Abstract
Objective
To evaluate UAE in normotensive and non-diabetic women with PCOS in relation to their clinical, endocrine and metabolic profiles. Polycystic ovary syndrome (PCOS) is associated with increased cardiovascular risks including evidence of altered endothelial function. Urinary albumin excretion (UAE) is closely related to endothelial function and closely correlates with nephropathy and adverse cardiovascular endpoints.
Design
Observational study.
Setting
University fertility center.
Patient(s)
Sixty-three women with PCOS were evaluated.
Intervention(s)
Clinical assessments and urine and blood testing.
Main Outcome Measure(s)
UAE, systolic and diastolic blood pressure, serum levels of LH, FSH, prolactin, testosterone, 17-hydroxyprogesterone, glucose, insulin, lipids, C-reactive protein and 24-hour urinary free cortisol.
Result(s)
In univariate and multivariate correlation analysis, UAE correlated with diastolic blood pressure, insulin area under the curve during glucose tolerance test, prolactin and 17-hydroxyprogesterone. Overt microalbuminuria was detected in a significant proportion of subjects.
Conclusion(s)
Urinary albumin excretion in women with PCOS correlates well with other cardiovascular risk factors. Since the relationship between UAE and adverse cardiovascular events is continuous, evaluation of UAE in the presence of PCOS may provide clinically relevant information and may aid in selecting appropriate patients for more aggressive treatment of likely aggravating factors, such as hyperinsulinemia or borderline hypertension.
Keywords: Polycystic ovary syndrome, urinary albumin, endothelial function
Introduction
Polycystic ovary syndrome (PCOS) is a complex and heterogeneous disorder affecting 5–10% of women in reproductive age (1, 2). While definitions of PCOS focus on reproductive-endocrine dysfunction, a majority of women with this condition also display a broad range of metabolic derangements associated with increased risk of developing type 2 diabetes, atherosclerosis, hypertension and, ultimately, cardiovascular events (3–6).
Cardiovascular risk factors common in PCOS include dyslipidemia, insulin resistance, systemic inflammation and endothelial dysfunction (7–10). Growing evidence points at endothelial dysfunction as an important early finding predicting development of atherosclerosis.
Urinary excretion of albumin (UEA) reflects renal function and is thought to be directly related to endothelial function, or more specifically, endothelial “leakiness”. The concept of UEA as a marker of endothelial dysfunction was initially developed based on studies of patients with diabetes; in these patients, even modest increases in UAE have been shown to predict adverse cardiovascular outcomes (11). However, UEA also predicts cardiovascular events in non-diabetic subjects independently from traditional risk factors (12). Excessive UEA is considered to be a risk factor for all-cause mortality and cardiovascular morbidity (13, 14). Even very low levels of microalbuminuria have been associated with coronary artery disease and death; this association was independent of renal function, hypertension or diabetes (15).
In view of the above considerations, this study was conducted to evaluate UEA in women with PCOS to determine whether it was increased above the reported normal range and to determine whether UEA correlates with clinical, endocrine and metabolic parameters relevant to PCOS.
Methods
Patients
Sixty-three women with PCOS (mean age, 24.7±7.9 years) were enrolled in the study; all subjects were evaluated at Fertility and Reproductive Endocrinology Center at Yale University School of Medicine. Fifty subjects were Caucasian (79%), nine African American (14%), two Asian (3%), one Hispanic and one Native Indian. The study protocol was approved by the Ethics Committee for Human Studies of Yale University in 2006. PCOS was defined as the presence of two of the following three features after the exclusion of other etiologies (16): (i) oligo- or anovulation (fewer than six menstrual periods in the preceding year); (ii) hyperandrogenism and/or biochemical signs of hyperandrogenism and/or (iii) polycystic ovaries. Exclusion criteria were: pregnancy, diabetes mellitus or blood pressure above 140 mmHg systolic or 90 mmHg diastolic; treated hypertension; hyperprolactinemia, adrenal dysfunction and thyroid dysfunction. Oligomenorrhea or amenorrhea was present in 56 patients (88.9%). All subjects had either clinical or biochemical evidence of hyperandrogenism/hyperandrogenemia; hirsutism was observed in 56 patients (88.9%) and acne was documented in 24 patients (38.1%). Polycystic ovarian morphology was detected (by transvaginal ultrasound) in 47 subjects (74.6%). Relevant clinical, hormonal and biochemical parameters evaluated in this study are summarized in Table 1.
