Abstract
Early life stress has effects on behavior and stress reactivity which are linked to enhanced sensitivity to stimulants in rodents. The present study investigated whether rhesus monkeys that experienced early life stress would show altered sensitivity to the reinforcing effects of stimulants as compared to controls. Control (n=5) and maternally-separated (n=4) monkeys were trained to self-administer cocaine (0.1mg/kg/injection) under a second order schedule of i.v. drug delivery. The rate of acquisition and subsequent dose-effect determinations for cocaine (0.01-1.0 mg/kg/injection) and amphetamine (0.003-0.3 mg/kg/injection) provided complementary measures of reinforcing effectiveness. In addition, stimulant-induced increases in home cage activity and dopamine D2 receptor binding potential were quantified with positron emission tomography (PET) neuroimaging. Compared to controls, maternally-separated monkeys showed lower responding during the acquisition of self-administration and in the dose-response curves for both stimulants and significantly lower response rates during maintenance of cocaine self-administration. Maternally-separated monkeys also failed to exhibit stimulant–induced increases in motor activity. Groups did not differ in dopamine D2 receptor binding potential in the caudate nucleus or the putamen. Taken together the results of the present study do not provide support for early life stress leading to enhanced vulnerability to stimulant use in the nonhuman primate model employed.
Keywords: maternal separation, rhesus monkey, drug self-administration, psychostimulants, motor activity, dopamine
Introduction
Drug addiction is among the leading public health concerns in the United States. In 2007 it was estimated that 1.6 million Americans could be classified as dependent on or abusing cocaine (NSDUH, SAMHSA 2007). With no effective treatments currently available, much focus has been directed toward identifying vulnerability factors and developing prevention strategies. Epidemiological studies have shown that early life events can serve to define populations at high or low risk of drug abuse during adulthood (Dobkin et al., 1997). Clinical studies have also pointed to stress as being an important variable in the initiation and maintenance of cocaine self-administration and in triggering relapse (Johanson and Fischman 1989; Gawin 1991).
Early life stress has been shown to have profound, long lasting effects on behavior and hypothalamic-pituitary-adrenal (HPA) axis function in animal models. Periodic maternal separation has been used in both rodents and nonhuman primates as an animal model of early life stress. Studies in rodents undergoing maternal separation have shown conflicting results depending on the timing and duration of the separations (van Oers et al.,1998, 1999; Plotsky and Meaney 1993). Squirrel monkeys that have experienced periodic maternal separations in infancy show robust increases in cortisol following the separation challenges, but exhibit decreased stress response following a social isolation challenge at 2-3 years of age (Levine and Mody 2003). When tested 3-5 years after the separations, maternally-separated squirrel monkeys have also shown an enhanced sensitivity to glucocorticoid negative feedback (Lyons et al., 2000). Recent studies in the rhesus monkey have demonstrated that repeated maternal separations, as employed in the present study, produce increased cortisol release in response to acute separation challenges; the same monkeys showed a flattened diurnal cortisol rhythm when tested at a later age (Sanchez et al., 2005). Flattened diurnal cortisol rhythms later in life have also been seen in humans exposed to early life stressors (Gunnar and Vazquez 2001).
Studies in rodent models have consistently shown that acute exposure to stressors can enhance the acquisition of drug self-administration and can induce reinstatement of previously extinguished drug seeking behaviors (Goeders and Guerin 1994, 1996; Goeders 2002). Research examining repeated maternal separation as a stressor has also shown enhanced drug effects in rodents. Maternally-separated rats exhibited increased dopamine release in the nucleus accumbens in response to psychostimulant drug challenges (Hall et al., 1999) and these effects were long lasting and persisted into adulthood (Kehoe et al., 1996). Maternally-separated rats also exhibited greater acquisition of cocaine self-administration than control animals (Kosten et al., 2000). While these data implicate exposure to early life stress as a possible factor that increases the propensity to abuse psychostimulant drugs, it has not been explored outside of the rodent model. Research in the nonhuman primate is particularly lacking. Accordingly, the present study investigated whether rhesus monkeys that experienced early life stress associated with maternal separation would show altered sensitivity to the reinforcing effects of stimulants as compared to controls.
Methods
Subjects
A group of nine female rhesus monkeys (Macaca mulatta) comprised of 5 controls (RJj-7, RUj-7, RRq-7, RFj-7, RRk-8) and 4 maternally-separated monkeys (RMq-8, RWp-8, RZo-7, RJu-7) served as subjects. Three of the 5 control monkeys were involved in drug self-administration experiments due to catheter problems in subjects RFj-7 and RRk-8. Four of the 5 control monkeys were involved in imaging experiments due to complications with isoflurane anesthesia in subject RFj-7. Monkeys were housed in individual home cages with access to food (monkey chow, Ralston Purina, St. Louis, MO) and water between experimental sessions. Animal care procedures strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University.
