Abstract
NADPH oxidase (Nox)–dependent reactive oxygen species production is implicated in the pathogenesis of cardiovascular diseases, including hypertension. We tested the hypothesis that oxidase subunits are differentially regulated in renal proximal tubules from normotensive and spontaneously hypertensive rats. Basal Nox2 and Nox4, but not Rac1, in immortalized renal proximal tubule cells and brush border membranes were greater in hypertensive than in normotensive rats. However, more Rac1 was expressed in lipid rafts in cells from hypertensive rats than in cells from normotensive rats; the converse was observed with Nox4, whereas Nox2 expression was similar. The D1-like receptor agonist fenoldopam decreased Nox2 and Rac1 protein in lipid rafts to a greater extent in hypertensive than in normotensive rats. Basal oxidase activity was 3-fold higher in hypertensive than in normotensive rats but was inhibited to a greater extent by fenoldopam in normotensive (58±3.3%) than in hypertensive rats (31±5.2%; P<0.05; n=6 per group). Fenoldopam decreased the amount of Nox2 that coimmunoprecipitated with p67phox in cells from normotensive rats. D1-like receptors may decrease oxidase activity by disrupting the distribution and assembly of oxidase subunits in cell membrane microdomains. The cholesterol-depleting reagent methyl–β-cyclodextrin decreased oxidase activity and cholesterol content to a greater extent in hypertensive than in normotensive rats. The greater basal levels of Nox2 and Nox4 in cell membranes and Nox2 and Rac1 in lipid rafts in hypertensive rats than in normotensive rats may explain the increased basal oxidase activity in hypertensive rats.
Keywords: NADPH oxidase, dopamine receptor, reactive oxygen species, lipid rafts
The NADPH oxidase (Nox) enzyme family is a major source of reactive oxygen species (ROS), eg, superoxide anion, hydrogen peroxide, and the hydroxyl radical.1,2 Nox-dependent ROS regulate diverse cellular processes, including angiotensin II–mediated renal growth and cardiovascular remodeling.3–6 The increased generation of ROS by Nox contributes to human5–7 and animal hypertension.8–15 Nox activity and superoxide formation are increased in vascular smooth muscle,5,13 endothelial,4,14 and neural cells15 in genetic and acquired hypertension. The renal contribution to the pathogenesis of hypertension has also been ascribed to increased ROS production.7–10,12,14
Lipid rafts (LRs) are membrane microdomains composed of glycosylphosphatidylinositol-linked proteins, glycosphingolipids, and cholesterol.16–19 Caveolae and LRs have been implicated in protein trafficking and signal transduction.16–19 They also serve as compartments for the recruitment of cell signaling components and enzymes to increase the efficient and rapid coupling of receptors to >1 effector system. G protein– coupled receptors, including dopamine receptors, are associated with caveolar and noncaveolar LRs.16–20 We and others have reported the presence of noncaveolar LRs in immortalized rat renal proximal tubule (RPT) cells, which do not express caveolin-1.20,21 Nox isoforms and subunits are expressed in LRs in phagocytic22–24 and nonphagocytic cells, such as rat vascular smooth muscle, bovine endothelial,11,25,26 and human RPT cells.27 In addition, Nox-dependent ROS production is differentially regulated in LRs and non-LRs.22–27
Dopamine is an important regulator of blood pressure, sodium balance, and renal and adrenal function through an independent peripheral dopaminergic system.28,29 Abnormal signaling of D1-like receptors is involved in rodent models of hypertension and in humans with essential hypertension.28,29 Moreover, D1-like receptors have been shown to inhibit oxidative stress in vascular smooth muscle and RPT cells.30–32 However, with the exception of our recent study,27 previous reports have not taken into consideration the membrane microdomain localization of Nox subunits in the cellular biological mechanism(s) for the D1-like receptor–mediated inhibition of Nox activity. The current studies demonstrate for the first time a differential targeting of Nox4 and Rac1 in LRs in RPT cells from Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHRs). In these cells, the D1-like receptor agonist fenoldopam decreased Nox activity in RPT cells from WKY and SHRs, accompanied by the dispersion of Nox and subunits in LRs and non-LRs. We have reported that, in human RPT cells, LRs constrain the activity of Nox.27 In the current studies using rat RPT cells, we found that LRs abet oxidase activity. Regardless of the species, D1-like receptors inhibit oxidase activity, in part by dispersing Nox subunits in LRs and non-LRs.
Methods
To read materials and some methods, please see the online data supplement at http://hyper.ahajournals.org.
Cell Culture and Cell Treatment
Immortalized RPT cells from WKY and SHRs (20 to 30 passages) were maintained in DMEM/F12 supplemented with 5% FBS, epidermal growth factor (10 ng/mL), insulin, transferrin, and selenium (5 µg/mL each) at 37°C in humidified 5% CO2 and 95% air.33 The effects of the D1-like receptor D1R on signal transduction and sodium transporters in these cells are similar to those observed in freshly obtained rat RPTs.34
Immortalized rat RPT cells prestarved in serum-free DMEM/F12 medium for 1 hour were treated with vehicle, the D1-like receptor agonist fenoldopam (1 µmol/L per 20 minutes), the D1-like receptor antagonist Sch23390 (5 µmol/L), and/or the protein kinase A (PKA) activator Sp-cAMP (50 µmol/L) for 20 minutes. For the combined D1-like receptor agonist and antagonist study, Sch was added 5 minutes before the addition of fenoldopam. For the PKA inhibition studies, the cells were preincubated with Rp-cAMP (50 µmol/L) for 40 minutes before the addition of fenoldopam. In these combination studies, the incubation was continued for another 20 minutes after the addition of fenoldopam.
