Abstract
Pseudoxanthoma elasticum (PXE) is an autosomal recessive disorder characterized by ectopic mineralization of connective tissues, and shows considerable intra- and inter-familial phenotypic variability. PXE is caused by mutations in the ABCC6 gene, and targeted ablation of Abcc6 in mouse recapitulates PXE. In this study, we examined the hypothesis that the GGCX gene encoding γ-glutamyl carboxylase may interfere with the mineralization process in Abcc6−/− mice. Thus, Abcc6−/− and Ggcx+/− mice were generated on 129S1;C57 and 129S1;129X1;C57 genetic backgrounds, respectively, and backcrossed with C57BL/6J for 5 generations. Thus, these strains differ by the 129X1 contribution to the background of the mice. We then generated Abcc6−/−;Ggcx+/+ and Abcc6−/−;Ggcx+/− mice by crossing Abcc6−/− and Ggcx+/− mice. The degree of mineralization of connective capsule of vibrissae, a biomarker of the mineralization process in PXE, was evaluated by computerized morphometric analysis and quantified colorimetrically by calcium and phosphate levels in tissues. The mineralization of the vibrissae in Abcc6−/− mice takes place at ~5-6 weeks of age and is significantly enhanced at 3 months of age in comparison to wild-type mice (>10-fold, p<0.001). However, the onset of mineralization in Abcc6−/−;Ggcx+/+ mice was delayed until between 3-4 months of age, suggesting that the genetic background plays a role in modifying the mineralization process. The mineralization in the Abcc6−/−;Ggcx+/− mice was accelerated in comparison with age-matched Abcc6−/−;Ggcx+/+ mice, with ~3-fold difference at 3, 4 and 9 months of age (p<0.01). The mineralization process was also accelerated in these mice by a special custom-designed diet with mineral modifications. These findings suggest a role for both the GGCX gene and the genetic background as well as dietary factors in modulating the phenotypic severity of PXE caused by loss-of-function mutations in ABCC6.
Keywords: Pseudoxanthum elasticum, ectopic mineralization, heritable skin diseases, ABC transporters
Introduction
Pseudoxanthoma elasticum (PXE) is a multi-system disorder characterized by extensive mineralization of the peripheral connective tissues (for review, see refs. [1, 2]). While a number of organs demonstrate ectopic mineralization, the clinical findings primarily involve three different organ systems, viz. the skin, the eyes, and the cardiovascular system. The characteristic cutaneous finding is a small, yellowish papule on the lateral side of the neck, axillae, and the antecubital fossae. The cutaneous lesions then coalesce into plaques of inelastic, leathery skin that depicts loss of recoil and elasticity. With the progression of the disease, essentially all of skin can become involved with the major cosmetic impact on the patient. The ocular manifestations characteristically consist of angioid streaks, associated with neovascularization and bleeding into the eye [3]. The eye involvement then leads to a progressive loss of the central vision and occasionally causes blindness as early as the fourth decade of life. The cardiovascular manifestations include intermittent claudication, bleeding from the gastric arteries and, occasionally, myocardial infarcts in early fourties. However, there is considerable clinical, both intra- and inter-familial, phenotypic variability so that one of the organ systems may be predominant in some families.
The characteristic histopathologic finding in PXE is accumulation of pleiomorphic elastotic material which becomes progressively mineralized [1, 2]. Similarly, the angioid streaks reflect mineralization of Bruch’s membrane, a thin elastin-rich layer behind the pigmented epithelium of the retina. Mineralization also affects the cardiovascular system primarily by deposition of calcium/phosphate complexes in the elastic lamellae in the arterial blood vessels. Thus, PXE was initially postulated to be a connective tissue disorder with primary abnormalities in the elastic fibers [4]. Subsequent genetic linkage analyses and sequencing of the genes encoding the principal components of the elastic structures, elastin and the elastin-associated microfibrillar proteins, excluded the elastic fibers as the primary sites of genetic lesions [5, 6]. Subsequent positional cloning analyses mapped the PXE locus to the short arm of human chromosome 16, to the region 16p13.1 [7, 8], and sequencing of the putative candidate genes within the critical interval identified mutations in the ABCC6 gene, a member of the ATP–binding casette family C, member 6 (for review, see [9]). The ABCC6 gene encodes a 1,503 amino acid transmembrane protein, ABCC6 (also known as MRP6), which is postulated to serve as an efflux pump, but the precise molecules transported under physiologic conditions are currently unknown.
