Abstract
Knowledge of the rates at which macrophages and epithelial cells synthesize NO is critical for predicting the concentrations of NO and other reactive nitrogen species in colonic crypts during inflammation, and elucidating the linkage between inflammatory bowel disease, NO, and cancer. Macrophage-like RAW264.7 cells, primary bone marrow-derived macrophages (BMDM), and HCT116 colonic epithelial cells were subjected to simulated inflammatory conditions, and rates of formation and consumption were determined for NO, O2, and O2−. Production rates of NO were determined in either of two ways: continuous monitoring of NO concentrations in a closed chamber with corrections for autoxidation; or NO2− accumulation measurements in an open system with corrections for diffusional losses of NO. The results obtained using the two methods were in excellent agreement. Rates of NO synthesis (2.3 ± 0.6 pmol s−1 106 cells−1), NO consumption (1.3 ± 0.3 s−1), and O2 consumption (59 ± 17 pmol s−1 106 cells−1 when NO is negligible) for activated BMDM were indistinguishable from those of activated RAW264.7 cells. NO production rates calculated from NO2− accumulation data for HCT116 cells infected with Helicobacter cinaedi (3.9 ± 0.1 pmol s−1 106 cells−1) were somewhat greater than those of RAW264.7 macrophages infected under similar conditions (2.6 ± 0.1 pmol s−1 106 cells−1). Thus, RAW264.7 cells have nearly identical NO kinetics to primary macrophages, and stimulated epithelial cells are capable of synthesizing NO at rates comparable to macrophages. Using these cellular kinetic parameters, simulations of NO diffusion and reaction in a colonic crypt during inflammation predict maximum NO concentrations of about 0.2 µM at the base of a crypt.
Introduction
Chronic increases in the rates of endogenous synthesis of NO have been implicated in the development of several human diseases, including cancer (1). In the gastrointestinal tract, NO or its metabolites have been linked to the pathogenesis of inflammatory bowel diseases (IBD), which often precede colon cancer (2,3). Immunohistochemical staining of samples from patients with active ulcerative colitis show that significant inducible nitric oxide synthase (iNOS) activity is localized in the crypt epithelium and in macrophages aggregated around crypt abscesses (4,5). However, it remains unknown what concentrations of NO in the colon are pathophysiological. The rates at which macrophages and epithelial cells synthesize NO is critical for predicting NO concentrations in a colonic crypt during inflammation (6), and the levels of NO are needed to estimate the intracellular concentrations of other reactive nitrogen species (7). Thus, a knowledge of the synthetic capacities of macrophages and epithelial cells is needed to improve the design of experiments to probe the cytotoxic and mutatgenic effects of NO, and thereby clarify the mechanisms by which NO exposure is linked to carcinogenesis in IBD.
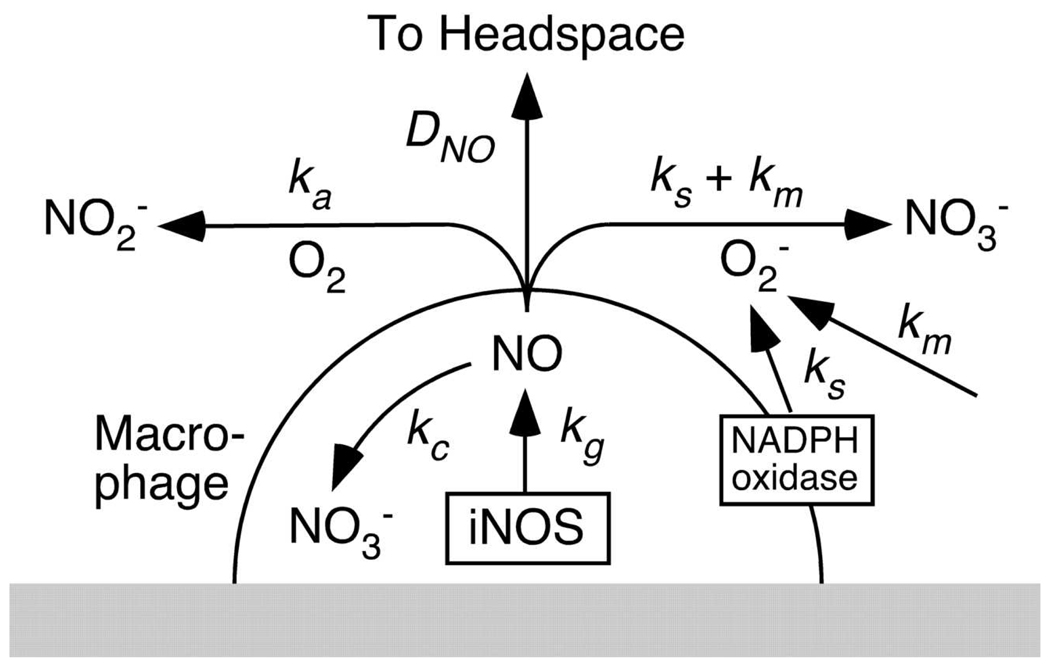
Two strategies have been used previously to measure cellular rates of NO production. Usually, the rate of NO synthesis is inferred from the rates of accumulation of stable end products of NO oxidation (8, 9, 10). As shown in Figure 1, NO produced by cells in a typical culture experiment will experience one of four principal fates: consumption by cells, reaction with O2 in the media to form NO2−, reaction with O2− in the media to form NO3−, or escape to the headspace by diffusion. The relatively slow, multistep reaction with O2, termed autoxidation, is written overall as (11)
| (1) |
The very fast reaction of NO with O2− is (12, 13)
| (2) |
As indicated, the rearrangement of peroxynitrite (ONOO−) to NO3− is catalyzed by CO2 (14). In an open system, the extent to which the diffusional loss of NO competes with Reactions 1 and 2 is determined by the liquid depth and the availability of O2 and O2− in the medium. Extracellular O2− is synthesized by macrophages via a membrane-bound NADPH oxidase (15, 16), and may also be generated in culture media (17). The fraction of the NO lost to the headspace can be significant, so that using NO2− and NO3− accumulation rates without correcting for NO loss can greatly underestimate NO production rates (18). The second strategy is to continuously monitor NO and O2 concentrations in a closed chamber (19). There is no physical loss of NO in this case, but extracellular Reactions 1 and 2 still must be considered when calculating cellular rates of NO synthesis. With either approach, separate experiments are needed to quantify extracellular O2− production, and the cellular consumption rate for O2 also must be measured or estimated to obtain reliable results.
Figure 1.
The four major fates of NO generated by cells in a typical plate culture experiment: oxidation to NO3− via intracellular pathways, oxidation to NO2− in the medium via Reaction 1, oxidation to NO3− in the medium via Reaction 2, or diffusional loss to the headspace. With macrophages (as shown), some of the O2− in the medium is produced by a membrane-bound NADPH oxidase; with colonic epithelial cells, that source of O2− is absent. The rate constants shown for each process are defined later, in connection with the kinetic models.
Another consideration in measuring rates of NO synthesis is that cells also consume NO. Reaction 2 is fast enough to allow NO to compete with superoxide dismutase for O2− (20), so that it will occur to some extent in virtually all cells. Other pathways for intracellular consumption of NO involve nitrogen dioxygenases (21) and reactions with oxygen-ligated reduced metals (22). Each of these reaction pathways involves NO oxidation to NO3−, so a 1:1 stoichiometry can be assumed for O2 usage in the intracellular consumption of NO. In NO-producing cells the net rate is the difference between the rates of synthesis and consumption, whereas in other cells there will be consumption only. This motivates experiments to measure NO consumption rates.
Reported here are rates of formation and consumption of NO, O2, and O2− for macrophage-like RAW264.7 cells, primary bone marrow-derived macrophages (BMDM), and HCT116 colonic epithelial cells under simulated inflammatory conditions. A combination of open-system and closed-system experiments was used to determine the rates of NO synthesis. With the former, a diffusion-reaction model was used to correct for NO losses to the headspace, and with the latter a batch reactor model was used to account for the various NO consumption pathways. Cellular rates of O2 consumption, NO consumption, and O2− production were measured in separate experiments. The open-system method with loss corrections and the more direct closed-system method have both been applied previously to RAW264.7 cells (19), but not to BMDM or HCT116 cells. Inflammatory conditions were simulated either by the addition of cytokines or by infection with Helicobacter cinaedi. H. cinaedi colonizes the lower bowel of various hosts, inducing intestinal inflammation with a pathology similar to that in human IBD, and has been found to upregulate iNOS expression in the cecum of mice (23). The NO synthetic capacity of HCT116 cells was tested also by exposing them to resveratrol and capsaicin; resveratrol has been shown to increase NOS expression in human adenoma carcinoma cells (SNU-1) (24) and the injection of capsaicin into Sprague Dawley rats has been found to upregulate all three NOS isoforms in the subnucleus caudalis (25). The cellular kinetic results were combined with a previously described model for NO diffusion and reaction in colonic crypts (6) to provide improved estimates of NO concentrations in inflamed crypts.