Table 1.
Clinical, endocrine and biochemical parameters evaluated in women with PCOS (n=63).
| Variable | Mean ± SD | Median (10th to 90th percentile) |
|---|---|---|
| Age | 24.7±7.9 | 24.0 (16–35) |
| BMI | 32.9±7.4 | 32.7 (22.5–42.9) |
| Systolic blood pressure (mm Hg) | 115.3±12.4 | 117 (98–130) |
| Diastolic blood pressure (mm Hg) | 68.7±9.8 | 70 (58–80) |
| Total testosterone (ng/dL) | 61.9 ± 22.5 | 61.5 (34.0–88.4) |
| DHEAS (g/dL) | 244.9 ± 127.1 | 236.0 (99–401) |
| 17-OH Prog (ng/dl) | 56.4 ± 36.4 | 47.0 (26.2–104) |
| SHBG (nmol/L) | 40.8.4 ± 38.6 | 25.0 (10.4–89.6) |
| Urinary 24-hour free cortisol (μg) | 31.2 ± 16.5 | 29.8 (12.2–56.5) |
| LH (mIU/mL) | 5.7 ± 3.4 | 5.3 (2.3–11.1) |
| FSH (mIU/mL) | 4.4 ± 1.7 | 4.3 (2.2–6.6) |
| PRL (ng/mL) | 12.7 ± 5.3 | 11.2 (7.2–105) |
| Fasting glucose (mg/dl) | 91.3 ± 10.0 | 89 (82–105) |
| Fasting insulin (uIU/mL) | 15.2 ± 9.5 | 13.0 (4.2–28.6) |
| LDL cholesterol (mg/dL) | 113 ± 30.8 | 112 (70–155) |
| HDL cholesterol (mg/dL) | 52.7 ± 15.2 | 49.0 (38.6–76.8) |
| Triglycerides (mg/dL) | 103 ± 57.8 | 87 (50.3–181) |
| C-reactive protein (mg/L) | 5.6 ± 7.7 | 3.2 (0.4–13.3) |
| Urinary albumin (μg/mL) | 17.2± (20.1) | 10.0 (3.7–51.9) |
| Urinary albumin to creatine ratio (μg/mg) | 13± (20.1) | 5.7 (3.2–32.5) |
Assays
Blood collections were carried out, following an overnight fast, in the morning, between cycle day three and nine of a spontaneous or medroxyprogesterone-induced menstruation. The serum concentrations of FSH, LH, prolactin (PRL), total testosterone and sex hormone-binding globulin were measured by chemiluminescent enzyme immunoassay (Chiron Diagnostics, Terry Town, New York, USA). Serum 17-hydroxyprogesterone was measured by RIA (Biermann Inc and Diagnostic Products Corp, Terry Town, New York, USA). Dehydroepiandrosterone sulfate (DHEAS) was measured by RIA (Diagnostic Products Corporation, Los Angeles, CA). All patients underwent a 2-hour glucose tolerance test (2h GTT) using a 75-g glucose load with determinations of both glucose and insulin at baseline and then at 30, 60, 90, and 120 minutes after glucose load. Serum glucose was measured by hexokinase calorimetric method using a Bayer Dax-48 system analyzer (Bayer Diagnostics Corporation, West Haven, CT, USA). Plasma insulin levels were measured by chemiluminescent enzyme (a solid-phase, two-site sequential chemiluminescent immunometric assay) using commercial kits from Bayer Diagnostic Corporation (West Haven, CT, USA). Lipid analysis in fasting serum was performed for all patients. The lipid profile included measurement of the levels of total cholesterol; triglyceride, high- and low-density lipoprotein (HDL and LDL). Cholesterol and triglyceride were measured