Abstract
Studies into the pathophysiology of schizophrenia have consistently demonstrated a dysfunction of dopamine (DA) system regulation in this disorder. This includes hyper-responsivity to DA agonists, the therapeutic efficacy of DA antagonists, and augmented striatal DA release in response to amphetamine. Nonetheless, there is little evidence for a pathological alteration with the DA system itself in schizophrenia. Instead, it is suggested that the disturbance lies in the manner by which the DA system is regulated. Recently, rodent models of schizophrenia have been advanced based on developmental disruption that recapitulates many of the symptoms observed in human schizophrenia patients. We found that administration of the mitotoxin methylaxosymethanol acetate (MAM) to rats at gestational day 17 leads to adult rats that exhibit neuroanatomical, pharmacological, and behavioral characteristics consistent with schizophrenia. These rats also exhibit hyperactivity within the ventral subiculum of the hippocampus that corresponds to a loss of parvalbumin-containing interneurons. This hyperactivity causes an increase in the population activity of the DA neurons (i.e., more DA neurons are firing spontaneously), thus increasing the responsivity of the DA system to stimuli. When the ventral subiculum is inactivated, DA neuron population activity is restored to baseline, and the hyper-responsivity to amphetamine is normalized to that observed in control rats. These findings demonstrate a direct link between the hippocampal pathophysiology, interneuronal alterations, and hyperdopaminergic state observed in the schizophrenia patient. Moreover, this suggests an alternate pharmacotherapeutic approach based on the normalization of hippocampal activity in the treatment of schizophrenia in humans.
Keywords: Schizophrenia, Dopamine, Hippocampus, MAM, GABA, Parvalbumin
Introduction
Schizophrenia is a psychotic disorder characterized by a wide variety of symptoms, which can be defined into three groups; cognitive, positive and negative symptoms (Andreasen, 1995). The cognitive symptoms are largely deficits related to memory and executive function. The positive symptoms include psychosis and are characterized by delusions and hallucinations, while the negative symptoms largely relate to blunted affect, avolition and anhedonia (Andreasen, 1995). Given the wide range of neuropsychiatric symptoms, it is perhaps not surprising that the neuropathology of schizophrenia is both subtle and widespread, involving both cortical and subcortical deficits. Although there are a number of pathologies consistently observed in the schizophrenia patient, as yet there is no clear primary origin of the disease.
The Dopamine Hypothesis of Schizophrenia
The dopamine hypothesis suggests that the over-activity of mesolimbic dopamine transmission underlies the positive symptoms of the disease (Abi-Dargham, 2004; Carlsson et al., 2000; Laruelle & Abi-Dargham, 1999). This hypothesis is based upon a number of observations, the most prominent being that all current antipsychotic drugs bind to and block the dopamine D2 receptor(Miyamoto et al., 2004). Further evidence for dopamine hyperfunction stems from the ability of dopamine agonists to exacerbate psychosis, and imaging studies demonstrating increased amphetamine-induced dopamine release in schizophrenia patients that correlates with exacerbation of psychosis (Abi-Dargham, 2004; Laruelle & Abi-Dargham, 1999).
Although there is significant well established data in support of the dopamine hypothesis, a major caveat of this theory is that there appears to be no primary pathology in the midbrain dopamine system of schizophrenia patients. This has led researchers to propose that, although the dopamine system appears normal in the schizophrenia patient, the primary disease pathology lays upstream of the ventral mesencephalon. Moreover, it is pathology in these regions that results in the aberrant regulation of ascending dopamine transmission (Abi-Dargham, 2004; Carlsson et al., 2000; Grace, 1991, 2000; Laruelle & Abi-Dargham, 1999). At present the regions associated with this dysregulation are unknown; however, there is a significant literature demonstrating that, in addition to dopamine hyperactivity, schizophrenia is associated with alterations in afferent structures to the VTA with two primary regions being the medial prefrontal cortex (thought to be largely associated with cognitive deficits (Lewis & Gonzalez-Burgos, 2007)) and the hippocampus, a temporal lobe structure associated with learning and memory(Moses et al., 2002; Squire et al., 2004).