Experimental Methods
Mammalian Cell Culture
Cells of the mouse macrophage-like RAW264.7 line, obtained from the American Type Culture Collection (Rockville, MD), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing L-glutamine supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% (v/v) heat-inactivated fetal bovine serum (FBS) (BioWhittaker, Walkersville, MD). Cells of the human colon cancer HCT116 line (courtesy of G. N. Wogan, Department of Biological Engineering, MIT) were cultured in McCoy’s 5A medium (BioWhittaker, Walkersville, MD) and supplemented with 10% heat-inactivated FBS and one per cent each of 100 U/mL penicillin and 100 µg/mL streptomycin. All cells were maintained at 37 °C in a humidified 5% CO2 atmosphere.
Growth of Helicobacter Cinaedi
H. cinaedi (strain CCUG18818) obtained from the Culture Collection, University of Göteborg, Sweden, were plated from freezing stock onto blood agar plates (Remel, Lenexa, KS). Bacteria were maintained in microaerobic chambers that were evacuated to −20 mm Hg and then equilibrated with a gas mixture consisting of 80% N2, 10% H2, and 10% CO2. After 72 hr, bacteria were gently loosened from the plate, transferred to a shaker flask containing 20 mL of Brucella broth (BD Diagnostics, Franklin Lakes, NJ) with 10% FBS, and grown to log-phase. The concentration of H. cinaedi was determined by optical density measurements. Bacterial suspensions were centrifuged at 3000 g for 10 min and resuspended in antibiotic-free McCoy’s media with 10% FBS. Cells cultured in antibiotic-free media for at least one week were infected by adding 1 mL of appropriately concentrated bacterial suspensions (see below).
Killed bacteria were prepared by treating the H. cinaedi (grown as described above) with 0.5% formalin for 24 h and storing them at 4°C. On the day of the experiment, the formalin-killed bacteria were washed three times with PBS and resuspended in antibiotic-free McCoy’s media with 10% FBS.
Isolation of Primary Macrophages
Primary bone-marrow-derived macrophages (BMDM) were isolated from mice as described in Tomczak et al. (26). Approximately 98% of the cultured cells obtained by this method are macrophages, as assessed by flow cytometry. Briefly, BMDM were obtained from C57/Bl6 mice (Jackson Laboratories, Bar Harbor, ME), two to four months old. After flushing the femurs with cell culture media, single-cell suspensions were incubated on tissue culture plates in bone marrow macrophage medium, consisting of DMEM with 10% fetal bovine serum, 5% horse serum, penicillin 100 U/mL, streptomycin 100 µg/mL, 2 mM L-glutamine, and 10% L cell-conditioned medium. Macrophages were cultured for 5 days before use in experiments and were removed from plates by scraping.
Materials
Additional reagents used include recombinant mouse IFN-γ from R&D Systems (Minneapolis, MN) as well as recombinant human IFN-γ and TNF-α from Invitrogen (Camarillo, CA). Escherichia coli lipopolysaccharide (LPS), bovine erythrocyte superoxide dismutase (SOD), capsaicin, resveratrol, rotenone, cytochrome c from horse heart, HEPES buffer solution (pH 7.4), and trypan blue (4% in saline) were all from Sigma Aldrich (St. Louis, MO). N-methyl-L-arginine monoacetate (NMA) was from CalBiochem Research (Salt Lake City, UT), and beef liver catalase was from Roche Molecular Biochemicals (Indianapolis, IN).
Closed Chamber for Measurement of NO and O2 Concentrations
An apparatus similar to that described in Nalwaya and Deen (19) was used to continuously monitor NO and O2 concentrations. A polycarbonate insert equipped with a stirring motor (Instech Laboratories, Plymouth Meeting, PA), NO electrode (World Precision Instruments, Sarasota, FL), and fiberoptic O2 sensor (Instech Laboratories, Plymouth Meeting, PA) was machined to fit within standard 60 mm polystyrene tissue culture dishes. After assembly, the chamber had a total liquid volume of 8.8 mL. All measurements were conducted in a 37°C warm room in the dark, to eliminate NO consumption by light-sensitive riboflavin-derived O2− in the medium.
O2 Consumption by Unactivated Cells in Closed Chamber
Cells (RAW264.7, BMDM, or HCT116) were detached from stock plates and resuspended in media into a homogeneous suspension through vigorous pipetting. The number of viable cells was determined by trypan blue exclusion. Between 5 × 106 and 8 × 106 cells were plated on 60 mm dishes and allowed to adhere for 2–3 h. To begin an experiment, the medium in a given dish was replaced with 15 mL of DMEM with 25 mM HEPES preheated to 37 °C and the dish was assembled with the closed-chamber insert. The O2 concentration was then monitored for 20–30 min and the rate of consumption calculated from the slope of the curve.
NO Synthesis and O2 and NO Consumption by Activated Cells in Closed Chamber
Between 5 × 106 and 8 × 106 cells were seeded onto 60 mm dishes. After the cells were allowed to adhere for 2–3 h, the media in plates containing RAW264.7 or BMDM was replaced with fresh DMEM containing LPS (20 ng/mL) and IFN-γ (20 U/mL); HCT116 cultures were replenished with McCoy’s media containing capsaicin (100 µM) and resveratrol (50 µM). Because it takes 6–8 h after the addition of LPS and IFN-γ for macrophages to produce NO (27), the concentration measurements to determine rates of NO production and consumption for RAW264.7 and BMDM were performed after 12 h. Experiments with HCT116 cells were performed at 24 h after the addition of capsaicin and resveratrol. At the start of each experiment, fresh, preheated media with 25 mM HEPES was added to the dish and the apparatus was assembled. The NO and O2 concentrations were monitored until a steady NO concentration was achieved, typically 15–20 min. Calculations were done using the kinetic model described below. O2 concentrations were also monitored for activated RAW264.7 cells in the presence of rotenone, a respiratory inhibitor. After the 12 h stimulation with IFN-γ and LPS, the cells were exposed to 2 µM of rotenone in the media for 1 h.
NO Synthesis in Open System
To avoid bacterial contamination of the NO electrode, rates of NO synthesis by RAW264.7 or HCT116 cells infected with H. cinaedi were assessed by measuring concentrations of NO2− and NO3− as a function of time in an open system. The number of viable cells was determined by trypan blue exclusion and cells were seeded at concentrations of 1 × 106 cells/well into 6-well plates. RAW264.7 cells were infected by adding 1 mL of H. cinaedi suspension containing either 2 × 107 cells/mL or 2 × 108 cells/mL to each well, giving a multiplicity of infection (MOI, the ratio of bacteria number to mammalian cell number) of 10 or 100, respectively. HCT116 cells were subjected to a pre-treatment of TNF-α (50 ng/mL) and IFN-γ (100 U/mL) for 24 h prior to infection, and then were infected using the same procedure with either live or killed H. cinaedi. The concentration of killed bacteria corresponded to an MOI of 100. Bacteria-free media was added to the control cells (total liquid volume of 2 mL/well).
Supernatant samples (1 sample per well) were collected at 24 and 48 h after infection and centrifuged at 12,000 rpm before analysis. Nitrite and nitrate accumulation was quantified using a Griess assay kit (R&D Systems, Minneapolis, MN). Briefly, 50 µL of culture supernatant was allowed to react with 100 µL of Griess reagent and incubated at room temperature for 10–30 min. For measurement of NO3−, NADH and nitrate reductase were added before reaction with the Griess reagents. Absorbance was measured at 540 nm using a microplate reader.
Nitrite and nitrate production was also measured for BMDM and RAW264.7 cells activated with IFN-γ (20 U/mL) and LPS (20 ng/mL). This allowed NO production rates in the open system to be compared with those in the closed chamber. Between 2 × 105 and 4 × 105 macrophages were seeded into each well of a 24-well plate with or without IFN-γ and LPS (total liquid volume of 1 mL/well) and supernatant samples were collected at 0, 4, 8, 12, 24, 36, 48, 60, and 72 h for Griess analysis.