by enzymatic colorimetric method in the Quest laboratory (Wallingford, CT, USA) using Olympus AU 600 auto analyzer and reagents from Olympus Diagnostics (New York, USA). High-density lipoprotein was separated by precipitating apolipoprotein-B (Boehringer Mannheim, Germany). Low-density lipoprotein was calculated using the Friedwald formula (17). Plasma hsCRP concentrations were measured by immunoturbidimetry with an image analyzer (Hitachi 917; Quest Laboratories, Wallingford, CT, USA) with intra-assay CV of 8.7% and sensitivity of 0.03 mg/L. Twenty-four hour urinary free cortisol was measured by radioimmunoassay (DPT Coat-A-Count Diagnostic Product Corporation, Los Angeles, CA, USA) in the Quest laboratory (Wallingford, CT, USA). Urinary albumin was measured by spectrophotometry (Perk&Elmer Corporation, Oak Brook, IL, USA); intra-assay and inter-assay CV was 4%. Creatinine was assayed using an Ectachem 400 Analyzer (Eastman Kodak Co, Rochester, New York, USA).
Statistical analysis
Statistical analysis was performed using JMP program version 7 (SAS Institute, Cary, NC, USA). Associations between variables were analyzed by Pearson correlation and multiple linear regression modeling. Normality of distribution was assessed by Shapiro-Wilk W test. In the absence of a normal distribution, variables were logarithmically transformed. Results are expressed as means ± S.D.
Results
Characteristics of the studied population of women with PCOS are summarized in Table 1. Women with PCOS excreted albumin in urine over a wide range of concentrations from 2 to 97 μg/mL with a median of 10 μg/mL. The ratio of urinary excretion of albumin per mg of creatinine ranged from 2 to 145.9 μg/mg with a median of 5.7 μg/mg. Microalbuminuria, defined in women as excretion of >25 μg of albumin per mg of creatinine (18), was observed in 16% of subjects. When applying another commonly used criterion of microalbuminuria (>20 μg of albumin per liter of urine) (19); 24% of subjects would be classified as displaying microalbuminuria.
Since the distribution of excretion of albumin was not normal, subsequent correlations were carried out on logarithmically transformed data. Correlation of urinary albumin to creatinine ratio was determined for each of the variables listed in Table 1. These variables were selected for analysis in view of their likely or postulated relationship to urinary albumin excretion and/or their prominent role in the pathophysiology of PCOS. Variables with Pearson correlation at P<0.1 are listed in Table 2.
Table 2.
Univariate analysis evaluating correlation of logarithm of urinary albumin (μg/mg urinary creatinine) with other variables.* Only variables correlating at P<0.1 are presented.
