SUMMARY
SUMO-specific protease 2 (SENP2) has a broad de-SUMOylation activity in vitro. However, the biological function of SENP2 is largely unknown. Here, we show that deletion of SENP2 gene in mouse causes defects in the embryonic heart and reduces the expression of Gata4 and Gata6, which are essential for cardiac development. SENP2 regulates transcription of Gata4 and Gata6 mainly through alteration of occupancy of Pc2/CBX4, a Polycomb Repressive Complex 1 (PRC1) subunit, on its promoters. We demonstrate that Pc2/CBX4 is a target of SENP2 in vivo and that SUMOylation is essential for Pc2/CBX4-mediated PRC1 recruitment to methylated histone 3 at K27 (H3K27me3). In SENP2 null embryo, SUMOylated Pc2/CBX4 accumulates and Pc2/CBX4 occupancy on the promoters of PcG target genes is markedly increased, leading to repression of Gata4 and Gata6 transcription. Our results reveal a critical role for de-SUMOylation in the regulation of PcG target gene expression through a novel mechanism.
INTRODUCTION
SUMOylation is implicated in multiple cellular processes through its ability to alter protein localization or protein-protein interaction (Geiss-Friedlander and Melchior, 2007; Hay, 2005; Yeh, 2009). SUMOylation is catalyzed by SUMO-specific E1, E2, and E3s and can be reversed by a family of Sentrin/SUMO-specific proteases (SENPs). Studies have shown that SENPs are important determinants of SUMO modification status in cells (Cheng et al., 2007; Hay, 2007; Mukhopadhyay and Dasso, 2007). There are six human SENPs, each with different subcellular locations and substrate specificities (Hay, 2007; Mukhopadhyay and Dasso, 2007; Yeh, 2009). These SENPs can be divided into three sub-families based on their sequence homology, substrate specificity, and cellular localization. The first sub-family consists of SENP1 and SENP2 that share similar substrate specificity in vitro, i.e. they are able to de-conjugate either SUMO-1 or SUMO-2/3-modified proteins. However, the second sub-family members, SENP3 and SENP5, and third sub-family members, SENP6 and SENP7, prefer SUMO2/3 as substrates (Hay, 2007; Mukhopadhyay and Dasso, 2007; Yeh, 2009). SENP1 is localized in the nucleoplasm (Gong et al., 2000), whereas SENP2 was reported to be tethered to the nuclear pore through binding to Nup153 nucleoporin (Hang and Dasso, 2002; Zhang et al., 2002). SENP2 is also localized in a yet undefined nuclear speckle that is distinct from the nuclear body (Best et al., 2002). Both SENP3 and SENP5 are nulceolar proteins (Gong and Yeh, 2006). However, SENP3 can shuttle from the nucleolus to nucleus under mild oxidative environment (Huang et al., 2009). SENP6 and SENP7 have additional insertion in the catalytic domain and mainly localized in the nucleus (Shen et al., 2009). Many, if not all, of the SENP knockout mouse embryos do not survive to birth (Cheng et al., 2007; Chiu et al., 2008), suggesting that the SENPs are not redundant and must have specific substrate specificity during development.
Polycomb group (PcG) proteins are transcriptional repressors that play an essential role in the epigenetic regulation of cell differentiation and cell fates during embryonic development (Kohler and Villar, 2008; Margueron et al., 2005; Schuettengruber et al., 2007; Schwartz and Pirrotta, 2008; Sparmann and van Lohuizen, 2006; Spivakov and Fisher, 2007). Genome-wide mapping of PcG proteins in mammalian cells has shown occupancy of a large set of genes, mainly developmental transcription factors (e.g., Hox, Gata, Tbx, and Sox genes), whose gene products control cell fate and embryonic development (Boyer et al., 2006; Bracken et al., 2006; Lee et al., 2006; Ringrose, 2007). PcG proteins form two distinct complexes: Polycomb repressive complex 1 (PRC1) and 2 (PRC2), the core components of which are conserved from fruit fly to human. Biochemical characterization of PRC complexes has shown that PRC2 possess histone methyltransferase activity and is involved in the initiation of gene repression by catalyzing trimethylation of K27 on histone H3 (H3K27me3) (Cao et al., 2002). H3K27me3 is well-documented as a repressive chromatin modification marker and provides a platform for binding of PRC1 complex to chromatin (Bernstein et al., 2006a; Cao et al., 2002; Fischle et al., 2003; Min et al., 2003; Shi, 2007). The binding of PRC1 to H3K27me3 is mainly mediated by PRC1 subunit Pc protein (Cao et al., 2002; Fischle et al., 2003; Min et al., 2003). This binding is proposed to target PRC1 to the appropriate genomic locations to suppress expression of target genes in this locus (Boyer et al., 2006; Lee et al., 2006).
Several mammalian PcG proteins, such as Pc2/CBX4 in PRC1 and Ezh2 and Suz12 in the PRC2 complex, were reported to be SUMOylated (Kagey et al., 2003; Riising et al., 2008; Roscic et al., 2006). However, it is unknown whether SUMOylation of these PcG proteins is functionally relevant to PcG-mediated gene silencing. In C. elegans, PcG-like protein SOP-2, functionally analogous to mammalian Polyhomeotic (Ph) in PRC1, is SUMOylated and SUMOylation is essential for in vivo PcG target Hox gene regulation (Zhang et al., 2004). Here, we found that mutation of the SENP2 gene in mouse caused defects in cardiac development and accumulation of SUMOylated Pc2/CBX4. We further showed that Pc2/CBX4 is a target for SENP2’s catalytic activity. SUMOylation facilitates binding of Pc2/CBX4 to H3K27me3. This binding is required for the Pc2/CBX4-contained PRC1 complex to suppress the transcription of Gata4 and Gata6, which are essential for cardiac development. These results reveal a critical role for de-SUMOylation in the regulation of mammalian PcG target gene expression through a novel molecular mechanism.
RESULTS
SENP2−/− embryos have a defect in cardiac development
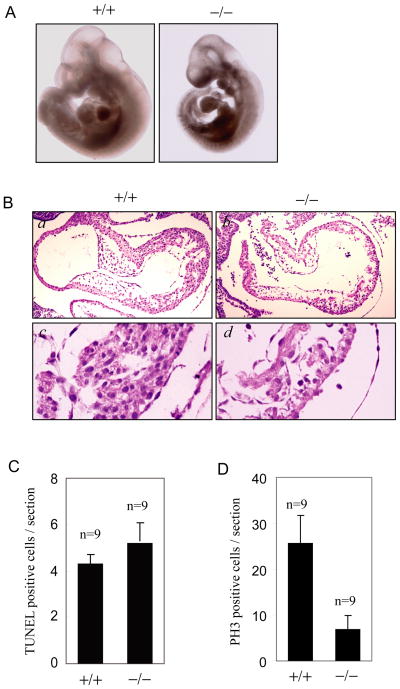
In order to elucidate the biological functions of the mammalian SENP2 gene, we generated SENP2 mutant mice with gene trap strategy (Cheng et al., 2007) (Figure S1A, B, C). SENP2 heterozygous mice develop normally with no gross differences from wild-type mice in viability and fertility. However, the null mutant of SENP2 was embryonic lethal and died at around embryonic day 10 (Figure S1D). The most dramatic abnormity of these SENP2−/− embryos was smaller cardiac chambers with pericardial effusion (Figure 1A). On histological sections, SENP2−/− embryos displayed marked myocardial thinning (Figure 1B). The atrioventricular endocardial cushions were also thinner and contained few mesenchymal cells (Figure 1B).
