Abstract
Objectives
Prevalence estimates for several liver cancer risk factors–hepatitis C, hepatitis B, and history of alcohol abuse–are substantially higher in U.S. prison populations than in the general population. However, liver cancer mortality data from these populations are lacking. The primary aims of this study were to examine trends in liver cancer mortality rates from 1992 to 2003 among male prisoners in the Texas Department of Criminal Justice (TDCJ) and to compare these rates to general population rates.
Methods
TDCJ data on male decedents (N = 4026) were linked with Texas Vital Statistics multiple-cause-of-death data. Crude average annual liver cancer death rates, average annual percent changes, and standardized mortality ratios were estimated.
Results
Crude liver cancer death rates increased by an average annual 6.1% among male prisoners, which was considerably higher than the average annual percent change among similarly aged males in Texas (2.0%) and the U.S. (2.9%). The number of liver cancer deaths among male prisoners was 4.7 (4.0–5.6) and 6.3 (5.3–7.5) times higher than the expected number of deaths estimated using age-specific rates from these reference populations.
Conclusions
From 1992 to 2003, liver cancer death rates and rate increases were elevated among Texas male prisoners. Findings support previous recommendations for targeted prevention, screening, and treatment of liver cancer risk factors in prison populations.
Keywords: Liver diseases, Cancer, Malignancies, Hepatitis, Prison, Incarceration, Time trend
Introduction
Liver cancer incidence and mortality rates have increased steadily in the United States over the last two decades, largely as a result of increases in the prevalence of chronic hepatitis C virus (HCV) infection and its sequelae (McGlynn et al., 2006; El-Serag et al., 2003). The U.S. HCV epidemic began in the 1960s and peaked in the 1980s, during which the prevalence of HCV risk factors such as injection drug use, needle sharing, and high-risk sexual behavior increased (El-Serag et al., 2000, 2003). These risk factors and, consequently, HCV infections are highly prevalent in U.S. prison populations, with prevalence estimates of HCV infection in U.S. prison populations have ranged from 10-times to more than 20-times that of the general, non-institutionalized U.S. population (Ruiz et al., 2002; Baillargeon et al., 2003; Macalino et al., 2004; Alter et al., 1999; Armstrong et al., 2006). Hepatitis B virus (HBV) infection and history of alcohol abuse–both of which are risk factors for liver cancer–are also highly prevalent in prison populations (Macalino et al., 2004; Fazel et al., 2006). Recent studies have suggested that liver cancer mortality may be elevated in prison populations compared to the general population (Baillargeon et al., 2009; Mathew et al., 2005), but no published studies have examined how liver cancer mortality rates have changed over time in this population or how these rates compare to rates from the general population. The aims of this study were to examine trends in liver cancer mortality rates from 1992 through 2003 among male prisoners in custody of the Texas Department of Criminal Justice (TDCJ), one of the nation’s largest state prison system (Pew Center on the States, 2008), and to compare these rates to state and national rates. In the context of a prison population that exceeds 2.2 million and that continues to grow and to age (Sabol et al., 2007; Mitka, 2004), reliable data describing trends in liver cancer mortality may provide vital information for correctional healthcare planners and policy-makers in assessing, predicting, and addressing the burden of liver cancer among prisoners.
Methods
Prisoner death and census data
A database containing information on all prisoners who died in TDCJ’s custody during 1992–2003 was maintained by the director of TDCJ’s Office of Preventive Medicine. From this database, demographic and other identifying information on decedents were extracted and linked to a multiple-cause-of-death database obtained from the Texas Bureau of Vital Statistics. Multiple-cause-of-death data had been drawn from death certificates, and then corrected for common data entry errors and for certifier errors in delineating multiple causes of death according to current selection rules (National Center for Health Statistics, 2008). Causes of death had been recorded on the death certificate by TDCJ healthcare providers after being ascertained by autopsy, chart reviews, narrative death summaries, and/or findings of the Mortality Review Committee. As in non-correctional medical settings, autopsy rates declined over the study period from ≥90% of prisoner deaths for 1989–1997 to an average of 50% for 1998–2003 (unpublished data, AJ Harzke, December 2008); however, these autopsy rates exceeded those reported in non-correctional medical settings for the entire study period (Shojania et al., 2003). Use of TDCJ and vital statistics data was approved by two institutional review boards (university and state) with prisoner advocate members and subsequently approved by TDCJ.