| Variable | Correlation coefficient | P-value |
|---|---|---|
| Diastolic blood pressure (mm Hg) | 0.05 | 0.07 |
| 17-Hydroxyprogesterone (ng/dL) | 0.07 | 0.03 |
| Prolactin (ng/mL) | 0.09 | 0.03 |
| LH (mIU/mL) | 0.05 | 0.08 |
| Insulin AUC (μU/mL/min) | 0.11 | 0.01 |
Outlier with urinary albumin of 146 μg per mg of creatinine was excluded from this analysis
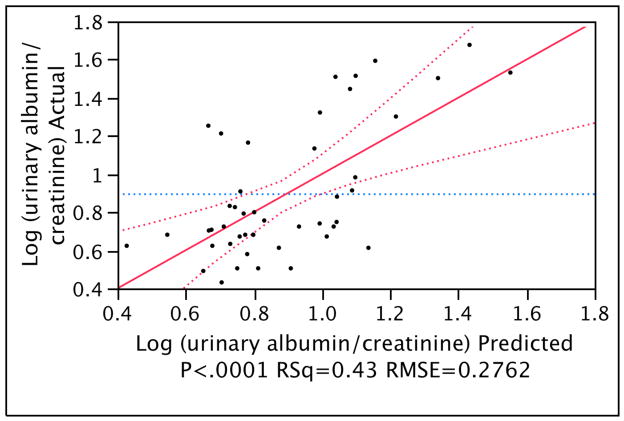
Multiple linear regression modeling was then carried out to characterize the relationship between several predictors and the primary studied measure of UAE: the ratio of urinary albumin excretion to creatinine. In addition to variables identified as predictors in univariate analysis (Table 2), all other variables (listed in Table 1) were also tested but were excluded from the final model when their respective P-values in the model exceeded 0.1. As shown in the final model (Table 3 and Fig. 1), four independent predictors were: diastolic blood pressure, 17-hydroxyprogesterone, prolactin and insulin area under the curve (during 2 h GTT). The R2 of the final model was 0.43 indicating that it explained forty-three percent of the variation in the response variable.
Table 3.
Multiple regression model for logarithm of urinary albumin (μg/mg urinary creatinine). The R2=0.43
| Variable | Estimate ± standard error | P-value |
|---|---|---|
| Diastolic BP (mm Hg) | 0.0112 ± 0.0047 | 0.02 |
| 17-Hydroxyprogesterone (ng/dL) | 0.0035 ± 0.0011 | 0.003 |
| Prolactin (ng/mL) | 0.0197 ± 0.0073 | 0.01 |
| Insulin AUC (μU/mL/min) | 1.32e-5 ± 6.69e-6 | 0.056 |
Figure 1.
Multiple regression model as presented in Table 3: actual by predicted plot of the logarithm of urinary albumin to creatinine ratio; RSq represents the square of the multiple correlation coefficient; RMSE represents the root mean square error.
Discussion
This report presents new findings describing UAE in normotensive and non-diabetic women with PCOS. First, UAE appears to be independently related to several clinical and endocrine parameters. Second, a high rate of overt microalbuminuria has been detected.
Among the four independent predictors of UAE identified in this study, diastolic blood pressure is the best, established parameter, which can mechanistically explain albumin excretion as a function of increased intraglomerular pressure. As in the present study, a close correlation of blood pressure and UAE has been described previously in various populations of both hypertensive and normotensive subjects (20, 21). The likely contribution of blood pressure to UAE in women with PCOS is highly relevant, since these patients are at increased risk of developing hypertension (22). However, importantly, in this study, a correlation of blood pressure with UAE was detected among normotensive subjects.
Another predictor of UAE identified in this report is the insulin area under the curve during a 2h GTT. A large proportion of women with PCOS are insulin resistant and have compensatory hyperinsulinemia (8). As in the present study, a correlation of insulin resistance and hyperinsulinemia with UAE has been described in various other populations (23, 24). Effects of insulin on UAE may be related to both acute and long-term actions. An acute injection of insulin has been shown to immediately increase UAE (25). In the long-term, hyperinsulinemia may act by inducing mesangial hyperplasia (26). Postulated mechanisms of actions of insulin on UAE include increased glomerular filtration (27) and increased vascular permeability (28). However, the role of insulin is still not fully understood and in some studies an association between UAE and insulin was not observed (29, 30).
A third factor found to be associated with UAE in women with PCOS is prolactin. This observation correlates well with a report that hyperprolactinemic pre-menopausal women had impaired endothelial function, as detected by flow-mediated dilatation of the brachial artery and decreased insulin sensitivity (31). Furthermore, treatment of these patients with bromocriptine resulted in improvement of endothelial function and insulin. Notably, in the present study of women with PCOS, UAE correlated with prolactin in the absence of hyperprolactinemia and, as determined by multiple regression analysis (Table 3), independently of insulin level. While the mechanistic link between prolactin and UAE is not readily apparent, it is interesting to note that endothelial cells express prolactin mRNA and there is evidence for autocrine actions of prolactin on endothelium (32, 33). There is also evidence that prolactin may modulate angiogenesis and affect endothelial cell proliferation, apoptosis and migration (34).