Figure 1. SENP2−/− embryos have a defect in myocardial development.
(A) Appearance of SENP2+/+ and SENP2−/− embryos at E10.5. The SENP2−/− embryo has a smaller heart with pericardial effusion.
(B) H& E-stained sections of heart from E10.5 wildtype (a, c) and SENP2−/− (b, d) embryos. The SENP2−/− embryos showed hypocellular endocardial cushions and myocardial hypoplasia with thinner myocardium.
(C) TUNEL staining of sections of hearts from SENP2 +/+ or −/− embryos at E10.5 showed no significantly increased in apoptosis in the mutant embryos (P>0.01). “n” indicates the number of sections examined.
(D) Phosphohistone H3 (PH3) staining of sections of hearts from SENP2 +/+ or −/− embryos at E10.5 revealed reduced proliferation in the mutant embryos (P<0.01). “n” indicates the number of sections examined.
To determine the mechanism underlying defective cardiac development in the SENP2 −/− embryos, we assessed apoptosis and proliferation in the cardiac sections. The TUNEL staining positive cells in both embryonic heart sections were very low, and there was no significant increase in SENP2−/− hearts at E10.5 (Figure 1C). However, SENP2−/− hearts displayed markedly reduced cellular proliferation at E10.5 as assessed by phosphor-histone H3 staining (Figure 1D) and PCNA staining (Figure S1E), suggesting that the defect in cardiac development resulted from reduced cellular proliferation.
Down-regulation of Gata4 and Gata6 expression in SENP2−/− embryos
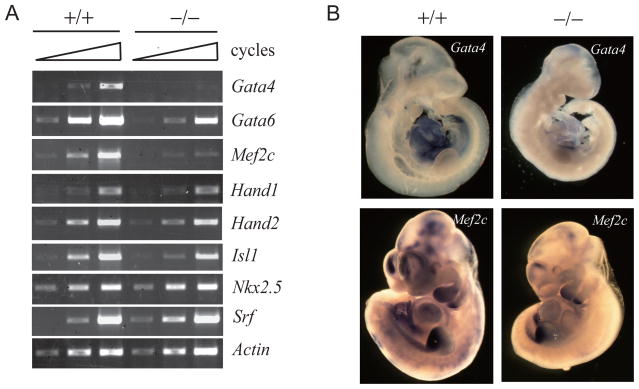
Cardiac development is controlled by a core set of conserved transcription factors, such as Nkx2.5, Gata4 and Gata6, Mef2c, and Hand (Olson, 2006). To determine the molecular mechanism underlying the defect in cardiac development in the SENP2 −/− embryos, we analyzed the expression of these cardiac transcription factors. RT-PCR analysis showed that Gata4 and Mef2c were significantly reduced in E10.5 SENP2 −/− embryos compared to that in wildtype, and Gata6 expression was down regulated to a lesser extent when compared to Gata4 and Mef2c (Figure 2A and 3A). In situ hybridization confirmed the reduction of Gata4 and Mef2c in SENP2−/− embryos (Figure 2B). As the mutation of Gata4 has been shown to be associated with the reduction of Mef2c expression (Dodou et al., 2004; Olson, 2006; Xin et al., 2006), the down-regulation of Gata4 and Gata6 may contribute to the cardiac phenotype observed in the SENP2−/− embryos.
Figure 2. Reduced expression of Gata4, Gata6, and Mef2c in SENP2−/− embryos.
(A) RT–PCR analysis of genes that are involved in cardiac development in E10.5 SENP2+/+ or −/− embryos. Samples were amplified for 32, 34, and 36 cycles for Hand1; 30, 32, and 34 cycles for Hand2 and Isl1; 28, 30, and 32 cycles for Gata4, Gata6, Nkx2.5, and Srf; 26, 28, and 30 cycles for Mef2c. β-Actin mRNA levels (amplified for 18, 21, and 24 cycles) were measured as control.
(B) SENP2+/+ or −/− embryos at E10.5 days were stained by whole mount in situ hybridization for the indicated transcripts.
Figure 3. SENP2 is essential for expression of PcG target genes.
(A) Quantitative expression level of PcG target genes in wildtype and SENP2−/− embryos. Transcript levels were measured by real-time PCR, normalized to a wildtype control and depicted as a fold change between SENP2−/− and wild-type embryos (set as 1, red dotted line). Error bars are based on the standard deviation derived from triplicate PCR reactions in three independent experiments.
(B) Quantitative expression level of PcG target genes in SENP2+/+ or SENP2−/− MEF cells, or SENP2−/− MEF cells infected with retroviral vector containing SENP2 or SENP2 catalytic mutant (SENP2m). Transcripts of indicated genes were measured by real-time PCR, normalized to a SENP2+/+ control (set as 1). Error bars are based on the standard deviation derived from triplicate PCR reactions in three independent experiments.
(C) Silencing of SENP2 down-regulates expression of PcG target genes. The transcripts of PcG target gene were analyzed by real-time PCR in 293 cells transfected with si-NS or si-SENP2. The mRNA level is shown in means±s.d. of three independent transfection experiments.
Indeed, the cardiac defect in SENP2−/− embryos described above was similar to that observed in Gata4−/− or Gata4/Gata6 double heterozygous embryo (Xin et al., 2006; Zeisberg et al., 2005). The early cardiac-specific deletion of Gata4 has been shown to cause myocardial thinning, abnormal endocardial cushion development, and right ventricular hypoplasia (Zeisberg et al., 2005). The Gata4/Gata6 double heterozygous mutant embryos display thin-walled myocardium, ventricular and aortopulmonary septal defects (Xin et al., 2006). Interestingly, myocardial hypoplasia in these Gata4/Gata6 double heterozygous mutant embryos is also due to the reduced proliferation of cardiomyocytes (Xin et al., 2006). Given the crucial role of Gata4 and Gata6 in the myocardial proliferation during the early heart development, the reduction of Gata4 and Gata6 may account for the myocardial hypoplasia observed in SENP2−/− hearts.
