Abstract
Encounters with stimuli associated with drug use are believed to contribute to relapse. To probe the neurobiology of environmentally triggered drug use, we have conducted single-unit recordings in rhesus monkeys during presentation of two distinct types of drug paired cues that differentially support drug-seeking. The animals were highly conditioned to these cues via exposure during self-administration procedures conducted over a 4 year period. The cues studied were a discriminative cue that signaled response-contingent availability of cocaine, and a discrete cue that was temporally paired with the cocaine infusion (0.1 or 0.5 mg/kg). Two cortical regions consistently activated by cocaine-associated cues in human imaging studies are the orbitofrontal (OFC) and anterior cingulate cortex (ACC), though little is known about cortical neuronal activity responses to drug cues. We simultaneously recorded single-unit activity in OFC and ACC as well as in dorsal striatum in rhesus monkeys during cocaine self-administration. Dorsal striatal neurons were less engaged by drug cues than cortical regions. Between OFC and ACC, distinct functionality was apparent in neuronal responses. OFC neurons preferentially responded to the discriminative cue, consistent with a role in cue-induced drug-seeking. In contrast, the ACC did not respond more to the discriminative cue than to the discrete cue. Also distinct from the OFC, ACC showed sustained firing throughout the 18 s duration of the discrete cue. This pattern of sustained activation in ACC is consistent with a role in reward expectation and/or in mediating behavioral effects of discrete cues paired with drug infusions.
Introduction
Discriminative cues that indicate availability of a drug for self-administration, and discrete cues that have been paired with the actual drug infusion, exert distinct influences over behavior. Noncontingent presentation of discriminative cues causes drug-seeking more than discrete cues (Weiss et al., 2000; Di Ciano and Everitt, 2003; Yun and Fields, 2003). Thus, to gain insight into how chance encounters with cocaine-associated cues could trigger relapse, it is crucial to examine neuronal activity responses to discriminative cues. Discrete cues exert a different control over self-administration behavior in animals. Specifically, they have the ability to support very high rates of responding during self-administration under second-order schedules of reinforcement in which they are intermittently presented, contingent on operant responses (Goldberg et al., 1981; Everitt and Robbins, 2000; Di Ciano and Everitt, 2003). Aspects of the environment associated with actual use could be serving as strong discrete cues, and thus they could play a role in maintaining drug consumption after relapse has occurred. Preferential engagement of different brain regions by these two distinct cue types could provide insight into neurobiological underpinnings of different aspects of addiction behavior, such as relapse and binge consumption.
Addiction is increasingly considered to involve maladaptations in orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Kalivas and Volkow, 2005; Garavan and Hester, 2007; Schoenbaum and Shaham, 2008). These regions are known to mediate cognitive function related to goal-directed behavior (Rushworth et al., 2007) and are particularly reactive to drug cues in humans (Garavan et al., 2000; Goldstein et al., 2009) and animals (Neisewander et al., 2000; McLaughlin and See, 2003). There is also a great deal of evidence that cognitive function associated with these regions is impaired in addicts (Aron and Paulus, 2007; Garavan and Hester, 2007; Robbins et al., 2008). While both OFC and ACC have been shown to be responsive to drug-associated cues, a comparison of how these areas respond to cues that regulate drug taking behavior has not been conducted at the neuronal activity level to evaluate whether there are differences in how distinct cue types are encoded.
Because the emphasis on cortical dysfunction in addiction derives primarily from clinical imaging studies, it is important to examine cortical involvement in a clinically relevant behavioral model. The animal model we have used to study neuronal activity responses to cocaine cues is the rhesus monkey with a long (4.5 years) history of cocaine self-administration associated with the specific cues examined. Cortical structure and the functions associated with cortical subregions in rhesus monkeys are highly similar to humans, and there is substantial knowledge regarding cortical and striatal neural correlates of cognition and reward processing. Thus, studies of neural responses to drug-associated cues in this model are particularly valuable in translational research (Passingham, 2009). To address the question of how distinct types of environmental cues differentially impact neuronal activity in OFC, ACC, and dorsal striatum, we conducted multielectrode recordings of single-unit activity in these structures simultaneously, during cue exposure and cocaine self-administration.
Materials and Methods
Animals.
Two female rhesus macaques weighting 6.0–7.5 kg were used for these studies. Following acquisition of lever pressing behavior for food pellets, a subcutaneous vascular access port was placed mid-scapula from which a catheter extended to either the internal jugular or femoral vein. Cocaine was then introduced as the reinforcer for responding. These procedures have been previously described (Bradberry et al., 2000). Both animals had a history of reinforcement by both cocaine and a psychoactive metabolite of cocaine, cocaethylene, from a study comparing reinforcement efficacy of the two compounds using progressive ratio procedures. Cocaethylene is very similar to cocaine in that it is equipotent at inhibiting the dopamine transporter (Iyer et al., 1995) and also shares cocaine's local anesthetic properties (Tokuno et al., 2004). Cumulative exposure was as follows: animal A: cocaine, 253 mg/kg, cocaethylene, 104 mg/kg; animal B: 470 mg/kg cocaine, 120 mg/kg cocaethylene.
Cocaine self-administration.