Superoxide Synthesis
Net cellular O2− synthesis of activated and unactivated macrophages was quantified using a cytochrome c assay. After seeding macrophages (RAW264.7 or BMDM) into 24-well plates as described above, 1 mL of media with or without IFN-γ (20 U/mL), LPS (20 ng/mL) was added to each well. NMA (2 mM), an iNOS inhibitor, was added to wells containing macrophages stimulated with IFN-γ and LPS because NO competes very effectively with ferricytochrome c for O2− (28). After stimulation of the macrophages with IFN-γ and LPS for 4 or 8 h, cytochrome c solution (80 µM) was added to the cell supernatant and the mixture was centrifuged at 4000 g for 1 min. Absorbance at 550 nm was measured immediately on 0.5 mL samples and converted to nanomoles of ferrocytochrome c concentrations using the molar extinction coefficient for the reduction of cytochrome c with ascorbic acid (21.0 × 103 M−1 cm−1 (29)). Plots of nanomoles of ferrocytochrome c vs. time were fitted linearly. The difference in the slopes between samples incubated with SOD (1000 U/mL) and without SOD was used to calculate the O2− production rates for activated and unactivated cells (ks and kso, respectively).
Model for Closed Chamber
The closed chamber was modeled as a well-stirred batch reactor, as described previously (19). The variation in NO concentration (CNO) with time (t) is given by
| (3) |
where m is the number of viable cells, V is the liquid volume, v is the volume of a single cell, (modeled as a hemisphere with radius = 7.5 µm), ks is the rate of extracellular NO consumption by macrophage-generated O2− (which equals the cellular rate of O2− synthesis), ka is the rate constant for autoxidation of NO, and km is the rate of extracellular NO consumption by culture medium-generated O2−. The values used for V, v, ka, and km are given in Table 1. Separate experiments (described above) yielded ks. The kinetic parameters for NO that were evaluated from the closed-chamber experiments were kg, the rate of generation of NO, and kc, the rate constant for intracellular consumption of NO.
Table 1.
Parameters used in the kinetic model for the closed chamber apparatus and in the reaction-diffusion model for the open system
| Parameter | Value | Reference |
|---|---|---|
| V | 8.8 × 10−3 L | see text |
| v | 8.8 × 10−13 L | see text |
| a | 1.77 × 10−10 m2 | see text |
| ka | 2.6 × 106 M−2 s−1 | (11) |
| km | 0.4 × 10−9 M s−1 | (19) |
| KM | 7 × 10−6 M | (19) |
| KI | 18 × 10−9 M | (19) |
| DNO | 3.0 × 10−5 cm2 s−1 | (35) |
| DO2 | 2.8 × 10−5 cm2 s−1 | (37) |
| C0 | 223 µM | (36) |
Symbols: V, liquid volume in the closed chamber; v, volume of a single cell; a, area of a flat surface occupied by a single cell; ka, rate constant for autoxidation of NO; km, rate of NO consumption by media-generated O2−; KM, the O2 concentration at which respiration is halved in the absence of NO; KI, constant describing inhibition of respiration by NO; DNO and DO2, aqueous diffusivities of NO and O2 respectively; C0, liquid-phase O2 concentration in equilibrium with the incubator.
The O2 concentration was described by
| (4) |
The bracketed quantity is the rate of O2 consumption per cell. The first term is the rate at which O2 is consumed by iNOS in synthesizing NO, assuming a 2:1 stoichiometry (30), and the second term is the rate of O2 usage during intracellular consumption of NO, with a 1:1 stoichiometry as discussed earlier. In the third term, which corresponds to O2 consumption from respiration, Rmax is the maximum rate, KM is the O2 concentration at which respiration is halved (in the absence of NO), and KI accounts for the inhibition of respiration by NO (31). The one O2 parameter obtained from the closed-chamber experiments was Rmax. The values of KM and KI used (Table 1) were those determined previously from data for RAW264.7 cells (19). Because they are intrinsic properties of the respiratory enzymes, they were assumed to be the same for all cells (macrophages and epithelial). In applying equations 3 and 4 to NO and O2 measurements for HCT116 cells, the radius of a single cell was assumed to be the same as that for macrophages (32, 33). The ks term was omitted because epithelial cells do not synthesize O2− extracellularly (discussed later).
The NO and O2 kinetic parameters (kg, kc, and Rmax) were calculated by fitting the NO and O2 concentration data for RAW264.7, BMDM, and HCT116 cells from appropriate sets of experiments; the root-mean-square-difference between the measured concentrations and those predicted by Equations 3 and 4 was minimized using the simplex method in MATLAB (MathWorks, Natick, MA).
Model for Open System
In the open-system experiments the rate of accumulation of NO2− was used to infer cellular rates of NO production for macrophages and epithelial cells. A model like that previously described (18, 34) was used to account for extracellular reactions, rates of diffusion, and the effects of liquid depth. Because the duration of these experiments was long enough to achieve steady-state levels of NO and O2, the concentrations were assumed to depend only on height within the liquid (z). In this model the bottom of the well and the gas-liquid interface correspond to z = 0 and z = L, respectively. The NO concentration in the liquid is governed by
| (5) |
| (6) |
| (7) |
| (8) |
The parameters not already defined are: DNO, the diffusivity of NO in water (Table 1); α, the fraction of the plate area covered by cells (based on the initial number of cells seeded into a well); β, the ratio of the O2− to the NO flux; and NNO, the rate of NO release per unit area from a hypothetical confluent monolayer of cells (units of mol m−2 s−1). The cellular flux of NO is equivalent to the net rate of NO synthesis per cell divided by the planar area per cell (a = πr2, with r = 7.5 µm). The NO consumption rate from media-generated O2− (km) was assumed to be the same as that used in the closed chamber model (Table 1). The value of km is determined largely by exposure of the culture medium to light (19), which was minimal in the open-system experiments and absent in the closed-chamber experiments.
The autoxidation reaction is too slow to have a significant effect on the O2 concentration (35), which is given by
| (9) |
| (10) |
Here, C0 is the liquid-phase O2 concentration in equilibrium with the incubator (Table 1), DO2 is the diffusivity of O2 (Table 1), and NO2 is the rate of O2 consumption per unit area for a confluent monolayer (units of mol m−2 s−1). Similar to the closed chamber model, O2 consumption in Equation 10 includes consumption by iNOS, O2 usage during intracellular consumption of NO, and competitive inhibition of respiration by NO. The other parameters are as defined for the chamber model. The NO flux and kg are related by Equation 7 and kc and Rmax were obtained from the closed-chamber experiments. Thus, the one unknown parameter in this model was NNO.
Equation 5 (with Equations 6–10) was solved using a commercial finite element package (COMSOL v.3.0, Stockholm, Sweden). Rates of NO2− accumulation were calculated by numerically integrating the local rate of NO2− formation (given by ) over the entire solution volume. The value of NNO was adjusted until the fractional difference between the calculated and measured rates of NO2− accumulation was <10−6.
Results
Oxygen Consumption by Unactivated Cells
A representative plot of O2 concentration as a function of time for unactivated BMDM in the closed chamber is shown in Figure 2. For this and the other curves (to be discussed shortly), t = 0 is the moment the test chamber was assembled and readings begun. The linear decline implies that the rate of O2 consumption was constant over the range of concentrations examined. Nearly identical plots were generated for unactivated RAW264.7 and HCT116 cells (data not shown). In each of these experiments NO was undetectable, so that respiratory inhibition was absent. The average rates of O2 consumption calculated from the slopes of such plots for RAW264.7, BMDM, and HCT116 cells were Rmaxo = 38 ± 4, 30 ± 3 and 26 ± 7 pmol s−1 106 cells−1, respectively. As indicated in Table 2, oxygen consumption rates for HCT116 cells were not statistically different from those of BMDM, and Rmax values for BMDM and RAW264.7 cells were also statistically indistinguishable from one another.
Figure 2.