SENP2 is essential for the expression of PcG target genes
Interestingly, the expression of Gata4 and Gata6 is tightly controlled by PcG proteins during development. During ES cell differentiation, the transcripts of Gata4 and Gata6 are up-regulated as the activity of PcG proteins is decreased (Boyer et al., 2006). Therefore, we hypothesized that the reduction of Gata4 and Gata6 might result from increased repressive activity of PcG in SENP2−/− embryos. If this is true, we would expect to observe decreased transcripts of other PcG target genes in SENP2−/− embryos. Indeed, the mRNA levels of some Hox genes, the well-characterized PcG target genes, assessed by real-time PCR were significantly reduced in SENP2−/− embryos in comparison with the wildtype embryos (Figure 3A). Down-regulation of some Hox genes, Gata4, and Gata6 was also observed in SENP2−/− MEF cells (Figure 3B). More importantly, re-introduction of SENP2, but not SENP2 catalytic mutant, could rescue the expression of these PcG target genes, including Gata4 and Gata6, in SENP2−/− MEF cells (Figure 3B). We further confirmed the role of SENP2 in the regulation of PcG target genes by using a siRNA approach. SENP2 siRNA decreased the expression of SENP2 by 78% in 293 cells (Figure 3C). Silencing of SENP2 resulted in significant down-regulation of transcript abundance in 8 out of 11 of the putative PcG target genes tested (Figure 3C). These results suggest a crucial role of SENP2 in regulation of the expression of PcG target genes.
SENP2 has a crucial role in regulation of Pc2/CBX4 occupancy on the promoter of Gata4 and Gata6
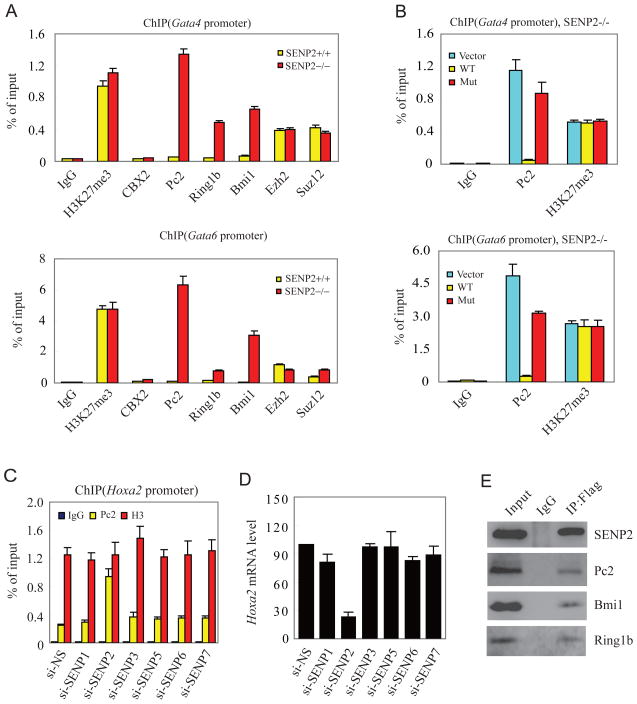
The silencing of gene expression by PcG proteins requires trimethylation on histone H3 K27 (H3K27me3) by PRC2 and the recruitment of PRC1 to chromatin at H3K27me3, which is always enriched in loci of PcG target genes (Cao et al., 2002; Schuettengruber et al., 2007; Sparmann and van Lohuizen, 2006; Whitcomb et al., 2007). The level of H3K27me3 measured by western blotting with anti-H3K27me3 antibody showed no difference in SENP2+/+ and SENP2−/− MEF cells (Figure S2). ChIP assay also revealed that mutation of SENP2 gene did nor alter the level of H3K27me3 and occupancy of PRC2 components Ezh2 and Suz12 on promoter of Gata4 and Gata6 genes (Figure 4A). These data suggest that SENP2 does not control the PRC2 activity. Thus, we reasoned that SENP2 might regulate PcG activity by altering recruitment of PRC1 to promoter of target genes at H3K27me3. Indeed, ChIP analysis demonstrated that occupancy of PRC1 components, including Pc2/CBX4, Ring1b, and Bmi1, on promoters of Gata4 and Gata6 genes was significantly increased in SENP2−/− MEF cells in comparison to that in SENP2+/+ MEF cells (Figure 4A).
Figure 4. SENP2 plays a crucial role in the regulation of PRC1 occupancy on the promoter of PcG target genes.
(A) Mutation of SENP2 gene enhances binding of PRC1 to the promoter of Gata4 and Gata6. Eight antibodies (anti-IgG, -H3K27me3, -CBX2, -Pc2, -Ring1b, -Bmi1, -Ezh2, and -Suz12) were used in the qChIP assays in SENP2+/+ or −/− MEF cells. Data are shown in means±s.d. of three independent experiments.
(B) Over-expression of SENP2, but not SENP2 catalytic mutant, reduces Pc2 recruitment in SENP2−/− MEF cells. Two antibodies (anti-Pc2, and -H3K27me3) were used in the qChIP assays in SENP2−/− MEF cells infected with empty retroviral vector, retroviral vector containing Flag-SENP2, or Flag-SENP2 catalytic mutant (SENP2m). Data are shown in means±s.d. of three independent transfection experiments.
(C) Pc2 and histone 3 (H3) occupancy at the Hoxa2 promoter were analyzed by a qChIP assay in the 293 cells transfected with siRNA against SENPs (si-SENP) or non-specific control (si-NS) as indicated. Data are shown in means±s.d. of three independent transfection experiments.
(D) Silencing of SENP2 reduces the expression of Hoxa2. The expression of Hoxa2 was analyzed by real-time PCR in the cells described in (C). The mRNA level is shown in means±s.d. of three independent transfection experiments.
(E) SENP2 binds to PRC1. Flag-SENP2 was immunoprecipitated with anti-Flag antibody or IgG from chromatin fraction of Flag-SENP2-transfected 293 cells. The precipitates were detected with anti-Flag, -Pc2, -Bmi1, or -Ring1b antibodies.
It is well known that the Pc/CBX protein of the PRC1 complex binds to H3K27me3 on chromatin, which is crucial for PcG proteins to silence its target genes (Cao et al., 2002). We confirmed that H3K27me3, not H3K9me3, was enriched at Gata4 and Gata6 gene loci (Figure S3A). To further determine whether H3K27me3 is essential for SENP2 to regulate Pc2/CBX4 occupancy, we knocked down Ezh2 expression in SENP2+/+ and −/− MEF cells to disrupt H3K27 methylation. ChIP assay showed that silencing of Ezh2 decreased the enrichment of H3K27me3 and the occupancy of Pc2/CBX4 on the promoter of Gata4 and Gata6 in SENP2−/− MEF cells (Figure S3B and C), suggesting that the increase of Pc2 occupancy in SENP2−/− MEF cells is via H3K27me3. Furthermore, there is a marked decrease in Pc2 binding to the promoters of Gata4 and Gata6 in the SENP2+/+ MEF cells due to reduced SUMOylation of endogenous Pc2 secondary to intact SENP2 activity (Figure S3C, also see Figure 5). Taken together, SENP2 plays an important role in regulating Pc2/CBX4 occupancy on the promoter of Gata4 and Gata6.