PXE-like cutaneous manifestations have also been described in a number of other clinical situations, including β-thalassemia and sickle cell anemia, as well as a sequela of long-term treatment with D-penicillamine [9-11]. More recently, it has also been demonstrated that patients with mutations in the GGCX gene develop not only vitamin K-dependent coagulation factor deficiency but also PXE–like cutaneous findings [12, 13]. Most interestingly, we have recently reported a family in which patients with PXE harbored heterozygous mutations in ABCC6 and GGCX genes, suggesting digenic inheritance of PXE in these individuals [14]. The latter studies suggested that GGCX may serve as a modifier gene that complements the loss-of-function mutations in the ABCC6 gene, thus enhancing the connective tissue mineralization and leading to PXE-like cutaneous findings.
Well over 300 distinct mutations have been disclosed in the ABCC6 gene in different families with PXE. Careful examination of the mutation database in the context of clinical manifestations in different families with PXE has not provided clear-cut genotype/phenotype correlations that would explain the considerable phenotypical variability [9]. We have recently developed an Abcc6−/− mouse which recapitulates the genetic, histopathologic and ultrastructural features of PXE [15]. Specifically, these mice demonstrate mineralization in the skin, eyes, and the arterial blood vessels similar to that in patients with PXE. In this study, we have utilized this mouse model to explore the contribution of genetic background, and particularly the expression of the GGCX gene, to the mineralization process.
Materials and methods
Mice and the special diets
The PXE mouse model was developed by targeted ablation of the Abcc6 gene, as described previously [15]. Heterozygous alleles were backcrossed for five generations into the C57BL/6J background (N5) and interbred to generate Abcc6−/− knock-out (KO) and Abcc6+/+ wild-type (WT) mice. The Ggcx heterozygous mice were obtained from University of Michigan (courtesy of Dr. David Ginsburg) [16]. Heterozygous alleles for Ggcx were backcrossed into C57BL/6J mice for five generations (N5). Compound Abcc6; Ggcx transgenic mice were generated by intercrossing Abcc6−/− mice with Ggcx+/− mice. The mice, fostered in the Animal Facility of the Thomas Jefferson University, had free access to water and were maintained in a temperature- and humidity-controlled environment under 12-hour light/dark cycles.
The mice were fed either a standard rodent diet (Laboratory Rodent Diet 5010; PMI Nutrition, Brentwood, MO, USA) or an experimental diet with specific mineral modifications (Harlan Teklad, Rodent diet TD.00442, Madison, WI, USA). In comparison with the standard rodent diet, the percentage of absorbable phosphorus (non-phytate) was increased by 2-fold (from 0.43% to 0.85%), vitamin D3 was increased by 22-fold (from 4.4 IU/g to 100 IU/g). In addition, there was a decrease in calcium (from 1.0% to 0.4%) and magnesium (from 0.22% to 0.04%).
The animal studies were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Histopathology and quantification of tissue mineralization by computerized morphometric analysis
For histopathological analysis of mineralization of vibrissae, muzzle skin biopsies were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin, Alizarin Red, or von Kossa using standard methods. Computerized morphometric analysis of hematoxylin and eosin-stained sections of muzzle skin was performed as described elsewhere [17]. The sections were examined with a Nikon model Te2000 microscope furnished with an Auto Quant Imaging system (Watervliet, New York, NY). The number of vibrissae with and without evidence of mineralization was determined in all sections, and the extent of mineralization was described as the percentage of area of mineralization per total area of vibrissae.
Quantification of calcium and phosphate deposition
To quantify the calcium/phosphate depositions in mouse vibrissae, the muzzle skin which contains the vibrissae, was harvested and decalcified with 0.15 N HCl for 48 hours at room temperature. The calcium content was determined colorimetrically by the o-cresolphthalein complexone method (Calcium (CPC) Liquicolor; Stanbio Laboratory, Boerne, TX). The phosphate content was determined with Malachite Green Phosphate Assay kit (BioAssay Systems, Hayward, CA). The values for calcium and phosphate were normalized to tissue weight. Calcium and phosphorus in the serum samples were quantitatively assayed as above.
Statistical analysis
The results in different groups of mice receiving various diets were first analyzed for normal distribution using Hamilton’s test. Comparisons of continuous measures across all groups were completed using two-sided Kruskal–Wallis non-parametric tests [18]. The Kruskal–Wallis test is comparable to one-way analysis of variance, but without the parametric assumptions. For each of the paired group comparisons, an exact two-sided Wilcoxon’s test was computed. All statistical computations were completed using SPSS version 15.0 software.