Maternal separation paradigm
The group of maternally-separated monkeys experienced repeated, periodic maternal separations as infants, as described previously (Sanchez et al., 2005). Briefly, the infants were born in stable social groups consisting of 4-7 adult females and 1 adult male sire per group. These social groups were maintained in indoor/outdoor run-type facilities at the Yerkes National Primate Research Center Field Station. Maternal separations took place between 3 and 6 months postpartum and consisted of repeated separations of systematically varied durations (0.5, 3.0 and 6.0 hours) following a counterbalanced design. Separations occurred once per day between 08:00 and 11:00h (onset of separation was variable/unpredictable within this time window), 2-3 times per week, to yield a total of 36 separations per infant in 90 days. Separations were achieved by herding the entire social group into a small pre-capture area and then releasing individuals back into their home run through a capture tunnel. Every member of the social group was disturbed during separation, but only one animal (mother) was removed from the group at a time.
Surgery
Monkeys were implanted with chronic indwelling venous catheters under sterile surgical conditions, as described previously (Howell and Wilcox 2001). Preoperative antibiotics [Rocephin (ceftriaxone, 25 mg/kg i.m.)] were given on the day of the surgery to prevent infection. A silicone catheter (0.65 mm i.d., 1.75 mm o.d.; Access Technologies, Skokie, IL) was implanted under a combination of Telazol (tiletamine hydrochloride and zolazepam hydrochloride, Fort Dodge Animal Health, Fort Dodge, IA; 4.0 mg/kg i.m.) and isoflurane anesthesia, using aseptic techniques. The proximal end of the catheter was implanted into the femoral or external jugular vein and terminated at the vena cava above the right atrium, and the distal end was routed under the skin and attached to a subcutaneous vascular access port (Access Technologies) located in the center of the lower back. After surgery, the monkey was returned to its home cage and received Banamine (flunixin meglumine, 1.0 mg/kg i.m.) every 6 h for 24 h postoperatively to reduce pain and discomfort associated with surgery. Catheters were flushed daily with 1.0 ml of heparinized (100 U/ml) saline to maintain patency.
Apparatus
Monkeys were seated in a commercially available primate chair (Primate Products, Miami, FL) with a panel outfitted with a response lever and stimulus lights attached to the front of the chair. A Huber needle infusion set was inserted into the vascular access port. The polyvinyl chloride tubing of the infusion set was attached to a motor driven syringe pump (Harvard Apparatus PhD 2000) containing the drug solution located outside of the chamber (Med Associates, St. Albans, VT). The pump delivered a volume of 2 ml/infusion over 7s. Daily sessions took place in a ventilated, sound-attenuating chamber.
Drug self-administration
Monkeys were trained to respond on a second-order schedule of drug reinforcement. Responding was initiated using a 1-response fixed-ratio schedule (FR 1) such that each response in the presence of the red light produced an i.v. drug injection and a 15 s illumination of a white light. There was a 2 h limited hold in which the subject could complete the ratio requirement. The ratio value was increased gradually as responding increased, from the initial FR 1 to FR 2, FR 5, FR 10 and ultimately FR 20. When the schedule value reached FR 20, drug injections no longer followed the completion of each FR but instead followed an increasing number of FR components during an initial fixed interval (FI) duration of 30 s. A 2 s white light was presented upon completion of each FR 20 component. Once response criteria were met at this duration, the FI was increased to 60 s, and from that point on, increased in 60 s intervals until the FI duration reached 600 s. Ultimately, the schedule was a second-order schedule of FR 20 components, with drug injection following the first ratio requirement completed after 600 s had elapsed (FI 600 s [FR 20:S]). Monkeys had the opportunity to take 10 injections in each daily session and the unit dose of cocaine remained constant at 0.1 or 0.03 (RMq-8) mg/kg/infusion. All subjects progressed through the same acquisition paradigm with set criteria for moving on to the next level. The criterion for the initial phase of the training (FR 1) was set such that the monkeys were required to take 9 of 10 infusions for 5 consecutive sessions before the ratio requirement was increased. For subsequent FR and FI values the monkey had to take all 10 infusions for 3 consecutive sessions before schedule parameters were changed.
Following acquisition, subjects were maintained on the terminal schedule (FI 600 s [FR 20:S]). However, the limited hold was reduced to 300s and a 30s time out, in which the stimulus lights were extinguished and responding had no consequence, was scheduled after each component until behavior was stable (<20% variance in response rates with animals taking all 10 infusions) for 10 consecutive sessions. Responding was then extinguished by substituting saline for cocaine and eliminating the presentation of the drug-paired stimulus (white light) that accompanied both the completion of an FR and the drug infusion. Otherwise, extinction sessions were identical to those described for the terminal second-order schedule. Extinction sessions continued until responding decreased to <20% of the response rate maintained by cocaine self-administration for 3 consecutive sessions. Subsequently, cocaine-maintained responding was reinitiated on the second-order schedule, and the number of components within a session was reduced from 10 to 5. The unit dose of cocaine was then manipulated in random order to establish a dose-response curve that encompassed 0.01-1.0 mg/kg/infusion. Each dose was tested until responding was stable (<20% variance in response rates over 5 consecutive sessions). Upon completion of the dose-response function for cocaine, stable responding was re-established (<20% variance in response rates over 5 consecutive sessions) with the maintenance dose of cocaine. A dose-response curve for amphetamine was then established by manipulating the unit dose in random order to encompass 0.003-0.3 mg/kg/infusion. Each dose was tested until responding was stable.