Hippocampal Dysfunction in Schizophrenia
Evidence for abnormal hippocampal structure and function in schizophrenia has come largely from postmortem and neuro-imaging studies (Harrison, 1999a; Heckers, 2004; Heckers & Konradi, 2002; Nelson et al., 1998; Shenton et al., 2001). More specifically, a postmortem reduction in hippocampal volume is one of the more consistent structural abnormalities observed in schizophrenia patients (Harrison, 1999a; Heckers, 2004; Heckers & Konradi, 2002; Nelson et al., 1998; Shenton et al., 2001). How this affects hippocampal information processing is not entirely known; however, recent functional imaging studies have suggested augmented hippocampal activity at rest and an abnormal information processing during the performance of memory retrieval tasks (Heckers et al., 1998; Lahti et al., 2006; Medoff et al., 2001; Meyer-Lindenberg et al., 2001; Nordahl et al., 1996; Weiss et al., 2006). Thus, hippocampal dysfunction has been demonstrated to contribute not only to impaired information processing but also to the positive symptoms of the disease. Furthermore, the role for the hippocampus in psychosis suggests an interaction with the midbrain dopamine system. Indeed the ventral subiculum of the hippocampus is a region that potently modulates dopamine neuron activity (Floresco et al., 2003; Legault & Wise, 1999; Lodge & Grace, 2006). Thus, rodent studies have demonstrated that NMDA activation of the ventral subiculum significantly increases dopamine neuron population activity (the number of dopamine neurons firing spontaneously) which is correlated with increased extrasynaptic DA efflux in the nucleus accumbens. Since the hippocampus doesn’t directly innervate the VTA, it has been suggested that the subiculum modulation of DA neuron activity is dependent on a polysynaptic (vHipp-NAc-VP) projection (Floresco et al., 2003; Lodge & Grace, 2006). Given evidence correlating hippocampal dysfunction with psychosis in schizophrenia (Harrison, 2004), we propose that aberrant hippocampal activity may underlie the DA dysregulation in this disorder.
The pathogenesis of the hippocampal abnormalities in schizophrenia is still largely unknown. Furthermore, although polymorphisms in a number of hippocampal genes have been identified as potential risk factors, there is no selective genetic dysfunction associated with the disease (Meyer-Lindenberg & Weinberger, 2006). Thus, increasing evidence has suggested that developmental alterations, specifically during cortical development, lead to prefrontal and hippocampal dysfunctions (Lewis & Levitt, 2002; Marenco & Weinberger, 2000; Moore et al., 2006; Waddington et al., 1999). The cause of the developmental alterations is not known; however, suggestions have included prenatal viral infections (Hornig & Lipkin, 2001; Murray et al., 1992; Pearce, 2001), trauma and birth complications (McNeil, 1995; McNeil et al., 2000). Nonetheless it seems likely that schizophrenia is, as least in part, a developmental disorder resulting in aberrant cortical (prefrontal and hippocampal) regulation and associated with increased dopaminergic activity.
The MAM-treated Rat as a Developmental Model of Schizophrenia
Recent studies have made use of these observations to produce a developmental disruption animal model of schizophrenia to further enable research into the pathophysiology of this disorder (Flagstad et al., 2004; Grace & Moore, 1998; Moore et al., 2006). One model that has substantial face validity utilizes the administration of the DNA methylating agent, methylazoxymethanol acetate (MAM) to pregnant dams on gestational day (GD) 17(Flagstad et al., 2004; Goto & Grace, 2006; Grace & Moore, 1998; Lodge & Grace, 2007; Moore et al., 2006). This rodent model intentionally utilizes a non-selective developmental disruption and as such has a high degree of construct validity, in that there is no selective genetic manipulation or loss of a specific brain structure; consistent with that observed in the human schizophrenia patient. Furthermore, the deficits observed in this model parallel those observed in schizophrenia patients including; anatomical changes (thinning of limbic cortices with increased packing density (Moore et al., 2006)), behavioral deficits (decreased prepulse inhibition of startle, disruption in learning new response contingencies, increased responses to PCP and amphetamine (Flagstad et al., 2004; Moore et al., 2006), an increased sensitivity to stress (Goto & Grace, 2006), executive behavioral impairment (Gourevitch et al., 2004), perseverative errors and deficits in latent inhibition (Flagstad et al., 2004), and social impairment (Talamini et al., 2000; Talamini et al., 1998)), and disruption of rhythmic activity in frontal cortex (Goto & Grace, 2006). As such, the MAM GD17 model has been reported to produce anatomical and behavioral disruptions that exhibit a high degree of face and construct validity with that observed in schizophrenia in humans.