Measurement of NO synthesis, NO consumption, and O2 consumption by bone marrow-derived macrophages (BMDM) and RAW264.7 cells. Shown are NO concentrations (black) and O2 concentrations (gray) as a function of time for representative closed-chamber experiments. The linear decline in O2 concentration in the absence of NO for unactivated macrophages (8 × 106 cells) indicates that the rate of O2 consumption is constant. Measurements with unactivated RAW264.7 and HCT116 cells resulted in similar plots (not shown). NO and O2 concentrations were also measured for 8 × 106 BMDM after activation by 12 h exposure to IFN-γ and LPS. The rate of O2 consumption slowed as the NO concentration increased. The kinetics of NO synthesis and consumption are reflected in the plateau concentration for NO and the rate at which the plateau is reached. Plots of NO and O2 concentrations measured for activated RAW264.7 cells were nearly identical (not shown). O2 concentrations are also depicted for activated RAW264.7 cells after 1 h exposure to rotenone, a respiratory inhibitor; the reduced slope corresponds to about an 85% reduction in the overall rate of O2 consumption.
Table 2.
Cellular kinetic parameters for NO, O2, and O2− measured in the closed-chamber experiments.
| Kinetic Parameter | RAW264.7 | BMDM | HCT116 |
|---|---|---|---|
| Rmaxo (pmol s−1 106 cells−1) | 38 ± 4 (n = 4) | 30 ± 3 (n = 4) | 26 ± 7 (n = 4)† |
| Rmax (pmol s−1 106 cells−1) | 62 ± 14 (n = 6) | 59 ± 17 (n = 6) | 41 ± 11 (n = 6) ‡ |
| kg (pmol s−1 106 cells−1) | 2.1 ± 0.3 (n = 6) | 2.3 ± 0.6 (n = 6) | 1.1 ± 0.2 (n = 6) †‡ |
| kc (s−1) | 1.6 ± 0.4 (n = 6) | 1.3 ± 0.3 (n = 6) | 4.1 ± 2.3 (n = 6) †‡ |
| (pmol s−1 106 cells−1) | 0.07 ± 0.02 (n = 4) | 0.05 ± 0.01(n = 4) | - |
| ks (pmol s−1 106 cells−1) | 0.3 ± 0.09 (n = 4) | 0.3 ± 0.08 (n = 4) | - |
Symbols: Rmaxo and Rmax, maximum respiration rates for unactivated and activated cells, respectively; kg, rate of generation of NO; kc, rate constant for intracellular consumption of NO for activated cells; kso and ks, rates of extracellular O2− generation by unactivated and activated macrophages, respectively. All values are mean ± SD for the number of experiments indicated.
p < 0.05 versus RAW264.7 values,
p < 0.05 versus BMDM values.
NO Synthesis and O2 and NO Consumption by Activated Cells in Closed Chamber
Figure 2 also shows NO and O2 concentrations in a typical closed-chamber experiment 12 h after stimulation (i.e., addition of IFN-γ and LPS to the media). As seen, there was an initial increase in CNO that leveled off at 0.45 µM at ~800 s. At early times (t < 200 s), when lower concentrations of NO were present, the decline in O2 concentration was nearly linear. The initial slope of O2 consumption for activated cells is greater than that observed for unactivated cells and is reflected in the higher values of Rmax for activated cells (Table 2). As NO concentrations increased, the inhibitory effects of NO on respiration were observed, and the rate of O2 consumption gradually decreased. Similar plots were generated for NO and O2 measurements with RAW264.7 cells (not shown). The addition of rotenone to activated RAW264.7 cells decreased the rate of O2 consumption by roughly 85%, as shown, confirming that most O2 consumption was respiratory. Applying the kinetic model to the data from such experiments, we calculated that kg = 2.3 ± 0.6 pmol s−1 (106 cells)−1, kc = 1.3 ± 0.3 s−1, and Rmax = 59 ± 17 (n = 6) for activated BMDMs (Table 2), all of which were not statistically different from the same kinetic parameters measured for RAW264.7 cells.
A representative plot of NO and O2 concentrations for HCT116 cells measured 24 h after incubation with capsaicin and resveratrol is shown in Figure 3. Similar to the experiments with macrophages, a steady NO concentration was reached about 800 s after the experiment started, the value in this case being 0.16 µM. Again, inhibition of respiration by NO was observed in the plot of CO2 versus time. Applying the kinetic model, kg = 1.1 ± 0.2 pmol s−1 (106 cells)−1, kc = 4.1 ± 2.3 s−1, and Rmax = 41 ± 11 (n = 6) for activated HCT116 cells. For the HCT116 cells kg was lower and kc higher than for macrophages (p < 0.05).
Figure 3.
Measurement of NO synthesis, NO consumption, and O2 consumption by activated HCT116 cells stimulated with capsaicin and resveratrol. Results are shown for 7 × 106 cells in the closed chamber. Induction of NO synthesis, as reflected by the increasing NO concentration, inhibited respiration in a manner similar to that seen with macrophages in Figure 2.
Nitrite and Nitrate Accumulation for Activated Cells
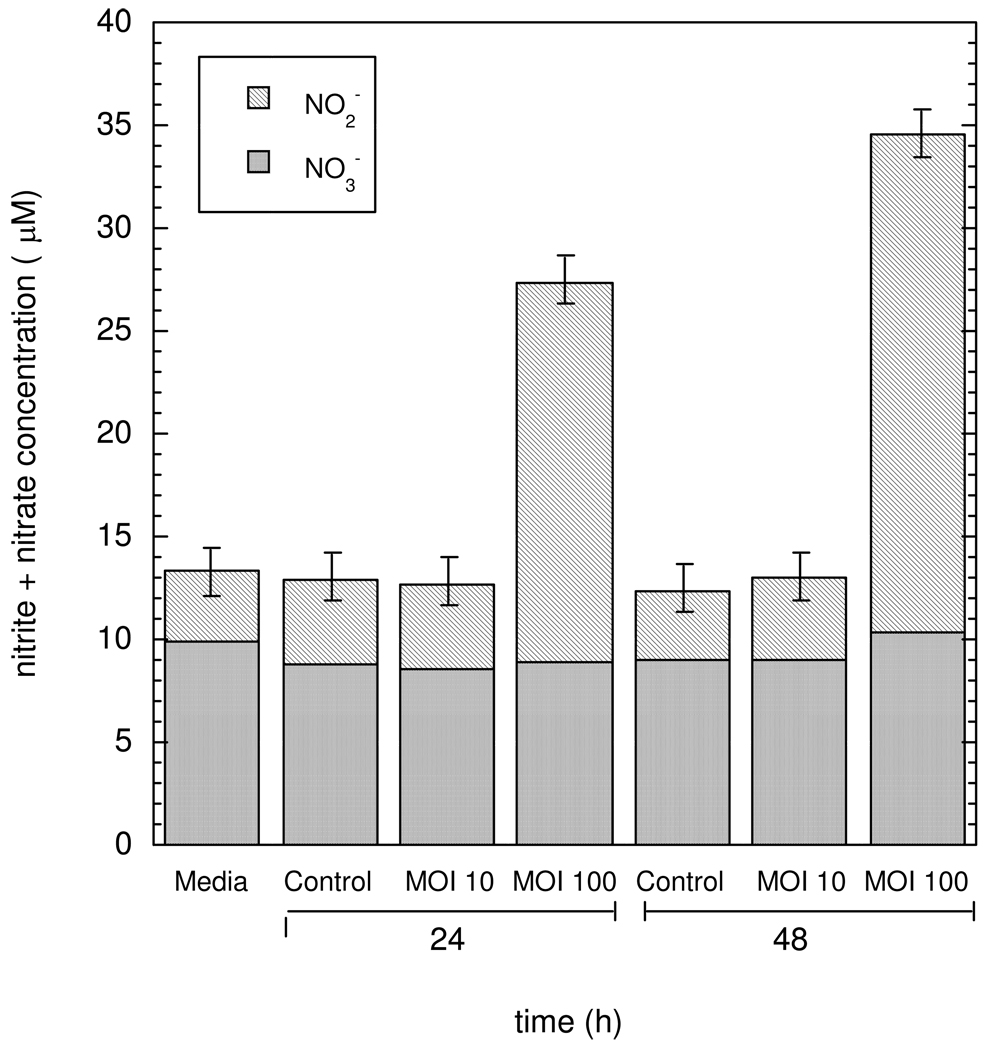
Figure 4 shows NO2− and NO3− concentrations measured for HCT116 cells pretreated with TNF-α (50 ng/mL) and IFN-γ (100 U/mL) and infected with live H. cinaedi. In this plot, 24 and 48 h correspond to the duration of the exposure to the bacteria. The NO3− concentrations in all samples remained nearly constant and were statistically indistinguishable from those detected in media alone. Likewise, uninfected cells and HCT116 infected with H. cinaedi at MOI = 10 yielded NO2− concentrations which were not different from those in the absence of cells. In contrast, a 4-fold increase in NO2− concentrations was measured after 24 h for HCT116 cells infected with H. cinaedi at MOI = 100, 18.5 ± 1.0 µM as compared to 4.7 ± 0.6 µM for uninfected cells. The rate of NO2− accumulation for MOI = 100 was lower for 24–48 h than for 0–24 h, probably due to a reduced number of viable cells. Experiments in which HCT116 cells were exposed in the same manner to killed bacteria did not result in NO2− accumulation (data not shown). Moreover, Griess assays on suspensions containing only H. cinaedi at concentrations corresponding to MOI = 10 or 100 were negative, indicating that the bacteria were not the source of NO2− accumulation in the media.