Results
Connective tissue mineralization in Abcc6−/− mice
In this study, we have utilized Abcc6−/− knock-out mice (KO) as a model system for ectopic mineralization in PXE. These mice were developed by targeted ablation of the mouse Abcc6 gene resulting in deletion of exons 15-18 [15]. The background of the initial KO mice was 129S1/SvImJ;C57BL/6J, and they were backcrossed for 5 generations into C57BL/6J background (N5). As reported previously [15], these mice demonstrate extensive ectopic mineralization of connective tissues in a number of organs (not shown). In particular, an early, reproducible biomarker of mineralization was found to be the presence of calcium/phosphate deposits in the connective tissue capsules surrounding the vibrissae, and this is reliably noted to begin at 5-6 weeks after birth and progresses so that at 3 months of age, mineralization in Abcc6−/− mice is obvious (Fig. 1b) [19]. No mineralization in the vibrissae of the corresponding wild-type mice is noted at 3 months of age (Fig. 1a) or during the subsequent follow-up to two years of age [15]. Thus, the Abcc6−/− mice demonstrate ectopic connective tissue mineralization recapitulating the histopathologic features of PXE.
Fig. 1.
Histopathologic demonstration of mineralization in the vibrissae of Abcc6−/− mice crossed with Ggcx+/− heterozygous mice. The resulting Abcc6−/− mice either on Ggcx+/+ (columns c, e and g) or Ggcx+/− (d, f, h) background as well as the original Abcc6−/− mice (b) and their corresponding wild-type littermates (a) were biopsied at 3, 4 or 9-months of age, and the muzzle skin samples were processed for histopathology with staining with H & E (top row), or with Alizarin Red or von Kossa stains (middle and lower rows, respectively) for calcium/phosphate.
The role of the Ggcx gene in the mineralization process
Recent studies have revealed that the GGCX gene, when deficient, can result in vitamin K-dependent coagulation factor deficiency as well as PXE-like cutaneous findings [12, 13]. Furthermore, we have demonstrated that individuals heterozygous for an ABCC6 mutation when combined with a heterozygous GGCX mutation also can result in PXE-like cutaneous findings [14]. To examine the role of the Ggcx gene as a potential modifier gene for mineralization, the Abcc6−/− mice were crossed with Ggcx+/− mice which were initially developed at 129S1/SvImJ; 129X1/SvJ; C57BL/6J background [16]. These mice were kept on standard control diet. The mineralization in the vibrissae was quantitated by computerized morphometric analysis of hematoxylin and eosin (H & E) stained sections (Fig. 1, top row), and the presence of calcium/phosphate deposits was demonstrated by special stains (Alizarin Red and von Kossa stains; Fig. 1, middle and bottom rows, respectively). Examination of Abcc6−/−;Ggcx+/+ mice revealed no mineralization at 3 months of age (Fig. 1c) while the original Abcc6−/− mice displayed distinct mineralization at the same age (Fig. 1b). Furthermore Abcc6−/− mice with haploinsufficiency of the Ggcx gene, i.e. Abcc6−/−; Ggcx+/−, demonstrated mineralization of vibrissae at 3 months of age (Fig. 1d) while the Abcc6−/−;Ggcx+/+ littermates did not (Fig. 1c). Thus, mineralization in the Abcc6−/− mice, when on Ggcx+/+ background, was delayed as compared to the original Abcc6−/− mice. However, deficiency in the Ggcx gene (Ggcx+/−) accelerated the mineralization at three months, indicating a role for the Ggcx gene in this process. Nevertheless, the Abcc6−/−;Ggcx+/+ mice demonstrated mineralization at four months of age (Fig. 1e), and this progressively increased between four and nine months of age (Fig. 1g). At the same time, mineralization in the Abcc6−/−;Ggcx+/− also progressively increased between three and nine months of age (Fig. 1d,f,h) and exceeded that noted in Abcc6−/−;Ggcx+/+ littermates (Table 1).
Table 1.
Quantitation of connective tissue mineralization of vibrissae1
| Mouse | Diet | Age (months) |
No. of mice |
Mineralized vibrissae (per mouse) |
Total vibrissae examined (per mouse) |
Percent mineralized2 |
Area of mineralization per total area of vibrissae (%)2 |
Fold3 | P-value4 | P-value5 |
|---|---|---|---|---|---|---|---|---|---|---|
| wild-type | control | 3 | 10 | 0 | 0 | 0 | 0.00 | |||
| Abcc6 −/− | control | 3 | 15 | 5.8 | 10.7 | 60.7 ± 5.2 | 0.73 ± 0.14 | 1.00 | ||
| Abcc6 −/− ;Ggcx +/+ | control | 3 | 10 | 0.1 | 12.3 | 1.4 ± 1.4 | 0 | 0.00 | ||
| Abcc6 −/− ;Ggcx +/− | control | 3 | 8 | 2.1 | 9.3 | 27.7 ± 6.8 | 0.34 ± 0.11 | 0.47 | 0.006 | |
| Abcc6 −/− ;Ggcx +/+ | control | 4 | 6 | 4.3 | 9.0 | 52.2 ± 9.4 | 0.88 ± 0.23 | 1.21 | ||
| Abcc6 −/− ;Ggcx +/− | control | 4 | 6 | 5.7 | 7.8 | 73.9 ± 11.3 | 2.45 ± 0.66 | 3.36 | 0.001 | |
| Abcc6 −/− ;Ggcx +/+ | control | 9 | 6 | 5.3 | 8.8 | 63.8 ± 9.6 | 1.04 ± 0.61 | 1.43 | ||
| Abcc6 −/− ;Ggcx +/− | control | 9 | 4 | 5.8 | 8.5 | 71.1 ± 10.0 | 3.49 ± 0.49 | 4.78 | 0.011 | |
| Abcc6 −/− ;Ggcx +/+ | experimental | 3 | 5 | 7.2 | 20.0 | 39.1 ± 5.3 | 1.03 ±0.46 | 1.41 | 0.001 | |
| Abcc6 −/− ;Ggcx +/− | experimental | 3 | 4 | 8.0 | 14.3 | 61.4 ± 6.7 | 3.10 ±0.78 | 4.25 | 0.065 | 0.006 |
Skin sections containing vibrissae were stained with H&E and examined by computerized morphometric analysis.