Subcellular Fractionation
LR and non-LR fractions from cells treated with vehicle, fenoldopam, or methyl-β-cyclodextrin (βCD) were obtained by sucrose gradient centrifugation using a detergent-free protocol, as described.20,27
Immunoblotting of Sucrose Gradient Fractions
Twelve fractions collected from each tube were electrophoresed in precast 26-well 4% to 20% gradient gels. The samples from WKY and SHRs were electrophoresed in 1 gel to compare the basal levels of Nox2, Nox4, and Rac1 proteins. The transfer of the protein (from gel onto the cellulose membrane) was performed using a constant current and duration to get the same transfer efficiency from gel. The proteins of interest were normalized to control groups, with the control given a value of 100%, when comparing vehicle and drug treatments.
Measurement of NADPH Oxidase Activity by Lucigenin Chemiluminescence
Oxidase activity of whole cell membranes, prepared as reported, was measured using lucigenin chemiluminescence in the presence of NADPH.27,31 Equal amounts of cell membranes were incubated with lucigenin (5 µmol/L) for 10 minutes at 37°C in a final volume of 1 mL of assay buffer. The dynamic tracing of chemiluminescence was started on the injection of NADPH (final concentration: 100 µmol/L) and recorded for 180 seconds (Autolumet Plus LB953, EG&G Berthold). The activity, arbitrary light units (ALUs) per second per 100 µg of protein, was converted to a percentage of change from control.
Immunoprecipitation and Immunoblotting
Immunoprecipitation and immunoblotting were performed, as described previously.20 Equal amounts of cell lysates (500 µg of protein) mixed with a polyclonal antip67phox antibody (1 µL) were incubated at 4°C overnight. Protein A beads (30 µL) were added to each sample with rocking for 2 hours. The immune complexes were washed (3 times) with PBS. The bound proteins were eluted by the addition of Laemmli buffer (30 µL) and boiled for 5 minutes. The samples were subjected to immunoblotting with a monoclonal anti-Nox2 antibody. Normal rabbit IgG was used as negative control. The bands were quantified by densitometry, as described previously.20,27
Statistical Analysis
Data are expressed as mean±SE. Significant differences between 2 groups were determined by Student t test. Significant differences among several groups (>2) were determined by factorial ANOVA, followed by Newman-Keuls test; P<0.05 was considered significant.
Results
Expression of Nox and Subunits
The expression of Nox2 but not Nox4 mRNA in RPT cells and freshly prepared RPTs was greater in SHRs than in WKY rats (Figure S1A). The basal level of Nox2 mRNA, quantified by real-time PCR (Figure S1B), was 2-fold higher in SHRs than in WKY rats. The basal total cellular levels of Nox2 (55 kDa) and Nox4 (75 kDa) proteins, semiquantified by immunoblotting (Figure 1, left, and Table S1), were greater in SHR than in WKY cells. In addition, Nox2 and Nox4 protein levels in brush border membranes (BBM) were greater in SHRs (Nox2=+27.8±9.1 and Nox4=+62.8±14.2, percentage difference relative to WKY) than in WKY rats (P<0.01, t test; Figure 1, right). The basal levels of total cellular Rac1 were similar in the 2 cell lines (Table S1).
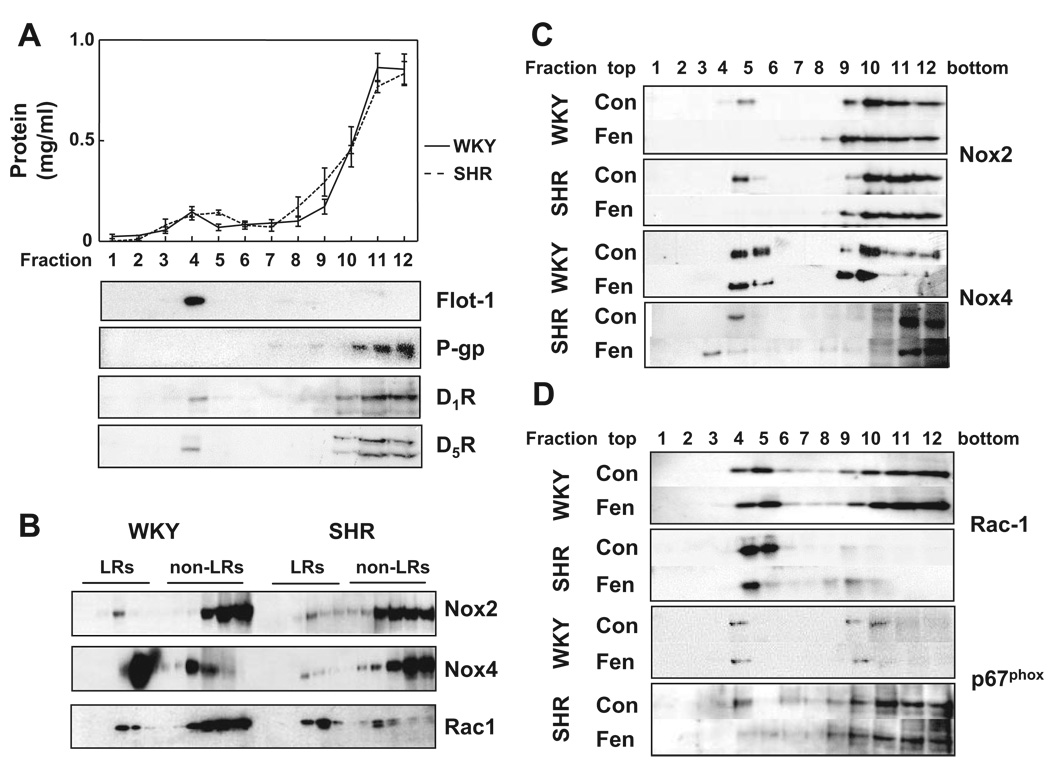
Figure 1.
