Abstract
Background
A crucial gap in the development of microbicides for HIV prevention is the absence of models predictive of safety. Previous studies have demonstrated an increased susceptibility to genital herpes in mice following repeated applications of nonoxynol-9 (N-9). This study was designed to explore the underlying mechanisms, focusing on the effects that N-9 has on genital tract epithelium and to apply this expanded model to evaluate the safety of microbicides that have been advanced to clinical trials.
Methods
Mice were treated intravaginally with formulated 3.5% N-9, 1% tenofovir, 0.5% or 2% PRO 2000, hydroxyethylcellulose (HEC) placebo or no treatment and the effect on herpes simplex virus 2 (HSV-2) susceptibility, epithelial cell architecture, junctional proteins and inflammation were assessed.
Results
Mice treated with seven daily doses of N-9, but not tenofovir, PRO 2000 or HEC, were significantly more susceptible to challenge with low doses of HSV-2; confocal microscopy demonstrated increased numbers of viral particles deep within the genital tract. N-9 disrupted the epithelium with loss of tight and adherens junctional proteins. By contrast, the epithelium was relatively preserved following tenofovir, PRO 2000 and HEC exposure. Additionally, N-9, but not the other microbicides, triggered a significant inflammatory response relative to untreated mice.
Conclusions
These findings indicate that disruption of the epithelium contributes to increased HSV-2 susceptibility and might provide a biomarker predictive of increased risk for HIV acquisition. The results are consistent with the safety outcomes of the recently completed Phase IIb clinical trial with 0.5% PRO 2000 gel, and predict that tenofovir gel will not adversely affect the genital tract.
Introduction
A major challenge in the development of microbicides that prevent HIV infection is the identification and validation of surrogate markers that are predictive of safety and efficacy. Meeting this challenge is crucial because large-scale clinical trials are difficult to design, pose risk to participants and require extensive resources. Recent microbicide clinical trial failures highlight the obstacles facing prevention initiatives and underscore the need to develop biomarkers predictive of safety prior to conducting large-scale clinical trials [1–5].
Preclinical assessment of safety has relied primarily on examining cytotoxicity in vitro and evaluating the histological response to microbicides in the rabbit vaginal irritation model [6]. Phase I clinical trials have focused primarily on colposcopy as a safety end point, although recent studies have also incorporated measurement of a limited array of inflammatory cytokines and changes in select vaginal flora [7]; however, the clinical trial failures suggest that these approaches are inadequate.
An inexpensive animal model predictive of safety would provide a crucial resource in the preclinical development of candidate microbicides. We have previously reported that Advantage-S, which contains 3.5% nonoxynol-9 (N-9), triggered a significant inflammatory response in mice compared with hydroxyethylcellulose (HEC) placebo gel. Moreover, N-9-treated mice were significantly more susceptible to intravaginal challenge with a low dose of herpes simplex virus 2 (HSV-2) administered 12 h after seven daily N-9 applications compared with mice who received HEC. Although an inflammatory response would be anticipated to increase HIV acquisition by recruiting and activating target cells [8], it is unclear whether inflammation contributes to the increased susceptibility to HSV-2, particularly because inflammatory responses are important for controlling infection [9]; therefore, this current study explored the additional mechanisms that might contribute to increased HSV-2 susceptibility.
The multilayered squamous epithelium in the lower genital tract provides the first line of defence against infection [10–13]. Disruption of this barrier might increase susceptibility to HSV by increasing exposure of nectin-1, a major coreceptor for HSV entry that localizes to adheren junctions [14,15]. Disruption would also increase the risk for HIV transmission by allowing virus to reach the submucosa, where target immune cells reside. An additional mechanism that could also contribute to enhanced risk for infection is down-regulation of antimicrobial peptides, such as defensins and secretory leukocyte protease inhibitor (SLPI), which have been shown to contribute to HIV and HSV inhibition [16–20]; thus, we expanded the murine model to assess the effect that N-9 products have on the epithelial barrier and the expression of antimicrobial peptides. We then applied this model to test tenofovir and 0.5% and 2% PRO 2000, microbicides that have been advanced to Phase II/III clinical trials.
Tenofovir is a potent reverse transcriptase inhibitor, which has been formulated as a 1% gel and is currently being evaluated in several international clinical trials [21,22]. PRO 2000, a naphthalene sulfonated polymer that inhibits HIV and HSV entry [23,24], was advanced to clinical trials as 0.5% and 2% formulations. In the recently completed Phase IIb trial (HPTN035), 0.5% PRO 2000 reduced the risk for HIV acquisition by 30% compared with HEC (P=0.10) [25]. Women used the gel for 12–30 months and no safety concerns were identified; however, in the ongoing Microbicide Development Program trial (MDP301), which was designed to compare 0.5% and 2% PRO 2000, the 2% arm was discontinued because the interim analysis indicated that it had little chance of showing efficacy. This prompted speculation that higher concentrations might trigger changes in the mucosal environment that counterbalanced the protective activity of the drug.
Methods
Microbicides
The tested gels were Encare (containing 3.5% N-9; Columbia Laboratories, Inc., Miami, FL, USA) or Advantage S (containing 3.5% N-9; Blairex Laboratories, Inc., Columbus, IN, USA), PRO 2000 (0.5% and 2%; Indevus Pharmaceuticals Inc., Lexington, MA, USA), 1% tenofovir and HEC placebo (International Partnership for Microbicides, Silver Spring, MD, USA).
Murine model
Female BALB/c mice (8–10 weeks old) were pretreated subcutaneously with 2 mg of medroxyprogesterone (Sicor Pharmaceuticals, Irvine, CA, USA) 5 days before gel application. To assess susceptibility to HSV-2, 40 μl of gel was delivered intravaginally daily for 7 days. At 12 h after the seventh application, groups of five mice were inoculated with HSV-2 strain G (HSV-2[G]) delivered in a volume of 15 μl/mouse equivalent to 103, 104 and 105 plaque-forming units [PFU]/mouse. Mice were evaluated daily for evidence of erythema, oedema, genital ulcers, hair loss around the perineum and hind-limb paralysis and were euthanized if symptoms of severe ulceration, hair loss or hind-limb paralysis developed [26]. Controls included mice that received no gel.