Values presented as mean ± S.E.
Calculated using the 3-month-old Abcc6−/− mice on the control diet as 1.00.
Compared with age-matched Abcc6−/−;Ggcx+/+ mice on the same diet.
Compared with age-matched Abcc6−/−;Ggcx+/+ or Abcc6−/−;Ggcx+/− mice on control diet.
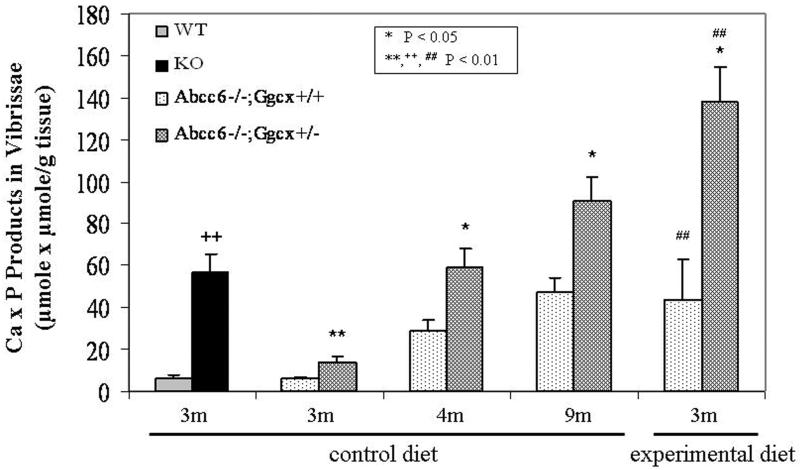
To verify the accumulation of calcium and phosphate in the mineralized areas of the vibrissae, the amounts of calcium and phosphate were separately determined by chemical assays and normalized to the tissue weight of the biopsies of the muzzle skin. The amounts of calcium and phosphate (expressed as Ca x P products in vibrissae per g tissue) confirmed the results of histopathologic analysis of Abcc6−/−; Ggcx+/+ and Abcc6−/−; Ggcx+/− mice at three, four, and nine months of age (Fig. 2).
Fig. 2.
Quantitation of the mineralization by chemical assay of calcium and phosphate in the vibrissae of mice. A group of mice was kept on standard control diet, while others were placed at 4 weeks of age on experimental diet. The genetic background of WT and KO mice was C57BL/6J, while the other mice have a genetic contribution from 129X1/SvJ. The values for Ca x P were normalized to the weight of the tissue. The statistical significance was calculated by Wilcoxon non-parametric ranking test (n = 4-15 mice). Statistical significance: *, **, = in comparison to age-matched Abcc6−/−; Ggcx+/+ mice on the same diet. +,++ = in comparison to age-matched WT mice. #,## = in comparison to age-matched Abcc6−/−; Ggcx+/+ or Abcc6−/−; Ggcx+/− mice on control diet.
Modulation of mineralization by genetic background (129X1/SvJ)
As indicated above, the genetic background of the Ggcx+/− mice seemed to delay the mineralization process in Abcc6−/− mice apparently due to differences in the genetic make-up of the embryonic stem (ES) cells used in development of the original KO mice. Specifically, the development of Ggcx+/− mice utilized R1 ES cells while the Abcc6−/− mice were developed using W9.5 ES cells. These two ES cells differ by the presence of 129X1/SvJ in R1 cells. To examine the influence of the genetic background in these mice, apart from the Ggcx deficiency, we crossed the Abcc6−/− mice with mice of 129X1/SvJ strain (Fig. 3a). The heterozygous mice were then interbred to generate Abcc6−/− mice with a contribution from the 129X1/SvJ genetic background, i.e. Abcc6−/−; 129X1/SvJ. These mice were compared with the original Abcc6−/− mice on C57BL/6J background. As shown in Fig. 3b, mineralization of the connective tissue in vibrissae was delayed in Abcc6−/− with 129X1/SvJ background contribution, and took place between the third and fourth month of age, as compared to at 5-6 weeks in the original Abcc6−/− mice (Fig. 1a,b).
