Abstract
Introduction
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT) is a psychoactive indolealkylamine substance that has been used for recreational purpose and may lead to fatal toxicity. While 5-MeO-DMT is mainly inactivated via deamination, it is O-demethylated to an active metabolite, bufotenine. Quantitation of 5-MeO-DMT and bufotenine is essential to understand the exposure to and the effects of drug and metabolite. This study, therefore, aimed to develop and validate a LC-MS/MS method for simultaneous analysis of 5-MeO-DMT and bufotenine in mouse serum.
Methods
A simple protein precipitation method coupled with an optimal gradient elution was used for sample preparation and separation. Detection of 5-MeO-DMT and bufotenine was accomplished using multiple reaction monitoring of m/z 219.2→174.2 and 205.2→160.2, respectively, in the positive ion mode. 5-Methyl-N,N-dimethyltrypamine (m/z 203.2→158.3) was used as internal standard for quantification. Accuracy and precision were determined after the analyses of quality control samples. Validated assay was then employed to determine drug and metabolite concentrations in serum samples collected from mice at different time points after intraperitoneal administration of 5-MeO-DMT (2 mg/kg).
Results
With a total run time of 9 min, 5-MeO-DMT and bufotenine were eluted at 2.8 and 5.6 min, respectively. The assay was linear over the range 0.90–5,890 ng/mL (1.12–7,360 pg on-column) for 5-MeO-DMT and 2.52–5,510 ng/mL (3.14–6,890 pg) for bufotenine. Intra- and inter-day precision and accuracy were within 15% for both analytes. The recovery of each analyte from 20 µL of serum containing 8.08, 72.7 and 655 ng/mL of 5-MeO-DMT and 7.56, 68.1 and 613 ng/mL of bufotenine was more than 75%. Pharmacokinetic analysis revealed that the systemic exposure (area under the curve) to metabolite bufotenine was about 1/14 of that to 5-MeO-DMT.
Conclusion
This LC-MS/MS method is a sensitive and reliable assay for quantitation of blood 5-MeO-DMT and bufotenine. Given the fact that bufotenine acts on 5-HT2A receptor with an affinity about 10-fold higher than 5-MeO-DMT, the active metabolite bufotenine may significantly contribute to the apparent pharmacological and toxicological effects of 5-MeO-DMT.
Introduction
Indolealkylamine (IAA) drugs are 5-hydroxytryptamine (5-HT or serotonin) analogs that act primarily on serotonergic system. Some IAAs are clinically utilized for antimigraine therapy, whereas other substances have high impact as drugs of abuse [1]. They are psychoactive ingredients of a wide range of plant, fungus and animal preparations that have been used for social and religious cultures in history. Currently, IAA drugs are readily synthesized chemically and they are sold via the internet; with the epidemic in abuse, intoxications due to IAA substances have been frequently reported in recent years [2–6].
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT; Fig. 1) is one of these abused IAA agents. 5-MeO-DMT and its O-desmethyl derivative, bufotenine (Fig. 1), are the major ingredients of the venom of psychoactive toads such as Colorado River Bufo alvarius [7,8]. 5-MeO-DMT is also a psychoactive agent in a variety of plant preparations including Virola snuffs, and an important active constituent of the beverage Ayahuasca [9]. 5-MeO-DMT was even referred to as the next generation designer drug to replace “ecstasy”. It is often abused with monoamine oxidase (MAO) inhibitor such as harmaline [2]. Recently, a fatal intoxication following the ingestion of Ayahuasca preparation and possibly some synthetic 5-MeO-DMT has been documented [6]. Although the cause of death remains unknown [6,10], 5-MeO-DMT was considered as a major factor because of its strikingly high concentration (1.88 mg/L) in the heart blood of that decedent.
Figure 1.
Chemical structures of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), bufotenine and 5-methyl-N,N-dimethyltryptamine (5-Me-DMT).
Like other tryptamines, 5-MeO-DMT is primarily deaminated via MAO-A. Besides oxidative deamination, O-demethylation followed by glucuronidation was shown as another major metabolic route for 14C-labeled 5-MeO-DMT in rats [11]. In vitro studies using human liver microsomes and recombinant cytochrome P450 (CYP) isoforms revealed that O-demethylation of 5-MeO-DMT is primarily catalyzed by CYP2D6 [12], a polymorphic drug-metabolizing enzyme well recognized for its clinical importance [13]. Of particular note, the O-demethylated metabolite, bufotenine, has been shown as a potent ligand for 5-HT2A receptor with an affinity up to 10-fold higher than 5-MeO-DMT itself [14,15]. On the other hand, bufotenine penetrates the blood-brain barrier poorly, as manifest by a low brain over blood ratio (1/15) of drug concentration in rats 1 hour after the administration of bufotenine (30 mg/kg, s.c.) [16].