Additional studies were conducted in the absence of viral challenge to further assess the mucosal response. Medroxyprogesterone-treated mice were treated daily for up to 14 days with each microbicide or were left untreated. Vaginal washes were collected for detection of cytokines and chemokines from groups of five mice by washing with normal saline (100 μl) at baseline and prior to microbicide application on days 3, 7, 14 and 21. In addition, five mice from each treatment group were sacrificed at each time point and genital tract tissue excised and processed for confocal microscopy, extraction of RNA for quantitative real-time PCR (RT-PCR) or evaluation of transcription factors in nuclear extracts.
Confocal microscopy
The vagina, cervix and uterine vault up to the uterine bifurcation were excised. The tissue was opened parallel to the vaginal axis to expose the apical surface and then quartered with the lower quadrants containing the vagina and cervix and the upper quadrants containing the uterus. The resulting sections were washed and processed immediately for confocal imaging. To determine if the apical epithelial surface was intact, the tissue was stained for 30 min with EZ-link sulfosuccinimidobiotin reagent (sulfo-NHS-biotin; 1:1,000 dilution; Pierce, Rockford, IL, USA), which reacts with primary amines on cell surface proteins. This reagent does not penetrate the cell membrane; therefore, only primary amines exposed on the surface will be biotinylated in intact cells. The tissue was fixed and permeabilized with 4% paraformaldehyde and 1% Triton-X, respectively. Non-specific antibody binding sites were blocked by overnight incubation at 4°C with phosphate-buffered saline (PBS) containing 10% goat serum and 1% bovine serum albumin. Bound EZ-link sulfo-NHS-biotin was detected by treatment with streptavidin conjugated to Alexa Fluor 647 (1:1,000) for 1 h at room temperature; tight junctions were detected with rabbit anti-zonula occludens protein 1 (ZO-1; 1:500) and Alexa Fluor 488-conjugated secondary antibody (1:1,000), and adheren junctions were detected with mouse anti-desmoglein-1 (1:500) and Alexa Fluor 555-conjugated secondary antibody (1:1,000). All antibodies were diluted in PBS with 1% bovine serum albumin and were obtained from Invitrogen (Carlsbad, CA, USA). Nuclei were detected by staining with 4′,6′-diamidino-2-phenylindole nucleic acid stain (DAPI; Molecular Probes, Inc., Eugene, OR, USA). The entire tissue was mounted to glass slides using ProLong Gold Antifade reagent (Invitrogen). Confocal images were obtained and analysed by an investigator who was blind to the treatment group on either a Zeiss LSM 510 meta confocal microscope fitted with a 100× objective or a Zeiss Live DuoScan confocal microscope fitted with a 100× objective (Carl Zeiss Micro-Imaging, Inc., Thornwood, NY, USA). Z-stack images were obtained starting from the first detected fluorescent signal and continued in 1 μm increments until no further fluorescence was detected in any channel. Image analysis, three-dimensional images and axial images were generated using Volocity Software (Perkin Elmer, San Jose, CA, USA). Pixel intensity on axial images was quantified with Image J Densitometric Software (National Institutes of Health, Bethesda, MD, USA).
To localize HSV particles, additional confocal microscopy studies were performed. Mice were infected with 5×105 PFU/mouse 12 h after the day 7 application of microbicide gel and then sacrificed 4 h later. The tissue was processed as described above and incubated with mouse anti-VP16 (HSV viral capsid protein; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Alexa Fluor-488 secondary antibody and with mouse anti-nectin-1 (1:100; gift from Claude Krummenacher, University of Pennsylvania, Philadelphia, PA, USA) and Alexa Fluor-555 secondary antibody. Nuclei were stained with DAPI.
Cytokine and chemokine analyses
Protease inhibitors (Complete Protease Inhibitor Cocktail; Roche Applied Science, Indianapolis, IN, USA) were added to each pooled vaginal wash sample before centrifugation at 210 g for 10 min at 4°C. The supernatants were stored at −80°C and assayed for cytokines and chemokines using the Fluorokine MultiAnalyte profiling system (R&D Systems, Minneapolis, MN, USA), measured with a Bioluminex 100 system (Bioluminex, Austin, TX, USA) and analysed with StarStation (version 2.0; Applied Cytometry Systems, Sheffield, UK).
Real-time PCR
Vaginal tissue was homogenized and total RNA was extracted using the Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA, USA). Reverse transcription was performed using 400 ng of RNA and the StrataScript cDNA Synthesis Kit (Applied Biosystems, Foster City, CA, USA). Quantitative RT-PCR was conducted in duplicate with 50 ng of complementary DNA and with 1 μl of 20× FAM-labelled probes in a 20 μl final reaction volume of 2× TaqMan® PCR Master Mix (Applied Biosystems). Probes were purchased from Applied Bio-systems. PCR cycling conditions on an ABI PRISM 7700 (Applied Biosystems) were: 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, 45 cycles at 95°C for 15 s and 1 cycle at 60°C for 1 min. Relative expression levels were calculated using the comparative Ct method (2[−ΔΔCT]), where sample values were compared with RNA from HEC-treated mice and the Ct values of both were normalized to β-actin RNA levels as an endogenous housekeeping gene.
Transcription factor assays
Nuclear extracts were prepared using Active Motif’s Nuclear Extract Kit (Carlsbad, CA, USA). Nuclear protein was quantified by the Quick Start Bradford Protein Assay (Bio-Rad, Hercules, CA, USA) and nuclear extracts were stored at −80°C. These samples were analysed for nuclear factor (NF)-κB (p65) levels using Active Motif’s TransAm ELISA-based assay kits.
Statistical analyses
GraphPad Prism (version 4; GraphPad Software, La Jolla, CA, USA) was used for statistical analyses. Cytokine, RT-PCR and transcription factor results were analysed by one-way analysis of variance with Tukey’s post hoc test to compare groups. Kaplan–Meier survival curves were assessed by log-rank test. A P<0.05 value was considered to be significant.