Fig. 3.
Schematic presentation of the crossing of Abcc6−/− mice on C57BL/6J background with mice of strain 129X1/SvJ. Note the agouti coat color in heterozygotes while Abcc6−/−;129X1/SvJ mice showed variable color (Box) (a). Histopathologic examination of the mineralization in the vibrissae of Abcc6−/− mice with 129X1/SvJ genetic contribution (b). Note that the mineralization, as visualized by H & E, Alizarin Red or von Kossa stains (top, middle and bottom rows, respectively) reveals that mineralization takes place between 3 and 4 months of age.
Experimental diet with mineral modifications alters the mineralization process
Early retrospective analyses of patient cohorts with PXE have suggested that ingestion of high quantities of dairy products, enriched in calcium and phosphate, during childhood and adolescence may accelerate the phenotypic manifestations of PXE [20, 21]. More recently, we have demonstrated that diet with enrichment of magnesium counteracts the mineralization in Abcc6−/− mice [22]. Thus, we proceeded to explore the effects of a special diet with mineral modifications in our PXE mouse model system, consisting of Abcc6−/−; Ggcx+/+ mice, with the hypothesis that this special custom-designed diet may enhance the mineralization process. The mice were placed on the special experimental diet (see Materials and methods) at four weeks of age at the time of weaning and continued on this diet for another two months. At three months of age, the degree of mineralization in the vibrissae was first examined by computerized morphometric analysis. As shown in Fig. 4a, the mice on experimental diet demonstrated extensive mineralization of the connective tissue capsule of the vibrissae which was significantly more extensive than that noted in the corresponding mice kept on normal control diet (Fig. 1c and Table 1).
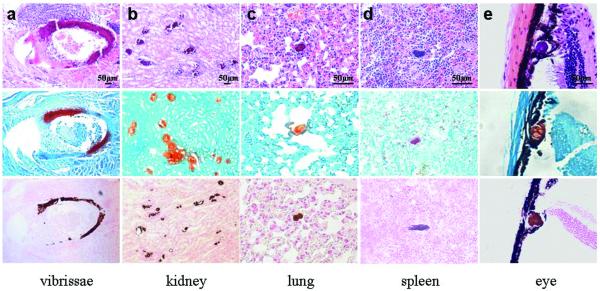
Fig. 4.
Illustration of extensive mineralization in various organs of Abcc6−/−; Ggcx+/+ mice, on C57BL/6J genetic background with contribution from 129X1/SvJ, at three months of age when placed on experimental diet. H & E, Alizarin Red and von Kossa stains (upper, middle, and bottom rows, respectively).
The acceleration of mineralization in Abcc6−/−;Ggcx+/+ mice on the experimental diet, as compared to those on control diet, was also confirmed with chemical assay of calcium and phosphate contents of the muzzle skin containing the vibrissae (Fig. 2). Furthermore, comparison of Abcc6−/−; Ggcx+/+ mice with Abcc6−/−; Ggcx+/− mice, both kept on experimental diet, revealed that the latter mice showed a 3-fold enhancement of mineralization, as determined by calcium and phosphate assays (Fig. 2, right hand columns) (p<0.05). Necropsy of the Abcc6−/−;Ggcx+/+ mice kept on experimental diet revealed extensive mineralization in the kidneys, lungs, spleen and the eyes attesting to the multi-organ involvement in this mouse model of PXE, and similar results were noted in Abcc6−/−;Ggcx+/− mice kept on the experimental diet. Finally, assay of the calcium and phosphorus concentrations in the serum of mice placed on experimental diet revealed values comparable to those measured in mice kept on control diet, and the serum Ca/P ratio was unaltered (Table 2).
Discussion
PXE is a pleiotropic, multi-system disorder with considerable intra- and inter-familial heterogeneity [1, 2, 20]. While the cardinal clinical manifestations revolve around three organ systems, i.e., the skin, the eyes, and the cardiovascular system, there is considerable variability in terms of the age of onset of the disease and extent of the clinical findings. While PXE is an autosomal recessive disease with complete penetrance [23], the manifestations are not present at birth, and the average age of diagnosis is around 13 years of age [20]. In some families, the skin manifestations are predominant with relatively little involvement of the eyes or the cardiovascular system, while in other families, the ocular findings or cardiovascular problems predominate [20]. The reasons for this phenotypic variability are currently unknown. In this study, we have explored the reasons for the phenotypic variability utilizing a mouse model that recapitulates features of human PXE, i.e., the Abcc6−/− mouse [15]. This mouse model reliably demonstrates progressive, late-onset mineralization of skin, retina, and arterial blood vessels after 3-6 months of age, but the first signs of mineralization are evident in the connective tissue capsule of vibrissae of the mice around 5-6 weeks of age. This mineralization process, which is progressive, can be followed by quantitative computerized morphometric analysis of histopathologic sections of the muzzle skin containing the vibrissae, and can also be quantitated by chemical assay of calcium and phosphate in the corresponding skin biopsies.