Locomotor Activity
To quantify locomotor activity, the monkey's collars were outfitted with Actiwatch (Mini Mitter, Bend, OR) activity monitors for a period of one week. The Actiwatch contains an omni-directional sensor that records motion in all directions. Monkeys were anesthetized with Ketaset (ketamine hydrocholride Fort Dodge Animal Health, Fort Dodge, IA; 3-10.0 mg/kg i.m.) in order to attach the Actiwatch to the collar and were given the day to recover and acclimate to the change. Normal baseline activity was measured for the next two days. On the third day, an i.m. injection of cocaine (1.0 mg/kg), amphetamine (0.3 mg/kg) or saline was administered in the home cage and the locomotor response to the drug challenge was recorded. All injections were given at the same time of day (0900h Sunday) in order to minimize disturbances from routine animal care and maintenance. The monkeys were anesthetized with Ketaset (3-10.0 mg/kg i.m.) again and the Actiwatch was removed from the collar at the end of the week.
Radiochemistry
[18F]fallypride is a selective, high affinity D2/D3 PET radioligand that is readily taken up in the striatum and is rapidly cleared from the cerebellum (Christian et al. 2000). [18F]fallypride was synthesized according to published methods (Mukherjee et al. 1995). Briefly, the tosylate precursor (S)-N-[(1-Allyl-2-pyrrolidinyl) methyl]-5-(3-toluene-sulfonyloxypropyl)-2,3-dimethoxybenzamide was prepared following general procedures described previously (Mukherjee 1991). The yield of the DCC coupling reaction of 2,3-dimethoxy-5-(3-hydroxypropyl)benzoic acid and (S)-2-(aminomethyl)-N-allylpyrrolidine to provide the alcohol (S)-N-[(1-Allyl-2-pyrrolidinyl)methyl]-5-(3-hydroxy-propyl)-2,3-dimethoxybenzamine was moderate (Mukherjee et al. 1995). Compound (S)-N-[(1-Allyl-2-pyrrolidinyl) methyl]-5-(3-hydroxy-propyl)-2,3-dimethoxybenzamine was converted to the tosylate in 70% yield. Radiolabelling was carried out by nucleophillic displacement of the tosylate precursor by no-carrier-added 18F to provide [18F]fallypride (Mukherjee et al. 1995). Purification was carried out by reverse phase HPLC to yield radiochemically pure [18F]fallypride (Mukherjee et al. 1995).
PET Imaging
On the day of the PET study, monkeys were anesthetized in their home cage with Telazol (4.0 mg/kg) and transported to the Yerkes Imaging Center to be scanned on the MicroPET Focus scanner. Subjects were intubated and anesthesia was maintained with isoflurane (1-2%). Monkeys were positioned in the tomograph and a 10-20 minute transmission scan was obtained for attenuation detection. Once the transmission scan was complete and verified a slow bolus of approximately 6 mCi [18F]fallypride was injected over 5 min at a rate of 1.0 ml/min. A dynamic PET sequence was obtained over the 2 h following injection of the radioligand. At the conclusion of the scan, monkeys were returned to their home cage to recover.
Drugs
Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD) and d-amphetamine H2SO4 (Sigma Aldrich, St. Louis, MO) were dissolved in 0.9% saline, and all doses are reported as salts.
Data Analysis
For acquisition studies, the mean total number of responses for each monkey was averaged from the three days (5 days for the FR1) in which they met the criteria set for the schedule parameter. Mean rates of responding for the maintenance phase of cocaine self-administration were determined for each monkey by averaging the data from the last five days of the ten-day maintenance phase. Mean rates of responding for each dose in the dose-response functions were determined for each monkey by averaging the data from the five days in which responding was stable. Acquisition data were analyzed using a two-way analysis of variance (ANOVA) with repeated measures on one factor. Response rate data during maintenance, days to meet extinction criteria, and response rate data at the peak dose on each dose response function were analyzed using a two-tailed Student's t test for unpaired samples. Group comparisons of dose-response functions for cocaine and amphetamine data did not meet the assumptions required for t-tests or ANOVA (equal variance and normally distributed data) so the Mann-Whitney U test was used. For activity measures the Actiwatch was programmed to record activity with an epoch length of 15 sec. The order of the experimental sessions was randomized across monkeys. Drug-induced locomotor activity is represented as a % of the activity level during the same time period following a saline injection. Time-course of stimulant-induced activity is shown for the 60 minutes following the injection. Group comparisons of locomotor activity following stimulant challenge did not meet the assumptions required for t-tests or ANOVA (equal variance and normally distributed data) so the Mann-Whitney U test was used.