Results
N-9, but not tenofovir or PRO 2000, increased the susceptibility to HSV-2 and promoted migration of viral particles across the epithelium
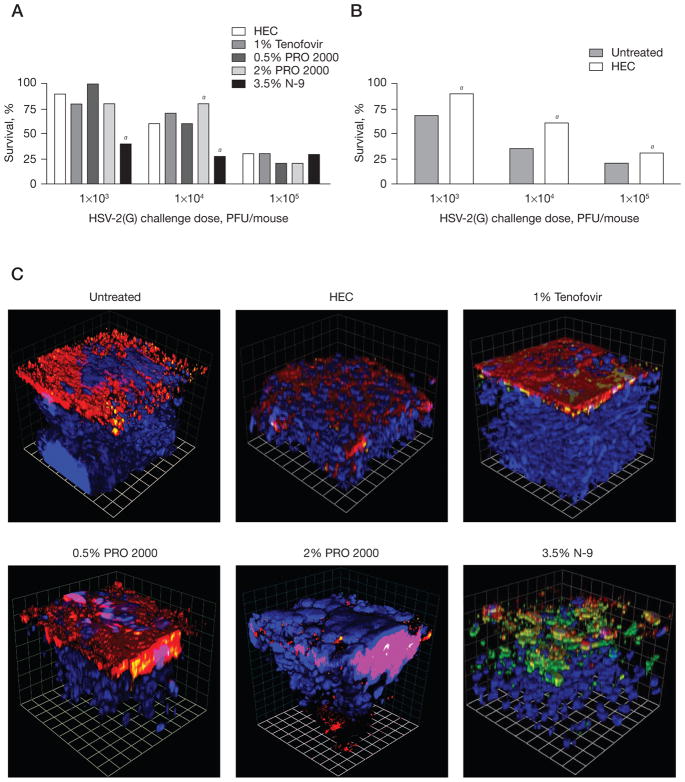
Disruption of the multilayered squamous epithelium might increase the susceptibility to HSV by facilitating the migration of viral particles across the keratinized superficial epithelium and increasing access to nectin coreceptors. To test this notion, mice were treated intra-vaginally with each microbicide for 7 days and then 12 h after the final dose, the mice were challenged with 103, 104 or 105 PFU/mouse of HSV-2(G) (Figure 1). Treatment with N-9 (Advantage-S or Encare), but not tenofovir or PRO 2000, resulted in a shift in the dose–response with significantly increased susceptibility to HSV-2(G) observed following challenge with 103 and 104 PFU/mouse (Figure 1A). Notably, 2% PRO 2000 provided some protection following challenge with 104 PFU/mouse, suggesting that some drug might persist in the genital tract 12 h after the final gel application to block HSV-2(G) infection. No differences in susceptibility were observed following infection with 105 PFU/mouse. HEC, which provides a physical barrier against infection (Figure 1B), provided significant protection relative to untreated mice (which might reflect the rheological properties of the gel).
Figure 1. N-9, but not tenofovir or PRO 2000, increases the susceptibility to HSV-2 infection.
Mice were challenged with 103, 104 or 105 plaque-forming units (PFU)/mouse of herpes simplex virus 2 strain G (HSV-2[G]) (A) at 12 h after receiving the seventh daily dose of nonoxynol-9 (N-9), tenofovir, 0.5% PRO 2000, 2% PRO 2000 or hydroxyethylcellulose (HEC) or (B) after being left untreated and were observed daily for 15 days for signs and symptoms of disease. Symptoms were scored on a 0–5 point scale: 0, no apparent infection; 1, slight redness of the vagina; 2, moderate redness and swelling of the vagina and surrounding tissue; 3, severe redness and swelling; 4, genital ulceration or hair loss of genital and surrounding tissue; and 5, evidence of hind-limb paralysis. Mice reaching stage 4 or 5 disease were euthanized. Results show the percentage of survival pooled from at least two independent experiments (n=20 mice/group). aP<0.05. To assess whether changes in susceptibility were associated with disruption of the epithelial barrier, confocal images of extracted lower genital tract tissue were examined. Mice were infected with HSV-2(G) at 12 h after the seventh daily gel application and were then sacrificed 4 h after infection. Tissues were stained for viral capsids (green), nectin-1 (red) and nuclei (blue). (C) Three-dimensional reconstruction representative of images taken from at least four animals per treatment group. At least six independent randomly selected images were acquired per mouse and each grid mark represents 10 μm.
To address whether the increase in susceptibility was associated with alterations in the epithelial architecture, mice that had received seven daily doses of each gel were again challenged with HSV-2(G); 4 h after viral challenge, the animals were sacrificed for confocal microscopy. Representative images of the lower genital tract, in which nectin-1 was stained red, nuclei were stained blue and viral capsids were stained green, are shown in Figure 1C [27,28]. Complete disruption of the architecture with viral capsids extending an average of 42 μm into the basal epithelial layers was observed following exposure to N-9. By contrast, viral capsids were difficult to detect and found primarily colocalized with nectin (yellow) in tissue extracted from untreated mice or from mice treated with other microbicides. Viral particles extended an average of 6 μm in untreated mice and 11–16 μm in tenofovir- and PRO 2000-treated mice.
Nectin appeared tightly packed towards the apical surface of the tissue in untreated mice as well as in mice that were treated with tenofovir or 0.5% PRO 2000. By contrast, nectin was more dispersed in HEC-treated mice and was detected within the deeper epithelial layers in mice treated with 2% PRO 2000 gel, suggesting some disruption in the integrity of the epithelial cell barrier; however, these changes were not associated with an increase in the detection of free viral capsids. The differences in response to the two doses of PRO 2000 might reflect not only the drug concentration, but also differences in formulation. The 2% PRO 2000 gel contains more carbomer gelling agent than the 0.5% gel (1.7% carbomer versus 1.35%) and is approximately 40% more viscous than the 0.5% gel (Al Profy, Indeus Pharmaceuticals, personal communication).