Basal expression of Nox2 and Nox4 protein in rat RPT cells and rat renal BBMs. To assess the basal level of Nox2 and Nox4 expression, rat RPT cell lysates (15 µg per lane; left) and rat renal BBMs (15 µg per lanel right) were immunoblotted with the indicated antibodies. The densities of the bands were quantified using β-actin as the standard. The values are mean±SEM (n=4 to 5 per group). #P<0.05 vs WKY, t test. The insert is 1 of 4 to 5 separate blots.
Sucrose Density Gradient Analysis of Nox Isoforms and Subunits
The majority of the membrane proteins (≈92%) were in non-LRs (fractions 7 to 12); only ≈8.0% of the proteins were in LRs (fractions 2 to 6) (Figure 2A). Flotillin-1, a LR marker protein,35 was mainly located in LRs. P-glycoprotein, a non-LR marker,36 was found mainly in fractions 10 to 12 and minimally in fractions 7 to 9 of non-LRs but not found in LRs, indicating that the LR fractions were not contaminated by non-LR proteins. Rat D1AR and D1BR (homologs of human D1R and D5R) were mainly found in non-LRs, although a small amount cofractionated with flotillin-1 in LRs.
Figure 2.
Sucrose gradient analysis of LR marker proteins and Nox isoforms. A, The protein concentrations (mg/mL) from each of the 12 fractions were measured and plotted (top). Proteins from the sucrose gradient fractions (1 to 12) were immunoblotted with the indicated antibodies, as described in the Methods section. Flotillin-1 (Flot-1) and P-glycoprotein (P-gp) are shown in the middle panels and D1R and D5R in the bottom panels. Fractions 2 to 6 are the LR fractions and fractions 7 to 12 are the non-LR fractions. The immunoblots from 1 of 3 separate experiments are shown. B, To compare the expression of Nox isoforms from each set of WKY and SHRs, the sucrose gradient fractions were electrophoresed in the same gel as described in the Methods section. The proteins were immunoblotted with the indicated antibodies, and the immunoreactive bands were semiquantified as reported.27 C and D, Proteins (30 µL per lane) from the sucrose gradient fractions were probed with antibodies against Nox2, Nox4, Rac1, and p67phox. The distribution of Nox2 and Nox4 is shown in C and Rac1 and p67phox in D. The immunoblots from 1 of 3 separate experiments are shown.
The percentage distribution of the basal levels of Nox2, Nox4, and Rac1 proteins in LRs and non-LRs in RPT cells from WKY and SHRs is shown in Table 1, and the immunoblots are shown in Figure 2B. The percentage of Nox2 expression in LRs was similar in the 2 cell lines, although the absolute total cellular level of Nox2 protein was greater in SHRs than in WKY rats. The percentage of Nox4 expression in LRs was greater in WKY than in SHR cells, although the total cellular Nox4 protein expression was greater in SHR than in WKY cells. The total cellular Rac1 protein expression was similar in SHR and WKY cells. However, the percentage of Rac1 expression in LRs was 1.7-fold higher in SHR than in WKY cells.
Table 1.
Distribution of Nox Proteins in LRs
| WKY | SHR | |||
|---|---|---|---|---|
| Variable | LRs | Non-LRs | LRs | Non-LRs |
| Nox 2 | 27.0±6.0* | 73.2±5.8 | 21.8±4.5* | 78.2±4.5 |
| Nox 4 | 55.4±2.8* | 44.6±2.7 | 27.1±5.1*† | 72.9±5.1 |
| Rac1 | 32.4±2.5* | 67.6±2.5 | 55.0±5.6† | 45.0±6.7 |
Proteins from sucrose gradient fractions were probed with the indicated antibodies as shown in Figure 2B and quantified, as described previously.27 The results are expressed as the percentage of the combined 12 fractions set at 100%. The values are mean±SEM (n=3 to 4 per group).
P<0.05, LRs vs non-LRs.
P<0.05, vs WKY-LRs, ANOVA, Newman-Keuls test.