Figure 4.
Nitrite and nitrate concentrations in the supernatant of HCT116 cells which were infected with H. cinaedi. Means ± SD are shown for n = 5. The initial cell numbers were 1 × 106 for HCT116 and 1 × 107 or 1 × 108 for H. cinaedi, yielding multiplicity of infections (MOI) of 10 or 100. The times (24 or 48 h) correspond to the duration of exposure to the bacteria. The increased NO2− concentrations for MOI = 100 are evidence of NO synthesis by the HCT116 cells.
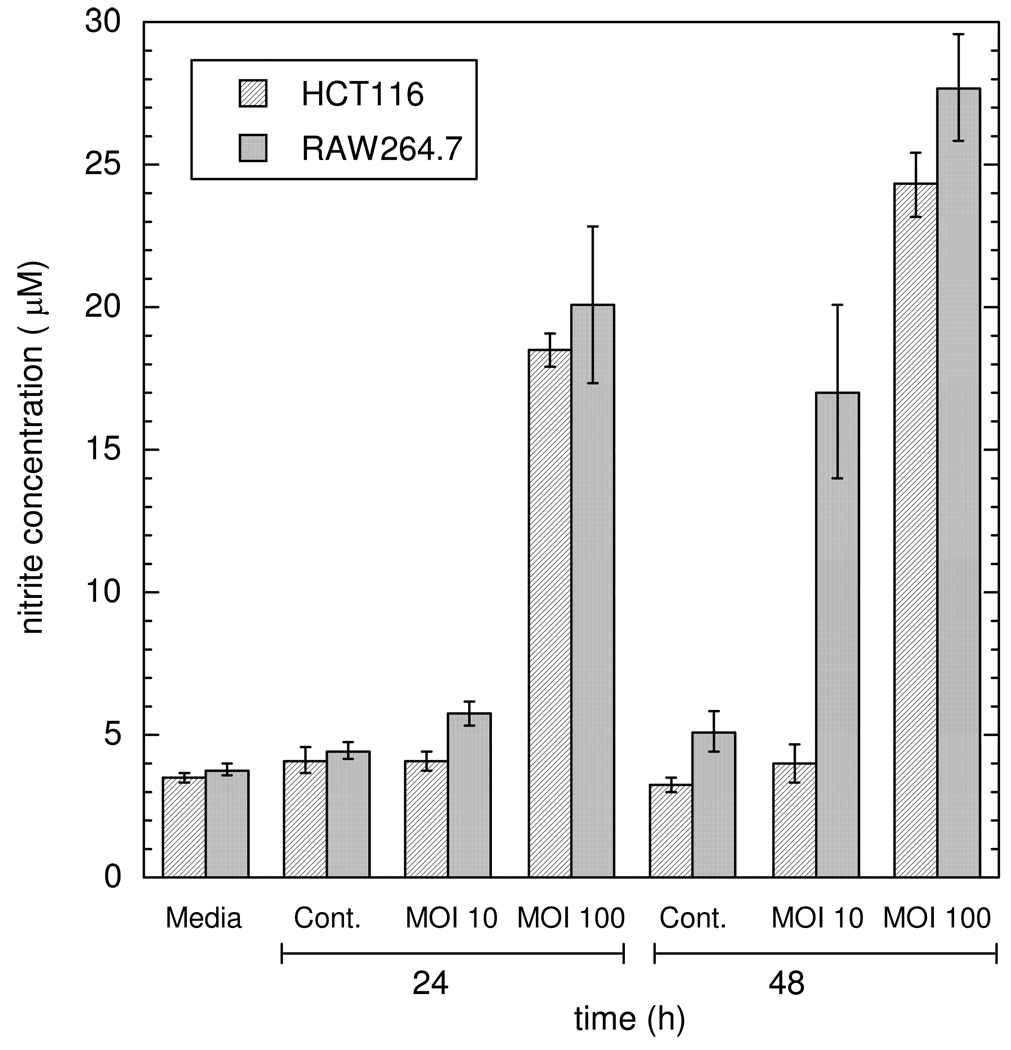
Figure 5 shows NO2− accumulation for RAW264.7 cells infected with H. cinaedi for 24 h at MOI = 10 or 100. The 24 h data for infected HCT116 cells are shown again for comparison. The NO2− concentrations measured for RAW264.7 cells infected at MOI = 100 (20.1 ± 2.7 µM and 27.7 ± 1.9 µM for samples collected at 24 and 48 h, respectively) were not statistically different from those measured for HCT116 cells. However, unlike HCT116 cells, which were unresponsive to H. cinaedi infections at MOI = 10, samples collected from infected RAW264.7 cells showed increased NO2− accumulation after 48 h (17.0 ± 3.0 µM) when compared to uninfected cells (5.1 ± 0.7 µM).
Figure 5.
Comparison of NO2− concentrations for RAW264.7 cells and HCT116 cells infected with H. cinaedi. Means ± SD are shown for n = 5. See Figure 4 for additional explanation.
The NO2− concentrations measured 24 h after infection with H. cinaedi at MOI = 100 were used to infer rates of NO production for macrophages and epithelial cells, using the open-system model. NO synthesis rates for HCT116 cells and RAW264.7 cells were found to be 3.9 ± 0.1 and 2.6 ± 0.1 pmol s−1 106 cells−1, respectively (Table 3). Thus, under these conditions, the rate of NO synthesis of HCT116 cells was 50% higher than that of RAW264.7 cells.
Table 3.
Conditions in the open-system experiments and comparative rates of NO synthesis and loss to the headspace.
| Parameter | RAW264.7 | BMDM | HCT116 | RAW264.7 |
|---|---|---|---|---|
| Stimulant | IFNγ + LPS | IFNγ + LPS | TNF-α + IFN-γ, H.Cinaedi |
H.Cinaedi |
| α | 0.35 | 0.2 | 0.2 | 0.2 |
| β | 0.15 | 0.15 | 0 | 0.15 |
| L | 5 mm | 5 mm | 2 mm | 2 mm |
| Fraction NO lost | 0.32 | 0.43 | 0.82 | 0.71 |
| kg . pmol s−1 (106 cells)−1) | 2.5 ± 0.6 (n = 2) |
2.3 ± 0.9 (n = 2) |
3.9 ± 0.1 (n = 5) |
2.6 ± 0.1 (n = 5) |
Symbols: α, fraction of plate area covered by cells; β, ratio of O2− to NO flux at cells; L, liquid depth; kg, rate of NO generation by cells. Values for kg are mean ± SD.
Similarly, rates of NO synthesis were calculated from NO2− accumulation data for BMDM and RAW264.7 cells stimulated with IFN-γ and LPS. After 8 h, NO2− and NO3− concentrations increased linearly with time for t = 8 – 60 h (data not shown). The average slopes obtained by linear regression over that interval were used to determine the accumulation rates of NO2−. The calculated rates of NO synthesis (kg) for RAW264.7 cells in the open system were indistinguishable from those for BMDM (Table 3), and the rates for either type of macrophage in the open system agreed with those in the closed chamber (compare with Table 2).
Superoxide Synthesis
Figure 6 shows representative plots of ferrocytochrome c concentration as a function of time in experiments designed to measure rates of O2− synthesis by BMDM. Data are shown for unactivated and activated BMDM (with and without SOD present) as well as for culture medium alone, with t = 0 being the moment at which fresh medium with or without NMA, SOD, LPS, and IFN-γ was added. In each case, the linear increase in concentration is evidence of a constant rate throughout the 8 h period. Activated BMDM yielded the largest slope, corresponding to a substantial amount of cellular O2− production. The slope for activated BMDM with SOD was greatly reduced, and was indistinguishable from that for cell-free medium with SOD. The residual ferrocytochrome c formation with SOD present is attributable to reduction of ferricytochrome c by media components other than O2−. The intermediate slope shown for medium without SOD is evidence that some of the O2− synthesis in the cell experiments came from the media. The very similar slopes shown for unactivated BMDM and media (each without SOD) suggest that O2− generation by unactivated macrophages is minimal. Although for the examples shown in Fig. 6 these slopes were nearly identical, analysis of all the data revealed a slightly greater slope for the cells, the difference being statistically significant. This small rate of O2− production by unactivated BMDM is consistent with previous work showing low NADPH oxidase activity in untreated macrophages (38). The O2− production rates for activated and unactivated macrophages (ks and kso), based on the data for all experiments, are given in Table 2. As shown, the net cellular O2− synthesis rate by activated BMDM was only 15% of the rate of NO synthesis.