Animals were restrained in a chair (Primate Products, Redwood City, CA) by a collar, and placed in a behavioral chamber (Med-Associates, GA, VT) fitted with an operant panel constructed from 1/4 inch aluminum to which the chair was attached. On the panel was an operant lever, and a red and green lamp that served as visual cues. The vertical and horizontal distance between the lamps was 10.3 cm. Animals could receive five infusions of cocaine under a fixed ratio 10 (FR10) lever press contingency, with a 10 min time out between infusions. Onset of a red lamp on the operant panel served as the discriminative cue that signaled availability of cocaine. Upon reaching the FR10 contingency, the red lamp was extinguished, and a green lamp was turned on. The green lamp discrete cue was maintained throughout the 18 s infusion used to flush the 0.1 ml (infusions 1–4) or 0.5 ml (infusion 5) bolus of cocaine through the intravenous line. There was no time limit for responding to the discriminative stimulus cue. Figure 1a shows the sequence of events for the self-administration task.
Figure 1.
Behavioral paradigm and lever pressing behavior. a, Self-administration and associated cues. A red lamp on the operant panel served as the discriminative cue (Discrim) that signaled availability of cocaine under an FR10 contingency. Upon meeting that contingency, the discrete cue (a green lamp) was presented during the 18 s infusion period (Coc Inf). b, Lever pressing behavior. Response rate was greater during the discriminative cue than discrete cue, or baseline. c, Distribution of time taken to the first lever press after the onset of the discriminative cue. The boundaries of the box indicate the 75th/25th percentile and the whiskers indicate the 90th/10th percentiles. The center line in the box represents the median value and the two dots, located above and below the box, represent 95th/5th percentiles. Statistics are calculated from 370 trials (5 in each of 74 sessions).
Extinction procedure.
After recording sessions with drug-associated cues were complete, in one animal, extinction sessions in which cocaine was replaced by saline were conducted. The green bulb used as a discrete cue was illuminated during the infusion of saline for 18 s if the FR10 contingency was met within 30 s of discriminative cue onset. The intertrial interval was shortened (random, centered on 2 min) compared with the 10 min intertrial interval during cocaine self-administration because of the lack of drug infusion. Extinction sessions were 40 min in duration, and could range from 14 to 17 trials per session.
Single-unit recording.
After a long history of drug self-administration, a craniotomy for placement of a recording chamber and head immobilizing apparatus was conducted under isoflurane anesthesia (1–3%). In monkey A, a regular cannula design (Bradberry et al., 2000) was used, for animal B, a commercial recording cylinder was used (Crist Instrument). The recording chamber was placed overlying the cortical and striatal regions and was centrally positioned along the medial lateral axis. The calculated placement sites from magnetic resonance (MR) images are shown in Figure 2.
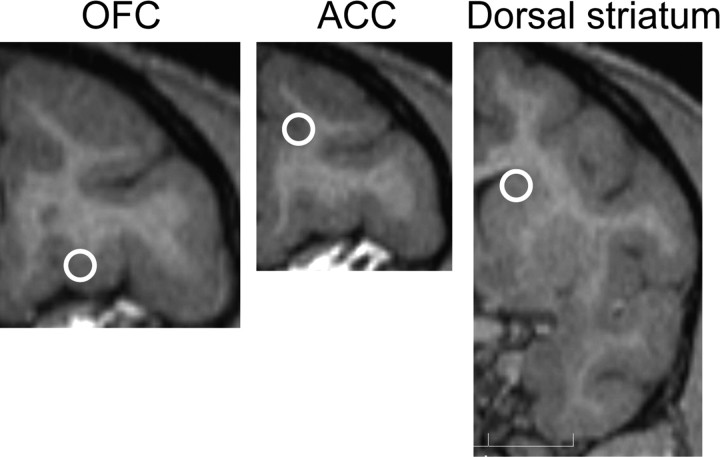
Figure 2.
Recording sites. Each circle marks the approximate center on a representative MR image from animal B of the calculated recording sites. Over 90% of units in OFC and ACC were targeted to within 1 mm of this center. For dorsal striatum, neurons were located in a more extended area on the anterior–posterior axis, with >80% of units targeted to within 2 mm of the indicated region.
Activity of single units was recorded extracellularly with acutely placed bundles of Teflon insulated tungsten microwires (50 μm diameter; California Fine Wire) from OFC, ACC and dorsal striatum while monkeys were performing the task. Bundles consisting of four wires, were lowered through a 23 G sharpened guide tube using either a manual (Crist Instrument) or electronically controlled microdrive (Plexon) to the target regions according to the MR images as previously described for microdialysis probe placement (Bradberry et al., 2000). Up to six independently placed bundles were used in a session. There was no prescreening for any selectivity of neurons. The standard procedure was to take 30 min to isolate units, then allow 30 min for stabilization before beginning the task. Neural activity was recorded using a Plexon Multichannel Acquisition Processor systems and sorted using Offline Sorter (Plexon). Further processing was conducted using Neuroexplorer (Nex Technologies) and Matlab (MathWorks).
Data analyses.
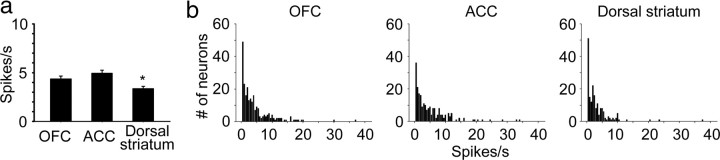
To ensure that behavioral responding was consistent, we did not use six sessions during which the time to complete the contingency was 3 SDs greater than the mean (i.e., >150 s, leaving a total of 74 self-administration sessions, and 71 extinction sessions). We focused our analysis on neurons with a basal firing rate between 0.5 and 20 Hz as determined over a 12 s period before the onset of the discriminative cue. The lower cutoff ensured an adequate number of spikes for statistical comparison, while the upper cutoff avoided the excessive influence of a very few neurons on statistical comparisons because of their very high firing rates. The complete distribution of basal firing rates for each region is shown in Figure 3.