The D1-like receptor agonist fenoldopam shifted Nox2 from LRs toward higher density fractions in both WKY (fraction 5 to fractions 6 to 9) and SHR cells (fraction 5 to fractions 8 and 9; Figure 2C) and decreased Nox2 protein in LRs to a greater extent in SHR than in WKY cells (P<0.05, WKY versus SHR; Table 2). Fenoldopam shifted Nox4 from fraction 5 to fraction 9 in WKY, whereas it shifted Nox4 from fraction 4 to fraction 3 in SHRs (Figure 2C). Fenoldopam decreased Nox4 protein expression in LRs to a similar extent in WKY and SHR cells (Table 2).
Table 2.
Effect of Fenoldopam on Nox Proteins in LRs
| WKY | SHR | |||
|---|---|---|---|---|
| Variable | Con | Fen | Con | Fen |
| Nox2 | 100±1.0 | 76.2±5.8* | 100±2.1 | 48.7±7.9*† |
| Nox4 | 100±5.4 | 65.3±5.1* | 100±10.0 | 77.1±9.9* |
| Rac1 | 100±5.7 | 114.8±11.1 | 100±2.1 | 58.5±5.6*† |
Proteins from sucrose gradient fractions were probed with the indicated antibodies and quantified, as described previously.27 The results are expressed as the percentage of control, set at 100%. The values are mean±SEM (n=3 to 4 per group). Con indicates control; Fen, fenoldopam.
P<0.01 vs Con, t test.
P<0.05 vs Fen WKY, ANOVA, Newman-Keuls test.
Fenoldopam redistributed Rac1 from fraction 4 to fraction 3 and to fractions 8 to 10 in SHRs (Figure 2D) and also markedly decreased its expression in LRs (Table 2). In contrast, fenoldopam did not affect Rac1 distribution in WKY cells.
Similar to Nox2, the majority of p67phox was in non-LRs in both rat strains (Figure 2D). Fenoldopam minimally affected p67phox expression in WKY cells but decreased its expression in LRs in SHR cells. We were unable to probe for p22phox and p47phox, because the antibodies did not recognize specific bands in lysates from rat kidney cells and tissues.
NADPH Oxidase Activity
The dynamic tracings of oxidase activity (Figure 3A, top) and the absolute activity in ALUs/s per 100 µg of protein (Figure 3A, bottom) are shown in Figure 3A. The basal oxidase activity was 3-fold higher in SHR cells (203.4±8.2 ALUs×100) than in WKY cells (58.4±1.7 ALUs×100; Figure 3A, bottom right). Fenoldopam decreased the absolute value of oxidase activity to a greater extent in SHR than WKY cells (P<0.01; n=6 per group; Table S2). However, the percentage of inhibition of oxidase activity (percentage of change from control) by fenoldopam was greater in WKY cells (58.0±3.3%) than in SHR cells (31.0±5.2%). A D1-like receptor antagonist (Sch23390) abrogated the inhibitory effect of fenoldopam on oxidase activity in both cell lines (Figure 3A), indicating that the inhibitory effect of fenoldopam on oxidase activity was via D1-like receptors.
Figure 3.
Measurement of oxidase activity. A, Immortalized rat RPT cells were treated for 20 minutes with vehicle (Con), D1-like receptor agonist fenoldopam (Fen; 1 µmol/L), D1-like receptor antagonist Sch23390 (Sch; 5 µmol/L), or the combination of Sch+Fen (S+F). Oxidase activity was measured in the presence of 5 µmol/L of lucigenin and 100 µmol/L of NADPH. The top graph shows the dynamic tracings of NADPH-dependent chemiluminescence recorded for 180 seconds. The bottom left graph shows the absolute activity (ALU/s per 100 µg of protein), and the bottom right graph shows the fold change in activity with WKY set at 1.0. Values are mean±SEM (n=6). *P<0.01 vs others in WKY and SHRs; #P<0.01 vs WKY, ANOVA, Newman-Keuls test. B, Effect of fenoldopam on oxidase activity in freshly prepared RPTs from WKY and SHRs. The right renal artery was infused with saline (control) or fenoldopam (1 µg/kg of body weight per minute×30 minutes at 40 µL/min), as described in the Methods section in the online data supplement). The absolute value of oxidase activity (ALU/s per 100 µg of protein) is shown in the left graph, and the fold change from WKY control set at 1.0 is shown in the right graph. Values are mean±SEM (n=6 per group). *P<0.01 vs control; #P<0.01 vs WKY, ANOVA, Newman-Keuls test.