PET images were co-registered to a standard adult rhesus monkey MRI image. Regions of interest (ROIs) were manually drawn over the striatum (caudate and putamen) and cerebellum on the frames where these structures could be seen clearly. The cerebellum was used as a reference region (representing a concentration of free and non-specifically bound [18F]fallypride with negligible specific binding). The regions of interest were then transferred to all frames to obtain time activity curves (TACs) decay-corrected to time of [18F]fallypride injection. Logan analysis was used to determine binding potential (Logan et al.,1990, 1996). Binding potentials for the caudate nucleus and putamen were derived for each monkey. Group comparisons for dopamine D2 binding potential were analyzed using the two-tailed Student's t-test for unpaired samples.
For all comparisons, probability of significance was set at p<0.05. All statistical analyses were conducted using Sigma Stat 3.0 for Windows.
Results
Drug self-administration
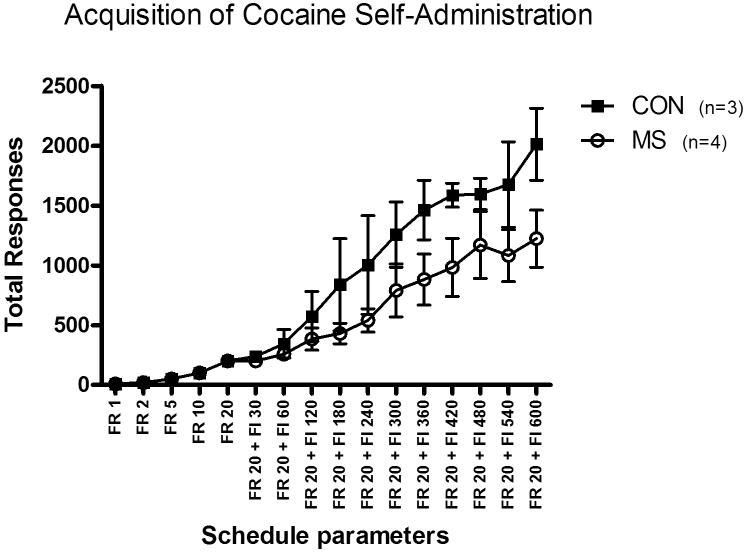
Rate of acquisition was determined by the number of days needed to meet specific acquisition criteria for each schedule parameter. After the initial FR 1 sessions, all subjects completed the criteria of the schedule condition (10 out of 10 infusions) in the minimal amount of time required (3 consecutive sessions). There was nosignificant group difference in rate of acquisition. However, maternally-separated monkeys consistently demonstrated less behavioral output as compared to control animals once the FI schedule was introduced. Figure 1 presents the mean total number of responses per session for each schedule condition during the acquisition phase. Due to the long limited hold during acquisition and variability in session length, response rate was not the appropriate dependent measure in this experiment so total number of responses was examined. No significant group difference was found for responding during acquisition (F(10,50)= 1.50, NS). Subsequently, subjects were maintained on the terminal schedule (FI 600 s [FR 20:S]) and these sessions continued until response rates were stable (<20% variance) for ten consecutive sessions. The mean response rate for the last five days of the maintenance phase was 0.35 ± 0.08 responses/sec for controls and 0.17 ± 0.02 responses/sec for maternally-separated monkeys and the group difference was statistically significant (t(5)=2.69 p=0.04). Following acquisition and maintenance of cocaine self-administration, subjects underwent a series of extinction sessions, which continued until responding was less than 20% of cocaine maintained responding for 3 consecutive sessions. On average, control monkeys reached extinction criteria in 10.7± 6.2 days while the average for experimental monkeys was 7.0± 1.5 days. The group difference was not statistically significant (t(5)=.67, NS). When response rates were stable, groups did not differ in their responding for saline.
Figure 1.
Mean total number of responses per session for each schedule condition by schedule parameter for control (n=3) and maternally-separated (n=4) monkeys during the acquisition of cocaine self-administration. No significant differences between groups were found for responding during acquisition of cocaine self-administration (F(10,50)=1.50, NS).