Figure 3.
Regional comparison of basal firing rates. a, Mean basal firing rate of analyzed neurons during the baseline period in each region ± SEM. Dorsal striatum firing rate was significantly lower than both OFC and ACC. b, Firing rate distribution for all neurons recorded in each region. Step size for histogram bars is 0.5 spikes/s.
Mean firing rates for the population responses were calculated using a bin size of 0.5 s. To subdivide the population response (see Fig. 5) (which represents the mean firing rate across all analyzed neurons) into population responses for neurons that increased or decreased their firing (see Fig. 8), the baseline firing rate (12 s before discriminative cue onset) was compared with the mean firing rate during the entire cue on period.
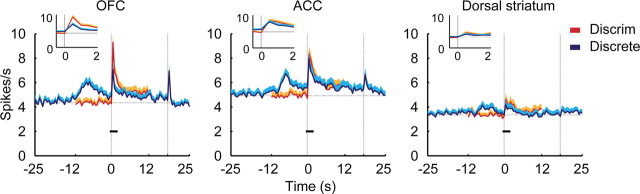
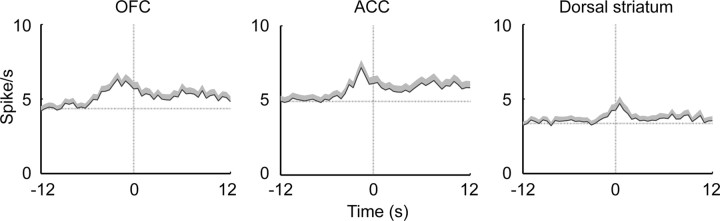
Figure 5.
Mean firing rate plots for all analyzed neurons across regions. Onset of the discriminative cue and discrete cue was at time = 0. (Increases before time = 0 for the discrete cue are due to the preceding discriminative cue.) The small black bars represent the time period used for the expanded insets. The horizontal dotted line represents the mean firing rate during 12 s of baseline before onset of the discriminative cue. The two vertical dotted lines indicate the onset time of both cues at time = 0, and the offset of the discrete cue at time = 18 s. Shaded area above each line is +SEM.
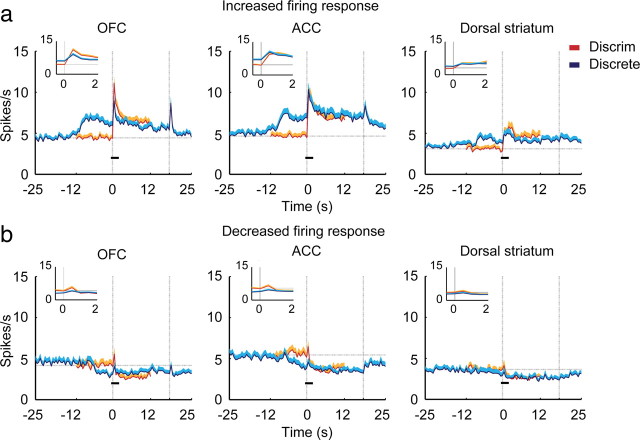
Figure 8.
Population responses of all neurons from Figure 5, subdivided according to whether their firing rate increased (a) or decreased (b) relative to baseline following cue onset. Note the more sustained response of ACC neurons that either increased or decreased firing following onset of the discrete cue. For the discriminative cue, the breakdown by region was: OFC, 127 increased, 61 decreased; ACC, 102 increased, 55 decreased; STR, 72 increased, 62 decreased. For the discrete cue, the breakdown by region was: OFC 116 increased, 72 decreased; ACC, 92 increased, 65 decreased; dorsal striatum, 74 increased, 60 decreased.
To determine whether neurons showed a significant response to a cue, the number of spikes during baseline (2 s before the onset of the discriminative cue) and 2 s after the onset of the discriminative cue or the discrete cue were compared (Mann–Whitney U test, p < 0.05). We chose the 2 s window to obtain a sufficient number of spikes for analysis before lever pressing behavior, which did not occur within this window on >85% of trials (Fig. 1c shows complete statistics for time to first lever press). We note also, as shown in Figure 4 that lever pressing behavior was not associated with sharp changes in firing in any region. A χ2 test was used for comparing regional differences in the proportion of responsive neurons. To compare changes in firing rate between regions during the last 2 s of the discrete cue, an absolute Z-score was used because of the large proportion of both increases and decreases in firing rate relative to baseline. Z-score was defined as the change from baseline as a multiple of the SD for that neuron.
Figure 4.
Firing was not tightly linked to lever pressing behavior. Each panel shows the mean firing rate plots by region, time locked to the time of the first lever press on each trial. Shaded area above each line is +SEM.
To examine the time course of the neuronal differentiation between the discriminative cue and discrete cue, a sliding receiver operating characteristic (ROC) analysis was used. This measures the degree of overlap of the neuronal response distributions between two conditions. (For a brief description, see Wallis and Miller, 2003; Johnston et al., 2007.) We used area under the ROC curve as an index of differential response of a neuron between the two conditions (discriminative cue and discrete cue). In this analysis, a value of 0.5 indicates there is no differentiation between the two conditions, whereas a value of 1.0 indicates no overlap in the response distributions. We restricted our comparison to a 2 s period following onset of the discriminative cue and discrete cue. Neuronal responses in a 0.5 s window sliding in 0.01 s steps were compared between the two cues. To evaluate differences in the mean ROC value, a Bonferroni-corrected one-way ANOVA was used to determine regional differences in 0.5 s windows incremented at 0.01 s steps.
