compare skeletal structure and growth dynamics in conditional mutants lacking components required for primary cilium structure (e.g., Kif3a) against those lacking specific ciliary signaling pathway components (e.g., Patched) to determine the contribution of each signaling pathway to overall primary cilium-mediated signaling during bone development and homeostasis.
UNANSWERED QUESTIONS
How are Mechanical Signals Sensed by Primary cilia?
As observed in time-lapse movies of cultured kidney tubule cells, primary cilia exposed to unidirectional fluid flow bend in the direction of flow. However, it is not known whether mechanosensitive proteins are distributed asymmetrically around the cilium, which would maximize the sensitivity to this bending. Although the primary cilium has been modeled as a cantilevered beam (Schwartz et al., 1997), the minimum degree to which it must be deflected in order to elicit a response is not known. Furthermore, it is not known whether the cilium functions as a switch or a rheostat: is there a threshold level of mechanostimulation that toggles an on–off signal from the cilium, or is the ciliary signaling output graded in proportion to the degree of mechanical stimulation? If the signaling activity is switch-like, there might also be a recalcitrant period immediately after activation during which the cilium cannot re-respond to stimulation. Combining high-resolution imaging of primary cilia and a signaling output (e.g. calcium flux) with careful control of the timing and strength of an experimental mechanical stimulus should allow definition of some of these parameters. In the case of bone cells, combining current models of fluid flow in bone with these parameters would provide useful constraints on models for how primary cilia function in vivo.
Also unknown is the extent to which the cilium’s connection to cytoplasmic microtubules might play a role in its function as a mechanosensor. The only characterized mechanosensory mechanism in the cilium involves the polycystins, which form a stretch-sensitive membrane channel complex (Nauli et al., 2003), but as noted above, the basal body is directly connected to a subset of cytoplasmic microtubules via its appendages. These connections could serve to anchor the cilium in place and provide it with structural support, much like the roots of a tree support its trunk. However, they might also play a role in mechanical signaling; bending of the primary cilium could translate into movement of the attached microtubules, which could then lead to downstream signaling activity or changes in the transport of materials along the cytoplasmic microtubules. The cilium and the basal body could thus act as an extracellular extension of the cytoplasmic microtubule cytoskeleton and provide it with information about the extracellular environment, much as integrins link extracellular matrix proteins to the actin cytoskeleton. Because the microtubules of the primary cilium and centrioles are less sensitive to low concentrations of microtubule-destabilizing drugs, such as nocodazole, than cytoplasmic microtubules (unpublished observations), it should be possible to study the mechanosensitivity of the primary cilium in the presence or absence of cytoplasmic microtubules. Furthermore, imaging of cytoplasmic microtubules during primary cilium deformation may give insight into the strength and extent of the connections between these two microtubule systems.
Is there Feedback Between Mechanical Stimulus and Ciliary Structure?
In human umbilical cord cells, fluid flow has been shown to cause loss of primary cilia from the endothelial surface (Iomini et al., 2004). It is possible that mechanical stimuli could lead to remodeling of ciliary structure, either through changes in its length or membrane composition. As mentioned above, application of Shh to cells causes the relocalization of Smoothened to the primary cilium (Corbit et al., 2005; Rohatgi et al., 2007), indicating that trafficking of proteins to the cilium in response to external signals occurs. Analysis of primary cilium components before and after exposure to fluid flow or other chemical or molecular signals might uncover a mechanism by which bone cells remodel their primary cilia in response to mechanical or chemical stimulation.
What are the Connections Between Mechanical Stimuli and Cellular Behavior in Bone Cells?