N-9, but not tenofovir, induced the loss of junctional proteins
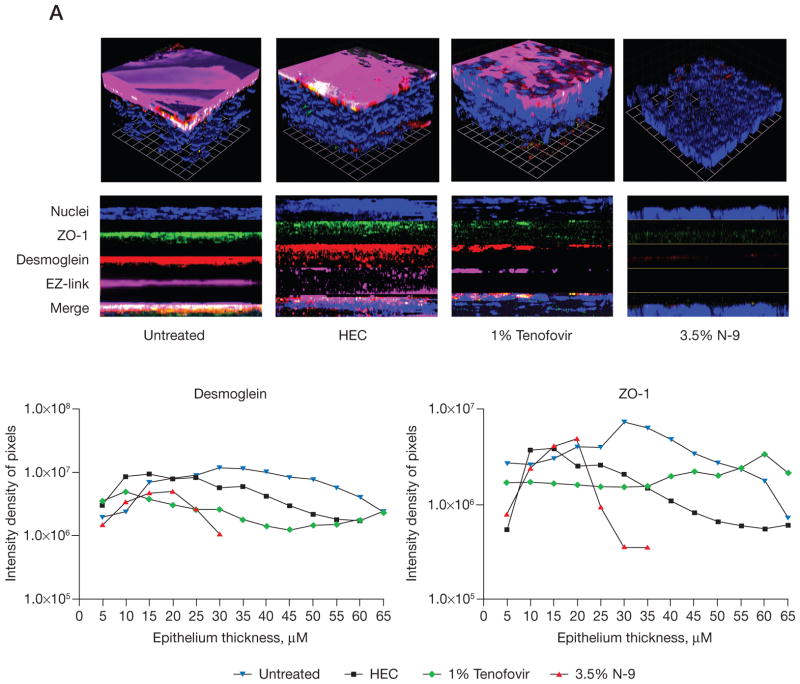
Additional confocal studies were conducted in mice treated with microbicides in the absence of HSV-2(G) challenge (Figure 2). These images were obtained 12 h after the day 7 gel application to coincide with the time point at which HSV-2(G) challenges were performed. Thinning and disruption of the epithelium was observed with reduction in bound EZ-link sulfo-NHS-biotin (magenta) at the apical surface in the lower genital tract of mice treated with N-9 (Figure 2A). N-9 treatment also led to a loss in ZO-1 (green) and desmoglein (red). Pixel intensity results obtained for successive z-stack sections confirmed the loss in tissue, which was reflected by the decreased number of sections and the lower intensity density of pixels within the sections (Figure 2A). The thickness of the lower tract was 92–158 μm in untreated mice compared with 35–70 μm in N-9-treated mice. Changes were less pronounced in the upper gentital tract and following three daily doses, indicating that the response reflected the cumulative effects of repeated exposure (data not shown). By contrast, tenofovir triggered little or no change in epithelial tissue thickness (similar number of sections) or loss of junctional proteins (pixel intensity; Figure 2A and 2C).
Figure 2. N-9 causes disruption of the epithelium with loss of junctional proteins.
Mice were treated daily for 7 days with microbicides and at 12 h after the final application, the mice were sacrificed and the entire vaginal canal up to the uterine bifurcation was excised. (A & B) The tissue was then fixed and stained with EZ-link sulfo-NHS-biotin (EZ-link; Pierce, Rockford, IL, USA) to detect the apical surface (magenta; [A] only) 4′,6′-diamidino-2-phenylindole nucleic acid stain to detect nuclei (blue), anti-zonula occludens protein 1 (ZO-1; green) and desmoglein (red) and viewed by confocal microscopy. Representative three-dimensional (upper panel) images are shown. Images were taken from at least six animals per treatment group and at least six independent randomly selected images were acquired per animal. Pixel intensity results were obtained for successive μm thick axial sections from the apical to the basal membrane for Z0-1 and desmoglein-1 and are shown in the graphs below each panel set. (C) After seven daily drug applications, gene expression of E-cadherin, ZO-1, desmoglein and nectin-1 were measured from RNA extracted from genital tract tissue by quantitative competitive real-time PCR. Results represent the β-actin normalized values compared with untreated samples (n= at least five mice/group in at least two independent experiments) and are presented as log10 change in expression (mean ± SE). aP<0.05. No change in E-cadherin expression was observed in response to hydroxyethylcellulose (HEC) or tenofovir. N-9, nonoxynol-9.
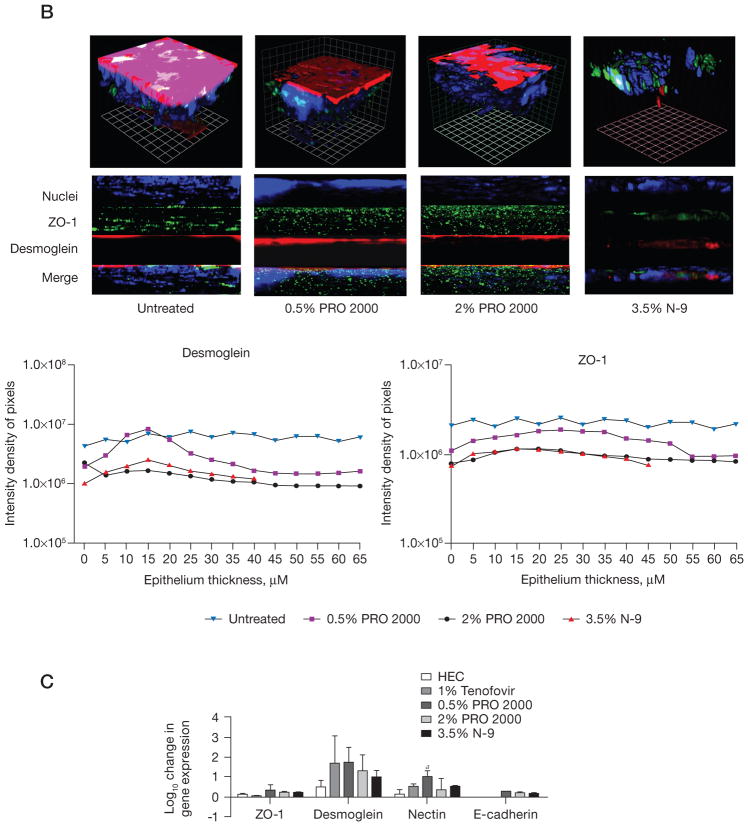
Parallel studies were conducted in mice treated for 7 days with 0.5% and 2% PRO 2000 using a different microscope and stained for desmoglein (red), ZO-1 (green) and nuclei (blue); N-9 and untreated mice were included as positive- and negative-controls, respectively (Figure 2B). A loss in tissue and junctional proteins was again observed in response to N-9, reflected in a decrease in sections and pixel intensity (Figure 2B). By contrast, the tissue thickness was relatively preserved in mice treated with PRO 2000 (number of sections); however, the pixel intensity for both ZO-1 and desmoglein was reduced in mice treated with 2% PRO 2000 (Figure 2C).