Aberrant Dopamine Signaling in the MAM Model
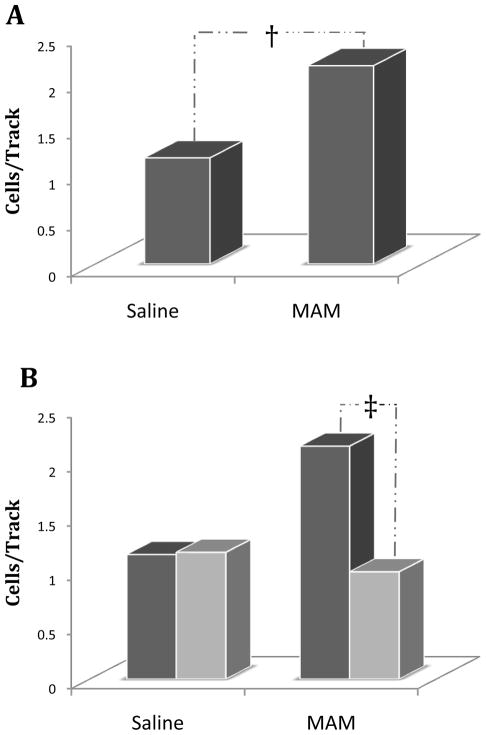
Using this highly validated experimental model, we recently attempted to determine the factors responsible for the aberrant dopamine system responsivity consistently observed in rodent models of psychosis as well as in schizophrenia in humans (Lodge & Grace, 2007). Specifically, the activity of the midbrain dopamine system was examined by single unit in vivo extracellular recordings from identified dopamine neurons of the rat VTA (Lodge & Grace, 2007). Such an approach in untreated rats has provided a model of sub-cortical dopamine function that posits the mesolimbic dopamine system is regulated via two independent mechanisms: 1) transient or “phasic” dopamine release driven by dopamine neuron burst firing, and 2) extrasynaptic or “tonic” levels dopamine dependent on basal dopamine neuron activity and regulated via presynaptic inputs (Grace et al., 2007). Dopamine neuron burst firing induces a large transient increase in perisynaptic dopamine(Chergui et al., 1994)and is considered to be the functionally relevant signal that encodes reward prediction or incentive-salience (Berridge & Robinson, 1998; Schultz, 1998), whereas tonic dopamine transmission occurs on a much slower scale and is proposed to set the background level of dopamine system activation (Grace, 1991). Using this approach we have recently determined that rats treated prenatally with MAM and examined as adults, display a pathologically enhanced dopamine neuron drive (Lodge & Grace, 2007). More specifically, this hyperactivity is observed as an increase in the number of spontaneously active dopamine neurons (Fig 2A), an index of tonic dopamine transmission that is highly correlated with changes in extrasynaptic dopamine levels in forebrain structures (Floresco et al., 2003). Such an increase in dopamine transmission not only produces an augmented dopamine release in terminal regions, but may also result in a considerably amplified phasic response when the animal is presented with otherwise non-salient events or objects (Kapur, 2003; Lodge & Grace, 2006).
Figure 2. Aberrant hippocampal activity is responsible for the DA hyper-responsivity in the MAM rodent model of schizophrenia.