In bone cells, primary cilia are required for cytokine release and changes in gene transcription in response to dynamic flow (Malone et al., 2007). However, the connections between mechanical stimulation of the cilium and cellular responses are not fully known. The best-studied case of a downstream signal resulting from mechanical stimulation of the primary cilium is that of calcium influx into kidney tubule cells after exposure to fluid flow, which leads to intracellular calcium release (Praetorius and Spring, 2001). Although calcium influx might also be involved in other settings, it is apparently only one of multiple mechanically activated second messenger systems in bone (Malone et al., 2007). There is growing evidence that the primary cilium can be coupled to other signaling pathways. Adenylyl cyclases have also been found in the primary cilium (Masyuk et al., 2006), suggesting that cAMP could be a second messenger in some forms of ciliary signaling. In addition, IFT could traffic activated signaling proteins from the ciliary compartment to the cytoplasm, as has been proposed for Shh signaling (May et al., 2005).
An important unanswered question is the extent to which primary cilium-mediated mechanosensation leads to changes in cellular communication and proliferation in bone. In kidney tubules, loss of primary cilia leads to overproliferation of tubule cells and the formation of cysts (Yoder et al., 2002), and primary cilia have been linked to cell cycle progression (Morgan et al., 2002; Pan and Snell, 2007). Although osteocytes themselves do not divide, it is possible that mechanical stimulation of osteocyte primary cilia could lead to cell proliferation at the bone surface, which would require cell–cell communication via the canalicular network. Conditional knockout of genes required for primary cilium in specific bone cell populations, which has already begun (Haycraft et al., 2007), should allow the dissection of primary cilium function in osteocytes, osteoblasts, and osteoclasts. An important caveat of these experiments is that primary cilia might play different signaling roles (chemosensory, mechanosensory, or both) depending on developmental stage, and the ability to control ciliogenesis temporally as well as in a tissue- or cell type-specific fashion would allow more detailed characterization of primary cilium function in bone.
The primary cilium has emerged as an important organelle for sensing environmental signals, and its function in diverse tissues is just beginning to be understood. One research goal that lies ahead is to integrate ciliary signaling with other cellular sensory systems to provide a comprehensive picture of how cells sense and respond to environmental stimuli, and bone, with its architectural complexity and plasticity, provides a challenging but exciting arena in which to accomplish it.
LITERATURE CITED
- Alieva IB, Vorobjev IA. Vertebrate primary cilia: a sensory part of centrosomal complex in tissue cells, but a “sleeping beauty” in cultured cells? Cell Biol Int. 2004;28:139–150. doi: 10.1016/j.cellbi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA. 2004;101:16689–16694. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Helms JA, Brugmann SA, Cordero DR. Inborn errors of development: the molecular basis of clinical disorders of morphogenesis. 2nd ed. New York, USA: Oxford University Press; 2007. Shh and other genes and the holoprosencephaly malformation sequence. [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Knothe U, Niederer P. Experimental elucidation of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. Am J Med Sci. 1998;316:189–195. doi: 10.1097/00000441-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, Pacifici M. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53 Suppl 1:S102–S106. doi: 10.1007/BF01673415. discussion S106–S107. [DOI] [PubMed] [Google Scholar]
- Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca21 concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289:F978–F988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci USA. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca21 and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JL, Martin JH An electron microscope study. Intracellular transport of calcium and its relationship to homeostasis and mineralization. Am J Med. 1971;50:589–597. doi: 10.1016/0002-9343(71)90114-8. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007;26:234–246. doi: 10.1016/j.matbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet. 2002;11:3345–3350. doi: 10.1093/hmg/11.26.3345. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich KM, Frangos JA. Protein kinase C mediates flow-induced prostaglandin E2 production in osteoblasts. Calcif Tissue Int. 1993;52:62–66. doi: 10.1007/BF00675628. [DOI] [PubMed] [Google Scholar]
- Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6:354. [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–936. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonna EA, Lampen NM. Electron microscopy of aging skeletal cells. I. Centrioles and solitary cilia. J Gerontol. 1972;27:316–324. doi: 10.1093/geronj/27.3.316. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- Whitfield JF. Primary cilium—is it an osteocyte’s strain-sensing flowmeter? J Cell Biochem. 2003;89:233–237. doi: 10.1002/jcb.10509. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281:30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn. 2003;227:78–90. doi: 10.1002/dvdy.10289. [DOI] [PubMed] [Google Scholar]