Apocynin and diphenylene iodonium, specific Nox inhibitors, decreased oxidase activity, as expected (Figure S2). The absolute decrease in Nox activity caused by diphenylene iodonium was greater in SHRs than in WKY rats, but the percentage of inhibition was similar. The absolute and percentage of inhibition of oxidase activity was greater with diphenylene iodonium than with apocynin in both WKY and SHRs. The absolute decrease in oxidase activity with apocynin was similar in WKY and SHRs, but the percentage of inhibition of oxidase activity with apocynin was greater in WKY than in SHRs. The apocynin-mediated inhibitory effect could be related to the expression of the myeloperoxidase in RPT cells (Figure S3); apocynin impairs Nox subunit assembly in the presence of myeloperoxidase.37
Additional studies in vivo were performed to determine the effect of fenoldopam on renal oxidase activity using freshly obtained RPTs. Similar to the results using RPT cells, the basal oxidase activity was higher in membranes of RPTs from SHRs (3.5-fold) than those from WKY rats (Figure 3B, left). Similar to the results obtained in immortalized RPT cells, fenoldopam decreased the absolute value of oxidase activity to a greater extent in RPTs from SHRs than from WKY rats. Also similar to the results obtained in RPT cells, the percentage of inhibition of oxidase activity by fenoldopam was greater in RPTs from WKY rats than from SHRs (Figure 3B, right, and Table S2).
To determine whether the effect of fenoldopam on Nox activity was regulated via PKA in RPT cells, the PKA inhibitor Rp-cAMP and the PKA activator Sp-cAMP were used (Figure 4 and Table S3). The pattern of the effect of fenoldopam on the oxidase activity in this experiment was the same as in Figure 3. However, direct activation of PKA with Sp-cAMP decreased oxidase activity to a similar degree in WKY and SHR cells. The effect of fenoldopam on oxidase activity was partially inhibited by Rp-cAMP in WKY but not in SHRs.
Figure 4.
Effect of fenoldopam and PKA on oxidase activity. Immortalized rat RPT cells were treated with vehicle (Con), fenoldopam (Fen; 1 µmol/L), PKA activator Sp-cAMP (50 µmol/L), and PKA inhibitor Rp-cAMP (50 µmol/L). Cell membranes were prepared and assayed for oxidase activity (absolute activity, ALU/s per 100 µg of protein). Values are Mean±SEM (n=6/group), *P<0.01 Con vs Fen, Sp-cAMP, or Rp+Fen-SHR; &P<0.05 Rp+Fen vs Fen, WKY; #P<0.01 SHR vs WKY, ANOVA, Newman-Keuls test.
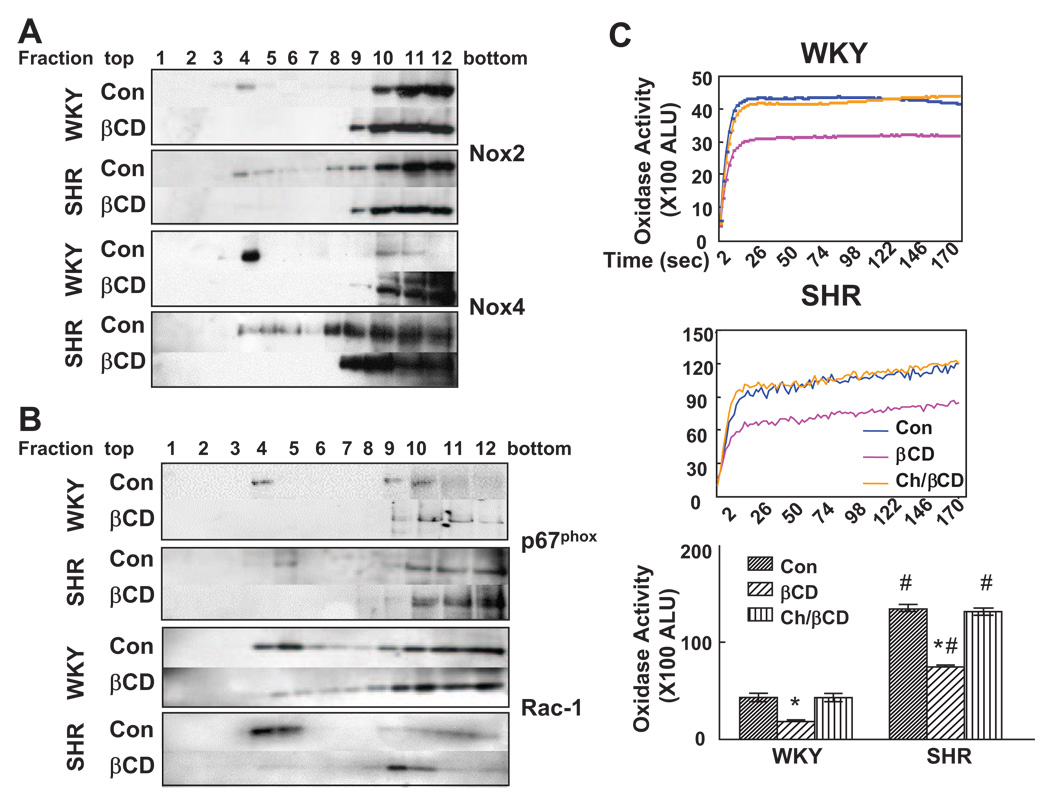
Effect of βCD on Nox Distribution and NADPH Oxidase Activity
To determine the effect of LRs on oxidase activity, βCD was used to remove cholesterol selectively from the plasma membrane and disrupt LRs.17,24,35 Treatment of cells with βCD (2%/1 hour) decreased cholesterol content in RPT cells from both rat strains (Figure S4 and Table S4), consistent with other reports,24,35 but the absolute and percentage decreases was greater in SHR than in WKY cells. βCD treatment shifted the distribution of Nox2, Nox4, p67phox, and Rac1 from LRs to non-LRs in RPT cells from both WKY and SHRs (Figure 5A and 5B). βCD treatment decreased oxidase activity to a greater extent in SHRs than in WKY rats (Figure 5C), consistent with the greater ability of βCD to decrease cholesterol content in SHR than in WKY cells (Figure S4). The inhibitory effect of βCD on oxidase activity was reversed by cholesterol repletion in both cell lines (Figure 5C).