Prenatal (GD17) MAM administration results in an increased number of spontaneously active VTA dopamine neurons (A) compared to prenatal saline treated rats. This appears to be attributed to vHipp hyperactivity since inactivation of the vHipp by tetrodotoxin (light bars) normalizes the aberrant increase in DA neuron population activity in MAM rats (B), while having no observable effect in control rats (dark bars). (Adapted from Lodge & Grace, 2007)
Aberrant Hippocampal Regulation of Dopamine Signaling in the MAM Model
Given that the alteration in dopamine activity purportedly occurs secondary to cortical disruption in human schizophrenia patients, we sought to determine the cause of the increased dopamine neuron activity in the MAM treated rats. Furthermore, given significant evidence of hippocampal disruption in schizophrenia and the role of the ventral subiculum in the regulation of dopamine neuron activity states, we suggest that aberrant hippocampal activity may underlie the augmented dopamine neuron function in the MAM model. This purported involvement of the ventral hippocampus in the aberrant dopamine neuron activity in the MAM model of schizophrenia has been recently examined (Lodge & Grace, 2007). Specifically, inactivation of the ventral hippocampus by infusion of the sodium channel blocker, tetrodotoxin (TTX) normalized the pathologically enhanced dopamine neuron population activity observed in MAM treated rats (Fig 2B). Moreover, this manipulation had no significant effect on any other parameter of dopamine neuron activity in MAM rats nor did it have any observable effects on DA neuron activity in control animals (Lodge & Grace, 2007). As such, it stands to reason that pathology within the hippocampus may result in an increased hippocampal output. Indeed, we have recently demonstrated an enhanced ventral hippocampal activity in MAM-treated rats using in vivo extracellular recordings (Lodge & Grace, 2007). More specifically, the spontaneous activity of ventral hippocampal neurons is increased in MAM rats, expressed as a significantly greater baseline firing rate with no significant change in patterned activity at the single cell level (Lodge & Grace, 2007). Thus, we propose that the augmented dopamine transmission observed in MAM animals is attributable to a pathologically enhanced hippocampal transmission and further suggest this is consistent with observations in human schizophrenia patients.
A Role for Hippocampal Hyperactivity in Human Schizophrenia
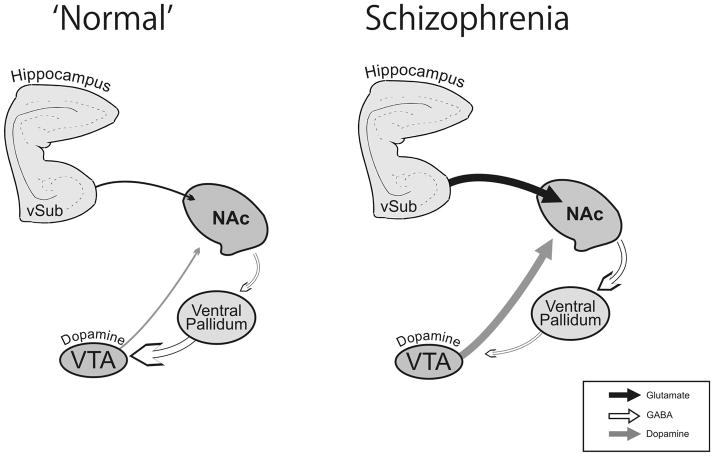
The model advanced here is consistent with hippocampal dysfunction observed in schizophrenia patients(Harrison, 1999b; Saykin et al., 1991; Shenton et al., 2001). Furthermore, recent functional imaging studies have suggested an abnormally high level of activity both during resting states (Heckers et al., 1998; Lahti et al., 2006; Nordahl et al., 1996) and during task performance (Medoff et al., 2001; Meyer-Lindenberg et al., 2001; Weiss et al., 2006). In addition, hyperactivity in hippocampal regions has been proposed to underlie the positive symptoms of the disorder, including abnormal thought processes, hallucinations, and delusions (Krieckhaus et al., 1992; Venables, 1992). Finally, it is well-known that temporal lobe epilepsy, a type of hyperactivity of the hippocampus, has been associated with schizophrenia-like positive symptoms in humans (Ounsted & Lindsay, 1981). Taken together, these data are consistent with the model suggested here in which hyperactivity within the hippocampus drives the dopamine hyperfunction(Fig 1).
Figure 1. Schematic depicting the aberrant regulation of the dopamine system in Schizophrenia.
Hippocampal hyperactivity (suggested to be associated with a decrease in GABA transmission) results in an increased activation of the nucleus accumbens (NAc). The subsequent increase in NAc output inhibits the ventral pallidum (VP) resulting in the disinhibition of VTA dopamine neurons.(Adapted from Grace et al., 2007)).