The time course of response to the discrete cue relative to precue baseline was also calculated using sliding ROC analysis. To calculate the ROC values, a 0.5 s window was compared with each of the five 0.5 s windows comprising a 2.5 s baseline period before the discriminative cue. The ROC was the mean of those five comparisons. This was repeated for each 0.5 s window created by 0.01 s steps.
Data analysis for extinction.
To compensate for the different number of trials per session, five trials (to match the number of cue presentations during self-administration sessions) were randomly selected from the total of 14–17 cue presentations in each extinction session. Whether the neuron had a significant response was then evaluated in the same way as described above, i.e., by Mann–Whitney comparison of the number of spikes in the 2 s after cue onset to the 2 s precue baseline. If that comparison indicated a significant response, then we counted the neuron as responsive to the discriminative cue. This process was repeated 100 times, and the median value for the proportion of responsive neurons is what was used for the extinction condition for comparison to the self-administration condition.
Results
A discriminative cue and discrete cue which had been associated with cocaine self-administration over a period of 4.5 years were used. A red lamp on an operant panel served as the discriminative cue indicating that an infusion of cocaine was available following a fixed ratio of 10 lever presses (FR10 contingency), after which, an 18 s infusion commenced to flush a bolus of cocaine (0.1 mg/ml) through the intravenous line. During the infusion, a green lamp on the operant panel was lit which served as the discrete cue (Fig. 1a). Thus, the discriminative cue was always present just before onset of the discrete cue, necessitating the use of a prediscriminative cue baseline. In each session, 5 cocaine infusions were available, separated by a 10 min imposed time out. The first four infusions were 0.1 mg/kg each, while the fifth was 0.5 mg/kg. Given only one trial per session, it was not possible to evaluate whether cue responses differed for the 0.5 mg/kg infusion. We note that our measurements were not made at a time postinfusion when direct pharmacological effects of the higher dose would be apparent.
Consistent with well learned operant behavior, the response rate during the discriminative cue was significantly higher than during the baseline period (the 12 s before the onset of the discriminative cue) or during the discrete cue (Fig. 1b, one-way ANOVA, F(2,219) = 408, p = 1.2 × 10−74). This demonstrates the salience of the discriminative cue, and the understanding by the monkeys that responding during the discrete cue had no consequence. The mean time to reach contingency (duration of discriminative cue) was 12.9 ± 0.6 s. Figure 1c indicates complete statistics for the time to initiate the first lever press.
Neurons from OFC, ACC, and dorsal striatum were simultaneously recorded using up to six acutely placed, independently driven, four electrode arrays in two female rhesus monkeys during cocaine self-administration. We recorded a total of 242, 218, and 185 well isolated neurons from OFC, ACC, and dorsal striatum, respectively (70, 26, and 39 from OFC, ACC, and dorsal striatum in animal A, and 172, 192, and 146 in animal B). A comparison of the mean basal firing rate of all neurons from each region used for the analyses is shown in Figure 3a. Values were significantly different between regions (one-way ANOVA, F(2,476) = 6.65, p = 0.0014). Least significant difference (LSD) post hoc showed no difference between OFC and ACC, whereas basal firing rate in dorsal striatum was significantly lower than both OFC (p = 0.017) and ACC (p = 0.00032). We focused our analysis on neurons with a basal firing rate between 0.5 and 20 Hz, yielding 188, 157, and 134 for the three regions, respectively, with the vast majority of exclusions due to neurons with firing rates below the minimum (Fig. 3b).
Differential response of OFC to the two cues
The discriminative cue signaled the immediate availability of cocaine upon completion of the lever press response requirement. As shown in Figure 1b, the discriminative cue clearly controlled operant responding. The discrete cue, in contrast, did not increase lever pressing above baseline. Thus, the two cues are associated with cocaine reward, but under quite distinct behavioral contingencies. Given the function of OFC in encoding the salience of a reward-associated stimulus into a goal that requires an instrumental action (Rolls and Grabenhorst, 2008), it could be predicted that the OFC would have a greater response to the discriminative cue than to the discrete cue. This was found to be the case. Figure 5 depicts the mean firing rate across all neurons analyzed in each region, referenced to cue onset for both the discriminative cue and the discrete cue. Comparing the mean firing rate during the beginning (first 2 s) of the discriminative cue and discrete cue showed that OFC neuronal responses were significantly greater following the discriminative cue (paired t test, t(187) = 4.53, p = 1.0 × 10−5; Fig. 5 insets). In contrast, neither ACC nor dorsal striatum had differentially altered firing during the beginning of the cue on periods.
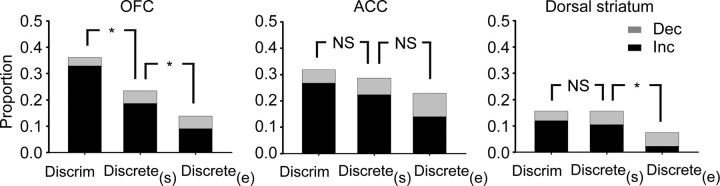
The greater mean firing rate in OFC during the discriminative cue could indicate a proportionately larger fraction of all neurons responding during the discriminative cue compared with the discrete cue. In support of this prediction, the proportion of neurons in OFC with a significant response to the discriminative cue was larger than to the discrete cue (χ2 test, p = 0.0068 for OFC), as shown in Figure 6, whereas the ACC and dorsal striatum showed no difference in proportion of neurons responding to the discriminative cue compared with the discrete cue.
Figure 6.