Our studies have previously demonstrated that the mineralization in the Abcc6−/− mice is progressive up to two years of age, while the corresponding wild-type littermates do not show any mineralization in the tissues affected in PXE [15, 19]. In this study, we first explored genetic factors that might alter the mineralization phenotype in Abcc6−/− mice. First, we examined the role of the Ggcx gene in modifying the mineralization in Abcc6−/− mice. The interest in the Ggcx gene is derived from recent demonstrations that loss-of-function mutations in the GGCX gene in patients can result in development of vitamin K-dependent coagulation factor deficiency and PXE-like skin manifestations [12, 13]. Even more strikingly, the presence of combined heterozygous mutations in the ABCC6 and GGCX genes have been shown to result in PXE-like cutaneous findings, suggesting digenic inheritance of PXE in selected patients [14]. The GGCX gene encodes γ-glutamyl carboxylase, an enzyme necessary for activation of vitamin K-dependent coagulation factors, and thus, homozygous or compound heterozygous mutations in this gene lead to bleeding tendency [24]. This γ-carboxylation activation of the coagulation factors is dependent on the presence of reduced vitamin K in hepatocytes. The activated coagulation factors are then secreted into circulation but in the absence of γ-carboxylation reaction, the coagulation factors remain inactive and bleeding diathesis ensues. γ-Glutamyl carboxylase is also expressed in peripheral cells, such as fibroblasts where it has been shown to activate matrix gla-protein (MGP) also by γ-carboxylation reaction [24-26]. The active form of MGP serves as a powerful anti-mineralization factor, as attested by the fact that MGP KO mice display profound mineralization of connective tissues, particularly in the cardiovascular system [27]. Thus, mutations in the GGCX gene can explain the bleeding disorder and PXE-like cutaneous findings in the same patients due to deficient γ-glutamyl carboxylation of Gla-proteins.
The physiologic substrate transported by ABCC6, which is expressed primarily in the baso-lateral surface of the hepatocytes [28, 29], is currently unknown. However, it has been postulated that one of the substrates might be a vitamin K derivative [30]. In the absence of ABCC6 transporter activity such derivative may not get transported from hepatocytes and becomes deficient in the peripheral connective tissue cells, such as in dermal fibroblasts. Consequently, the absence or reduced quantity of vitamin K co-factor in the peripheral tissue could result in reduced MGP activation and as a result, connective tissue mineralization takes place [2, 26]. Consequently, identification of the critical transport substrate for ABCC6 would allow the development of a treatment strategy to directly introduce the missing factor to the circulation bypassing the liver, thus resulting in potential amelioration, and perhaps cure, for PXE, a currently intractable disease.
In the present study, besides the specific modulation of the mineralization process in the Abcc6−/− mice by haploinsufficiency of the Ggcx gene, the genetic background associated with the Ggcx heterozygous mice, 129X1/SvJ, was shown to delay the onset of the mineralization in PXE mice. In fact, while the Abcc6−/− mice reliably demonstrate mineralization of the vibrissae at 5-6 weeks, the same mice with genetic contribution from 129X1/SvJ develop mineralization not until between 3 and 4 months of age. While the precise gene content of this mouse strain is largely unknown it is conceivable that such modifier genes may also exist in humans and they modulate both the age of onset and the extent of mineralization in patients with PXE, providing partial explanation for the phenotypic variability. In this context, it is of interest that a few recent studies have also suggested genetic modulation in PXE. For example, single nucleotide polymorphisms in the promoter of the secreted phosphoprotein 1 (SPP1, also known as osteopontin) were shown to be associated with PXE, contributing to the susceptibility to this disease [31]. In another study, polymorphisms in the xylosyltransferase genes were shown to be involved in the severity of the disease [32]. Specifically, variations in the XYLT were shown to associate with higher internal organ involvement, while a missense variation (p. A115S) in the XT-I gene resulted in high serum xylosyltransferase activity, potentially reflecting increased remodeling of the extracellular matrix. Finally, genetic variations in the antioxidant genes are risk factors for early disease onset of PXE [33]. This observation is in concurrence with our previous demonstrations that oxidative stress may play a role in the development of PXE [34].