TDCJ Executive Services provided the data for calculating the average prisoner census, which was used as the denominator for calculating annual death rates. TDCJ Executive Services takes a “snapshot” census of the in-custody population on the last day of each month. For each year, the average of the 12 monthly “snapshot” censuses for males within each stratum (race/ethnicity by 10-year age categories) was calculated.
Data linkage
Six personal identifiers (first, middle, and last names, race/ethnicity, dates of birth and death) representing 10 separate codes were used to link the TDCJ decedent data with the multiple-cause-of-death data. A successful link required a 90% match(9 of 10 codes). Of the total number of prisoner deaths recorded in the TDCJ database (N = 4423), 98% (N = 4343) were successfully linked with the state multiple-cause-of-death data.
Dependent variables
Prisoner deaths for which liver cancer, HCV, HBV, or chronic liver disease/cirrhosis (CLD) was indicated as the underlying cause of death were identified. ICD-9 and ICD-10 codes were: liver cancer = 155.0–155.2, C22.0, C22.2, C22.9; HCV = 070.4, 70.5, B17.1, B18.2; HBV = 070.2–070.3, B16.0–16.9, B18.0–B18.1; CLD = 571.0–571.3, 571.8, 571.9, K70.09, K74.6. Liver cancer included primary liver cancer, intrahepatic bile duct cancer, and unspecified liver cancer. Deaths with liver cancer identified as the underlying cause of death are referred to as “liver cancer deaths” or as deaths “due to liver cancer.” HCV, HBV, and CLD deaths were considered “liver cancer related” if liver cancer was identified as an intervening or contributing cause of death. CLD deaths were not further delineated according to whether or not they were alcohol-related. Preliminaryanalyses indicated that, from 1999 to 2003, nearly all CLD deaths were unspecified with respect to alcohol use. This limitation in the data may have resulted from the change from ICD-9 to ICD-10 codes in 1999.
Statistical analyses
Because very few prisoner deaths due to liver cancer occurred among females (N = 15) or outside the age range of 25–84 years (N = 2), analyses were limited to male prisoners who were 25–84 years of age, N = 4026. Counts and proportions of deaths due to liver cancer and other causes were calculated by categories of race–ethnicity, age, and year of death (SPSS 16.0, 2007). Age- and race-specific average annual liver cancer death rates with exact Poisson 95% confidence intervals (Ulm, 1990) were estimated (StatsDirect Statistical Software 2.7.2, StatsDirect Ltd., 2008). These rates were not further stratified by year of death, as this would have resulted in a large number of strata with 0 or very few deaths.
For deaths with liver cancer indicated as the underlying cause, Poisson regression models on a log normal scale were used to describe trends in crude annual death rates and to calculate an average annual percent change (AAPC) with 95% confidence intervals (Joinpoint 3.3, National Cancer Institute/Statistical Research and Applications, 2008). Average annual death rates for 3-year periods per 100,000 population along with 95% exact Poisson confidence intervals were estimated (Ulm, 1990). Average annual death rates were age-standardized using the indirect method and standardized mortality ratios (SMRs) were estimated (StatsDirect Statistical Software 2.7.2, StatsDirect Ltd., 2008). The reference populations were similarly aged males in Texas and the U.S. Age-specific death rates (10-year categories) for reference populations were obtained through CDC WONDER (United States Department of Health and Human Services, 2008). Because of a lack of age- and race-specific mortality data and because of small numbers of observed deaths when stratified by both age, race, and year of death, SMRs were not race-standardized and were based on 3-year average annual rates rather than annual rates.
Additionally, to allow for a more complete accounting of liver cancer related mortality, the proportion of HCV, HBV, and CLD, deaths that were liver cancer related was calculated. Also, to gain a better understanding of the contribution of these risk factors to liver cancer mortality in this population, we calculated the proportion of liver cancer deaths for which HCV, HBV, or CLD was identified as intervening or contributing cause of death (SPSS 16.0, 2007).