Determination of the concentrations of bufotenine produced from 5-MeO-DMT xenobiotics in a whole body system shall help understand the complex pharmacological and toxicological effects of 5-MeO-DMT and delineate the precise exposure-effect relationship for 5-MeO-DMT and bufotenine. Although other methods exist for the measurement of 5-MeO-DMT or/and bufotenine [6,12,17–19], an assay for simultaneous quantitation of blood 5-MeO-DMT and bufotenine toward pharmacokinetic analysis is lacking. Therefore, we have developed and validated a highly sensitive and specific liquid chromatography tandem mass spectrometry (LC-MS/MS) method for the analysis of 5-MeO-DMT and bufotenine in biological fluid. This assay was revealed to provide accurate quantification of 5-MeO-DMT and bufotenine in limited quantity of mouse serum, and facilitate 5-MeO-DMT and bufotenine pharmacokinetic study in mice.
Materials and Methods
Chemicals and Materials
5-MeO-DMT, 5-methyl-N,N-dimethyltryptamine (5-Me-DMT, Fig. 1) and HPLC-grade formic acid were purchased from Sigma-Aldrich (St. Louis, MO). Bufotenine standard was bought from Cambridge Isotope Laboratories, Inc. (Andover, MA). HPLC-grade methanol and acetonitrile were purchased from B&J (Muskegon, MI). The HPLC-grade water was purified by Milli-Q water purification system (Millipore Corp., Bedford, MA).
Stock Solutions
5-MeO-DMT and 5-Me-DMT were dissolved in 50% methanol to 2 mg/mL stock solutions. Purchased bufotenine (1 mg/mL in acetonitrile) and 5-MeO-DMT were diluted with HPLC-grade water and mixed to prepare the working solution. 5-Me-DMT, used as internal standard (IS), was diluted with acetonitrile to 10 ng/mL as precipitator. 5-MeO-DMT and bufotenine stock solutions were mixed and diluted with pooled blank mouse serum to five concentrations (8.08, 24.2, 72.7, 218.2 and 655 ng/mL of 5-MeO-DMT and 7.56, 22.7, 68.1, 204 and 613 ng/mL of bufotenine) as quality control (QC) samples. All the stock solutions, working solutions, and QC samples were stored at −80°C before use.
Animals
All procedures involving animal care and use were approved by the Institutional Animal Care and Use Committee (IACUC) at the author’s institution. All mice were maintained under the controlled temperature (20 ± 2°C), relative humidity (50–60%) and lighting (lights on 6:00 a.m. – 6:00 p.m.), with food and water provided ad libitum. Seven to nine weeks old, male FVB/N mice with body weights of 25–30 g were used for the experiment. A single dose of 5-MeO-DMT at 2 mg/kg was given intraperitoneally (i.p.) to individual mice. Blood were collected at 0, 1, 3, 5, 10, 20, 30, 60, and 90 min (N = 3 per time point) after drug administration. Serum was prepared using a serum separator (Becton Dickinson, Franklin Lakes, NJ), following the manufacturer’s instructions. Serum samples were stored at −80°C until analysis.
Sample Preparation
A simple and rapid protein precipitation method was used for sample preparation. Twenty microliters of serum sample was added into 60 µL of acetonitrile containing 10 ng/mL of 5-Me-DMT (internal standard) to precipitate protein. After centrifugation at 14,000 rpm for 10 minutes, 5 µL of supernatant was injected for LC-MS/MS analysis.
The recovery of each analyte was evaluated using three different concentrations of 5-MeO-DMT and bufotenine (8.08, 72.7 and 655 ng/mL for 5-MeO-DMT and 7.56, 68.1 and 613 ng/mL for bufotenine). Blank serum spiked with 5-MeO-DMT and bufotenine vs. saline spiked with the same concentrations of analytes was treated with acetonitrile as described above. The recoveries were calculated by comparing the peak area obtained from serum samples and that from saline samples.