The final and unexpected predictor of UAE in this study is serum level of 17-hydroxyprogesterone. The association with UAE was observed at levels of 17-hydroxyprogesterone well below those found in subjects with 21-hydroxylase deficiency. At this time no plausible explanation of the present observation is offered and it is possible that levels of 17-hydroxyprogesterone may co-variate with other not yet elucidated factors which may be linked to endothelial function and albumin excretion. PCOS is associated with excessive 17-hydroxyprogesterone responses to GnRH agonist and a recent study has found that women with an exaggerated 17-hydroxyprogesterone response to a GnRH analog also had greater insulin resistance and β-cell insulin secretion (35). Hence, one may propose that the level of 17-hydroxyprogesterone may be a surrogate marker for metabolic derangements such as excessive insulin secretion, which in turn may affect UAE. However, such an explanation is highly speculative and in the present study, the multivariate model of UAE excretion indicates that 17-hydroxyprogesterone was an independent predictor of UAE when accounting for insulin levels.
Surprisingly, UAE did not correlate with some parameters, which in view of their association with cardiovascular risks would be expected to predict UAE. Thus, in this study neither BMI, nor hsCRP had any significant correlation with urinary albumin concentration or with urinary albumin to creatinine ratio. Previous studies evaluating the relationship of microalbuminuria and BMI led to conflicting results. In some reports obesity was associated with microalbuminuria (36); however, surprisingly, in other studies an unexpected decrease of microalbuminura among obese individuals was observed (37). Similarly, the correlation of microalbuminuria and hsCRP has been previously evaluated and unexpectedly has been found to be quite weak (38).
While this study was not designed to compare the level of UAE among women with PCOS to general population, a high frequency of overt microalbuminuria was observed. In a large study evaluating a large sample from the general population, microalbuminuria (defined as UAE from 20 to 200 mg/L) was found in 7.2% of the subjects; among diabetics it was 16.4%, among hypertensives in 11.5% and among non-diabetics and non-hypertensives in 6.6% (19) of the subjects. When the same criteria of microalbuminuria are applied to the present study, then 24% of women with PCOS had overt microabluminuria. Thus, it may appear that excessive UAE may be even more common in PCOS than in subjects with overt diabetes and/or hypertension. However, comparisons of findings from different reports need to be interpreted with caution and verification of the present observations should be obtained from further studies on larger populations of women with PCOS.
The present understanding of the mechanisms underlying UAE is still incomplete. While traditionally UAE has been thought to be primarily related to blood pressure and glomerular endothelial integrity, several recent studies indicate that this process may be also affected by other factors such as tubular re-absorption of filtered albumin (39, 40).
Overall, the present findings indicate that normotensive and nondiabetic women with PCOS have significant UAE correlating well with other established cardiovascular risk factors. Notably, even high “normal” levels of UAE (<25 μg of albumin per mg of creatinine) in subjects without diabetes or hypertension predicts increased risk of subsequent development of hypertension (41). Since the boundary between normal albumin excretion and microalbuminuria is artificial, and the relationship between UAE and cardiovascular risks is continuous (42), evaluation of UAE in subjects with PCOS may provide new and clinically relevant information. Furthermore, since interventions lowering UAE have been shown to significantly decrease cardiovascular risks (43, 44), consideration may be given to more aggressive treatment of likely aggravating factors, such as hyperinsulinemia or borderline hypertension.