Results
From 1992 to 2003, the size of the prison population grew substantially, while the proportions of older prisoners increased. The average daily census of TDCJ male prisoners, aged 25–84 years, nearly tripled over the study period, growing from 40,994 to 117,147 prisoners. The proportion of prisoners aged 35–54 years increased from 42% of the prison population in 1992 to 56% in 2003, and prisoners aged 55–84 years from 3% in 1992 to 6% in 2003. Changes in the distribution of the population across categories of race–ethnicity were fairly modest, with the proportion of Hispanic prisoners increasing from 24% to 28% and the proportion of Black prisoners declining from 47% to 40%. Over the study period, the annual number of deaths grew steadily from 170 deaths in 1992 to peak at 452 deaths in 2000, and then declined to 360 deaths in 2003. However, the annual all-cause death rate remained fairly stable during the study period, ranging from 0.3% to 0.4% [data not shown].
Liver cancer was identified as the underlying cause of death for more than 3% of prisoner deaths (134/4026) and was recorded as an intervening or contributing cause for an additional 26 deaths, including 8.6% of HCV deaths (11/128) and 4.4% of CLD deaths (9/203). No HBV deaths were considered liver cancer related. Notably, of deaths for which liver cancer was identified as the underlying cause, HCV was identified as an intervening or contributing cause of death for 32% (43/134), CLD for 29% (39/134), and HBV for 4% (6/134) [data not shown].
Liver cancer deaths were distributed across categories of race–ethnicity, age, and year of death in a manner that differed from deaths due to other causes (Table 1). Seventy percent of liver cancer deaths occurred in the latter half of the study period, while slightly more than half of deaths due to other causes occurred during the same period. Compared to other causes of death, greater proportions of liver cancer deaths occurred among Hispanic prisoners and older prisoners (≥45 years). Similarly, age- and race-specific average annual liver cancer death rates were generally higher among older prisoners and Hispanic prisoners (Table 2).
Table 1.
Deaths due to liver cancera and other causes by race/ethnicity, age, and year of death among male inmates in Texas,b 1992–2003.
| Liver cancer deaths | Other deaths | All deaths | ||||
|---|---|---|---|---|---|---|
|
N = 134 |
N = 3892 |
N = 4026 |
||||
| n | % (column) | n | % (column) | n | % (column) | |
| Race/ethnicity | ||||||
| Black | 34 | 25.4 | 1685 | 43.3 | 1719 | 42.7 |
| Hispanic | 51 | 38.1 | 745 | 19.1 | 796 | 19.8 |
| White | 47 | 35.1 | 1441 | 37.0 | 1488 | 37.0 |
| Other | 2 | 1.5 | 21 | 0.5 | 23 | 0.6 |
| Age | ||||||
| 25–34 | 3 | 2.2 | 664 | 17.1 | 667 | 16.6 |
| 35–44 | 20 | 14.9 | 1116 | 28.7 | 1136 | 28.2 |
| 45–54 | 65 | 48.5 | 1028 | 26.4 | 1093 | 27.1 |
| ≥55 | 46 | 34.3 | 1084 | 27.9 | 1130 | 28.1 |
| Year of death | ||||||
| 1992–1994 | 11 | 8.2 | 677 | 17.4 | 688 | 17.1 |
| 1995–1997 | 32 | 23.9 | 973 | 25.0 | 1005 | 25.0 |
| 1998–2000 | 37 | 27.6 | 1160 | 29.8 | 1197 | 29.7 |
| 2001–2003 | 54 | 40.3 | 1082 | 27.8 | 1136 | 28.2 |
ICD-9 and ICD-10 codes for liver cancer were 155.0, 155.2, C22.0, C22.2, and C22.9.
Limited to males that were ages 25–84 years in the Texas Department of Criminal Justice (TDCJ).
Table 2.