Proportional responding of neurons in each region. The bars represent the proportion of neurons that showed a significant response by comparison of the number of spikes in the baseline period (2 s before discriminative cue onset) with the first 2 s of the discriminative cue, the first 2 s of the discrete cue (Discrete(s)) and the last 2 s of the discrete cue (Discrete(e)). The two shaded portions indicate the proportion of neurons with increased (black) or decreased firing (gray) relative to baseline, *Significantly different by χ2 test (p < 0.05), NS, not significant.
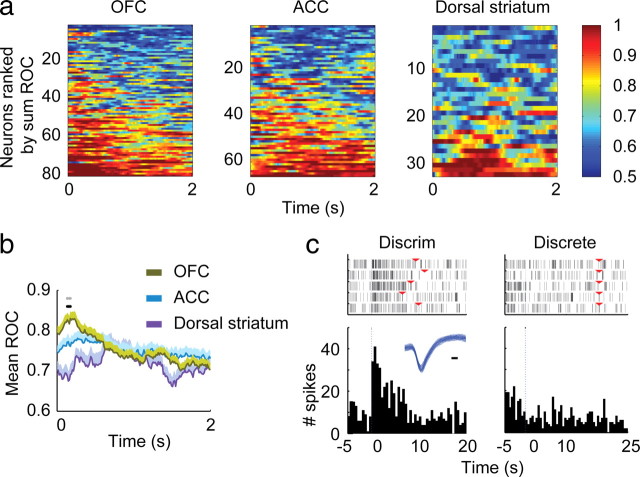
As can be seen in the mean firing rate curves in Figure 5, the time at which the firing rate of OFC neurons was most different between the two cue types was just after onset. The time course of differentiation between the two cues was evaluated using ROC analysis as shown in Figure 7. ROC analysis indicates the probability of separating a neuronal response between two conditions, with a value of 0.5 indicating no differentiation, and 1.0 indicating complete differentiation (Wallis and Miller, 2003). The analysis was restricted to the neurons that had a statistically significant response to at least one of the two cues. The ranked ROC curves of individual neurons (Fig. 7a) demonstrate the time course of the probability of differentiation between the two cues. Comparison of the mean ROC values across all neurons in each region (Fig. 7b) confirms that it is very early after cue onset that the mean ROC value is greater in OFC than the other two regions (black dots mark bins with a significant difference between regions by one-way ANOVA with Bonferroni correction, p < 0.00033; gray dots mark bins that differ between OFC and ACC, LSD post hoc, p < 0.05). The activity of a representative OFC neuron during presentation of the two cues is shown in Figure 7c.
Figure 7.
Selectivity between the discriminative cue and discrete cue using ROC analysis. Only responsive neurons (determined as in Fig. 6) to either one or both cue types were used for this analysis. a, A sliding window (0.5 s wide, 0.01 s steps) was used to compare the neuronal responses during the first 2 s of the two cues. Figures were plotted by ordering neurons from top to bottom by increasing sum of the ROC value. b, Mean ROC values for all neurons from a. Black dots indicate the time periods of differences among regions, the gray dots are the points that showed significant difference between OFC and ACC. c, Example of an OFC neuron which showed more responses to the discriminative cue (left) compared with responses to discrete cue (right). Inset shows the waveform of the neuron, black bar = 0.1 ms. The red triangles on the raster plots indicate the onset of the discrete cue on the left panel, and offset of the discrete cue on the right panel.
To summarize the OFC pattern of response to the two cue types: by both mean firing rate across all neurons analyzed, and proportion of neurons responding, the OFC responds more strongly to the discriminative cue than to the discrete cue. Comparison of mean ROC values indicates that the distinction by OFC occurs very early after cue onset.
Sustained response of the ACC to the discrete cue
The ACC showed a very different pattern of response to the cues compared with the OFC. The neuronal response to the discriminative cue and the discrete cue did not differ either in mean firing rate (Fig. 5) or proportion of neurons that were initially responsive to the discriminative cue and discrete cue (Fig. 6). What most distinguished the ACC was a pattern of sustained neuronal response throughout the discrete cue. This can be seen in the mean firing rate plot in Figure 5 in that the mean firing rate remains further above baseline throughout the entire cue on period, compared with either OFC or dorsal striatum. However, the mean firing rate plot somewhat obscures this effect because neurons showed both increases and decreases in firing rate in response to the cue. Long-lasting changes in ACC neuronal firing are much more apparent when broken down according to the direction of firing rate change (Fig. 8), in which all of the neurons analyzed to produce the population response shown in Figure 5, are broken down by direction of firing rate change. There, it can be seen that the responses of both excited and inhibited neurons to the discrete cue are clearly more extensive and prolonged than in the other regions.
The pattern of sustained response in ACC is also demonstrated by a comparison of the proportion of neurons that have a significantly different firing rate (from baseline) during the beginning (first 2 s) and end (last 2 s) of the discrete cue period. Figure 6 demonstrates that in the ACC, there was no difference in the proportion of neurons with a firing rate different from baseline between the beginning and end of the discrete cue (χ2 test, p = 0.24). In contrast, a significant decrease in the proportion of responsive neurons between the beginning and end of the cue is seen for both the OFC and dorsal striatum (Fig. 6, χ2 test, p = 0.017 for OFC, p = 0.035 for dorsal striatum). In addition to the ACC not showing a change in proportion of responsive neurons between the beginning and end of the discrete cue, two additional comparisons indicate that the ACC remains more responsive at the end of the discrete cue than either the OFC or dorsal striatum. First, a χ2 test indicates that there is a regional difference in proportion of responsive neurons during the end of the discrete cue, relative to baseline (overall effect, p = 0.00096; ACC vs OFC, p = 0.028, ACC vs dorsal striatum, p = 0.00031, OFC vs dorsal striatum, p = 0.074). A second positive indicator of a regional difference is ANOVA of the absolute Z-scores across regions (overall comparison, F(2,476) = 4.5, p = 0.012; LSD post hoc comparison: ACC vs OFC, p = 0.026; ACC vs dorsal striatum, p = 0.0045; OFC vs dorsal striatum, p = 0.40).