The loss in junctional proteins could reflect protein degradation and/or down-regulation of gene expression. To evaluate this, RNA was extracted and evaluated for ZO-1, desmoglein, E-cadherin and nectin-1 expression by quantitative RT-PCR. None of the junctional proteins were down-regulated. By contrast, a trend towards up-regulation of desmoglein and nectin-1 was observed both on day 3 (data not shown) and day 7 (Figure 2C). The up-regulation might represent the cellular response to protein degradation.
Effect of microbicides on antimicrobial peptides
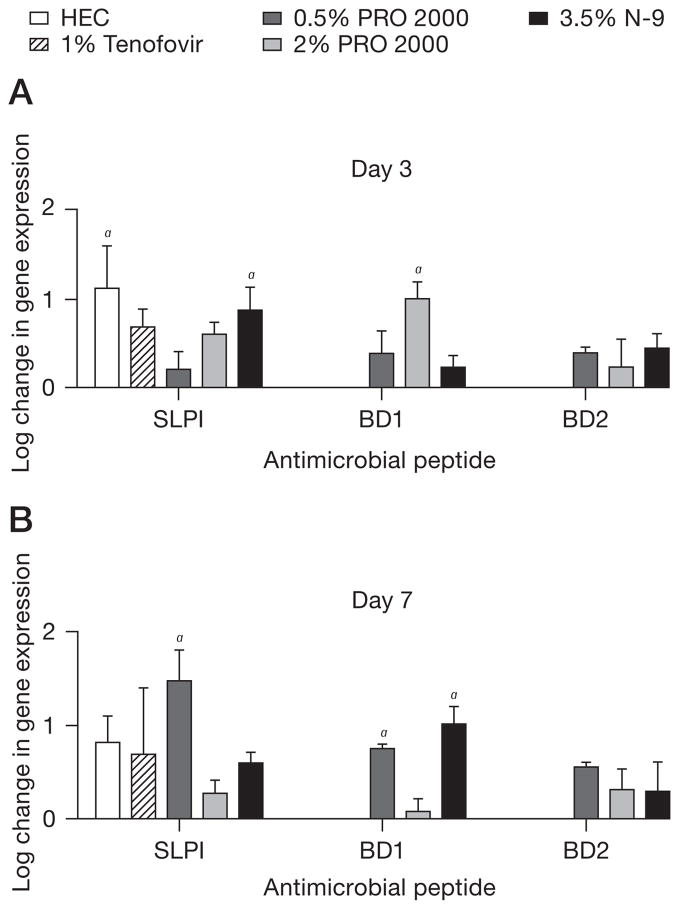
Genital tract epithelial and resident immune cells secrete antimicrobial peptides, including defensins and SLPI that inhibit HIV and HSV-2 in vitro and might contribute to host defence [19,29–31]. To explore whether microbicides modify the expression of murine antimicrobial peptides, the RT-PCR studies were extended to include β-defensin (BD)1, BD2 and SLPI. Significant increases in BD1 were observed in response to 2% PRO 2000 on day 3 and to 0.5% PRO 2000 and 3.5% N-9 on day 7 (Figure 3). There was also a trend toward increased BD2 in response to PRO 2000 and N-9. These findings are consistent with inflammation as defensins are induced by proinflammatory stimuli, including tumour necrosis factor (TNF)-α and interferon (IFN)-γ [32]. Surprisingly, SLPI, which is anti-inflammatory, was also up-regulated in response to all of the microbicides, including HEC, although this was only significant after N-9 and HEC treatment on day 3 and 0.5% PRO 2000 treatment on day 7. These results are in contrast to those obtained in vitro, where we found that microbicides down-regulate SLPI expression in human epithelial cell cultures [33,34]. The variance might reflect differences in species (human versus mouse), time points (h versus days) and the response of a single cell type in culture compared with the cumulative tissue response and interactions between cell types.
Figure 3. Effect of microbicides on SLPI and defensin gene expression.
After (A) three or (B) seven daily applications of each formulated microbicide, gene expression of β-defensin (BD)1, BD2 and secretory leukocyte protease inhibitor (SLPI) were measured from vaginal tissue by quantitative real-time PCR. Results represent the β-actin normalized values compared with untreated mice (n= at least five mice/group in at least two independent experiments) and are presented as log10 change in gene expression (mean ± SE). aP<0.05. There was no change in gene expression observed for BD1 or BD2 in response to hydroxyethylcellulose (HEC) or tenofovir. N-9, nonoxynol-9.