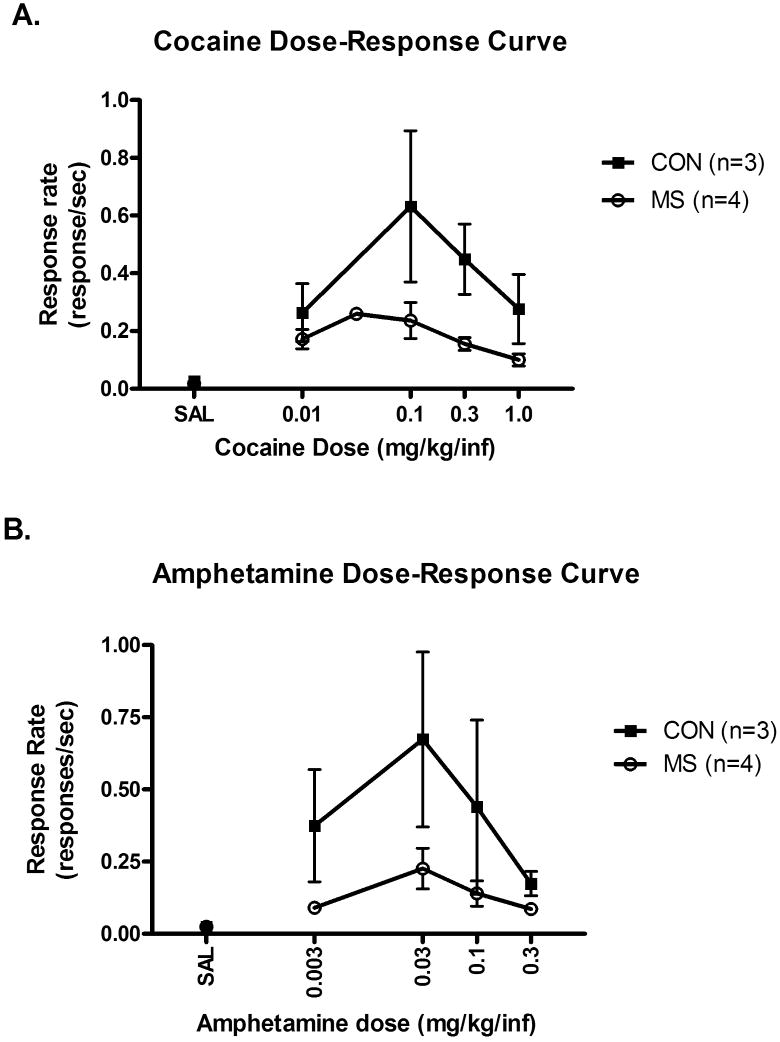
When a range of cocaine doses (0.01-1.0 mg/kg/injection) was substituted for the maintenance dose of cocaine, maternally-separated subjects demonstrated lower rates of responding at all doses tested compared to controls (Figure 2A). However, the dose that maintained peak rates of responding was the same in both groups. Control and maternally-separated groups differed significantly in responding across a range of doses of cocaine (Mann-Whitney U= 0, p=0.03). When a range of amphetamine doses (0.003-0.3 mg/kg/injection) was substituted for the maintenance dose of cocaine, maternally-separated monkeys demonstrated reduced rates of responding across all doses tested, compared to controls (Figure 2B). However, the dose that maintained peak rates of responding was the same for both groups. Control and maternally-separated groups showed a strong trend towards a difference in responding across a range of doses of amphetamine (Mann-Whitney U=1.0, p=0.06).
Figure 2.
(A) Doses of cocaine (0.01-1.0 mg/kg/injection) and (B) amphetamine (0.003-0.3 mg/kg/injection) were substituted for the maintenance dose of cocaine. Each dose was tested until response rates were stable for 5 consecutive sessions. Groups differed significantly in responding for a range of doses of cocaine (Mann-Whitney U=0, p=0.03) and there was a strong trend towards a difference in responding across a range of doses of amphetamine (Mann Whitney U=1.0, p=0.06).