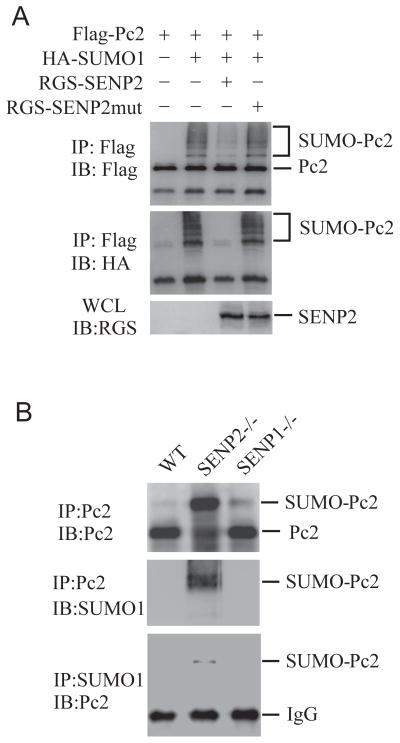
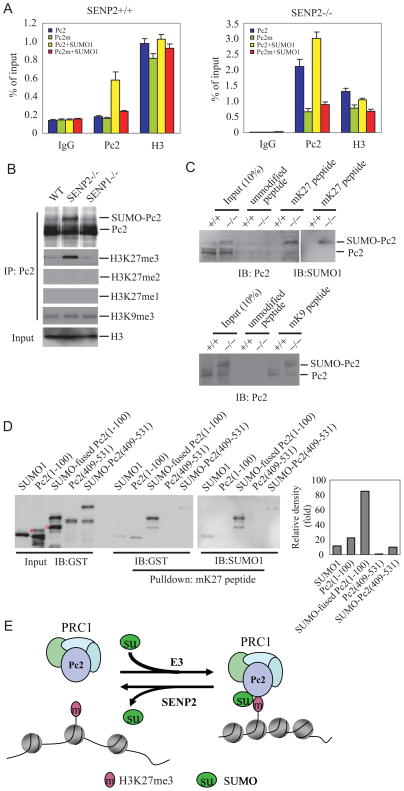
Figure 5. SENP2 de-conjugates SUMOylated Pc2.
(A) SENP2 de-SUMOylates Pc2 in vivo. COS-1 cells were transfected with indicated plasmids. The immunoprecipitates with anti-Flag (IP) from transfected cell lysates were detected by immunoblotting with anti-HA and anti-Flag (IB). Whole-cell lysates (WCL) were immunoblotted (IB) with anti-RGS antibody.
(B) SUMOylated Pc2 accumulated in SENP2−/−, not SENP2+/+ nor SENP1−/− MEF cells. Chromatin fractions from 5×107 MEF cells were treated with micrococcal nuclease, and then immunoprecipitated with Pc2 and SUMO1 antibodies. Bound proteins were detected by immunoblotting with anti-Pc2 or anti-SUMO1 (IB).
The mouse and human Pc/CBX family has five members (Whitcomb et al., 2007). Pc2/CBX4 is the only SUMOylated protein reported, thus is likely to be a target of SENP2 (Kagey et al., 2003; Roscic et al., 2006). ChIP assay showed that Pc2/CBX4 occupancy on the promoter of Gata4 and Gata6 is increased in SENP2−/− cells (Figure 4A). Interestingly, when a similar assay was performed by using anti-Pc1/CBX2, another member of mouse Pc family, there was no difference in Pc1/CBX2 occupancy between SENP2+/+ and −/− MEF cells (Figure 4A). These results suggest that SENP2 specifically regulate recruitment of Pc2/CBX4 to the promoter of Gata4 and Gata6. More importantly, over-expression of SENP2, but not SENP2 mutant, in SENP2−/− MEF cells decreased the Pc2/CBX4 occupancy on Gata4/Gata6 promoter (Figure 4B and S3D) and restored the transcription of Gata4 and Gata6 in SENP2−/− MEF cells (Figure 3B).
To directly show that the occupancy of Pc2/CBX4 regulated by SENP2 influenced the expression of PcG target genes, we performed an experiment by using the siRNA approach. SENP siRNA down-regulated the expression of individual SENPs by 70–80% in 293 cells (Figure S3E). Pc2/CBX4 occupancy on Hoxa2 locus, a well known human Pc2/CBX4 target gene, was determined in these SENP knock-downed cells. A quantitative ChIP assay showed that silencing of SENP2, but not other SENPs, significantly promoted Pc2/CBX4 occupancy on the promoter of Hoxa2 (Figure 4C). Consequently, the Hoxa2 transcript was markedly reduced in SENP2 but not other SENP siRNA transfected cells (Figure 4D). These results confirmed that Pc2 occupancy regulated by SENP2 was functionally relevant to PcG silencing.
To provide more evidence to support the link between SENP2 and Pc2/CBX4, we carried out an immunoprecipitation assay in SENP2-transfected 293 cells to demonstrate that SENP2 was able to precipitate Pc2/CBX4, Bmi1, and Ring1b, the core components of PRC1 complex (Figure 4E). SENP2 have been reported previously to localize in the nuclear envelope (Hang and Dasso, 2002; Zhang et al., 2002) and undefined nuclear structures (Best et al., 2002). The nuclear appearance of SENP2 was in the form of dots that are distinct from PML-containing nuclear bodies (Figure S4A). We asked whether SENP2 could co-localize with Pc2/CBX4 in these nuclear structures. Indeed, SENP2 and Pc2/CBX4 clearly co-localized in these nuclear foci when co-expressed in HeLa cells (Figure S4B). Furthermore, many endogenous Pc2/CBX4 foci (15 dots among total 17 foci in Figure S4C) also co-localized with endogenous SENP2 in human umbilical vein endothelial cells. These data indicate that SENP2 can co-localize with Pc2/CBX4 foci, thus is poised to regulate the activity of Pc2/CBX4-contained PRC1.
SUMOylated Pc2/CBX4 is accumulated in SENP2−/− cells
Above experiments suggest that the SENP2’s de-SUMOylation activity is essential for inhibition of Pc2/CBX4 recruitment as well as the expression of PcG target genes. Given that Pc2/CBX4 is a SUMOylated protein, we proposed that SENP2 could de-SUMOylate Pc2/CBX4 to alter Pc2/CBX4 occupancy on PcG target promoter. To test this possibility, we first performed an in vivo de-SUMOylation assay to determine whether SENP2 could de-conjugate SUMOylated Pc2/CBX4. Pc2/CBX4 was SUMOylated when co-expressed with SUMO1. The expression of SENP2, but not SENP2 catalytic mutant, removed the SUMO-conjugated Pc2/CBX4 bands (Figure 5A). We further confirmed the de-SUMOylation activity of SENP2 to Pc2/CBX4 in SENP2−/− MEF cells. As shown in Figure 5B, SUMOylated Pc2/CBX4 accumulated in SENP2−/−, but not in wildtype MEF cells, suggesting that mutation of SENP2 impaired the de-conjugation of SUMOylated Pc2/CBX4. Interestingly, we did not observe accumulation of SUMOylated Pc2/CBX4 in SENP1 −/− MEF cells, highlighting the specificity of SENP2 in regulating SUMO conjugation status of Pc2/CBX4.