Figure 5.
Effect of βCD on the distribution of Nox isoforms, subunits, and oxidase activity. A and B, Proteins from the sucrose gradient fractions were probed with the indicated antibodies. Nox2 and Nox4 immunoblots are shown in A and p67phox and Rac1 in B. The immunoblots from 1 of 3 separate experiments are shown. C, RPT cells were treated with vehicle (Con), βCD alone, or in combination with cholesterol (Ch/βCD). Dynamic tracings of NADPH-dependent activity were recorded for 180 seconds (top graphs). The bottom graph shows the absolute activity (ALU/s per 100 µg of protein) from 3 experiments. Values are mean±SEM (n=3); *P<0.001 βCD vs others; #P<0.01 vs WKY cells, ANOVA, Newman-Keuls test.
D1-Like Receptor Agonist Fenoldopam Impairs the Assembly of Nox2 With p67phox
In immortalized RPT cells from WKY rats, fenoldopam (1 µmol/L for 20 minutes) decreased the amount of Nox2 that coimmunoprecipitated with p67phox (64.5±9.1%; P<0.05, versus control: 100±4.3%) but did not affect the protein abundance in whole cell lysates (Figure 6).
Figure 6.
Effect of fenoldopam on the association of Nox2 with p67phox in RPT cells. RPT cells from WKY rats were treated with vehicle (Con) or fenoldopam (Fen; 1 µmol/L per 20 minutes) in serum-free DMEM/F12. Cell lysates were immunoprecipitated with polyclonal anti-p67phox antibody and probed with monoclonal anti-Nox2 antibody, and the immune reactive bands were semiquantified. The effect of fenoldopam on Nox2 association with p67phox (percentage change) is shown in the left graph, and the ratio (Nox2/p67phox) is shown in the middle graph. Cell lysates, aliquoted before immunoprecipitation, were probed with anti-Nox2 and anti-p67phox antibodies (right graph). Values represent mean±SEM (n=6 per group). *P<0.05 vs Con, t test. One blot is depicted in the inset.
Discussion
There are 3 novel findings in this report. First, there is a marked difference in the extent of the targeting of Nox4 and Rac1 to LRs in RPT cells from WKY and SHRs. Second, basal levels of Nox2 and Nox4 proteins in RPT cells and renal BBMs are greater in SHRs than in WKY rats. Third, the basal level of membrane oxidase activity in immortalized RPT cells and in freshly obtained RPTs is higher in SHRs than in WKY rats, and the ability of D1-like receptors to inhibit oxidase activity, expressed as a percentage from control, is greater in WKY than in SHRs.
The finding of Nox2, Rac1, and p67phox expression in LRs of rat RPT cells is in agreement with those findings reported in rat vascular smooth muscle, bovine aortic endothelial, and coronary arterial endothelial cells.24–26 Nox4 and Rac1 are differentially expressed in LRs and non-LRs in RPT cells from WKY and SHRs. Rac1 expression is 1.7-fold higher in LRs in SHR than in WKY cells. Nox4 expression is 2-fold higher in LRs in WKY than in SHR cells, whereas Nox2 distribution in LRs is identical. Nox4 has been reported to be the major Nox in the human and rat kidney.7,8 However, its localization in LRs in rats has not been reported. Our laboratory was the first to report the expression of Nox4 mainly in non-LRs in RPT cells from normotensive human subjects.27 The current studies also show that the total expression of Nox4 in BBMs and RPT cells is greater in SHRs than in WKY rats. Pedrosa et al38 also reported a greater expression of Nox4 in RPT cells from SHRs than from WKY rats. The greater basal expression of Nox2 and Nox4 in SHR cells should also result in a greater basal expression in LRs, although the percentage of expression of Nox2 in LRs is similar in the 2 rat strains, and the percentage of expression of Nox4 in LRs is lesser in SHRs than in WKY rats.
Rac1 has been found to be mainly expressed in cytosol of resting human neutrophils.2 However, in nonimmune cells, Rac1 has been shown to be constitutively concentrated in caveolae of quiescent rat-1B fibroblasts39 and in cholesterolrich membrane microdomains in rat neuron cells and human fibroblasts.40,41 Rac1 is also expressed in non-LRs in unstimulated rat aortic vascular smooth muscle11 and bovine coronary arterial endothelial cells.25 Thus, the expression of Rac1 in LRs or non-LRs may be cell-type dependent.