Age- and race-specific average annual liver cancera death rates per 100,000 populationb among male inmates in Texas,c 1992–2003.
| Black |
Hispanic |
White |
All |
|||||
|---|---|---|---|---|---|---|---|---|
| Rate | 95%CId | Rate | 95% CId | Rate | 95% CId | Rate | 95% CId | |
| Age | ||||||||
| 25–34 | 0.9 | 1.0–3.2 | 0 | 0–2.6 | 0.7 | 0.02–4.2 | 0.6 | 0.1–1.7 |
| 35–44 | 2.6 | 0.9–6.2 | 7.2 | 2.9–14.8 | 6.0 | 2.6–11.9 | 4.8 | 2.9–7.4 |
| 45–54 | 23.7 | 13.5–38.4 | 75.4 | 50.1–109.0 | 32.9 | 19.8–51.3 | 40.1 | 31.0–51.1 |
| ≥55 | 68.3 | 34.1–122.3 | 135.6 | 77.5–220.3 | 85.1 | 51.3–133.0 | 91.7 | 67.1–122.3 |
| All ages | 6.8 | 4.7–9.5 | 17.6 | 13.1–23.1 | 13.6 | 10.0–18.0 | 11.8 | 9.9–14.0 |
ICD-9 and ICD-10 codes for liver cancer were 155.0, 155.2, C22.0, C22.2, and C22.9.
Denominators for calculation of stratum-specific rates were based on cross-tabulations of the sum of the average annual censuses, 1992–2003.
Limited to males in custody, ages 25–84 years, Texas Department of Criminal Justice.
Confidence intervals are 95% exact Poisson (Ulm, 1990).
The crude average annual liver cancer death rate per 100,000 was 11.8 (9.9–14.0) for the study period, increasing from 7.0 (3.5–12.5) in 1992–1994 to 15.6 (11.8–20.4) in 2001–2003 (Table 3). From 1992 to 2003, crude liver cancer death rates increased by an average annual 6.1% among male Texas prisoners, which was considerably higher than the average annual percent change among similarly aged males in Texas and the U.S., which were 2.0% and 2.9%, respectively (Fig. 1). SMRs indicated that liver cancer deaths among male prisoners were 4.7 (4.0–5.6) and 6.3 (5.3–7.5) times higher than the expected number of deaths estimated using age-specific rates from these reference populations (Table 3).
Table 3.
Crude average annual liver cancer death ratesa,b and standardized mortality ratios (SMRs),c male inmates in Texas,d 1992–2003.
| Years | Rate per 100,000 | CIe | SMR—Texasc | CI | SMR—USc | CI |
|---|---|---|---|---|---|---|
| 1992–1994 | 7.0 | 3.5–12.5 | 3.6 | 1.8–6.4 | 4.7 | 2.4–8.5 |
| 1995–1997 | 11.1 | 7.6–15.6 | 4.7 | 3.2–6.6 | 6.1 | 4.1–6.4 |
| 1998–2000 | 10.8 | 7.4–14.9 | 3.5 | 2.5–4.8 | 4.5 | 3.2–6.3 |
| 2001–2003 | 15.6 | 11.8–20.4 | 3.8 | 2.9–5.0 | 5.1 | 3.8–6.6 |
| 1992–2003 | 11.8 | 9.9–14.0 | 4.7 | 4.0–5.6 | 6.3 | 5.3–7.5 |
ICD-9 and ICD-10 codes for liver cancer were 155.0, 155.2, C22.0, C22.2, and C22.9.
Because of the small number of observed events per year, average annual death rates over 3-year periods were calculated to improve stability and precision of estimates.
Reference populations were 25–84-year-old males in Texas and the United States.
Limited to males in custody, ages 25–84 years, Texas Department of Criminal Justice.
Confidence intervals are 95% exact Poisson (Ulm, 1990).
Fig. 1.