Locomotor Activity
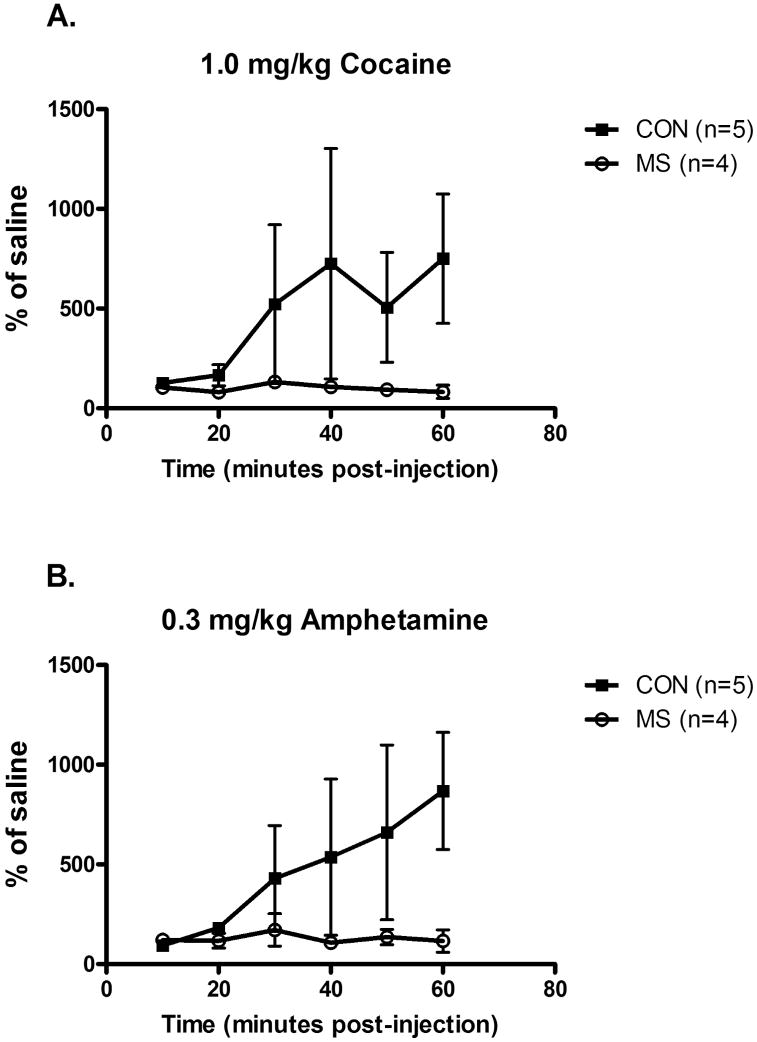
Gross motor activity was recorded following an i.m. injection of saline, cocaine (1.0 mg/kg) and amphetamine (0.3 mg/kg). Activity following drug injection was normalized to % of activity following saline injection (Figure 3). Maternally-separated animals showed smaller drug effects than controls. Control and maternally-separated groups differed significantly for cocaine-induced (Figure 3A) increases in motor activity (Mann- Whitney U=1.0, p=0.004) and there was a strong trend towards a difference for amphetamine-induced (Figure 3B) increases in motor activity (Mann-Whitney U=6.0, p=0.06). The onset of the increase in activity in control monkeys occurred approximately 30 minutes post-injection, reached peak levels within 60 minutes (Figure 3A,B) and returned to baseline by 120 minutes post-injection (data not shown).
Figure 3.
Gross motor activity following injection of (A) cocaine (1.0 mg/kg) or (B) amphetamine (0.3 mg/kg) was measured and data normalized to a % of motor activity following saline injection. Control and maternally-separated groups differed significantly in cocaine-induced increases in motor activity (Mann Whitney U=1.0, p=0.004) and there was a strong trend towards a difference for amphetamine-induced increases in motor activity (Mann Whitney U=6.0, p=0.06).
Dopamine D2 Receptor Binding Potential
PET imaging with the dopamine D2 receptor radioligand [18F]fallypride was used to determine dopamine D2 receptor binding potential in the caudate nucleus and putamen of each monkey. Mean binding potential in the caudate nucleus for controls was 47.99 ± 7.38 and 50.29 ± 3.48 for maternally-separated monkeys. In the putamen, mean binding potential for controls was 42.92 ± 6.20 and 46.09 ± 3.04 for maternally-separated monkeys. These differences were not statistically significant (caudate t(6)=0.28, p=0.78; putamen t(6)=0.46, p=0.66).
Discussion
Early life stress has been shown to have profound effects on the HPA axis and stress reactivity. A large body of literature derived from rodent studies has also shown that stressors early in life can lead to an increase in self-administration of psychostimulants and other drugs of abuse. The current set of experiments determined whether early life stress in nonhuman primates influenced the sensitivity to cocaine and amphetamine, including the propensity to self-administer each drug. Measures of D2 receptor binding potential with PET imaging did not reveal group differences. However, the behavioral effects of these psychostimulants were blunted in maternally-separated monkeys compared to controls. These results do not provide support for early life stress leading to enhanced vulnerability to stimulant use in the nonhuman primate model employed.
All monkeys acquired cocaine self-administration at the same rate, as they all met the acquisition criteria in the minimum amount of time required. However, the maternally-separated monkeys showed less behavioral output throughout the acquisition of cocaine self-administration as compared to controls. This reduced behavioral output was also seen during the maintenance phase of cocaine self-administration. Maternally-separated monkeys exhibited significantly lower rates of responding as compared with controls during the last 5 days of the 10 day self-administration maintenance period. When saline was substituted for cocaine and the cocaine-paired stimuli were removed in extinction sessions, there was no significant group difference in the average number of days to meet extinction criteria. When a range of drug doses of cocaine and amphetamine was substituted for the maintenance dose of cocaine, maternally-separated monkeys demonstrated reduced behavioral output and response rates across drug doses for both cocaine and amphetamine compared to controls. Hence, although maternally-separated monkeys did not differ significantly from controls on measures of acquisition and extinction, they consistently demonstrated reduced behavioral output during drug self-administration as compared to control monkeys, and they did not appear to be sensitive to changes in the unit dose of cocaine or amphetamine. In addition, control monkeys exhibited stimulant-induced increases in motor activity but maternally-separated monkeys did not, with activity in the latter group not increasing beyond that induced by an injection of saline. This lack of sensitivity to the motor effects of cocaine substantiates the attenuated responding found in the maternally-separated monkeys during drug self-administration and the lack of sensitivity to a change in unit dose for both cocaine and amphetamine. In fact, the results are consistent with an extensive literature showing a close correspondence between the behavioral-stimulant and reinforcing effects of psychomotor stimulants. It is reasonable to speculate that the group differences in reinforcing effectiveness observed in the current study were due, in part, to differences in sensitivity to the motor effects of cocaine and amphetamine.