Acknowledgments
Financial Support: This work was supported in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant RO1- HD050656 to Antoni J Duleba.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Pelusi B, Gambineri A, Pasquali R. Type 2 diabetes and the polycystic ovary syndrome. Minerva Ginecol. 2004;56:41–51. [PubMed] [Google Scholar]
- 4.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–42. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 5.Talbott EO, Zborowski J, Rager J, Stragand JR. Is there an independent effect of polycystic ovary syndrome (PCOS) and menopause on the prevalence of subclinical atherosclerosis in middle aged women? Vasc Health Risk Manag. 2008;4:453–62. doi: 10.2147/vhrm.s1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93:1276–84. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Wild RA, Applebaum-Bowden D, Demers LM, Bartholomew M, Landis JR, Hazzard WR, et al. Lipoprotein lipids in women with androgen excess: independent associations with increased insulin and androgens. Clin Chem. 1990;36:283–9. [PubMed] [Google Scholar]
- 8.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987;65:499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- 9.Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453–5. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Spina G, Kouli C, Migdalis I. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab. 2001;86:4666–73. doi: 10.1210/jcem.86.10.7904. [DOI] [PubMed] [Google Scholar]
- 11.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–8. [PubMed] [Google Scholar]
- 12.Pedrinelli R, Dell’Omo G, Penno G, Mariani M. Non-diabetic microalbuminuria, endothelial dysfunction and cardiovascular disease. Vasc Med. 2001;6:257–64. doi: 10.1177/1358836X0100600410. [DOI] [PubMed] [Google Scholar]
- 13.Yuyun MF, Khaw KT, Luben R, Welch A, Bingham S, Day NE, et al. Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: The European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol. 2004;33:189–98. doi: 10.1093/ije/dyh008. [DOI] [PubMed] [Google Scholar]
- 14.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17:2100–5. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 15.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–5. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 16.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–9. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 19.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–26. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 20.Redon J, Liao Y, Lozano JV, Miralles A, Baldo E, Cooper RS. Factors related to the presence of microalbuminuria in essential hypertension. Am J Hypertens. 1994;7:801–7. doi: 10.1093/ajh/7.9.801. [DOI] [PubMed] [Google Scholar]
- 21.Boulatov VA, Stenehjem A, Os I. Association between albumin:creatinine ratio and 24-hour ambulatory blood pressure in essential hypertension. Am J Hypertens. 2001;14:338–44. doi: 10.1016/s0895-7061(00)01278-4. [DOI] [PubMed] [Google Scholar]
- 22.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and Adverse Cardiovascular Risk Profile of Diagnosed Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2006;91:1357–63. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi S, Bigazzi R, Nenci R, Campese VM. Hyperinsulinemia, circadian variation of blood pressure and end-organ damage in hypertension. J Nephrol. 1997;10:325–33. [PubMed] [Google Scholar]
- 24.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–62. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 25.Mogensen CE, Christensen NJ, Gundersen HJ. The acute effect of insulin on heart rate, blood pressure, plasma noradrenaline and urinary albumin excretion. The role of changes in blood glucose. Diabetologia. 1980;18:453–7. doi: 10.1007/BF00261700. [DOI] [PubMed] [Google Scholar]
- 26.Conti FG, Striker LJ, Lesniak MA, MacKay K, Roth J, Striker GE. Studies on binding and mitogenic effect of insulin and insulin-like growth factor I in glomerular mesangial cells. Endocrinology. 1988;122:2788–95. doi: 10.1210/endo-122-6-2788. [DOI] [PubMed] [Google Scholar]