LRs are cell membrane microdomains rich in cholesterol, sphingolipids, and saturated fatty acids that are sensitive to cholesterol-depleting reagents.17,19,35 Cell membrane components, eg, phospholipids, free cholesterol, arachidonic and fatty acids, and sphingomyelin, are increased, and fluidity is decreased in plasma membranes of SHRs.42,43 In agreement with other reports, we also found that the basal level of cholesterol is higher in SHR than in WKY cells.42,43 The greater depletion of cell membrane cholesterol by βCD23–26 in SHR than in WKY cells is associated with a greater inhibition of oxidase activity. Cholesterol depletion also causes the redistribution of Nox2, Nox4, p67phox, and Rac1 from LRs to non-LRs, similar to previous reports from rodents.24,25 Rac1 in caveolae LRs may be in an activated state.39 Because Nox subunits may be preassembled in an active state in LRs, the increased amounts of Nox2 and Rac1 (and probably Nox4) in LRs could explain the increased basal oxidase activity and increased ROS production in SHRs. The increased oxidative stress in SHRs has been reported to precede the onset of hypertension.10
The current studies provide important insights into the mechanisms by which D1-like receptors negatively regulate oxidase activity. D1-like receptor–mediated inhibition of oxidase activity is associated with the dispersion of Nox isoforms and subunits in LRs and non-LRs in rat RPT cells. Fenoldopam causes the redistribution of Nox2, Nox4, and Rac1 from LRs and to non-LRs. The effect is similar to cholesterol depletion of the plasma membrane by βCD treatment, suggesting that, in rodents, the mechanism of D1-like receptor–induced inhibition of oxidase activity is, in part, mediated by redistributing Nox2, Nox4, and Rac1 of LRs into non-LRs. In addition, fenoldopam decreases the assembly of Nox2 with p67phox in WKY cells. The inhibitory effect of fenoldopam on oxidase activity is attenuated by an inhibitor of PKA activity (Rp-cAMP) in WKY rats, supporting the notion that the fenoldopam-mediated inhibition of oxidase activity is, in part, attributed to a fenoldopam-mediated increase in cAMP production/PKA activation, in agreement with studies in rat vascular smooth muscle cells.30
Stimulation of D1-like receptors (D1AR/D1R and D1BR/D5R) by fenoldopam decreases oxidase activity in both rat strains, in agreement with the reports.30,31 The inhibitory effect of fenoldopam on the absolute oxidase activity is greater in SHRs than in WKY rats, consistent with the greater inhibition of the expression of Nox2 and Rac1 in LRs in SHRs than in WKY rats. These results suggest that the inhibitory effect of fenoldopam on oxidase activity is mainly exerted by D1B/D5R in SHRs, because D1R function is impaired in SHRs.28,29,38 However, the percentage of inhibition of oxidase activity attributed to fenoldopam is greater in WKY than in SHRs. This is consistent with our report that the renal D1B/D5R expression is lesser in SHRs than in WKY rats.44 The failure of Rp-cAMP to attenuate the inhibitory effect of fenoldopam on oxidase activity in SHRs indicates a differential D1-like receptor signaling pathway in WKY and SHRs. The percentage of inhibition of oxidase activity by apocynin, which, like the D1-like receptor agonist fenoldopam, impairs the assembly of Nox subunits, is also greater in WKY rats than in SHRs.
In summary, we have demonstrated for the first time that endogenous Nox4 and Rac1 are differentially expressed in LRs and non-LRs in immortalized RPT cells from normotensive and hypertensive rats. D1-like receptors inhibit membrane oxidase activity by redistributing Nox isoforms and catalytic subunits in LRs and non-LRs and by interfering in the assembly of the Nox complex. Our studies also demonstrate that LRs abet the oxidase activity in rat kidney cells, the increased activity of which could contribute to the oxidative stress in the SHR.
Perspectives.
The results from the current study could enhance the understanding of the D1-like receptor signaling mechanism in membrane microdomains. Because LRs can constrain or abet oxidase activity depending on the species, understanding how D1-like receptors regulate LRs may provide insights in regulating or controlling oxidative stress. In rodents, D1-like receptors could decrease oxidase activity by altering the affinity of Nox subunits with LR components, eg, cholesterol or sphingolipids.45
Supplementary Material
Acknowledgments
Sources of Funding
These studies were supported in part by grants from the National Institutes of Health (HL023081, HL074940, DK039308, HL092196, HL068686, and RR020185).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None
References
- 1.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 2.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 3.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 4.Bolterman RJ, Manriquez MC, Ortiz Ruiz MC, Juncos LA, Romero JC. Effects of captopril on the renin angiotensin system, oxidative stress, and endothelin in normal and hypertensive rats. Hypertension. 2005;46:943–947. doi: 10.1161/01.HYP.0000174602.59935.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 7.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 8.Guzik TJ, Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of reNox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 11.Zuo L, Ushio-Fukai M, Hilenski LL, Alexander RW. Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts: role in redox signaling. Arterioscler Thromb Vasc Biol. 2004;24:1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. [DOI] [PubMed] [Google Scholar]