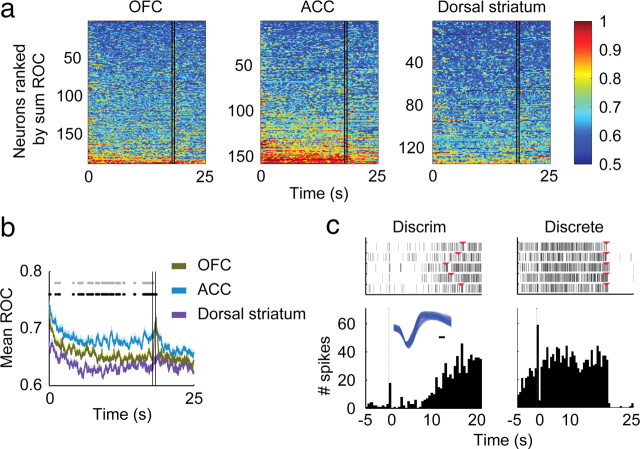
ROC analysis (Fig. 9a,b) reveals not only the greater number of individual neurons in ACC that differentiate discrete cue from baseline, but also how clearly the neuronal response in ACC is linked to the actual light-on period. This is shown by the sharp break at the 17.5/18 s window (marked by the two vertical lines) and can be seen as well in the response of the representative neuron shown in Figure 9c. Comparison of the mean ROC values across all neurons in each region (Fig. 9b) confirms that the increased neuronal response extends throughout the light-on period (by one-way ANOVA with Bonferroni correction, p < 2.0 × 10−5; gray dots mark bins that differ between OFC and ACC, LSD post hoc, p < 0.05). The ROC analysis reveals the pattern of differentiation of each analyzed neuron, and thereby demonstrates that a separate population is not becoming active just before cue offset. This is important because it is likely that subjective effects from the cocaine are beginning to occur toward the end of the discrete cue. The entry of cocaine into the animal was somewhat delayed from cue onset, given that a bolus was placed in the intravenous line some distance from the animal. Visual inspection of a bolus of dye using an identical set of lines and vascular access port showed that the 0.1 ml bolus placed in the line visibly exited the catheter tip across an interval from 7.5 to 12.5 s of the infusion. Based on human reports (Evans et al., 1996), cocaine subjective effects would have begun within a few seconds after exiting the catheter tip, and thus direct pharmacological effects should not have begun until the latter portion of the cue on period. Therefore, a developing subjective sensation of cocaine's effects could not be the cause of the sustained response in the ACC.
Figure 9.
Selectivity between the discrete cue and baseline using ROC analysis demonstrating the sustained response of ACC neurons to the discrete cue. a, A sliding window (0.5 s wide, 0.01 s steps) was used to compare the neuronal responses to baseline. Area under the ROC curve was calculated across all the neurons analyzed. The figures were constructed by sorting the neurons from top to bottom by increasing sum of the ROC value. The two vertical black lines (at T = 17.5 s and T = 18 s) represent the overlapping times of the sliding window and cue offset at 18 s. b, Mean ROC values for all neurons from a. Black dots represent the times of differences among regions and gray dots are the points that are significantly different between OFC and ACC. (c) Example of an ACC neuron which started its response toward the end of the discriminative cue (left) and continued to respond throughout the discrete cue (right). Inset shows the waveform of the neuron, black bar = 0.1 ms. The red triangles on the raster plots indicate the onset of the discrete cue on the left panel, and offset of the discrete cue on the right panel.
To summarize the ACC pattern of response to the two cue types, there is not a difference in initial response to the discriminative cue and discrete cue as shown by mean firing rate and proportion of neurons responding. The ACC neuronal response extended throughout the entire discrete cue period as shown by no change in the proportion of responsive neurons between the beginning and end of the cue period, a difference between regions in the proportion of responsive neurons at the end of the cue period, and a difference between regions in absolute Z-scores of the firing rate at the end of the cue period.
Diminished dorsal striatum responses relative to OFC and ACC
Compared with the two cortical regions examined, the dorsal striatum was less responsive to drug-associated cues. This can be seen in the mean firing rate graphs in Figure 5, and in the proportion of responsive neurons to each cue type in Figure 6. When we compared the proportion of responsive neurons across the three regions, there was a significant difference among regions for both the discriminative cue and discrete cue (p = 0.00021, and p = 0.031, respectively). More specifically, in response to the discriminative cue, dorsal striatum had a lower proportion of responsive neurons than both OFC (p = 5.0 × 10−5) and ACC (p = 0.0014). In response to the first 2 s of the discrete cue, the dorsal striatum response was smaller than ACC (p = 0.0083), and not different from OFC (p = 0.088, all comparisons by χ2 test).