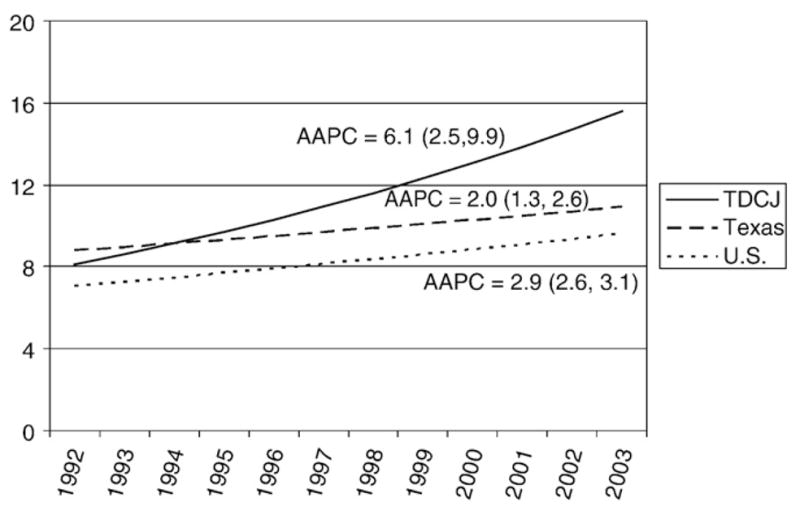
Trends in crude annual liver cancer death rates per 100,000 among males, ages 25–84 years, in the Texas Department of Criminal Justice (TDCJ), Texas, and the U.S., 1992–2003. Trends are described by the average annual percent change (AAPC) with 95% exact Poisson confidence intervals. AAPCs were based on Poisson modeled values on a log normal scale and produced using Joinpoint 3.3.
Discussion
These data reveal a substantial and growing burden of liver cancer related deaths among male prisoners in Texas. From 1992 to 2003, the average annual percent change in crude liver cancer death rates was higher in this prison population than among similarly aged males in Texas and the U.S., suggesting that these rates increased more rapidly in the prison population than in the reference populations. Age-standardized average annual crude liver cancer death rates were also considerably higher in this prison population compared to these reference populations.
The relatively high liver cancer mortality rates among male prisoners estimated in our study reflect the high prevalence of liver cancer in the Texas prison population found in other recent studies (Mathew et al., 2005; Baillargeon et al., 2009). In the U.S. population overall, the 5-year survival for liver cancer is a rather low 10% (American Cancer Society, 2009). The high prevalence of liver cancer morbidity and mortality in this population is likely due largely to a higher prevalence of HBV and HCV infections and history of alcohol abuse or dependence. During the study period, HCV seroprevalence estimates in prisoner samples ranged from 23.1% to 41.0% (Ruiz et al., 2002; Baillargeon et al., 2003; Macalino et al., 2004), while general population estimates ranged from 1.6% to 1.8% (Alter et al., 1999; Armstrong et al., 2006). HBV seroprevalence estimates ranged from 18% to 30% in prisoner samples (CDC, 2004; Solomon et al., 2004), while averaging about 5% in the general population. Notably, HBV or HCV was a causal factor in 37% of liver cancer deaths in our sample during the study period. Alcohol abuse may be particularly important in this prison population, because male inmates in Texas show higher rates of alcohol abuse or dependence than male inmates in other states (Fazel et al., 2006). About 30% of newly admitted male inmates in Texas meet diagnostic criteria for alcohol abuse or dependence (McClellan et al., 1997), as compared to the prevalence of alcohol abuse or dependence in the general population, which ranges from 3.8% to 5.5% (Caetano and Tam, 1995; Grant et al., 2004). Moreover, CLD was identified as a causal factor in 29% of liver cancer deaths in this sample.