Abnormal GABA Regulation of Hippocampal Activity in Schizophrenia
The mechanisms underlying the pathological enhancement of hippocampal output are not currently known; however, we propose that the augmented hippocampal output may be attributed to a reduction in inhibitory GABAergic transmission within the hippocampus. Evidence for GABA dysfunction in schizophrenia is abundant (Benes, 2002; Benes & Berretta, 2001; Benes et al., 2007; Heckers et al., 2002; Lewis et al., 2005; Reynolds et al., 2002). Thus, a number of post-mortem studies of cortical GABA signaling in schizophrenia patients have provided consistent evidence for diminished GABA activity. More specifically, a diminished expression of glutamic acid decarboxylase (GAD)-1 mRNA and associated decrease in GAD-67 protein are observed post mortem throughout the cortex of human schizophrenia patients (Akbarian et al., 1995; Hashimoto et al., 2003; Volk et al., 2000). GAD is an enzyme critical for GABA biosynthesis and as such a deficit in GAD expression in the hippocampus may result in an aberrant inhibition of hippocampal transmission. More recently, there has been increasing evidence that the GAD deficits in schizophrenia may be largely restricted to a specific class of GABAergic interneurons; specifically those containing the calcium binding protein parvalbumin (Lewis et al., 2005). Consistent with this, we have recent evidence for a selective decrease in parvalbumin positive neurons throughout the ventral subiculum of the hippocampus (but not in the dorsal subiculum) in the MAM model (Lodge et al., 2007). Furthermore, a decrease in hippocampal parvalbumin containing interneurons is a consistent observation in a diverse variety of animal models of schizophrenia, including the MAM model (Penschuck et al., 2006), the chronic phencyclidine (PCP) model (Abdul-Monim et al., 2007), and in rats reared in rearing isolation (Harte et al., 2007). As such, we propose that the augmented hippocampal output is a consequence of a diminished GABAergic regulation of pyramidal cell output.
Conclusion
The model advanced here is largely based on recent data obtained using a verified animal model of schizophrenia and correlated with observations of schizophrenia in humans. Thus, we have recently demonstrated that the pathological increase in tonic DA transmission observed in MAM rats is likely attributable to hyperactivity within the ventral hippocampus. Moreover, we propose that the hippocampal dysfunction consistently observed in schizophrenia patients is the basis for the dopamine dysregulation in this disorder. We posit that the hippocampal hyperactivity may be a consequence of a deficit in intrinsic GABA signaling. Such an understanding of the functional interactions among these systems and how disruption within these circuits affects information processing is central to gaining a better understanding of the disease pathophysiology and suggests an alternate pharmacotherapeutic approach based on the normalization of hippocampal activity in the treatment of schizophrenia.
References
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. Journal of Psychopharmacology. 2007;21(2):198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Internation Journal of Neuropsychopharmacology. 2004;7(Suppl 1):S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52(4):258–278. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346(8973):477–481. doi: 10.1016/s0140-6736(95)91325-4. [DOI] [PubMed] [Google Scholar]
- Benes FM. Is the GABA cell a final common pathway for the etiology and treatment of schizophrenia and bipolar disorder? Current Opinion in Psychiatry. 2002;15(3):277–278. [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Waters S, Carlsson ML. Network interactions in schizophrenia -therapeutic implications. Brain Research Reviews. 2000;31(2–3):342–349. doi: 10.1016/s0165-0173(99)00050-8. [DOI] [PubMed] [Google Scholar]
- Chergui K, Akaoka H, Charlety PJ, Saunier CF, Buda M, Chouvet G. Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. Neuroreport. 1994;5(10):1185–1188. doi: 10.1097/00001756-199406020-00006. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29(11):2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neuroscience. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biological Psychiatry. 2006;60(11):1259–1267. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, Jay TM. Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behavioral Pharmacology. 2004;15(4):287–292. doi: 10.1097/01.fbp.0000135703.48799.71. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grace AA, Moore H. Regulation of information flow in the nucleus accumbens: A model for the pathophysiology of schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia: Advances in experimental psychopathology. Washington D.C.: American Psychological Association Press; 1998. pp. 123–157. [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999a;122(4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999b;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174(1):151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. Journal of Neural Transmission. 2007;114(7):893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. Journal of Neuroscience. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. The hippocampus in schizophrenia. American Journal of Psychiatry. 