Impact of extinction on neuronal responding
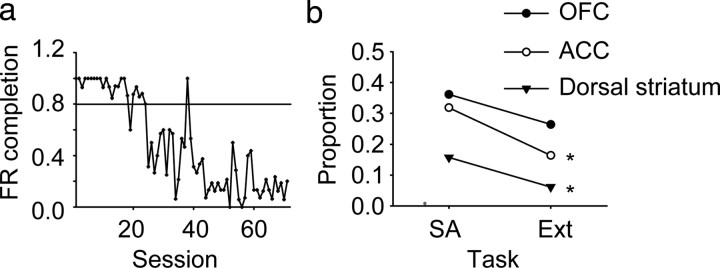
To confirm that neuronal responding to the cues was related to their reward significance, an extinction procedure was used in animal B. Substitution of saline for cocaine gradually reduced lever pressing behavior in response to discriminative cue presentation, though it took 24 sessions for the rate of successful completion of the FR10 contingency to drop below 80% over two consecutive sessions (Fig. 10a). Using all sessions (25–71) past that criterion, the proportion of responsive neurons before and after partial extinction were compared (Fig. 10b). There was a significant decrease in ACC and dorsal striatum (χ2 test, p = 0.0010 for ACC, p = 0.0385 for dorsal striatum), however, in the OFC, the proportion of responsive neurons was not significantly different (p = 0.07).
Figure 10.
Behavior and unit firing during cocaine extinction. a, FR completion represents the fraction of trials in which the FR10 lever press contingency was completed, resulting in saline infusion. A total of 71 extinction sessions were conducted. The horizontal line represents the cutoff of <80% of FR contingencies met over 2 successive sessions used for b. Although lever pressing behavior was not completely extinguished, it was reduced across extinction sessions (linear regression, p = 1.0 × 1022). b, Comparison of proportion of responsive neurons between sessions during cocaine self-administration (SA) and during extinction (Ext) according to region. Sessions after the criterion indicated on a (session 25–71) were used to calculate the proportion of responsive neurons during extinction. A significant difference was observed in ACC and dorsal striatum by χ2 test comparing SA to Ext (p = 0.0010 for ACC, p = 0.0385 for dorsal striatum). OFC was not significantly different (p = 0.07).
Discussion
We observed that in OFC and ACC, two cortical regions prominently implicated in addiction, there was a profound engagement of neuronal activity by drug-associated cues. Selective functionality of the two cortical regions was apparent in that the OFC was more engaged by the discriminative cue relative to the discrete cue. In contrast, while not differing in initial response to the two cue types, the ACC displayed a much more sustained response throughout the entire discrete cue associated with cocaine infusion. This study is the first to report cortical neuronal responses to drug-associated cues in nonhuman primates with a long history of conditioning that is typical of addiction in humans. These results define, at a neuronal activity level, the engagement of two key cortical regions by distinct types of cues known to differentially control drug reinforced behavior in animals.
The substantial primate electrophysiology literature on neuronal activity in cortical regions such as OFC and ACC during non-drug reward tasks has significantly shaped the interpretation of human imaging findings related to cue reactivity and cognitive dysfunction associated with addiction. The electrophysiological studies of dopamine neuronal responses in primates (Mirenowicz and Schultz, 1996) to rewards and reward cues have also been highly influential in guiding an understanding of how the dopaminergic properties of drugs of abuse contribute to their reinforcing effects. However, despite the contributions of primate electrophysiolgical studies to basic reward mechanisms, preclinical electrophysiological studies of neuronal activity responses to drugs of abuse and associated cues have, with one exception (Bowman et al., 1996), been conducted in the rodent. Within the rodent literature, there is relatively little work on electrophysiological measures of cortical neuronal activity responses to drug-associated cues (Chang et al., 1997, 1998, 2000; Sun and Rebec, 2006). Given that clinical imaging methods assess measures dependent on blood flow and metabolism which are influenced more by local synaptic processing than action potential generation (Goense and Logothetis, 2008), the current results complement imaging-based measures by assessing the actual neuronal firing that results from that processing. Thus, studying cortical single-unit activity in primates wherein the long term nature of human addiction can be modeled fills a significant gap in addiction research.
We used an unbiased sampling of neurons within each region, as there was no attempt to assess any task related responding before cue exposure. Thus, we are reporting comparisons of integrated responses between regions. We chose to use highly reinforcing doses of cocaine for these studies to more closely mimic actual patterns of consumption. Each infusion has an extended duration of action determined by the rate of metabolism (t1/2 for elimination in rhesus monkeys is 30–45 min) and acute tolerance to the pharmacological effects (Bradberry, 2000). Therefore, the number of trials that could be conducted within a session was limited. Consequently, we did not attempt to explicitly control for neural responses that could be selective for visual characteristics of stimuli such as color, size, etc. within session as done in the elegant studies from the many primate electrophysiological labs studying reward related function in these regions (Apicella et al., 1992; Schultz et al., 1992; Bowman et al., 1996; Roesch and Olson, 2004; Padoa-Schioppa and Assad, 2006; Rolls and Grabenhorst, 2008). Though previous work has shown that some unit activity in striatum is linked to movement execution such as in the case of visual saccades (Hikosaka et al., 1989), a general conclusion from the body of work on both striatal and cortical responses to instructional and reward-associated cues is that responding is primarily associated with their reward relevance rather than sensory processing or motor response preparation. Given that we used only five trials per session, the high proportion of statistically significantly responsive cortical neurons we observed is an indicator of how strongly these cues engaged the OFC and ACC.