SUMOylation facilitates the binding of Pc2/CBX4 to H3K27me3
The recognition and binding of PRC1 to H3K27me3 is mediated by the chromodomain of the Pc subunit of PRC1 complex in Drosophila (Cao et al., 2002). All the members (Pc1/CBX2, Pc2/CBX4, CBX6, CBX7, and Pc3/CBX8) in mammalian Pc family contain a highly conserved chromodomain at the amino terminus (Schuettengruber et al., 2007). The chromodomain of Pc2/CBX4 was reported to bind to H3K27me3 less than to H3K9me3 by an in vitro analysis (Bernstein et al., 2006b). As we have shown that Pc2/CBX4 occupancy was regulated by SENP2 in PcG-mediated gene silencing, we hypothesized that SUMOylation might facilitate binding of Pc2/CBX4 chromodomain to H3K27me3 in vivo. To prove it, we first compared the occupancy of Pc2/CBX4 wild-type and Pc2/CBX4 K492R mutant, a SUMOylation site mutant (Roscic et al., 2006), on Gata4 promoter in SENP2+/+ and −/− MEF cells by using a ChIP assay. As shown in Figure 6A and Figure S5A, the occupancy of Pc2/CBX4 wildtype (Pc2), but not Pc2/CBX4 K492R (Pc2m), on Gata4 promoter in SENP2+/+ MEF cells was significantly increased in the presence of SUMO1, indicating that SUMOylation is responsible for Pc2/CBX4 recruitment to PcG target genes. A similar assay in SENP2−/− MEF cells showed that the occupancy of Pc2 wildtype, but not Pc2 K492R mutant, was increased even without the presence of exogenous SUMO1 (Figure 6A). This is likely due to an increase in SUMOylated Pc2 in SENP2−/− MEF cells (Figure S5B). To determine whether SENP2 affects Pc2/CBX4 binding to H3K27me3, we performed in vivo binding assay and showed that Pc2/CBX4 from SENP2−/− MEF cells pulled down much more H3K27me3 than that from SENP1−/− or wildtype MEF cells (Figure 6B). This binding is very specific because Pc2/CBX4 could not pulled down H3K27me1 and H3K27me2 (Figure 6B). Moreover, H3K27me3, but not un-modified H3K27, peptide precipitated more SUMO-conjugated Pc2/CBX4 proteins than un-modified Pc2/CBX4 from SENP2 −/− MEF cell lysates (Figure 6C). Our above results suggest that SENP2 regulates binding of Pc2/CBX4 to H3K27me3 in a SUMOylation-dependent manner. It is not known whether SENP2 also regulate binding of Pc2/CBX4 to other methylated lysine residue on Histone 3. Thus, we tested the ability of SENP2 to regulate binding of Pc2/CBX4 to H3K9me3. As shown in Figure 6B, Pc2/CBX4 binding to H3K9me3 is identical in SENP2 wild type, SENP2−/−, or SENP1−/− MEF cells. Furthermore, H3K9me3 peptide could precipitate both SUMO-conjugated and un-modified Pc2/CBX4, but there were no significant difference in binding affinity to SUMO-conjugated and un-modified Pc2/CBX4 proteins (SUMO-Pc2: Pc2 = 1.2:1) from SENP2 −/− MEF cell lysates (Figure 6C, bottom panel). Taken together, SUMOylation significantly enhance Pc2/CBX4 binding to H3K27me3, but not H3K9me3 in vivo.
Figure 6. SUMOylation increases binding affinity of Pc2 to H3K27me3.
(A) Mutation of SUMOylation site decreases the ability of Pc2 to occupy the promoter of Gata4. Two antibodies (anti-Flag and -H3) were used in the qChIP assays using SENP2+/+ or −/− MEF cells transfected with indicated plasmids. Data are shown in means±s.d. of three independent transfection experiments.
(B) The immunoprecipitates with Pc2 (IP) from chromatin fraction of SENP2+/+ (WT), SENP2−/−, or SENP1−/− MEF cells were detected by immunoblotting with anti-Pc2 and anti-H3K27me3, H3K27me2, H3K27me1, and H3K9me3 (IB). Chromatin fractions were immunoblotted (IB) with anti-H3 as input.
(C) SUMOylated Pc2 binds to tri-methylated H3K27, but not H3K9, with higher affinity than un-conjugated Pc2. Biotinylated histone H3 peptides that were either unmodified or tri-methylated on K27 (upper panel) or on K9 (bottom panel), were incubated with the chromatin fractions from SENP2+/+ (WT) or SENP2−/− (Mut) MEFs in the presence of streptavidin-conjugated sepharose beads. The precipitates were detected by immunoblotting (IB) with anti-Pc2 or anti-SUMO1.
(D) SUMOylation facilitates binding of Pc2 chromodomain to tri-methylated H3K27. Biotinylated histone H3 peptides that were tri-methylated on K27 (mK27), were incubated with GST-SUMO1, GST-Pc2(1-100), GST-SUMO1-fused Pc2(1-100), GST-Pc2(409-531), and SUMOylated GST-Pc2(409-531) recombinant proteins in the presence of streptavidin-conjugated sepharose beads. Inputs and precipitates by mK27 peptides as detected with anti-GST were shown in the left panel. The precipitates by mK27peptidea as detected with anti-SUMO1 were shown in the middle panel. The relative binding affinity was shown as relative fold change of signal density of precipitates detected by anti-GST and standardized with input (Right panel). “*” indicates a non-specific band. “ ” indicates a degraded band.
” indicates a degraded band.
(E) A model depicting the role of SENP2 in the regulation of PRC1 recruitment to H3K27me3 through controlling the SUMOylation status of Pc2. E3 ligase for Pc2 may be Pc2 itself or an undefined E3 ligase.
To further determine the role of Pc2/CBX4 SUMOylation in binding to H3K27me3, we performed an in vitro binding assay to compare the binding affinity of Pc2 (1-100), SUMO-fused Pc2 (1-100), Pc2 (409-531), or SUMOylated Pc2 (409-531) for H3K27me3 peptide. As shown in Figure 6D and S5C, Pc2 (1-100), a fragment containing the chromodomain, but not Pc2 (409-531), a fragment without chromodomain, was bound by H3K27me3, but not un-modified, peptide, indicating that the chromodomain have a role for Pc2/CBX4 binding to H3K27me3. Interestingly, the amount of Pc2 (1-100) bound to H3K27me3 was increased by more than 4 folds when SUMO1 was tethered to the N-terminus of Pc2 (1-100) fragment. Although SUMOylated Pc2 (409-531) was also bound by H3K27me3, the amount was much lower than that of SUMO-fused Pc2 (1-100), indicating that SUMOylation could enhance the binding ability of chromodomain for H3K27me3 (Figures 6D, S5D and E). Thus, both SUMO and the chromodomain of Pc2/CBX4 are required for Pc2/CBX4 to bind to H3K27me3 efficiently. These biochemical results also lend support to the biological consequence of SENP2 mutation in embryos and MEF cells.