- 27.Cohen AJ, McCarthy DM, Stoff JS. Direct hemodynamic effect of insulin in the isolated perfused kidney. Am J Physiol. 1989;257:F580–5. doi: 10.1152/ajprenal.1989.257.4.F580. [DOI] [PubMed] [Google Scholar]
- 28.Catalano C, Muscelli E, Quinones Galvan A, Baldi S, Masoni A, Gibb I, et al. Effect of insulin on systemic and renal handling of albumin in nondiabetic and NIDDM subjects. Diabetes. 1997;46:868–75. doi: 10.2337/diab.46.5.868. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Jensen MD. Relationship between urinary albumin excretion, body composition, and hyperinsulinemia in normotensive glucose-tolerant adults. Diabetes Care. 1999;22:1728–33. doi: 10.2337/diacare.22.10.1728. [DOI] [PubMed] [Google Scholar]
- 30.Cubeddu LX, Hoffmann IS, Aponte LM, Nunez-Bogesits R, Medina-Suniaga H, Roa M, et al. Role of salt sensitivity, blood pressure, and hyperinsulinemia in determining high upper normal levels of urinary albumin excretion in a healthy adult population. Am J Hypertens. 2003;16:343–9. doi: 10.1016/s0895-7061(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 31.Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gozu H, Sezgin O, et al. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. Eur J Endocrinol. 2003;149:187–93. doi: 10.1530/eje.0.1490187. [DOI] [PubMed] [Google Scholar]
- 32.Clapp C, Lopez-Gomez FJ, Nava G, Corbacho A, Torner L, Macotela Y, et al. Expression of prolactin mRNA and of prolactin-like proteins in endothelial cells: evidence for autocrine effects. J Endocrinol. 1998;158:137–44. doi: 10.1677/joe.0.1580137. [DOI] [PubMed] [Google Scholar]
- 33.Corbacho AM, Macotela Y, Nava G, Torner L, Duenas Z, Noris G, et al. Human umbilical vein endothelial cells express multiple prolactin isoforms. J Endocrinol. 2000;166:53–62. doi: 10.1677/joe.0.1660053. [DOI] [PubMed] [Google Scholar]
- 34.Corbacho AM, Martinez De La Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J Endocrinol. 2002;173:219–38. doi: 10.1677/joe.0.1730219. [DOI] [PubMed] [Google Scholar]
- 35.Pasquali R, Patton L, Pocognoli P, Cognigni GE, Gambineri A. 17-hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4208–17. doi: 10.1210/jc.2007-0870. [DOI] [PubMed] [Google Scholar]
- 36.Liese AD, Hense HW, Doring A, Stieber J, Keil U. Microalbuminuria, central adiposity and hypertension in the non-diabetic urban population of the MONICA Augsburg survey 1994/95. J Hum Hypertens. 2001;15:799–804. doi: 10.1038/sj.jhh.1001266. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen S, McCulloch C, Brakeman P, Portale A, Hsu CY. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics. 2008;121:37–45. doi: 10.1542/peds.2007-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakker SJ, Gansevoort RT, Stuveling EM, Gans RO, de Zeeuw D. Microalbuminuria and C-reactive protein: similar messengers of cardiovascular risk? Curr Hypertens Rep. 2005;7:379–84. doi: 10.1007/s11906-005-0075-3. [DOI] [PubMed] [Google Scholar]
- 39.Russo LM, Comper WD, Osicka TM. Mechanism of albuminuria associated with cardiovascular disease and kidney disease. Kidney Int Suppl. 2004:S67–8. doi: 10.1111/j.1523-1755.2004.09218.x. [DOI] [PubMed] [Google Scholar]
- 40.Clavant SP, Greive KA, Nikolovski J, Reeve S, Smith AI, Comper WD. Albumin fragments in normal rat urine are derived from rapidly degraded filtered albumin. Nephrology (Carlton) 2003;8:72–9. doi: 10.1046/j.1440-1797.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 41.Forman JP, Fisher ND, Schopick EL, Curhan GC. Higher Levels of Albuminuria within the Normal Range Predict Incident Hypertension. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int. 2006;70:1214–22. doi: 10.1038/sj.ki.5001729. [DOI] [PubMed] [Google Scholar]
- 43.Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–16. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 44.Geluk CA, Asselbergs FW, Hillege HL, Bakker SJ, de Jong PE, Zijlstra F, et al. Impact of statins in microalbuminuric subjects with the metabolic syndrome: a substudy of the PREVEND Intervention Trial. Eur Heart J. 2005;26:1314–20. doi: 10.1093/eurheartj/ehi253. [DOI] [PubMed] [Google Scholar]