2004;161(11):2138–2139. doi: 10.1176/appi.ajp.161.11.2138-a. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal neurons in schizophrenia. Journal of Neural Transmission. 2002;109(5):891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Archives of General Psychiatry. 2002;59(6):521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Hornig M, Lipkin WI. Infectious and immune factors in the pathogenesis of neurodevelopmental disorders: Epidemiology, hypotheses, and animal models. Mental Retardation and Developmental Disabilities Research Reviews. 2001;7(3):200–210. doi: 10.1002/mrdd.1028. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Krieckhaus EE, Donahoe JW, Morgan MA. Paranoid schizophrenia may be caused by dopamine hyperactivity of CA1 hippocampus. Biological Psychiatry. 1992;31(6):560–570. doi: 10.1016/0006-3223(92)90242-r. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31(1):221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31(4):241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of Neocortical Circuits in Schizophrenia. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annual Review Neuroscience. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens M, Grace AA. Diminished GABAergic regulation of hippocampal activity in an animal model of schizophrenia. 2007 In Preparation. [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31(7):1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. The Journal of Neuroscience. 2007 doi: 10.1523/JNEUROSCI.2847-07.2007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: Following a trail of evidence from cradle to grave. Development and Psychopathology. 2000;12(3):501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- McNeil TF. Perinatal risk factors and schizophrenia: Selective review and methodological concerns. Epidemiologic Reviews. 1995;17(1):107–112. doi: 10.1093/oxfordjournals.epirev.a036165. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Cantor-Graae E, Ismail B. Obstetric complications and congenital malformation in schizophrenia. Brain Research Reviews. 2000;31(2–3):166–178. doi: 10.1016/s0165-0173(99)00034-x. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. The Americal Journal of Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry. 2004;10(1):79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biological Psychiatry. 2006;60(3):253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SN, Sutherland RJ, McDonald RJ. Differential involvement of amygdala and hippocampus in responding to novel objects and contexts. Brain Research Bulletin. 2002;58(5):517–527. doi: 10.1016/s0361-9230(02)00820-1. [DOI] [PubMed] [Google Scholar]
- Murray RM, Jones P, O’Callaghan E, Takei N, Sham P. Genes, viruses and neurodevelopmental schizophrenia. Journal of Psychiatric Research. 1992;26(4):225–235. doi: 10.1016/0022-3956(92)90029-n. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analytic study. Archives of General Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O’Shora-Celaya L, et al. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;15(6):541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- Ounsted C, Lindsay J. In: Epilepsy and Psychiatry. Reynolds EH, Trinble MR, editors. Churchill Livingstone; Edinburgh: 1981. [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: A focus on mechanisms. Molecular Psychiatry. 2001;6(6):634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. European Journal of Neuroscience. 2006;23(1):279–284. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL, Zhang ZJ. Understanding the neurotransmitter pathology of schizophrenia: Selective deficits of subtypes of cortical GABAergic neurons. Journal of Neural Transmission. 2002;109(5–6):881–889. doi: 10.1007/s007020200072. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Talamini LM, Ellenbroek B, Koch T, Korf J. Impaired sensory gating and attention in rats with developmental abnormalities of the mesocortex. Implications for schizophrenia. Annals of the New York Academy of Sciences. 2000;911:486–494. doi: 10.1111/j.1749-6632.2000.tb06751.x. [DOI] [PubMed] [Google Scholar]
- Talamini LM, Koch T, Ter Horst GJ, Korf J. Methylazoxymethanol acetate-induced abnormalities in the entorhinal cortex of the rat; parallels with morphological findings in schizophrenia. Brain Research. 1998;789(2):293–306. doi: 10.1016/s0006-8993(98)00020-1. [DOI] [PubMed] [Google Scholar]
- Venables PH. Hippocampal function and schizophrenia. Experimental psychological evidence. Annals of the New York Academy of Sciences. 1992;658:111–127. doi: 10.1111/j.1749-6632.1992.tb22841.x. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical γ-aminobutyric acid neurons in subjects with schizophrenia. Archives of General Psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Lane A, Larkin C, O’Callaghan E. The neurodevelopmental basis of schizophrenia: Clinical clues from cerebro-craniofacial dysmorphogenesis, and the roots of a lifetime trajectory of disease. Biological Psychiatry. 1999;46(1):31–39. doi: 10.1016/s0006-3223(99)00055-4. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Goff D, Schacter DL, Ditman T, Freudenreich O, Henderson D, et al. Fronto-Hippocampal Function During Temporal Context Monitoring in Schizophrenia. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.06.025. [DOI] [PubMed] [Google Scholar]