In addition to the genetic modulation, we explored the causes of phenotypic variability in PXE by placing the Abcc6−/−;Ggcx+/− and Abcc6−/−;Ggcx+/+ mice on a special, custom-designed diet with mineral modifications. Our recent studies have demonstrated that high content of magnesium in the mouse diet abolishes the development of mineralization at least up to three months of age [22]. Consequently, we tested the possibility that an experimental diet with mineral modifications, including low magnesium content, might enhance the mineralization process. Indeed, feeding of the mice with this experimental diet resulted in acceleration of the mineralization process in Abcc6−/−; Ggcx+/− and Abcc6−/−;Ggcx+/+ mice as compared to the corresponding mice kept on standard control diet. These findings support the notion that changes in the diet can alter the age of onset and extent of mineralization in PXE. Specifically, our recent studies have shown that supplementation of the diet with magnesium can prevent and stop the progression of mineralization in the Abcc6−/− mouse model of PXE [22]. Collectively, our results of the study demonstrate that mineralization in a PXE mouse model can be modulated by genetic factors and diet, thus implying that PXE is a metabolic disease at the genome-environment interface [35].
Supplementary Material
Acknowledgements
We thank Dr. David Ginsburg (Howard Hughes Medical Institute, University Of Michigan, Ann Arbor, Mi) for providing the Ggcx heterozygous mice; Alix Grand-Pierre for mouse care and genotyping; Reid Oldenburg for assistance in histology; Gianpaolo Guercio for assistance in manuscript preparation; and Dr. Quijie Jiang for helpful discussions.
Funding This work was supported by the United States DHHS, NIH/NIAMS Grants R01 AR28450, R01 AR52627, and R01 AR55225.
Footnotes
Competing interests None
References
- 1.Neldner KH, Struk B. In: Pseudoxanthoma elasticum: In Connective tissue and its heritable disorders: Molecular, genetic and medical aspects. Royce PM, Steinmann B, editors. Wiley-Liss, Inc.; New York: 2002. pp. 561–83. [Google Scholar]
- 2.Li Q, Jiang Q, Pfendner E, Váradi A, Uitto J. Pseudoxanthoma elasticum: Clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgalas I, Papaconstantinou D, Koutsandrea C, Kalantzis G, Karagiannis D, Georgopoulos G, Ladas I. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag. 2009;5:81–9. [PMC free article] [PubMed] [Google Scholar]
- 4.McKusick VA. Heritable disorders of connective tissues. 4th Ed. Mosby; 1972. [Google Scholar]
- 5.Christiano AM, Lebwohl MG, Boyd CD, Uitto J. Workshop on pseudoxanthoma elasticum: molecular biology and pathology of the elastic fibers. J Invest Dermatol. 1992;99:660–3. doi: 10.1111/1523-1747.ep12668156. [DOI] [PubMed] [Google Scholar]
- 6.Raybould MC, Birley AJ, Moss C, Hultén M, McKeown CM. Exclusion of an elastin gene (ELN) mutation as the cause of pseudoxanthoma elasticum (PXE) in one family. Clin Genet. 1994;45:48–51. doi: 10.1111/j.1399-0004.1994.tb03990.x. [DOI] [PubMed] [Google Scholar]
- 7.Cai L, Struk B, Adams MD, Ji W, Haaf T, Kang HL, Dho SH, Xu X, Ringpfeil F, Nancarrow J, et al. A 500-kb region on chromosome 16p13.1 contains the pseudoxanthoma elasticum locus: high-resolution mapping and genomic structure. J Mol Med. 2000;78:36–46. doi: 10.1007/s001090000079. [DOI] [PubMed] [Google Scholar]
- 8.Le Saux O, Urban Z, Göring HH, Csiszar K, Pope FM, Richards A, Pasquali-Ronchetti I, Terry S, Bercovitch L, Lebwohl MG, et al. Pseudoxanthoma elasticum maps to an 820-kb region of the p13.1 region of chromosome 16. Genomics. 1999;62:1–10. doi: 10.1006/geno.1999.5925. [DOI] [PubMed] [Google Scholar]
- 9.Pfendner EG, Vanakker O, Terry SF, Vourthis S, McAndrew P, McLain MR, Fratta S, Marais AS, Hariri S, Coucke PJ, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–8. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamlin N, Beck K, Bacchelli B, Cianciulli P, Pasquali-Ronchetti I, Le Saux O. Acquired pseudoxanthoma elasticum-like syndrome in beta-thalassaemia patients. Br J Haematol. 2003;122:852–4. doi: 10.1046/j.1365-2141.2003.04484.x. [DOI] [PubMed] [Google Scholar]
- 11.Baccarani-Contri M, Bacchelli B, Boraldi F, Quaglino D, Taparelli F, Carnevali E, Francomano MA, Seidenari S, Bettoli V, De Sanctis V, et al. Characterization of pseudoxanthoma elasticum-like lesions in the skin of patients with beta-thalassemia. J Am Acad Dermatol. 2001;44:33–9. doi: 10.1067/mjd.2001.110045. [DOI] [PubMed] [Google Scholar]