Figure 6.
Rate of O2− production by primary macrophages (BMDM). Plotted are nmol of ferrocytochrome c detected as a function of time for representative experiments with 5 × 105 cells and with culture medium only. Results are shown for cells activated by incubation with IFN-γ and LPS beginning at t= 0 in the absence or presence of SOD, unactivated cells, untreated medium, and medium with SOD. Experiments performed with RAW264.7 resulted in similar plots (not shown).
Similar experiments to determine whether or not HCT116 cells are capable of producing extracellular O2− were also conducted. Supernatant samples collected from HCT116 cells, incubated with and without capsaicin and resveratrol, yielded ferrocytochrome c concentrations that were not statistically different from medium alone (data not shown), confirming that activated and unactivated epithelial cells produce little or no extracellular O2−.
Discussion
The present study provides the first relatively direct measurements of the rates of NO, O2, and O2− production and consumption by primary macrophages (BMDM) and a colonic epithelial cell line (HCT116) under simulated inflammatory conditions. Included also are new data for a macrophage-like cell line (RAW264.7) that had been studied previously using the same methodology. The results for activated RAW264.7 cells in the closed chamber (Table 2) are comparable to the previously reported values of kg = 4.9 ± 0.6 pmol s−1 (106 cells)−1, kc = 0.6 ± 0.8 s−1, ks = 0.32 ± 0.07 pmol s−1 (106 cells)−1, Rmax = 108 ± 17 pmol s−1 (106 cells)−1 (19), although there are some differences. The present rate of NO generation (kg) and maximum rate of respiration (Rmax) are each about one-half the previous values, and the rate constant for NO consumption (kc) is about twice that reported before, whereas the cellular rate of O2− generation (ks) is nearly identical to the previous value. The maximum NO concentrations generated by RAW264.7 cells in the closed chamber (0.2 – 0.4 µM) were also very similar to those found before (19). With one-half the rate of NO synthesis and the same rate of O2− generation, the present O2−/NO synthesis ratio is twice the previous value (0.15 vs. 0.07). The numerical differences aside, the present results confirm that NO is synthesized by RAW264.7 cells in large excess relative to O2−.
Respiration in unactivated RAW264.7 cells was similar to that in BMDM and HCT116 cells. The O2 consumption rates for each equaled Rmaxo, the measured values of that parameter (Table 2) being within the range reported for other mammalian cells (39, 40). Also consistent with findings for other cells is that the rate of O2 consumption in the absence of NO was constant (39, 40), as exemplified by the linear plot for unactivated cells in Figure 2. Activation of macrophages (both RAW264.7 and BMDM) did not change their actual rates of O2 consumption, an approximate doubling of the maximum rate of respiration (Rmax, Table 2) compensating almost exactly for the inhibitory effects of NO, once NO had reached its final concentration. The remarkable constancy of total O2 consumption suggests that iNOS expression may be linked to the respiratory needs of the cell. That is, the rate of NO synthesis, and therefore the NO concentration, may be capped at a level which does not depress the respiration rate below that of an unactivated cell. The increase in Rmzx in NO-producing cells may be another part of the same control system.
Despite the added demand for O2 associated with NO synthesis and NO consumption, O2 consumption by activated macrophages remained mostly respiratory. Assuming CO2 = 200 µM and CNO = 0.5 µM, which are representative of concentrations measured in the closed chamber experiments, the NO synthesis and consumption terms in Equation 4 are calculated to account for only about 10% of total O2 consumption. Consistent with this is that addition of a respiratory inhibitor (rotenone) to activated macrophage cultures reduced the rate of O2 consumption by 85–90%.
It is possible that lower, physiological O2 concentrations could affect iNOS gene expression and thus the rates of NO synthesis reported here, but the available findings concerning O2 are contradictory. Otto and Baumgardner (41) determined that iNOS activity was directly proportional to the O2 partial pressure over a wide range, whereas Palmer et al. (42) found an inverse relationship between iNOS protein and PO2. McCormick et al. (43) reported that the amount of iNOS protein was unaffected by PO2. Thus, more data are needed to examine the possible effects of O2 on total iNOS activity.
The apparent rate constant for NO consumption by either type of activated macrophage (kc = 1.3 – 1.6 s−1, Table 2) is in the range of reported values for other cell types, including NH32 (0.1 s−1) and βTC3 cells (1.7 s−1) (44). As already mentioned, the mechanisms for intracellular NO consumption include its reaction with O2− (in competition with intracellular SOD), nitrogen dioxygenases, and oxygen-ligated reduced metals. In cells which express iNOS, another possibility is oxidation to NO3− after rebinding to the enzyme (45). Whatever the pathway(s) involved, consumption of NO by macrophages is only marginally important relative to NO synthesis. For instance, at CNO = 0.5 µM, the cellular rate of NO consumption by BMDM would be 0.5 pmol s−1 (106 cells)−1, which is about 20% of the rate for NO synthesis (kg, Table 2). At higher NO concentrations, or with higher values for kc, as is the case with colonic epithelial cells, NO consumption becomes a larger fraction of NO synthesis and therefore has more impact on net synthesis. The rate constant for NO consumption by HCT116 cells (kc, Table 2) is approximately double that of macrophages, and reported rates for another colonic epithelial cell line (Caco-2) are even higher (37.7 s−1) (46). As suggested by the values of kg for HCT116 in Tables 2 and 3, the rate of NO synthesis by epithelial cells can be similar to that of macrophages. Thus, depending on the value of kc, colonic epithelial cells may consume half or more of the NO that they synthesize.
Plots of NO and O2 concentrations (Figure 2) and superoxide synthesis (Figure 6) for BMDM activated with IFN-γ and LPS were nearly identical to those of activated RAW264.7 cells (not shown). Accordingly, the NO, O2, and O2− synthesis and consumption rates for primary bone marrow-derived macrophages and RAW264.7 cells (Table 2) were statistically indistinguishable. This similarity in cellular kinetics is consistent with early work showing comparable rates of NO2− and NO3− formation in primary peritoneal macrophages and macrophage cell lines (47). The nearly identical kinetic behavior of RAW264.7 cells and primary macrophages confirms the suitability of the former for in vitro NO toxicity studies. In other words, co-culture of target cells with RAW264.7 should yield realistic concentrations of reactive nitrogen species. It should be noted, however, that RAW264.7 cells are not identical to primary macrophages in all respects; for instance, LPS-treated RAW264.7 cells produce much greater quantities of TNF-α than resident peritoneal macrophages (48).
Monitoring closed-chamber NO and O2 concentrations for HCT116 cells treated with capsaicin and resveratrol (as in Figure 3) demonstrated that colonic epithelial cells are capable of synthesizing NO at rates comparable to macrophages, quantified the ability of this cell line to consume NO, and confirmed the expected inhibitory effect of NO on respiration. To create conditions that would more closely mimic intestinal inflammation, additional experiments were performed in which HCT116 cells were infected with H. cinaedi. Due to potential contamination of the NO electrode by the bacteria, rates of NO synthesis by infected cells were calculated from NO2− concentrations measured by Griess assays in an open system. At a bacterium-to-mammalian cell ratio (MOI) of 100, NO2− concentrations measured for HCT116 cells were four-fold higher than with uninfected HCT116 cells (Figure 4). Samples infected at MOI = 10 did not show increased NO2− accumulation, indicating that a minimum cell number ratio is needed to stimulate NO production by epithelial cells. The use of killed H. cinaedi in the infection protocol did not induce NO production in HCT116 cells (data not shown), suggesting that viable, motile bacteria are necessary.