Apparent increases in liver cancer mortality over time similarly reflect increases in the incidence of liver cancer in the Texas prison population (Mathew et al., 2005). The increase in liver cancer can be explained, in part, by an increase in HBV or HCV infections over the study period, as the incidence of the diagnosis of these conditions increased sharply in the late 1999s (unpublished data, Harzke, 2009). The increase in liver cancer may also be explained by demographic shifts in the study population over time. The proportion of Hispanic prisoners, among whom higher rates of liver cancer have been shown (Baillargeon et al., 2009), increased slightly over the study period. Conversely, the proportion of Black prisoners, among whom lower liver cancer rates have been demonstrated (Baillargeon et al., 2009), decreased moderately over the study period. The proportion of older inmates increased substantially in the study population over the study period–due primarily to longer prison sentences (Beck and Mumola, 1999) and an aging prison population (Sabol et al., 2007; Mitka, 2004)–and this may play a key role in explaining the increase in liver cancer morbidity and mortality. Older inmates with chronic hepatitis infection or histories of alcohol abuse would have experienced these risk factors for a longer duration and, in turn, would be at increased risk for CLD and liver cancer and decreased survival time (Mathew et al., 2005). Notably, although crude average annual percent change values for liver cancer death rates were higher among male prisoners in Texas compared to males in Texas and the U.S., standardized mortality ratios did not increase over time. This could suggest that, after adjusting for age, increases in liver cancer death rates in the prison population were similar to those in the reference populations. However, because a number of factors can affect the magnitude of an estimated SMR, this interpretation may not fully explain the discrepancy (Reitsma et al., 2000).
Several study limitations should be considered in interpreting findings here. Study findings may not generalize to other male state prison populations because of variations in the prevalence of risk factors for liver cancer across these populations (Baillargeon et al., 2003; Macalino et al., 2004; Fox et al., 2005). For liver cancer deaths with CLD identified as a causal factor, we were unable to examine whether CLD was alcohol-related or not because of aforementioned limitations in the source data. However, given the prevalence of alcohol abuse in the population, it is reasonable to assume that a large proportion of liver cancer deaths with CLD identified as a causal factor was related to alcohol abuse—especially where viral hepatitis was not identified as a causal factor. Confidence intervals for average annual percent change in crude annual liver cancer death rates, crude average annual liver cancer death rates, and SMRs were somewhat wide, reflecting a lack of precision due to the small number of annual deaths. However, the direction of point estimates and confidence intervals for these values support our interpretations. Finally, because our analyses were limited to prisoners who died while incarcerated, our study reflects only a small fraction of the burden that liver cancer morbidity places on a correctional healthcare system. In a recent study of Texas prisoners (Baillargeon et al., 2009), about half as many Texas prisoners died from liver cancer while in custody (N = 213) than were treated for liver cancer and released to their communities (N = 484). Moreover, in a recent North Carolina study, age-standardized liver cancer deaths among former state inmates were 3 times that of the local non-incarcerated population (Rosen et al., 2008).
Despite these limitations, our study is the first to use vital statistics data to assess liver cancer as an underlying and contributing cause of mortality in a prison population. Additionally, by using multiple-cause-of-death data, we were able to assess the contribution of HBV and HCV to liver cancer deaths and, conversely, the contribution of liver cancer to HBV and HCV deaths. Our study represents an important step toward assessing the burden of liver cancer and related conditions on a correctional healthcare system.
Specifically, our findings point to the relatively high and growing burden of liver cancer among male prisoners. An implication of these findings is that the costs of liver cancer, as well as other HCV-related conditions, are substantial and can be expected to increase in prison populations if current trends continue (Tan et al., 2008; Salomon et al., 2003; Pisu et al., 2002). The annual per case costs of hepatocellular carcinoma in prison have been estimated at U.S. $42,255 per case (Tan et al., 2008).
To reduce the burden of liver cancer morbidity, mortality, and related costs, targeted prevention, screening, and treatment of liver cancer risk factors in prison populations have been recommended (Spaulding et al., 2006; Weinbaum et al., 2003). Targeted vaccination of prisoners for HBV has been found to be cost-saving from the perspective of the U.S. healthcare system (Pisu et al., 2002). HBV and HCV screening along with early intervention through alcohol abstinence or interferon treatment may reduce liver cancer related morbidity, mortality, and costs (Tan et al., 2008; Salomon et al., 2003; Pisu et al., 2002). However, policies regarding HBV vaccination and testing and treatment for chronic HBV and HCV infections vary widely across prison systems (Beck and Maruschak, 2004). It is possible that behavioral risk reduction education along with substance abuse treatment may lessen alcohol use and may decrease HCV prevalence in this population by decreasing unsafe injection drug use (Spaulding et al., 2006). However, protocols for alcohol and substance abuse screening and treatment also vary widely across prison systems, but generally, available programming is sufficient to serve only a fraction of prisoners meeting the diagnostic criteria for alcohol or drug dependence (Mumola, 1999).