- 12.Zhan CD, Sindhu RK, Vaziri ND. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: reversal by lifelong antioxidant supplementation. Kidney Int. 2004;65:219–227. doi: 10.1111/j.1523-1755.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 13.Touyz RM, Schiffrin EL. Reactive oxygen species and hypertension: a complex association. Antioxid Redox Signal. 2008;10:1041–1044. doi: 10.1089/ars.2007.2012. [DOI] [PubMed] [Google Scholar]
- 14.Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110:243–253. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- 15.Dai XL, Cao X, Kreulen DL. Superoxide anion is elevated in sympathetic neurons in DOCA-salt hypertension via activation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;290:H1019–H1026. doi: 10.1152/ajpheart.00052.2005. [DOI] [PubMed] [Google Scholar]
- 16.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 17.Smart EJ, Anderson RG. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 2002;353:131–139. doi: 10.1016/s0076-6879(02)53043-3. [DOI] [PubMed] [Google Scholar]
- 18.Ostrom RS, Post SR, Insel PA. Stoichiometry and compartmentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving GS. J Pharmacol Exp Ther. 2000;294:407–412. [PubMed] [Google Scholar]
- 19.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 20.Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, Felder RA, Jose PA. D1 Dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–2180. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- 21.Breton S, Lisanti MP, Tyszkowski R, McLaughlin M, Brown D. Basolateral distribution of caveolin-1 in the kidney: absence from H+-ATPase-coated endocytic vesicles in intercalated cells. J Histochem Cytochem. 1998;46:205–214. doi: 10.1177/002215549804600209. [DOI] [PubMed] [Google Scholar]
- 22.Shao D, Segal AW, Dekker LV. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 23.Guichard C, Pedruzzi E, Dewas C, Fay M, Pouzet C, Bens M, Vandewalle A, Ogier-Denis E, Gougerot-Pocidalo MA, Elbim C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J Biol Chem. 2005;280:37021–37032. doi: 10.1074/jbc.M506594200. [DOI] [PubMed] [Google Scholar]
- 24.Vilhardt F, van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 2004;23:739–748. doi: 10.1038/sj.emboj.7600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;7:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- 26.Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006;20:1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- 27.Han W, Li H, Villar VA, Pascua AM, Dajani MI, Wang X, Natarajan A, Quinn MT, Felder RA, Jose PA, Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51(part 2):481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- 28.Jose PA, Eisner GM, Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 2002;11:87–92. doi: 10.1097/00041552-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 30.Yasunari K, Kohno M, Kano H, Minami M, Yoshikawa J. Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D1-like receptors. Circulation. 2000;101:2302–2308. doi: 10.1161/01.cir.101.19.2302. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 32.White BH, Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G-protein coupling. J Hypertens. 1998;16:1659–1665. doi: 10.1097/00004872-199816110-00013. [DOI] [PubMed] [Google Scholar]
- 33.Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, Hopfer U. Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int. 1996;50:125–134. doi: 10.1038/ki.1996.295. [DOI] [PubMed] [Google Scholar]
- 34.Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, Jose PA. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1064–R1073. doi: 10.1152/ajpregu.2000.278.4.R1064. [DOI] [PubMed] [Google Scholar]
- 35.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radeva G, Perabo J, Sharom FJ. P-Glycoprotein is localized in intermediate-density membrane microdomains distinct from classical lipid rafts and caveolar domains. FEBS J. 2005;272:4924–4937. doi: 10.1111/j.1742-4658.2005.04905.x. [DOI] [PubMed] [Google Scholar]
- 37.Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys. 2007;457:134–141. doi: 10.1016/j.abb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Pedrosa R, Villar VA, Pascua AM, Simão S, Hopfer U, Jose PA, Soaresda-Silva P. H2O2 stimulation of the Cl-/HCO3-exchanger by angiotensin II and angiotensin II type 1 receptor distribution in membrane microdomains. Hypertension. 2008;51:1332–1338. doi: 10.1161/HYPERTENSIONAHA.107.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaely PA, Mineo C, Ying YS, Anderson RG. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J Biol Chem. 1999;274:21430–21436. doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- 40.Kumanogoh H, Miyata S, Sokawa Y, Maekawa S. Biochemical and morphological analysis on the localization of Rac1 in neurons. Neurosci Res. 2001;39:189–196. doi: 10.1016/s0168-0102(00)00211-x. [DOI] [PubMed] [Google Scholar]
- 41.del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 42.Dorrance AM, Graham D, Webb RC, Fraser R, Dominiczak A. Increased membrane sphingomyelin and arachidonic acid in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2001;14:1149–1153. doi: 10.1016/s0895-7061(01)02188-4. [DOI] [PubMed] [Google Scholar]
- 43.Vázquez CM, Mate A, Angeles de la Hermosa M, Planas JM, Ruíz-Gutiérrez V. Abnormalities in lipid composition of brush-border membranes isolated from renal cortex of spontaneously hypertensive rats. Am J Hypertens. 2001;14:578–584. doi: 10.1016/s0895-7061(00)01325-x. [DOI] [PubMed] [Google Scholar]
- 44.Zeng C, Yang Z, Wang Z, Jones JE, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of AT1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–810. doi: 10.1161/01.HYP.0000155212.33212.99. [DOI] [PubMed] [Google Scholar]
- 45.Polymeropoulos MH, Licamele L, Volpi S, Mack K, Mitkus SN, Carstea ED, Getoor L, Thompson A, Lavedan C. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr Res. 2009;108:134–142. doi: 10.1016/j.schres.2008.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.