DISCUESSION
SUMO modification has emerged as an important regulatory mechanism for protein-protein interaction (Geiss-Friedlander and Melchior, 2007; Hay, 2005; Yeh, 2009). In this report, we identified a novel functional interaction of PRC1 protein and methylated chromatin that is regulated by SUMOylation. Our initial study of the developmental defects in the SENP2 null embryos revealed that some of the PcG target genes, such as Hox, Gata4, and Gata6, are down regulated. We showed that mutation of SENP2 results in the accumulation of SUMOylated Pc2/CBX4, which increased Pc2/CBX4 occupancy on the promoters of PcG targets genes, such as Gata4 and Gata6. These results suggest an essential role for SUMOylation in maintaining transcription suppression-mediated by Pc2/CBX4-contained PRC1. Furthermore, biochemical studies revealed that SUMOylation of Pc2/CBX4 is required for high affinity binding of Pc2/CBX4 to H3K27me3. Based on these findings, we proposed that SENP2 plays a critical role in the regulation of the SUMOylation status of Pc2/CBX4, which is critical for Pc2/CBX4 binding to H3K27me3 to repress transcription of PcG target genes (Figure 6E).
Pc/CBX, a core component in PRC1 complex, has been shown to be a docking protein mediating the binding of PRC1 to the chromatin at H3K27me3 locus in Drosophila (Cao et al., 2002; Whitcomb et al., 2007). The chromodomain of Pc protein in Drosophila has been shown to bind to H3K27me3 by biochemistry and crystal structure studies (Cao et al., 2002; Fischle et al., 2003; Min et al., 2003). In contrast to the single Pc gene in Drosophila, the mouse has five Pc homologs, known as Pc1/Cbx2, Pc2/Cbx4, Cbx6, Cbx7, and Pc3/Cbx8 (Whitcomb et al., 2007). Although highly conserved, the chromodomains of five Pc homologs have significant difference in binding preferences towards H3K27me3 in vitro (Bernstein et al., 2006b). In fact, it has been shown that the chromodomain of mouse Pc2/Cbx4 binds poorly to H3K27me3 peptide (Bernstein et al., 2006b). We also find that the chromodomain of Pc2/CBX4 binds to H3K27me3 weakly and this binding is markedly enhanced by SUMOylation. Due to technical difficulties, we were not able to SUMOylate full length Pc2/Cbx4 in vitro. However, a SUMO-1-chromodomain fusion protein binds to H3K27me3 with much higher affinity than the chromodomain alone. Thus, it is clear that SUMOylation of Pc2/Cbx4 is required for high affinity binding of Pc2/Cbx4 to H3K27me3. The importance of Pc2 SUMOylation is revealed by the biological results in the SENP2 null embryos or MEF cells. In the MEF cells, we can restore transcription of PcG target genes by wild type SENP2, but not SENP2 catalytic mutants. Collectedly, the biochemical demonstration of the role of SUMOylation in Pc2 and H3K27me3 binding is corroborated with findings in the embryos and in MEF cells.
PcG proteins play important roles in embryonic development and ES cell differentiation (Boyer et al., 2006; Bracken et al., 2006; Lee et al., 2006). Several studies applied ChIP-on-chip analysis to identify developmental regulatory targets of PcG complexes. Young and colleagues used genome-wide tiling arrays and identified more than 1000 PRC2 target genes, including a large number of genes encoding developmental regulators such as Hox transcription factors and key signaling proteins (Lee et al., 2006). A parallel study in murine ES cells from Jaenisch and colleagues also identified a large number of developmental PRC1 and PRC2 target genes (Boyer et al., 2006). These targets are largely silent transcriptionally in ES cells, but many were activated when ES cell differentiated (Boyer et al., 2006; Bracken et al., 2006; Lee et al., 2006), suggesting that the activity of PcG is being precisely regulated during embryonic development. Such regulation may occur at multiple levels or targets, but at least the PRC complex assembly with inclusion of various PcG protein homologs may play an important role (Muller and Verrijzer, 2009; Whitcomb et al., 2007). As shown in the SENP2−/− embryos, we did not find all PcG target genes were affected by SENP2 mutation, suggesting that not all Pc/CBXs are targets of SENP2. Thus, it will be of interest to use genome-wide approach to determine the target gene profiles regulated by SENP2-Pc2/CBX4 pathway during embryonic development.
SENP2 was originally reported to be associated with the nuclear envelope through binding to Nup153 (Hang and Dasso, 2002; Zhang et al., 2002). We have previously shown that SENP2 contain both nuclear import and export signals that can shuttle between the nucleus and cytoplasm (Itahana et al., 2006). It is of interest to note that when SENP2 enter the nucleoplasm it can be co-localized with Pc2/CBX4. The regulation of SENP2 and Pc2/CBX4 co-localization is unknown as present. However, it is clear that SENP2 is the key regulator of Pc2/CBX4 function through regulation of the SUMOylation status of Pc2/CBX4. It is likely that association of Pc2/CBX4 with SENP2 would maintain Pc2/CBX4 in a hypo-SUMOylated state, which binds to H3K27me3 poorly. Thus, in the presence of SENP2, the PcG targets genes are not silenced. When SENP2 is down-regulated in cells, Pc2/CBX4 becomes hyper-SUMOylated, leading to enhanced binding to H3K27me3 and transcriptional repression. Pc2/CBX4 has been shown to be a SUMO E3 ligase (Kagey et al., 2003). It is currently unknown whether SUMOylation of Pc2/CBX4 is mediated by self ligase activity or by another SUMO E3.
Chiu et al. recently reported that the disruption of SENP2 leads to developmental defects in trophoblast stem cell niches and lineages through modulating p53-Mdm2 pathway in placental (Chiu et al., 2008). We also examined Mdm2 and p53 in our SENP2−/− embryos and did not observe any difference in Mdm2 and p53 expression level or Mdm2 SUMOylation status (Figure S6) between E10.5 SENP2+/+ and −/− embryos. The basis for this difference is not clear. It might reflect difference in cellular processes regulated by SENP2 in different cell types. We also noticed that Chiu et al. used gene knockout strategies different from ours. Their mice are generated by deletion of exon 3 to 5 of the SENP2 genes, while we used gene trapped vector inserted into intron 10 of SENP2 genomic DNA and disrupted the transcript of catalytic domain of SENP2. We have found that there are different SENP2 transcripts in different organs due to differential splicing (Unpublished data). Thus, the difference in phenotypes may be caused by disruption of different SENP2 splice variants existed in different organs.
In conclusion, our data support that SENP2 specifically controls Pc2/CBX4 contained PRC1 activity through regulation of the SUMOylation status of Pc2/CBX4, which facilitates its binding to H3K27me3 in mammalian cells to mediate transcriptional repression (Figure 6E). Our findings establish a new role for SUMOylation in mediating interaction between protein and protein methylation in epigenetic regulation.