OFC had a significantly greater response to the discriminative cue than the discrete cue, unlike the other regions examined. Previous work has shown that OFC is involved in reward evaluation and initiation of reward guided responses (Roesch and Olson, 2004; Padoa-Schioppa and Assad, 2006; Rolls and Grabenhorst, 2008). Our observation of preferential engagement of OFC by a discriminative cue suggests a role for OFC in relapse triggered by environmental cues indicating drug availability, given that discriminative cues support more drug seeking than discrete cues when presented noncontingently in animal models (Di Ciano and Everitt, 2003; Yun and Fields, 2003). During the extinction process which one animal underwent, the OFC response was the most resistant to extinction. This was surprising, because normally, one of the key functions of the OFC is an ability to rapidly adapt to changing reward contingencies and preferences following satiety (Rolls and Grabenhorst, 2008). However, chronic exposure to cocaine appears to seriously diminish this adaptive flexibility of stimulus reward associations (Schoenbaum and Shaham, 2008) in OFC. Our results are consistent with these observations. It appears plausible that a chronically enduring responsiveness to a discriminative cue by the OFC could represent a resilient entryway for discriminative cues to trigger relapse long after establishment of abstinence.
The other striking regionally selective cortical response was the prolonged engagement of neurons in ACC during the discrete cue which ceased when it was turned off. A prolonged response is consistent with the established role of the ACC in reward expectation and in guiding behavior for obtaining rewards. However, this involvement of the ACC in response selection and evaluation of outcome has generally been established over short term action outcome contingencies (Shidara and Richmond, 2002). In the present case, the ACC engagement was maintained even when responses were no longer necessary. ACC receives less sensory input than OFC, and is strongly connected with higher level motor processing centers (Carmichael and Price, 1995), consistent with the ACC being a key node in a cognitive control network devoted to response preparation (Cole and Schneider, 2007). What we observed was a high degree of activation throughout the discrete cue, despite the fact that it specifically signaled that no behavioral response was needed (and virtually none were made). This enduring engagement of ACC by discrete drug cues could be a mechanism through which those external cues maintain their prominence within motivational and motor planning networks.
The proportion of neurons engaged and the duration of the population responses to cues in the cortical regions far surpassed those in dorsal striatum. This is particularly significant in relation to what neural processes might be sustaining an enduring state of mind such as craving. Importantly, these are also cortical regions shown clinically to be highly responsive to drug-associated cues. In contrast to the cortex, striatal regions generally do not show activation with cue exposure (Grant et al., 1996; Maas et al., 1998; Wexler et al., 2001) (but see Garavan et al., 2000), unless the cues were based on internal imagery (Kilts et al., 2001). The dorsal striatum wherein we recorded neuronal responses is heavily innervated by dorsolateral prefrontal cortex and to a lesser extent by ACC and OFC (Haber et al., 2006). It is considered to be a likely site of convergence of a range of cortical inputs placing it in an excellent position to influence reward-based learning (Haber et al., 2006). A shift of mediation of drug reinforcement from ventral to more dorsal striatum has been proposed to accompany the transition from voluntary to compulsive drug consumption (Everitt et al., 2008). It is difficult to assess the functional homology of the dorsal striatal region we sampled with that from rodent-based preclinical studies given substantial differences in cortical inputs. However, if dorsal striatal neuronal responses are mediating behavioral responding to drug cues in these monkeys with an extensive history of self-administration, they are doing so with relatively less engagement than the cortical areas sampled.
We have examined neuronal activity responses to drug cues in animals with a long history of exposure to those cues. However, comparisons with non-drug reward-associated cues were not made. Consequently, we do not infer that the selective regional patterns of responding we observed only apply to drug rewards, nor that they necessarily are the key mechanisms underlying addiction or relapse. However, the present report does complement the substantial clinical literature indicating greater cingulate and orbitofrontal cortical reactivity to drug cues relative to striatum (Grant et al., 1996; Maas et al., 1998; Garavan et al., 2000; Wexler et al., 2001; Kosten et al., 2006; Goldstein et al., 2007, 2009). It also establishes unique functionality associated with the different prefrontal cortical regions in response to environmental cues that drive distinct aspects of drug-reinforced behavior in animals. In so doing, it will help to lay the foundation for future research to identify cognitive control mechanisms whereby drug cues exert such a profound influence over decisions and behavior, and potential neuronal activity metrics for pharmacotherapy development.
Footnotes
This work was supported by grant support from the National Institute of Drug Abuse (DA10331) and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. Thanks are extended to Carl Olson, Andrew Schwartz, and Nelson Totah for comments on earlier versions of this manuscript.
References
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. doi: 10.1152/jn.1992.68.3.945. [DOI] [PubMed] [Google Scholar]
- Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102(Suppl 1):33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Chang JY, Sawyer SF, Paris JM, Kirillov A, Woodward DJ. Single neuronal responses in medial prefrontal cortex during cocaine self-administration in freely moving rats. Synapse. 1997;26:22–35. doi: 10.1002/(SICI)1098-2396(199705)26:1<22::AID-SYN3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;99:433–443. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Evans SM, Cone EJ, Henningfield JE. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J Pharmacol Exp Ther. 1996;279:1345–1356. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacologia. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol. 1989;61:780–798. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- Iyer RN, Nobiletti JB, Jatlow PI, Bradberry CW. Cocaine and cocaethylene: effects on extracellular dopamine in the primate. Psychopharmacology (Berl) 1995;120:150–155. doi: 10.1007/BF02246187. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R. How good is the macaque monkey model of the human brain? Curr Opin Neurobiol. 2009;19:6–11. doi: 10.1016/j.conb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno HA, Bradberry CW, Everill B, Agulian SK, Wilkes S, Baldwin RM, Tamagnan GD, Kocsis JD. Local anesthetic effects of cocaethylene and isopropylcocaine on rat peripheral nerves. Brain Res. 2004;996:159–167. doi: 10.1016/j.brainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]