To determine correctional healthcare priorities and policy directions, of course, costs related to liver cancer must be considered alongside costs associated with other causes of morbidity and mortality in the prison population. Because state correctional healthcare systems are typically under-funded, other public funding may be required to provide preventive and therapeutic services that meet community standards. As prison populations continue to age and increase in size (Sabol et al., 2007; Mitka, 2004), the growing role of correctional healthcare systems in preventing, detecting, and treating liver cancer must be considered.
Conclusions
From 1992 through 2003, liver cancer death rates and rate increases were considerably higher among male prisoners in Texas than among similarly aged males in Texas and the U.S. These findings suggest that prison populations have experienced substantial increases in liver cancer mortality and that such increases may continue. Moreover, these findings lend support to previous recommendations for targeted prevention, screening, and treatment of liver cancer risk factors in prison populations.
Acknowledgments
Early phases of this work were funded by a Predoctoral Cancer Control and Prevention Fellowship from the National Cancer Institute (NIH/NCI R25 CA 57712). The fellow was Amy Jo Harzke and the grantee was Patricia Dolan Mullen, University of Texas School of Public Health. The research described herein was coordinated in part by the Texas Department of Criminal Justice (TDCJ), research agreement #470-RM05. The contents of this manuscript reflect the views of the authors and do not necessarily reflect the views or policies of the TDCJ.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Liver cancer overview: how is liver cancer treated. 2009 Available at: http://www.cancer.org/docroot/cri/content/cri_2_2_4x_how_is_liver_cancer_treated_25.asp.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Baillargeon J, Wu H, Kelley MF, Grady J, Linthicum L, Dunn K. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–48. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Baillargeon J, Snyder N, Paar D, Baillargeon G, Spaulding A, Pollock BH, et al. Hepatocellular carcinoma prevalence and mortality in a male state prison population. Public Health Rep. 2009;124:120–126. doi: 10.1177/003335490912400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AJ, Maruschak LM. Bureau of Justice Statistics Special Report (NCJ 199173C) US Department of Justice; Washington, DC: 2004. Hepatitis Testing and Treatment in State Prisons. Available at: http://www.ojp.usdoj.gov/bjs/pub/pdf/httsp.pdf. [Google Scholar]
- Beck AJ, Mumola CJ. Bureau of Justice Statistics Special Report (NCJ 175687) US Department of Justice; Washington, DC: 1999. Prisoners in 1998. Available at: http://www.ojp.usdoj.gov/bjs/pub/pdf/p98.pdf. [Google Scholar]
- Caetano R, Tam TW. Prevalence and correlates of DSM-IV and ICD-10 alcohol dependence: 1990 U.S. National Alcohol Survey. Alcohol Alcohol. 1995;30:177–186. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Hepatitis B vaccination of inmates in correctional facilities—Texas, 2000–2002. MMWR Morb Mortal Wkly Rep. 2004;53:681–683. [PubMed] [Google Scholar]
- El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Davila JA, Peterson NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006;101:181–191. doi: 10.1111/j.1360-0443.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence of and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2000–2001. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Macalino GE, Vlahov D, Sanford-Colby S, Patel S, Sabin K, Salas C, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94:1218–1223. doi: 10.2105/ajph.94.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew P, Elting L, Cooksley C, Owen S, Lin J. Cancer in an incarcerated population. Cancer. 2005;104:2197–2204. doi: 10.1002/cncr.21468. [DOI] [PubMed] [Google Scholar]
- McClellan DS, Farabee D, Crouch BM. Early victimization, drug use, and criminality: a comparison of male and female prisoners. Crim Justice Behav. 1997;24:455–476. [Google Scholar]
- McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–1203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- Mitka M. Aging prisoners stressing health care system. JAMA. 2004;292:423–424. doi: 10.1001/jama.292.4.423. [DOI] [PubMed] [Google Scholar]
- Mumola CJ. Bureau of Justice Statistics Special Report (NCJ 172871) US Department of Justice; Washington, DC: 1999. Substance Abuse and Treatment, State and Federal Prisoners, 1997. Available at: http://www.ojp.usdoj.gov/bjs/pub/pdf/satsfp97.pdf. [Google Scholar]
- National Cancer Institute/Statistical Research and Applications. Joinpoint regression program: average annual percent change. 2008 Available at: http://srab.cancer.gov/joinpoint/aapc.html.