Without IFN-γ and TNF-α pretreatment, live H. cinaedi infection did not elicit NO synthesis from HCT116 cells, indicating that one or more signaling components must be recruited in advance of bacterial exposure. The toll-like receptors (TLRs) serve as receptors for various microbial products; in particular, TLR4 functions as the main receptor for LPS from gram-negative bacteria and thus is required for effective LPS signal transduction. HCT116 cells have been shown to be non-responsive to LPS stimulation in terms of IL-8 production largely because of their limited TLR4 expression (49). Consequently, HCT116 cells may respond to LPS from H. cinaedi through a mechanism independent of surface expression of TLR4. In a murine small-intestinal epithelial cell line, Hornef et al. found TLR4 in the Golgi apparatus which colocalized with internalized LPS (50). Thus the combination of TNF-α/IFN-γ pretreatment and live, motile bacteria may augment the incorporation of LPS into cytoplasm in HCT116 cells, which can provide signals for NO production through intracellular TLR4 complexes. Additional studies are needed to clarify the mechanisms by which bacteria induce NO production in colonic epithelial cells during infection.
Infection with H. cinaedi also elicited NO production by macrophages. The rates of NO synthesis for infected RAW264.7 cells were remarkably similar to those obtained by using just IFN-γ and LPS to activate the macrophages in the open system (kg, Table 3). Also, the rates for RAW264.7 cells activated with IFN-γ and LPS were found to be the same in the open system as in the closed chamber (kg, Tables 2 and 3). Although NO2− accumulation after 24 h was nearly identical for infected RAW264.7 cells and infected HCT116 cells (Figure 6), the rate of NO synthesis by the latter was calculated to be about 50% greater than by the former. This is a reflection of the higher rate constant for NO consumption in HCT116 cells, measured in separate experiments (kc, Table 2). Previous estimates of epithelial NO synthesis rates span 3–4 orders of magnitude, ranging from 0.2 fmol s−1 (106 cells)−1 to 0.9 pmol s−1 (106 cells)−1 (9, 8). Our rate of 3.9 pmol s−1 (106 cells)−1 for infected HCT116 cells is much higher than any of the reported values. Contributing to this is that our open-system calculations accounted for NO loss to the headspace, which is commonly neglected. As shown in Table 3, the fraction of NO lost was calculated to range from 30% to 80% of that synthesized, depending on the liquid depth and cell type. The cell type is influential because cellular consumption of NO combines with extracellular autoxidation to compete with NO diffusion to the headspace. If the reaction-diffusion model had not been used to correct for NO losses, the rates of NO production in the open-system experiments would have been underestimated by as much as five-fold. The excellent agreement found between the closed-chamber and open-system values of kg for RAW264.7 cells provides confidence in the corrections made for NO loss.
A computational model designed to predict NO concentrations in a colonic crypt during inflammation was developed previously (6). By using differential equations to describe rates of diffusion and reaction of NO and O2 in an idealized representation of the crypt anatomy, concentrations are obtained as a function of position. In addition to the crypt shape and dimensions, key inputs in that model are the rates of NO synthesis and consumption by macrophages and epithelial cells. The rate constants for NO synthesis and consumption by BMDM and HCT116 cells in Tables 2 and 3 yield the results shown in Figure 7, in which the NO concentration is plotted as a function of height above the base of a crypt. Three cases are considered: (i) NO synthesis by macrophages only; (ii) NO synthesis by both macrophages and epithelial cells, using for the latter the capsaicin-resveratrol kg for HCT116 cells; and (iii) NO synthesis by both cell types, using for epithelial cells the kg for infected HCT116 cells. The localization of macrophages near the base of the crypt causes the NO concentration to be largest there, whether or not there is epithelial synthesis of NO. The greater the total rate of NO synthesis (macrophage plus epithelial), the larger the maximum NO concentration, which ranges from 0.11 µM to 0.21 µM for the three cases. Because epithelial cells line the entire crypt, their synthesis of NO tends to cause elevated NO concentrations to persist even in the upper part.
Figure 7.
Predicted NO concentrations in an inflamed colonic crypt. Concentrations at the crypt center are plotted as a function of height above the base. The calculations assumed that kc = 1.3 s−1 for macrophages and 4.1 s−1 for epithelial cells, based on the BMDM and HCT116 data in Table 2. The rate of NO synthesis used for macrophages was 2.3 pmol s−1 (106 cells)−1 (Table 2); using the cell volume (v) in Table 1, this corresponds to a volumetric rate of 2.4 µM/s. Results are shown for three assumed rates of NO synthesis by epithelial cells: 0, 1.2, and 4.2 µM/s. The latter values correspond to the HCT116 data for capsaicin-resveratrol in Table 2 and H. cinaedi in Table 3, respectively, again using v from Table 1. All other parameter values were as given in Chin et al (2008).
Even using the highest measured rates of epithelial NO synthesis (corresponding to infected HCT116 cells), the concentrations in Figure 7 are below the cytotoxic thresholds found when NO concentrations have been precisely controlled. The minimum NO concentration at which loss of viability was detected in human lymphoblastoid (TK6) cells was 0.5 µM (51, 52), and other cell types, including HCT116, have been shown to be more resistant to NO (53). Thus, even if epithelial cells are as sensitive to NO as TK6 cells, our model predicts that they will remain viable. The viability of epithelial cells may be compromised more by NO in vivo; due to reversible binding to cytochrome oxidase, even low (nM) concentrations of NO are sufficient to inhibit electron transport at physiological O2 levels (54). In whatever fraction of cells remains viable in a colonic crypt, exposure to NO and related reactive nitrogen species may initiate a mutagenic change that causes irregular proliferative behavior and ultimately leads to cancer. The concentration estimates for colonic crypts that we have obtained from cellular kinetic measurements and modeling provide guidance in selecting experimental conditions that should be useful in elucidating the molecular biological linkage between NO exposure and carcinogenesis in IBD.
Acknowledgments
We thank Dr. James Fox and Dr. Gerald Wogan for their helpful advice. Laura Trudel and Nisanart Charoenlap assisted with the mammalian cell culture and bacterial culture experiments, respectively. This work was supported by a grant from the National Cancer Institute (PO1 CA26731). MPC was supported in part by a graduate student fellowship from the National Science Foundation.