EXPERMENTAL PROCEDURES
Antibodies, plasmids, siRNA, and cell lines
Antibodies against H3K27me3 (ab6002) and Ring1B (ab3832) were purchased from Abcam. Anti-Histone3 (06-755) was from Upstate. Anti-Bmi1 (NB110-40823) was from NOVUS. Anti-MPc2 (sc-19299) was from Santa Cruz. Anti-SENP2 (GTX23660) was from Gene Tex. Anti–Flag (M2, F3165) was from Sigma. Anti–HA (16B12, MMS-101P) was from Covance. Anti-RGS(34610) was from Qiagen. Anti-SUMO1(18-2306) was from Zymed.
Plasmid Flag-Pc2 was generously provided by Dr. David Wotton (University of Virginia, USA) (Kagey et al., 2003), HA-SUMO-1, Flag-SENP2, RGS-SENP2, and RGS-SENP2m, a SENP2 catalytic mutant, were previously described (Gong et al., 2000; Kamitani et al., 1998). pbabe-SENP2, pbabe-SENP2m, pGEX-Pc2(1-100), pGEX-SUMO1-Pc2(1-100), pGEX-Pc2 (409-531), and Flag-Pc2 K492R were generated using standard cloning procedures and PCR-based mutagenesis.
siRNA against SENP1(J-006357-05), SENP2(J-006033-05), SENP3(J-006034-05), SENP5(J-005946-05), SENP6(J-006044-05), SENP7(J-006035-05), and non-specific siRNA(D-001810-01) were purchased from Dharmacon RNA Technologies.
SENP1 or SENP2 MEF cells were isolated from E10.5 embryos as previously described (Cheng et al., 2007).
Immunoprecipitation in chromatin fraction
Immunoprecipitations were performed in chromatin fraction of MEF cells following Wysocka et.al ’s method with few modifications (Wysocka et al., 2001). Briefly, 5×107 MEF cells were collected, washed with phosphate-buffered saline (PBS), and resuspended in 0.5ml buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 0.1% Triton X-100, 10% glycerol, 1 mM dithiothreitol, and protease inhibitor cocktail [Sigma]). After incubation on ice for 10 mins, the nuclei were collected by centrifugation (5 mins, 1,300 g, 4°C). The nuclei were washed once in buffer A and lysed for 30 mins in 0.5ml buffer B (3 mM EDTA, 0.2 mM EGTA, 1 mM dithiothreitol, 20mM NEM and protease inhibitor cocktail), and insoluble chromatin fraction was collected by centrifugation (5 mins, 1,700 g, 4°C). The fraction was washed once with buffer B, resuspended in 200μl buffer containing 20 mM Tris, 5mM NaCl, 2.5 mM CaCl2, 20mM NEM, protease inhibitor cocktail, and 5U of micrococcal nuclease (Mnase, Takara) and incubated for 10 mins in 37°C. The reaction was stopped by adding EGTA (1 mM final concentration). The soluble and insoluble fractions were separated by centrifugation (10 mins, 20,000 g, 4°C). The soluble supernatant was used for immunoprecipitation and western analysis.
Peptide pull-down assay
For peptide pull-down assays, 0.5μg of biotinylated histone H3 peptides (aa 21-44, Upstate), which were either un-methylated or methylated on K27, or biotinylated histone H3 peptides (aa 1-21, Upstate), which were either un-methylated or methylated on K9, were incubated with 20 μl streptavidin-conjugated Sepharose beads (Amersham Pharmacia Biotech ) in PBS for 2 hrs at 4oC. After washing three times with binding buffer (20 mM Hepes, pH 7.9, 150 mM KCl, 1 mM DTT, 1 mM PMSF, 10% glycerol, 0.1% NP-40 and protease inhibitors cocktail), the beads were mixed with 300μl soluble chromatin fraction from 5×107 MEF cells and rotated for 3 hrs at 4°C. After extensive washing with washing buffer (20 mM Hepes, pH 7.9, 150 mM KCl, 1 mM DTT, 1 mM PMSF, 0.1% NP-40), the bound proteins were eluted in SDS loading buffer, resolved by SDS-PAGE and subjected to western blot.
Chromatin Immunoprecipitation Assay
Formaldehyde-crosslinked chromatin was prepared from MEF cells or 293 cells and immunoprecipitations were performed using the ChIP Assay Kit according to the manufacturer’s recommended protocol (Upstate Biotechnology). Pairs of real-time PCR primers were used for amplification of the promoter segments of the human Hox2a gene (forward-AACTTATGTGGCTGGGACGCA, reverse- AGTCGGGAAGGAACGCCTCAT, 405 bps), mouse Gata4 gene (forward-CTCTCCCGAGCTCACTTCAAGG, reverse- GGAGAAGGTGACCTCGCACAC, 110bps) and mouse Gata6 gene (forward-CCTTCCCATACACCACAACC, reverse- CCCCTCCTTCCAAATTAAGC, 237bps) promoter region (Boyer et al., 2006).
PCR
For quantitative analysis of gene expression, total RNA was isolated by Trizol kit (Invitrogen). RNA was treated with DNase (Promega, Madison, WI). Complementary DNA was synthesized using the cDNA synthesis kit (Takara) according to the manufacturer’s instructions. Fluorescence real-time RT-PCR was performed with the double-stranded DNA dye SYBR Green PCR Core Reagents (PE Biosystems, Warrington, United Kingdom) using the ABI PRISM 7300 system (Perkin-Elmer, Torrance, CA). PCR was done in triplicate and standard deviations representing experimental errors were calculated. All data were analyzed using ABI PRISM SDS 2.0 software (Perkin-Elmer). This software, which is coupled to the instrument, allows the determination of the threshold cycle (Ct) that represents the number of the cycle where the fluorescence intensity is significantly above the background fluorescence intensity. Pairs of PCR primers used to amplification of the target genes were shown in Table S1.
RT-PCR was performed on 20 ng of total RNA with specific primers and a Qiagen onestep RT-PCR kit (Qiagen) according to the manufacturer’s protocol. The primers used for the expression analysis of genes in SENP2 wild-type and mutant embryos were as follows: Hand1: forward (5′-AAGACACACCCTTCCAACCCATCT-3′), reverse (5′-ATTTCCCTTGGAAAGCAGAGGTGC-3′). Hand2: forward (5′-CACAGTGAGCAGCAACGACAAGAA-3′), reverse (5′-TGTCTACACAGCCAAGAGGGAACA-3′). Gata4: forward (5′-TCAAATTCCTGCTCGGACTTGGGA-3′), reverse (5′-TGAACCCAGCACTTTGGAGTCAGA-3′). Gata6: forward (5′-ACCACAACCACTACCTTATGGCGT-3′), reverse (5′-TGCCATCTGGACTGCTGGACAATA-3′). Nkx2.5: forward (5′-AGCAACTTCGTGAACTTTGGCGTC-3′), reverse (5′-TGTGAAATCTGAGGGACAGGGCAT-3′). Mef2c: forward (5′-GTTTGGACAACAAAGCCCTCAGCA-3′), reverse (5′-ATCTCAAAGCTGGGAGGTGGAACA-3′). Is1l: forward (5′-AAGCTGTACGTGCTTGGTTAGGGA-3′), reverse (5′-ACTGGGTTAGCCTGTAAACCACCA-3′). Srf: forward (5′-ATCCCTGTCTCTGCAGTTCAGCTT-3′), reverse (5′-AAGCTAACCAGAGGTCGCCAAAGA-3′). Actin:forward (5′-TCTTGGGTATGGAATCCTGTGGCA-3′), reverse (5′-ACTCCTGCTTGCTGATCCACATCT-3′).