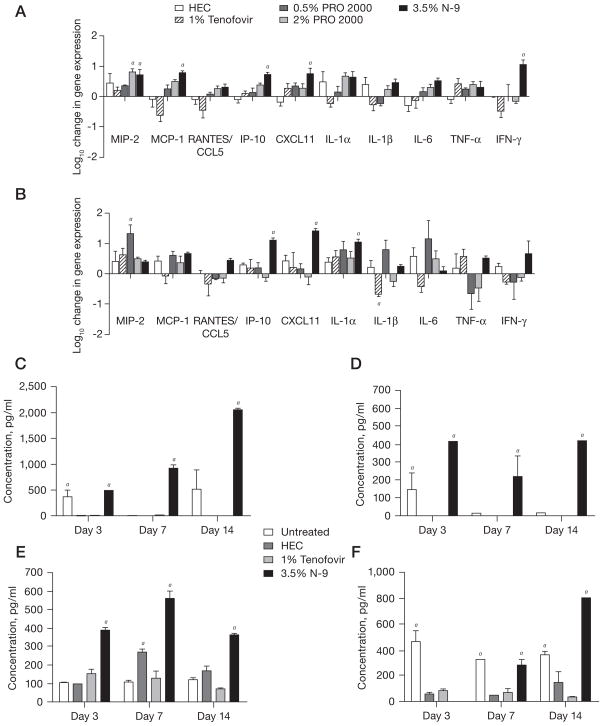
Tenofovir triggers little or no inflammatory response
Consistent with our prior studies, N-9 induced a significant increase in gene expression of multiple inflammatory cytokines and chemokines, including macrophage inflammatory protein (MIP)-2, monocyte chemotactic protein (MCP)-1, IFN inducible protein (IP)-10, C-X-C motif ligand (CXCL)11, interleukin (IL)-6, and IFN-γ on day 3 (Figure 4A), and IP-10, CXCL11 and IL-1α on day 7 (Figure 4B). By contrast, tenofovir triggered little inflammation and decreased the level of IL-1β on day 7. A trend towards increased expression of inflammatory cytokines and chemokines was also observed in response to PRO 2000, but this only reached statistical significance for MIP-2 on day 3 for 2% PRO 2000 and day 7 for 0.5% PRO 2000. No statistically significant differences in gene expression were observed between HEC-treated and untreated mice at any time point or in response to any of the drugs on days 14 or 21 (data not shown).
Figure 4. Changes in gene expression and protein levels of cytokines and chemokines in response to microbicides.
Levels of messenger RNA were quantified from tissue on (A) day 3 and (B) day 7 by quantitative real-time PCR. Results represent β-actin-normalized values relative to untreated samples (n= at least five mice/group in at least two independent experiments) and are presented as the log10 change in gene expression (mean ± SE). Vaginal washes pooled from at least five mice were collected before microbicide application in normal saline on days 3, 7, 14 and 21 from mice treated daily for 14 days with each microbicide formulation or untreated control mice. The levels of chemokines and cytokines were measured in the vaginal lavage by BioLuminex (Austin, TX, USA). The sensitivities for each analyte (in pg/ml) were as follows: (C) monocyte chemotactic protein (MCP)-1=0.95, (D) tumour necrosis factor (TNF)-α=0.42, (E) macrophage inflammatory protein (MIP)-2=2.2 and (F) interleukin (IL)-1β=3.3. In addition, the sensitivity for IL-6 was 0.71. Results for the detectable mediators (n = at least five mice/group in at least two independent experiments) are presented as mean ± SE. No protein was detected in the lavage samples for which no bar is observed. aP<0.05. CCL5, C–C motif ligand 5; CXCL11, C-X-C motif ligand 11; HEC, hydroxyethylcellulose; IP-10, interferon-inducible protein 10; N-9, nonoxynol-9; RANTES, regulated upon activation, normal T-cell expressed and secreted protein.
We also measured the secretion of cytokines and chemokines into genital tract secretions. We focused on tenofovir for these studies, as we have previously reported findings with PRO 2000 [35]. No increase in any of the mediators was observed in response to tenofovir (Figure 4C, 4D, 4E and 4F). Rather, lower concentrations of MCP-1, TNF-α and IL-1β were observed in mice treated with tenofovir or HEC compared with untreated mice and this persisted for IL-1β on day 7 and 14. By contrast, a significant increase in MIP-2, MCP-1/CCL2 and TNF-α was observed in vaginal washes obtained from mice treated with N-9, which is consistent with previous studies [35]. Additionally, HEC induced a significant increase in MIP-2 on day 7. Little or no IFN-γ or IL-6 was detected in any of the vaginal washes (data not shown).
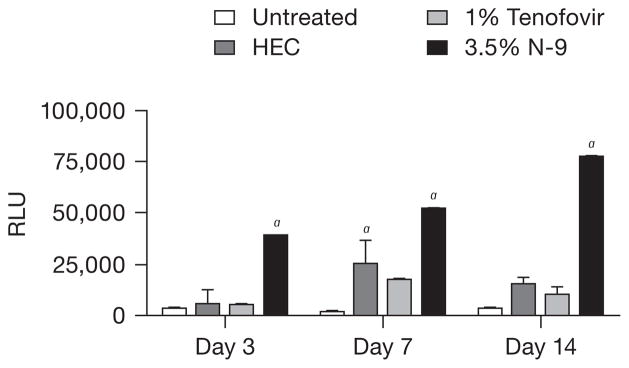
The inflammatory response is controlled, in part, by NF-κB, which also participates in transcriptional regulation of defensins [36,37]. For example, the human BD2 gene (DEFB2) promoter region contains three potential NF-κB binding sequences and is up-regulated by NF-κB activation [38]. Tenofovir had little or no effect on NF-κB, which is consistent with the absence of any up-regulation of cytokines, chemokines or defensins in the tenofovir-treated mice. By contrast, NF-κB (p65) was significantly increased in tissue obtained from N-9-treated animals on days 3, 7 and 14 (P<0.001; Figure 5) [35]. This effect was diminished by day 21 (data not shown). NF-κB was also activated in response to HEC exposure, but only on day 7. We have previously reported that 2% PRO 2000 did not activate NF-κB [35].
Figure 5. N-9, but not tenofovir, activates NF-κB.