- National Center for Health Statistics/Centers for Disease Control and Prevention. Instructions for Classifying the Underlying Cause-of-Death. U.S. Department of Health and Human Services; Hyattsville, MD: 2008. Available at: http://www.cdc.gov/nchs/data/dvs/2a2008Final.pdf. [Google Scholar]
- One in 100: Behind Bars in America. Washington, DC: Pew Center on the States; 2008. Available at: http://www.pewcenteronthestates.org/report_detail.aspx?id=35904. [Google Scholar]
- Pisu M, Meltzer MI, Lyerla R. Cost-effectiveness of hepatitis B vaccination of prison inmates. Vaccine. 2002;21:312–321. doi: 10.1016/s0264-410x(02)00457-7. [DOI] [PubMed] [Google Scholar]
- Reitsma JB, Bonsel GJ, Gunning-Schepers LJ, Tijssen JG. Lack of standards in direct standardization. Am J Public Health. 2000;90:139–140. doi: 10.2105/ajph.90.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DL, Schoenbach VJ, Wohl DA. All-cause and cause-specific mortality among men released from state prison, 1980–2005. Am J Public Health. 2008;98:2278–2284. doi: 10.2105/AJPH.2007.121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JD, Moliter F, Plagenhoef JA. Trends in hepatitis C and HIV infection among inmates entering prisons in California, 1994 versus 1999. AIDS. 2002;16:2236–2238. doi: 10.1097/00002030-200211080-00023. [DOI] [PubMed] [Google Scholar]
- Sabol WJ, Minton TD, Harrison PM. Bureau of Justice Statistics Bulletin (NCJ 217675) US Department of Justice; Washington, DC: 2007. Prison and Jail Inmates at Midyear 2006. Available at: http://www.ojp.usdoj.gov/bjs/pub/pdf/pjim06.pdf. [Google Scholar]
- Salomon JA, Weinstein MC, Hammitt JK, et al. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228–237. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- Shojania KG, Burton EC, McDonald KM, et al. Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA. 2003;289:2849–2856. doi: 10.1001/jama.289.21.2849. [DOI] [PubMed] [Google Scholar]
- Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81:25–37. doi: 10.1093/jurban/jth085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding AC, Weinbaum CM, Lau DT, Sterling R, Seef LB, Margolis HS, et al. A framework for management of hepatitis C in prisons. Ann Int Med. 2006;144:762–769. doi: 10.7326/0003-4819-144-10-200605160-00010. [DOI] [PubMed] [Google Scholar]
- StatsDirect Statistical Software, Version 2.7.2. StatsDirect Ltd; Cheshire, WA, UK: [Google Scholar]
- Tan JA, Joseph TA, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology. 2008;48:1387–1395. doi: 10.1002/hep.22509. [DOI] [PubMed] [Google Scholar]
- Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio. Am J Epidemiol. 1990;131:373–375. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Centers for Health Statistics (NCHS), Office of Analysis and Epidemiology (OAE) Compressed Mortality File (CMF) compiled from CMF 1968–1988, Series 20, No. 2A 2000, CMF 1989–1998, Series 20, No. 2E 2003 and CMF 1999–2003, Series 20, No. 2I 2006 on CDC WONDER On-line Database. 2008 Available at: http://wonder.cdc.gov/mortsql.html.
- Weinbaum C, Lyerla R, Margolis HS. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR Recomm Rep. 2003;52 (RR-1):1–36. [PubMed] [Google Scholar]