In situ hybridization
Whole-mount in situ hybridization was performed according to the procedure of Lu et al (Lu et al., 1999). E10.5 embryos were digested with protease K and hybridized overnight with digoxigenin-labeled probes (generously provided by Jim Martin, Texas A&M University System Health Science Center at Houston). Conjugated anti-digoxigenin antibody was used to detect bound probes. After staining with NBT/BCIP, embryos were photographed as a whole mount and stored in PBS. Gata4 and Mef2c probes were synthesized by RNA polymerase (Ambion) and labeled by digoxigenin (Roche).
Highlights
Mutation of SENP2 gene in mouse causes defect in embryonic heart development
SENP2 regulates transcription of Gata4 and Gata6 mainly through alteration of PcG activity
SENP2 specifically de-conjugates SUMOylated Pc2
SUMOylation is essential for Pc2 recruitment to H3K27me3 to mediate transcription repression
Supplementary Material
Acknowledgments
We thank D. Wotton for generous provision of plasmids and J. Martin for generous provision of probes. This work was supported in part by National Natural Science Foundation of China (30772462 to J.C.), National Basic Research Program of China (973 Program) (No. 2009CB918403 to J.C.), NIH grants (CA-239520 to E.T.H.Y and CA-16672 to MDACC). E.T.H.Y is the McNair Scholar of the Texas Heart Institute/St. Luke’s Episcopal Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006a;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Duncan EM, Masui O, Gil J, Heard E, Allis CD. Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol Cell Biol. 2006b;26:2560–2569. doi: 10.1128/MCB.26.7.2560-2569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JL, Ganiatsas S, Agarwal S, Changou A, Salomoni P, Shirihai O, Meluh PB, Pandolfi PP, Zon LI. SUMO-1 protease-1 regulates gene transcription through PML. Mol Cell. 2002;10:843–855. doi: 10.1016/s1097-2765(02)00699-8. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SY, Asai N, Costantini F, Hsu W. SUMO-Specific Protease 2 Is Essential for Modulating p53-Mdm2 in Development of Trophoblast Stem Cell Niches and Lineages. PLoS Biol. 2008;6:e310. doi: 10.1371/journal.pbio.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 2003;17:1870–1881. doi: 10.1101/gad.1110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gong L, Millas S, Maul GG, Yeh ET. Differential regulation of sentrinized proteins by a novel sentrin-specific protease. J Biol Chem. 2000;275:3355–3359. doi: 10.1074/jbc.275.5.3355. [DOI] [PubMed] [Google Scholar]
- Gong L, Yeh ET. Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J Biol Chem. 2006;281:15869–15877. doi: 10.1074/jbc.M511658200. [DOI] [PubMed] [Google Scholar]
- Hang J, Dasso M. Association of the human SUMO-1 protease SENP2 with the nuclear pore. J Biol Chem. 2002;277:19961–19966. doi: 10.1074/jbc.M201799200. [DOI] [PubMed] [Google Scholar]
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Hay RT. SUMO-specific proteases: a twist in the tail. Trends Cell Biol. 2007;17:370–376. doi: 10.1016/j.tcb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, Chen Y, Cang H, Li H, Shi G, et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009 doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahana Y, Yeh ET, Zhang Y. Nucleocytoplasmic shuttling modulates activity and ubiquitination-dependent turnover of SUMO-specific protease 2. Mol Cell Biol. 2006;26:4675–4689. doi: 10.1128/MCB.01830-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Melhuish TA, Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell. 2003;113:127–137. doi: 10.1016/s0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Kamitani T, Nguyen HP, Kito K, Fukuda-Kamitani T, Yeh ET. Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J Biol Chem. 1998;273:3117–3120. doi: 10.1074/jbc.273.6.3117. [DOI] [PubMed] [Google Scholar]
- Kohler C, Villar CB. Programming of gene expression by Polycomb group proteins. Trends Cell Biol. 2008;18:236–243. doi: 10.1016/j.tcb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15:163–176. doi: 10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Min J, Zhang Y, Xu RM. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 2003;17:1823–1828. doi: 10.1101/gad.269603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends Biochem Sci. 2007;32:286–295. doi: 10.1016/j.tibs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Muller J, Verrijzer P. Biochemical mechanisms of gene regulation by polycomb group protein complexes. Curr Opin Genet Dev. 2009;19:150–158. doi: 10.1016/j.gde.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riising EM, Boggio R, Chiocca S, Helin K, Pasini D. The polycomb repressive complex 2 is a potential target of SUMO modifications. PLoS ONE. 2008;3:e2704. doi: 10.1371/journal.pone.0002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose L. Polycomb comes of age: genome-wide profiling of target sites. Curr Opin Cell Biol. 2007;19:290–297. doi: 10.1016/j.ceb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Roscic A, Moller A, Calzado MA, Renner F, Wimmer VC, Gresko E, Ludi KS, Schmitz ML. Phosphorylation-dependent control of Pc2 SUMO E3 ligase activity by its substrate protein HIPK2. Mol Cell. 2006;24:77–89. doi: 10.1016/j.molcel.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. Polycomb complexes and epigenetic states. Curr Opin Cell Biol. 2008;20:266–273. doi: 10.1016/j.ceb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Shen LN, Geoffroy MC, Jaffray EG, Hay RT. Characterization of SENP7, a SUMO-2/3-specific isopeptidase. Biochem J. 2009;421:223–230. doi: 10.1042/BJ20090246. [DOI] [PubMed] [Google Scholar]
- Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829–833. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007;8:263–271. doi: 10.1038/nrg2046. [DOI] [PubMed] [Google Scholar]
- Whitcomb SJ, Basu A, Allis CD, Bernstein E. Polycomb Group proteins: an evolutionary perspective. Trends Genet. 2007;23:494–502. doi: 10.1016/j.tig.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Reilly PT, Herr W. Loss of HCF-1-chromatin association precedes temperature-induced growth arrest of tsBN67 cells. Mol Cell Biol. 2001;21:3820–3829. doi: 10.1128/MCB.21.11.3820-3829.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci U S A. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ET. SUMOylation and De-SUMOylation: wrestling with life’s processes. J Biol Chem. 2009;284:8223–8227. doi: 10.1074/jbc.R800050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Saitoh H, Matunis MJ. Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex. Mol Cell Biol. 2002;22:6498–6508. doi: 10.1128/MCB.22.18.6498-6508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Smolen GA, Palmer R, Christoforou A, van den Heuvel S, Haber DA. SUMO modification is required for in vivo Hox gene regulation by the Caenorhabditis elegans Polycomb group protein SOP-2. Nat Genet. 2004;36:507–511. doi: 10.1038/ng1336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.