Nuclear levels of nuclear factor (NF)-κB (p65) were determined by ELISA performed on nuclear extracts prepared from harvested genital tract tissue. Results are shown as mean ± SE values using tissue from at least five mice/group from at least two replicate experiments. aP<0.05 relative to untreated control mice. HEC, hydroxyethylcellulose; N-9, nonoxynol-9; RLU, relative luciferase units.
Discussion
The current studies suggest that the primary mechanism contributing to the increased susceptibility to HSV infection in the murine model is disruption of the epithelial barrier. Treatment of mice with seven daily doses of N-9 resulted in thinning of the genital tract epithelium, loss of apical membrane staining, disruption of intercellular junctions and degradation of junctional proteins. These structural changes were associated with increased migration of viral particles into deeper epithelial layers. We speculate that an intact epithelial barrier is also a crucial determinant of susceptibility to HIV and that the increase in HSV-2 susceptibility observed in this model in response to microbicides might provide a surrogate marker predictive of increased risk for HIV acquisition.
These studies were conducted with medroxyprogesterone-treated mice, which more closely resemble the progesterone dominant luteal phase of the menstrual cycle in humans. During diestrous, the murine cervical epithelium is thinner and is composed of several layers of cuboidal nucleated cells with cell junctions detected near the surface by electron microscopy [39]. Mice are more susceptible to genital herpes during diestrous, in part because the structural changes include increased expression and access to the HSV coreceptor, nectin-1 [14]. Moreover, the response to HSV is highly reproducible in medroxyprogesterone-treated mice compared with cycling mice [40]. Similarly, mice might also be more susceptible to microbicide-induced changes during diestrous, thereby providing a highly stringent and reproducible safety model. We found little variability in the response to N-9 in this model in multiple experiments involving approximately 100 mice and using different formulations. Studies are currently planned with cycling mice for comparison.
Whether this model will prove to be too stringent or not stringent enough will require correlating results with those obtained in ongoing clinical trials. The findings are consistent with a dual-chamber Transwell culture model that also examined the effect of microbicides on tight junctions and with clinical experiences [33]. N-9 increased the risk of HIV infection in women who used the study gel more than 3× a day compared with women in the placebo group in the Phase II/III clinical trial [41]. The mouse model would have predicted this outcome and demonstrated a cumulative response to repeated gel exposure with extensive changes observed following seven daily exposures compared with three daily exposures. Conversely, no increase in HSV-2 susceptibility and little or no change in the epithelial architecture was observed in response to 0.5% PRO 2000 or HEC. These findings are consistent with the HPTN035 study, in which women safely used 0.5% PRO 2000 or HEC gel for 12–30 months. Some changes in the epithelium were observed in response to 2% PRO 2000, but these were not sufficient to trigger an increase in HSV-2 susceptibility. If the results of the MDP301 trial demonstrate that 2% is less protective than 0.5% PRO 2000 gel, this would suggest that these modest changes might be relevant and that the murine model should be modified to achieve higher stringency. Increasing the frequency and/or duration of exposure to formulated microbicides prior to challenging with HSV-2 might unmask toxicities and demonstrate increased susceptibility.
These studies focused on the response to repeated gel application. Others have explored the effect of a single gel application of several microbicides [42–44]. For example, after exposure of female Swiss Webster mice to a single intravaginal application of Conceptrol (4% N-9), minimal epithelial disruption or inflammation of the vaginal mucosa was observed by histology. Regions of epithelial disruption were detected in the cervix 2–4 h post-application, but these completely resolved by 24 h, suggesting regeneration of the columnar epithelium [44]. By contrast, we observed greater changes at the more apical surfaces of the lower tract following repeated applications and the damage appeared to be cumulative, suggesting a loss in regenerative capacity over time. This could prove particularly problematic in the setting of inconsistent adherence to gel application. Modest epithelial cell damage might be tolerated when sufficient drug is present to inhibit infection; however, damage might promote infection with intermittent gel use or as the concentration in the genital tract wanes.
Although the results suggest that epithelial barrier disruption might be the primary mechanism underlying the increased susceptibility to HSV-2, inflammation might also contribute to overall toxicity and the increased risk for HIV infection. The importance of inflammation in facilitating HIV infection was highlighted in a recent macaque study. The initially infected founder cell population was small, but the virus (Simian immunodeficiency virus) overcame this by activating a signalling pathway that resulted in increased chemokine expression and the recruitment CCR5+ T-cell targets [45]. Notably, in these studies, N-9 triggered significant up-regulation of CXCL10 (IP-10) and CXCL11, which are ligands for the chemokine receptor CXCR3 expressed on CCR5+ T-cells, These chemokines have been implicated in the recruitment of HIV target cells and pathogenesis [46].
Limitations of this model are that the murine genital tract does not fully recapitulate the human tract and mice are not susceptible to HIV. Humanized bone marrow-liver-thymus (BLT) mice are fully susceptible to intravaginal HIV infection and provide the opportunity to test whether the increased HSV susceptibility predicts an increased risk for HIV infection [47]. Similar studies could also be performed with macaques; however, limited access and cost of BLT mice and macaques restrict the widespread use of these models for screening microbicide formulations. If continued testing of candidate microbicides demonstrates that increased HSV-2 susceptibility in this inexpensive and highly reproducible murine model is predictive of clinical outcomes, it should be incorporated into the safety algorithm for preclinical microbicide evaluation. The model predicts that tenofovir, which is currently in Phase II/III trials, will prove to be safe and will have no deleterious effects on epithelial integrity nor trigger any untoward inflammatory response.