References
- 1.Tamir S, Tannenbaum SR. The role of nitric oxide in the carcinogenic process. Biochim Biophys Acta. 1996;1288:31–36. doi: 10.1016/0304-419x(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 2.Levin B. Ulcerative colitis and colon cancer: biology and surveillance. J Cell Biochem Suppl. 1993;16G:47–50. doi: 10.1002/jcb.240501109. [DOI] [PubMed] [Google Scholar]
- 3.Farrell RJ, Peppercorn MA. Ulcerative colitis. Lancet. 2002;359:331–340. doi: 10.1016/S0140-6736(02)07499-8. [DOI] [PubMed] [Google Scholar]
- 4.Singer I, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H, Hokari R, Miura S, Shigetmatsu T, Hirokawa M, Akiba Y, Kurose I, Higuchi H, Fujimori H, Tsuzuki Y, Serizawa H, Ishii H. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180–187. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin MP, Schauer DB, Deen WM. Prediction of nitric oxide concentrations in the colonic crypt during inflammation. Nitric Oxide. 2008;19:266–275. doi: 10.1016/j.niox.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Lim CH, Dedon PC, Deen WM. Kinetic analysis of intracellular concentrations of reactive nitrogen species. Chem Res Toxicol. 2008;21:2134–2147. doi: 10.1021/tx800213b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witthoft T, Eckmann L, Kim JM, Kagnoff MF. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am J Physiol Gastrointest Liver Physiol. 1998;275:G564–G571. doi: 10.1152/ajpgi.1998.275.3.G564. [DOI] [PubMed] [Google Scholar]
- 9.Wright K, Kolios G, Westwick J, Ward SG. Cytokine-induced apoptosis in epithelial HT-29 cells is independent of nitric oxide formation. J Bio Chem. 1999;274:17193–17201. doi: 10.1074/jbc.274.24.17193. [DOI] [PubMed] [Google Scholar]
- 10.Chen B, Deen WM. Effect of liquid depth on the synthesis and oxidation of nitric oxide in macrophage cultures. Chem Res Toxicol. 2002;15:490–496. doi: 10.1021/tx010126p. [DOI] [PubMed] [Google Scholar]
- 11.Lewis RS, Deen WM. Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions. Chem Res Toxiol. 1994;7:568–574. doi: 10.1021/tx00040a013. [DOI] [PubMed] [Google Scholar]
- 12.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 13.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 14.Lymar SV, Hurst JK. Physiological catalyst for peroxynitrite-mediated cellular damage or cellular protectant? Chem Res Toxicol. 1996;9:845–850. doi: 10.1021/tx960046z. [DOI] [PubMed] [Google Scholar]
- 15.Babior BM. The respiratory burst of phagocytes. J Clin Invest. 1984;73:599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berton G, Dusi S, Bellavite P. The respiratory burst of phagocytes. In: Sbarra AJ, Strauss RR, editors. The Respiratory Burst and Its Physiological Significance. New York: Plenum Press; 1988. pp. 33–52. [Google Scholar]
- 17.Keynes RG, Griffiths C, Garthwaite J. Superoxide-dependent consumption of nitric oxide in biological media may confound in vitro experiments. Biochem J. 2003;369:399–406. doi: 10.1042/BJ20020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B, Deen WM. Analysis of the effects of cell spacing and liquid depth on nitric oxide and its oxidation products in cell cultures. Chem Res Toxicol. 2001;14:135–147. doi: 10.1021/tx000164t. [DOI] [PubMed] [Google Scholar]
- 19.Nalwaya N, Deen WM. Nitric oxide, oxygen, and superoxide formation and consumption in macrophage cultures. Chem Res Toxicol. 2005;18:486–493. doi: 10.1021/tx049879c. [DOI] [PubMed] [Google Scholar]
- 20.Nalwaya N, Deen WM. Analysis of cellular exposure to peroxynitrite in suspension cultures. Chem Res Toxicol. 2003;16:920–932. doi: 10.1021/tx025664w. [DOI] [PubMed] [Google Scholar]
- 21.Gardner PR, Gardner AM, Martin LA. Nitric oxide dioxygenase activity and function of flavohemoglobins. J Biol Chem. 2000;275:31581–31587. doi: 10.1074/jbc.M004141200. [DOI] [PubMed] [Google Scholar]
- 22.Eich RF, Li TS, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 23.Shen Z, Feng Y, Rogers AB, Rickman B, Whary MT, Xu S, Clapp KM, Boutin SR, Fox JG. Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice. Infection and Immunity. 2009;77:2508–2516. doi: 10.1128/IAI.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holian O, Wahid S, Atten MJ, Attar BM. Inhibition of gastric cancer cell proliferation by resveratrol: role of nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2002;282:G809–G816. doi: 10.1152/ajpgi.00193.2001. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Zhang Y, Ro JY. Involvement of neuronal, inducible and endothelial nitric oxide synthases in capsaicin-induced muscle hypersensitivity. European Journal of Pain. 2008;13:924–928. doi: 10.1016/j.ejpain.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomczak MF, Erdman SE, Poutahidis T, Rogers AB, Holcombe H, Plank B, Fox JG, Horwitz BH. NF-κβ is required within the innate immune system to inhibit microflora-induced colitis and expression of IL-12 p40. J. Immunol. 2003;171:1484–1492. doi: 10.4049/jimmunol.171.3.1484. [DOI] [PubMed] [Google Scholar]
- 27.Lewis RS, Tamir S, Tannenbaum SR, Deen WM. Kinetic Analysis of the Fate of Nitric Oxide Synthesized by Macrophages in Vitro. J. Biol. Chem. 1995;270:29350–29355. doi: 10.1074/jbc.270.49.29350. [DOI] [PubMed] [Google Scholar]
- 28.Deen WM, Tannenbaum SR, Beckman JS. Protein tyrosine nitration and peroxynitrites. Comment FASEB J. 2002;16:1144–1144. doi: 10.1096/fj.02-0133lte. [DOI] [PubMed] [Google Scholar]
- 29.Massey V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959;35:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- 30.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 31.Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 32.Galle J, Hoffmann M, Aust G. From single cells to tissue architecture – a bottom-up approach to modeling the spatio-temporal organization of complex multicellular systems. J Math Biol. 2009;58:261–283. doi: 10.1007/s00285-008-0172-4. [DOI] [PubMed] [Google Scholar]
- 33.Swietach P, Patiara S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem. 2009;284:20299–20310. doi: 10.1074/jbc.M109.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalwaya N, Deen WM. Analysis of effects of nitric oxide and oxygen on nitric oxide production by macrophages. J Theor Biol. 2004;226:409–419. doi: 10.1016/j.jtbi.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Zacharia IG, Deen WM. Diffusivity and solubility of nitric oxide in water and saline. Ann. Biomed. Eng. 2004;33:214–222. doi: 10.1007/s10439-005-8980-9. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Deen WM. Effect of liquid depth on the synthesis and oxidation of nitric oxide in macrophage cultures. Chem Res Toxicol. 2002;15:490–496. doi: 10.1021/tx010126p. [DOI] [PubMed] [Google Scholar]
- 37.Goldstick TK, Fatt I. Diffusion of oxygen in solutions of blood proteins. Chem. Eng. Prog. Symposium Ser. 1970;99:101–107. [Google Scholar]
- 38.Sasada M, Pabst MJ, Johnston RB. Activation of mouse peritoneal macrophages by lipopolysaccharide alters the kinetic parameters of the superoxide-producing NADPH oxidase. J Biol Chem. 1983;258:9631–9635. [PubMed] [Google Scholar]
- 39.Yamada T, Yang JJ, Ricchiuti NV, Seraydarian MW. Oxygen consumption of mammalian myocardial cells in culture: Measurements in beating cells attached to the substrate of the culture dish. Anal Biochem. 1985;145:302–307. doi: 10.1016/0003-2697(85)90365-3. [DOI] [PubMed] [Google Scholar]
- 40.Balis UJ, Behnia K, Dwarakanath B, Bhatia SN, Sullivan SJ, Yarmush ML, Toner M. Oxygen consumption characteristics of porcine hepatocytes. Metab Eng. 1999;1:49–62. doi: 10.1006/mben.1998.0105. [DOI] [PubMed] [Google Scholar]
- 41.Otto CM, Baumgardner JE. Effect of culture PO2 on macrophage (RAW 264.7) nitric oxide production. Am. J. Physiol. 2001;280:C280–C287. doi: 10.1152/ajpcell.2001.280.2.C280. [DOI] [PubMed] [Google Scholar]
- 42.Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type ii nos gene expression in pulmonary artery endothelial cells via hif-1. Am. J. Physiol. 1998;274:L212–L219. doi: 10.1152/ajplung.1998.274.2.L212. [DOI] [PubMed] [Google Scholar]
- 43.McCormick CG, Li WP, Calero M. Oxygen tension limits nitric oxide synthesis by activated macrophages. Biochem. J. 2000;350:709–716. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C. Controlled delivery of nitric oxide for cytotoxicity studies, Ph. D. Thesis. MIT; 2003. [Google Scholar]
- 45.Santolini J, Meade AL, Stuehr DJ. Differences in three kinetic parameters underpin the unique catalytic profiles of nitric-oxide synthases i, ii, and iii. J. Biol. Chem. 2001;276:48887–48898. doi: 10.1074/jbc.M108666200. [DOI] [PubMed] [Google Scholar]
- 46.Gardner PR, Martin LA, Hall D, Gardner AM. Dioxygen-dependent metabolism of nitric oxide in mammalian cells. Free Radic Biol Med. 2001;31:191–204. doi: 10.1016/s0891-5849(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 47.Stuehr DJ, Marletta MA. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Research. 1987;47:5590–5594. [PubMed] [Google Scholar]
- 48.Rouzer CA, Jacobs AT, Nirodi CS, Kinglsey PJ, Morrow JD, Marnette LJ. RAW264.7 cells lack prostaglandin-dependent autoregulation of tumor necrosis factor-a secretion. J Lipid Research. 2005;46:1027–1037. doi: 10.1194/jlr.M500006-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular toll-like receptor 4-MD-2 complex. Infect Immun. 2001;71:3503–3511. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Trudel L, Wogan GN, Deen WM. Thresholds of nitric oxide-mediated toxicity in human lymphoblastoid cells. Chem Res Toxicol. 2003;16:1004–1013. doi: 10.1021/tx0340448. [DOI] [PubMed] [Google Scholar]
- 52.Li Q, Pang B, Kiziltepe T, Trudel L, Engelward BP, Dedon P, Wogan GN. Threshold effects of nitric oxide-induced toxicity and cellular responses in wild-type and p53-null human lymphoblastoid cells. Chem Res Toxicol. 2006;19:399–406. doi: 10.1021/tx050283e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, Kim MY, Godoy LC, Thiantanawat A, Trudel LJ, Wogan GN. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc Natl Acad Sci. 2009;106:14547–14551. doi: 10.1073/pnas.0907539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;63:910–916. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]