Both the reinforcing and motor stimulating effects of psychostimulants have been attributed to their actions on dopamine. Given the diminished reinforcing and motor effects found in the maternally-separated monkeys, it is possible that their dopaminergic system is altered in some way. Therefore additional studies specifically designed to examine the dopamine system were performed in these animals in an attempt to elucidate the source of the blunted reinforcing and motor stimulating effects of psychostimulants.
Dopamine D2 receptors are thought to be involved in both the reinforcing effects (Volkow et al., 1999; Morgan et al., 2002; Nader et al., 2006) and motor stimulating effects (Baker et al., 1996) of cocaine and amphetamine. There appears to be an inverse relationship between D2 receptor availability and sensitivity to stimulant reinforcement. In humans, levels of dopamine D2 receptors were found to be associated with the subjective responses to methylphenidate (Volkow et al., 1999). In cynomolgus monkeys, dominant animals, with high levels of D2 receptors showed very little cocaine self-administration behavior and lower cocaine intake as compared to subordinate animals with lower D2 levels (Morgan et al., 2002). To assess whether differences in D2 receptors could explain the differences seen in drug sensitivity in the present study, PET imaging with the dopamine D2 receptor radioligand [18F]fallypride was used. Control and maternally-separated monkeys did not differ in dopamine D2 receptor binding potential in the caudate nucleus or the putamen. Therefore, D2 receptor levels cannot explain the group differences seen in behavioral output during acquisition of cocaine self-administration, in response rates during maintenance and across a range of doses of cocaine or amphetamine, or in drug-induced increases in locomotor activity.
Although the majority of literature derived from rodent studies has reported enhanced sensitivity to psychostimulants following early life stress, the results of the present study do not support an increase in sensitivity to cocaine or amphetamine in rhesus monkeys that underwent maternal separation. Maternally-separated monkeys showed a reduced behavioral output during the maintenance phase of self-administration and in response to a wide range of doses of both cocaine and amphetamine. One interpretation of the reduced behavioral output is that the maternally-separated group was less sensitive to psychostimulants. The blunted response to the locomotor-stimulant effects of cocaine and amphetamine is consistent with the latter interpretation. However the maternally-separated monkeys showed no increase in responding at the higher end of the dose range for cocaine and amphetamine, as would be expected if they had a reduced sensitivity to psychostimulants. Alternatively, these monkeys may have a reduced motivation that is leading to the overall lower behavioral output on all measures. It has been suggested that vertical downward shifts in the dose-response function predict a lower motivation to self-administer the drug (Piazza et al., 2000). Testing the maternally-separated monkeys on a progressive ratio schedule might provide more direct evidence of the reinforcing efficacy of cocaine and amphetamine. Operant tasks with alternative reinforcers may be another way to target the reinforcing efficacy of psychostimulants in this group of monkeys.
Reinforcing effectiveness is thought to be mediated to some extent by the level of dopamine D2 receptors (Volkow et al., 1999; Morgan et al., 2002; Nader et al., 2006). However, there were no differences in D2 receptor levels between control and maternally-separated monkeys so this cannot explain the differences in reinforcing effects seen in self-administration studies. The PET imaging studies were performed after the monkeys had participated in drug self-administration experiments, so it is possible that inherent differences in D2 levels did exist and were in some way normalized by stimulant exposure. Nader et al., (2006) demonstrated that cocaine self-administration decreased dopamine D2 receptor availability in rhesus monkeys. That study also found a marked inverse relationship between D2 receptor availability in drug-naive rhesus monkeys and future rates of cocaine self-administration, suggesting that low D2 receptor availability is a predisposing trait to cocaine abuse (Nader et al., 2006). Therefore, future experiments would benefit from the determination of dopamine D2 levels in drug-naive monkeys prior to drug self-administration experiments. Future studies would also benefit from examination of other aspects of the dopamine system, as well as other neurotransmitter systems such as serotonin and norepinephrine which have also been implicated in the effects of psychostimulants.