Acknowledgments
We thank the International Partnership for Microbicides for providing HEC and tenofovir gels and Indevus Pharmaceuticals for providing PRO 2000 gels. We thank the Analytical Imaging Facility at Albert Einstein College of Medicine (Bronx, NY, USA) and the Mount Sinai School of Medicine Microscopy Shared Resource Facility (New York, NY, USA) for assistance with confocal studies. We also thank the DNA core facility at Albert Einstein College of Medicine. This work was supported by the International Partnership for Microbicides and NIH grants AI079763, AI065309, AI069551 and AI077549.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. AIDS. 2000;14:85–88. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 2.Honey K. Microbicide trial screeches to a halt. J Clin Invest. 2007;117:1116. doi: 10.1172/JCI32291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Check E. Scientists rethink approach to HIV gels. Nature. 2007;446:12. doi: 10.1038/446012a. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. AIDS research. Promising AIDS vaccine’s failure leaves field reeling. Science. 2007;318:28–29. doi: 10.1126/science.318.5847.28. [DOI] [PubMed] [Google Scholar]
- 5.HIV vaccine failure prompts Merck to halt trial. Nature. 2007;449:390. doi: 10.1038/449390c. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein P, Jackson MC, Millman N, Sobrero AJ. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz JL, Mauck C, Lai JJ, et al. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind Phase I safety study. Contraception. 2006;74:133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184:418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 9.Svensson A, Bellner L, Magnusson M, Eriksson K. Role of IFN-α/β signaling in the prevention of genital herpes virus type 2 infection. J Reprod Immunol. 2007;74:114–123. doi: 10.1016/j.jri.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Serwadda D, Gray RH, Sewankambo NK, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 11.Celum C, Levine R, Weaver M, Wald A. Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ. 2004;82:447–453. [PMC free article] [PubMed] [Google Scholar]
- 12.Moss GB, Clemetson D, D’Costa L, et al. Association of cervical ectopy with heterosexual transmission of human immunodeficiency virus: results of a study of couples in Nairobi, Kenya. J Infect Dis. 1991;164:588–591. doi: 10.1093/infdis/164.3.588. [DOI] [PubMed] [Google Scholar]
- 13.Myer L, Wright TC, Jr, Denny L, Kuhn L. Nested case-control study of cervical mucosal lesions, ectopy, and incident HIV infection among women in Cape Town, South Africa. Sex Transm Dis. 2006;33:683–687. doi: 10.1097/01.olq.0000216026.67352.f9. [DOI] [PubMed] [Google Scholar]
- 14.Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J Virol. 2004;78:2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J Virol. 2006;80:12209–12218. doi: 10.1128/JVI.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quayle AJ, Porter EM, Nussbaum AA, et al. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 17.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John M, Keller MJ, Fam EH, et al. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis. 2005;192:1731–1740. doi: 10.1086/497168. [DOI] [PubMed] [Google Scholar]
- 19.Hazrati E, Galen B, Lu W, et al. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J Immunol. 2006;177:8658–8666. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- 20.Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199–230. [PubMed] [Google Scholar]
- 21.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 22.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 23.Cheshenko N, Keller MJ, MasCasullo V, et al. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob Agents Chemother. 2004;48:2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scordi-Bello IA, Mosoian A, He C, et al. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob Agents Chemother. 2005;49:3607–3615. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karim SA, Coletti A, Richardson B, et al. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO 2000/5 gel for the prevention of hiv infection in women: results of the HPTN 035 trial. 16th Conference on Retroviruses and Opportunistic Infections; 8–11 February 2009; Montreal, QC, Canada. [Google Scholar]
- 26.Hendrickson BA, Guo J, Brown I, et al. Decreased vaginal disease in J-chain-deficient mice following herpes simplex type 2 genital infection. Virology. 2000;271:155–162. doi: 10.1006/viro.2000.0303. [DOI] [PubMed] [Google Scholar]
- 27.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 28.Taylor JM, Lin E, Susmarski N, et al. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Owen SM, Rudolph DL, et al. Activity of alpha- and theta-defensins against primary isolates of HIV-1. J Immunol. 2004;173:515–520. doi: 10.4049/jimmunol.173.1.515. [DOI] [PubMed] [Google Scholar]
- 30.Quiñones-Mateu ME, Lederman MM, Feng Z, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 31.Guo CJ, Tan N, Song L, Douglas SD, Ho WZ. Alpha-defensins inhibit HIV infection of macrophages through upregulation of CC-chemokines. AIDS. 2004;18:1217–1218. doi: 10.1097/00002030-200405210-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albanesi C, Fairchild HR, Madonna S, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–992. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 33.Mesquita PM, Cheshenko N, Wilson SS, et al. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J Infect Dis. 2009;200:599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheshenko N, Herold BC. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J Gen Virol. 2002;83:2247–2255. doi: 10.1099/0022-1317-83-9-2247. [DOI] [PubMed] [Google Scholar]
- 35.Galen BT, Martin AP, Hazrati E, et al. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J Infect Dis. 2007;195:1332–1339. doi: 10.1086/513279. [DOI] [PubMed] [Google Scholar]
- 36.Mineshiba J, Myokai F, Mineshiba F, Matsuura K, Nishimura F, Takashiba S. Transcriptional regulation of β-defensin-2 by lipopolysaccharide in cultured human cervical carcinoma (HeLa) cells. FEMS Immunol Med Microbiol. 2005;45:37–44. doi: 10.1016/j.femsim.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser V, Diamond G. Expression of mammalian defensin genes. J Leukoc Biol. 2000;68:779–784. [PubMed] [Google Scholar]
- 38.Liu L, Wang L, Jia HP, et al. Structure and mapping of the human β-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 39.Corbeil LB, Chatterjee A, Foresman L, Westfall JA. Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell. 1985;17:53–68. doi: 10.1016/0040-8166(85)90015-1. [DOI] [PubMed] [Google Scholar]
- 40.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 42.Phillips DM, Zacharopoulos VR. Nonoxynol-9 enhances rectal infection by herpes simplex virus in mice. Contraception. 1998;57:341–348. doi: 10.1016/s0010-7824(98)00040-7. [DOI] [PubMed] [Google Scholar]
- 43.Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catalone BJ, Kish-Catalone TM, Budgeon LR, et al. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob Agents Chemother. 2004;48:1837–1847. doi: 10.1128/AAC.48.5.1837-1847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foley JF, Yu CR, Solow R, Yacobucci M, Peden KW, Farber JM. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J Immunol. 2005;174:4892–4900. doi: 10.4049/jimmunol.174.8.4892. [DOI] [PubMed] [Google Scholar]
- 47.Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]