- 12.Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali-Ronchetti I, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;27:581–7. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Schurgers L, Smith A, Tsokos M, Uitto J, Cowen E. Co-existent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency. Am J Pathol. 2009;174:534–40. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Grange D, Armstrong N, Whelan A, Hurley M, Rishavy M, Hallgren K, Berkner K, Schrugers L, Jiang Q, Uitto J. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2007;129:553–63. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klement JF, Matsuzaki Y, Jiang Q-J, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, et al. Targeted ablation of the ABCC6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu A, Sun H, Raymond RM, Jr, Furie BC, Bronstein M, Kaufman RJ, Westrick R, Ginsburg D. Fatal hemorrhage in mice lacking gamma-glutamyl carboxylase. Blood. 2007;109:5270–5. doi: 10.1182/blood-2006-12-064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaRusso J, Jiang Q, Li Q, Uitto J. Ectopic mineralization of connective tissue in Abcc6−/− mice: effects of dietary modifications and a phosphate binder – a preliminary study. Exp Dermatol. 2008;17:203–7. doi: 10.1111/j.1600-0625.2007.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel S, Castellan N. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill; Singapore: 1988. pp. 180–1. [Google Scholar]
- 19.Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: Systemic and local regulatory factors. J Invest Dermatol. 2007;127:1392–402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- 20.Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 21.Renie WA, Pyeritz RE, Combs J, Fine SL. Pseudoxanthoma elasticum: high calcium intake in early life correlates with severity. Am J Med Genet. 1984;19:235–44. doi: 10.1002/ajmg.1320190205. [DOI] [PubMed] [Google Scholar]
- 22.LaRusso J, Li Q, Jiang Q, Uitto J. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) J Invest Dermatol. 2009;129:1388–94. doi: 10.1038/jid.2008.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringpfeil F, McGuigan K, Fuchsel L, Kozic H, Larralde M, Lebwohl M, Uitto J. Pseudoxanthoma elasticum is a recessive disease characterized by compound heterozygosity. J Invest Dermatol. 2006;126:782–6. doi: 10.1038/sj.jid.5700115. [DOI] [PubMed] [Google Scholar]
- 24.Berkner KL. The vitamin K-dependent carboxylase. Ann Rev Nutr. 2005;25:127–49. doi: 10.1146/annurev.nutr.25.050304.092713. [DOI] [PubMed] [Google Scholar]
- 25.Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Mtab Care. 2000;3:433–8. doi: 10.1097/00075197-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gheduzzi D, Boraldi F, Annovi G, DeVincenzi CP, Schurgers LJ, Vermeer C, Quaglino D, Ronchetti IP. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest. 2007;87:998–1008. doi: 10.1038/labinvest.3700667. [DOI] [PubMed] [Google Scholar]
- 27.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartillage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 28.Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–8. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- 29.Iliás A, Urban Z, Seidl TL, Le Saux O, Sinkó E, Boyd CD, Sarkadi B, Váradi A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–7. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- 30.Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (Multidrug Resistance Protein 6) in patients with pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–9. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- 31.Hendig D, Arndt M, Szliska C, Kleesiek K, Götting C. SPP1 promoter polymorphisms: identification of the first modifier gene for pseudoxanthoma elasticum. Clin Chem. 2007;53:829–36. doi: 10.1373/clinchem.2006.083675. [DOI] [PubMed] [Google Scholar]
- 32.Schöen S, Schulz V, Prante C, Hendig D, Szaliska C, Kuhn J, Kleesiek K, Götting C. Polymorphisms in the xylosyltransferase genes cause higher serum XT-I activity in patients with pseudoxanthoma elasticum (PXE) and are involved in a severe disease course. J Med Genet. 2006;43:745–9. doi: 10.1136/jmg.2006.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarbock R, Hendig D, Szliska C, Kleesiek K, Götting C. Pseudoxanthoma elasticum: genetic variations in antioxidant genes are risk factors for early disease onset. Clin Chem. 2007;53:1734–40. doi: 10.1373/clinchem.2007.088211. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Jiang Q, Uitto J. Pseudoxanthoma elasticum: oxidative stress and antioxidant diet in a mouse model (Abcc6−/−) J Invest Dermatol. 2008;128:1160–4. doi: 10.1038/sj.jid.5701145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment-genome interface? Trends Mol Med. 2001;7:13–7. doi: 10.1016/s1471-4914(00)01869-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.