Studies demonstrating a link between early life stress and increased sensitivity to psychostimulants have primarily used rodent models. However, the present study utilized a stress and testing paradigm that is quite different from those commonly described in the literature derived from rodent studies. Rodents are typically only a few months old when tested for acquisition of drug self-administration, and thus only a few months removed from the stressors. In the present study, the monkeys were 5-6 years removed from the maternal separations when the acquisition of cocaine self-administration sessions began. Therefore, any differences observed in sensitivity to stimulants would be related to enduring changes in neurobiology and it is possible that whatever effects maternal separation may have had on these monkeys in the short-term have normalized with the passage of time.
Substantial individual differences were seen in responding during acquisition of cocaine self-administration and in dose-response functions for both cocaine and amphetamine, particularly in the control group. It is possible that group differences may have been overshadowed by this individual variability given the small group sizes. Another limitation in the present study was the use of only female subjects. As a result, effects of gender or the interaction of rearing and gender on self-administration of psychostimulants were not evaluated.
Overall, significant differences between maternally-separated monkeys and controls were not observed in the rate of acquisition for cocaine self-administration or in days to reach extinction criteria. However, rates of responding during the maintenance phase of self-administration were significantly lower in maternally-separated monkeys. Maternally-separated monkeys also consistently showed reduced behavioral output as compared to controls across the range of doses tested for cocaine and amphetamine, and failed to exhibit stimulant-induced motor activity to cocaine or amphetamine injection. However, these differences cannot be explained by dopamine D2 receptor binding potential in the caudate nucleus or the putamen. Collectively, these data do not provide support for early life stress, in the form of maternal separation, increasing sensitivity or vulnerability to the reinforcing effects of psychostimulants in the nonhuman primate model employed.
Acknowledgments
The authors would like to thank Lisa Neidert, Carol Nichols and Juliet Brown for their technical support.
This research was supported by U.S. Public Health Service grants MH058922, DA10344, DA00517 and RR00165 (Division of Research Resources, National Institutes of Health).
References
- Baker D, Khroyan T, O'Dell L, Fuchs R, Neisewander J. Differential effects of intra-accumbens sulpiride on cocaine-induced locomotion and conditioned place preference. J Pharmacol Exp Ther. 1996;279:392–401. [PubMed] [Google Scholar]
- Christian B, Narayanan T, Shi B, Mukherjee J. Quantitation of striatal and extrastriatal D-2 dopamine receptors using PET imaging of [(18)F]fallypride in nonhuman primates. Synapse. 2000;38:71–79. doi: 10.1002/1098-2396(200010)38:1<71::AID-SYN8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dobkin P, Tremblay R, Sacchitelle C. Predicting boys' early-onset substance abuse from father's alcoholism, son's disruptiveness, and mother's parenting behavior. J Consult Clin Psychol. 1997;65:86–92. doi: 10.1037//0022-006x.65.1.86. [DOI] [PubMed] [Google Scholar]
- Gawin F. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Goeders N. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders N, Guerin G. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Goeders N, Guerin G. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res. 1996;722:145–152. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hall F, Wilkinson L, Humby T, Robbins T. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Howell L, Wilcox K. Intravenous drug self-administration in nonhuman primates. In: Buccafusco J, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton FL: CRC Press; 2001. pp. 91–110. [Google Scholar]
- Johanson C, Fischman M. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Kehoe P, Shoemaker W, Triano L, Hoffman J, Arons C. Repeated isolation in the neonatal rat produces alterations in behavior and ventral striatal dopamine release in the juvenile after amphetamine challenge. Behav Neurosci. 1996;110:1435–1444. doi: 10.1037//0735-7044.110.6.1435. [DOI] [PubMed] [Google Scholar]
- Kosten T, Miserendino M, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neuroscience and biobehavioral reviews. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler J, Volkow N, Wolf A, Dewey S, Schlyer D, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler J, Volkow N, Wang G, Ding Y, Alexoff D. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lyons D, Yang C, Mobley B, Nickerson J, Schatzberg A. Early environmental regulation of glucocorticoid feedback sensitivity in young adult monkeys. Journal of neuroendocrinology. 2000;12:723–728. doi: 10.1046/j.1365-2826.2000.00505.x. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant K, Gage H, Mach R, Kaplan J, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader M, Morgan D, Gage H, Nader S, Calhoun T, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health. Substance Abuse and Mental Health Service Administration. 2007 www.DrugAbuseStatistics.samhsa.gov. [PubMed]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky P, Meaney M. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Noble P, Lyon C, Plotsky P, Davis M, Nemeroff C, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- van Oers H, de Kloet E, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res Dev Brain Res. 1998;111:245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- van Oers H, de Kloet E, Levine S. Persistent effects of maternal deprivation on HPA regulation can be reversed by feeding and stroking, but not by dexamethasone. Journal of neuroendocrinology. 1999;11:581–588. doi: 10.1046/j.1365-2826.1999.00329.x. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, Gifford A, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]