Liquid Chromatography Tandem Mass Spectrometry
The LC-MS/MS system consisted of a Shimadzu prominence HPLC (Kyoto, Japan) coupled to an API 3000 turbo ionspray ionization triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). The whole system was controlled by Analyst 1.4.2 software (Foster City, CA). Separation of analytes was achieved using a 3 µm Phenomenex phenyl-hexyl column, 50 × 4.6 mm (Torrance, CA). The mobile phase included Buffer A (0.1% formic acid in water) and Buffer B (0.1% formic acid in methanol). The gradient cycle consisted of an initial 2.3-min isocratic elution with 95% of Buffer A and 5% of Buffer B, a linear increase of Buffer B to 90% at 3 min and then elution with 90% of Buffer B for 3 min, followed by initial condition from 6.1 min. The total run time was 9 min for each injection. The mass spectrometer was operated in the turbo ion spray mode with positive ion detection. The detection and quantitation of 5-MeO-DMT, bufotenine and the IS (5-Me-DMT) were accomplished by multiple reaction monitoring (MRM) with the transitions m/z 219.2→174.2 for 5-MeO-DMT, 205.2→160.2 for bufotenine and 203.2→158.3 for 5-Me-DMT. The instrumental parameters were tuned to maximize the MRM signals. An online motorized six-port divert valve was used to introduce the LC eluent to the mass spectrometer over the period of 1.7–9 min for data acquisition, whereas eluent of 0–1.7 min was diverted to the waste.
Matrix Effects
Possible matrix effect was evaluated by post-column infusion and comparison of peak areas of each compound in two sets of samples. The acetonitrile-precipitated blank serum sample was injected during continuous post-column infusion of a solution (0.1 µg/mL of 5-MeO-DMT, 5-Me-DMT, and bufotenine mixture) at a flow rate of 10 µL/min. The change of signals by matrix was evaluated by comparing the MRM responses obtained with an injection of the pretreated blank serum sample and an equivalent injection of acetonitrile-treated water. For a quantitative estimation, the peak areas of 5-MeO-DMT and bufotenine (20 ng/mL) in the two sets of samples was compared (n = 4). One consisted of standard solution diluted with acetonitrile-water (3:1; v/v), the other consisted of solution diluted with the supernatant of acetonitrile-treated blank serum (3:1; v/v). In addition, nine samples (100 ng/mL) were prepared from nine blank serum samples obtained from different mice, in which the peak areas of analytes were determined for the evaluation of matrix variability.
Calibration Plots and Method Validation
Blank mouse serum was spiked with the standard working solution, and then serially diluted with pooled blank mouse serum to generate nominal concentrations (nine different concentrations from 0.90 to 5,890 ng/mL for 5-MeO-DMT and eight concentrations from 2.52 to 5,510 ng/mL for bufotenine). These matrix-based calibration standards were treated as the same as samples. The calibration plots were constructed using weighted (1/X2) linear regressions of the peak area ratio of analyte over IS (Y-axis) against the corresponding nominal concentration of the analyte (X-axis).
To determine the intra-day and inter-day variability of the LC-MS/MS assay, the QC samples of 5-MeO-DMT and bufotenine were evaluated at five different concentrations (8.08, 24.2, 72.7, 218 and 655 ng/mL of 5-MeO-DMT and 7.56, 22.7, 68.1, 204 and 613 ng/mL of bufotenine) that were close to drug/metabolite concentrations in serum samples from treated mice, according to a pilot study. Intra-day accuracy and precision were determined after experimental concentrations were calculated for triplicate samples containing the above nominal concentrations according to the calibration plots of 5-MeO-DMT (0.90–5,890 ng/mL) and bufotenine (2.52–5,510 ng/mL). Inter-day accuracy and precision were assessed after three repetitions of the intra-day assay (n=9).
Pharmacokinetic Analysis
Pharmacokinetic parameters for 5-MeO-DMT and its active metabolite, bufotenine, were estimated from the serum drug and metabolite concentration vs. time data by a non-compartmental model using WinNonLin (version 5.0, Pharsight, Mountain View, CA). The peak concentration in serum (Cmax) and the corresponding time of maximum concentration (Tmax) were obtained from the original data. The area under the serum concentration-time curve from time 0 to t min (AUC0-t) was calculated by the trapezoidal rule. The elimination rate constant (λ) was determined as the slope of linear regression for the terminal log-linear portion of the concentration versus time curve, and the elimination half-life (T1/2) was calculated from 0.693/λ. The mean residence time (MRT) value was determined as the ratio of the area under the first moment curve (AUMC) over AUC (AUMC/AUC).
Results
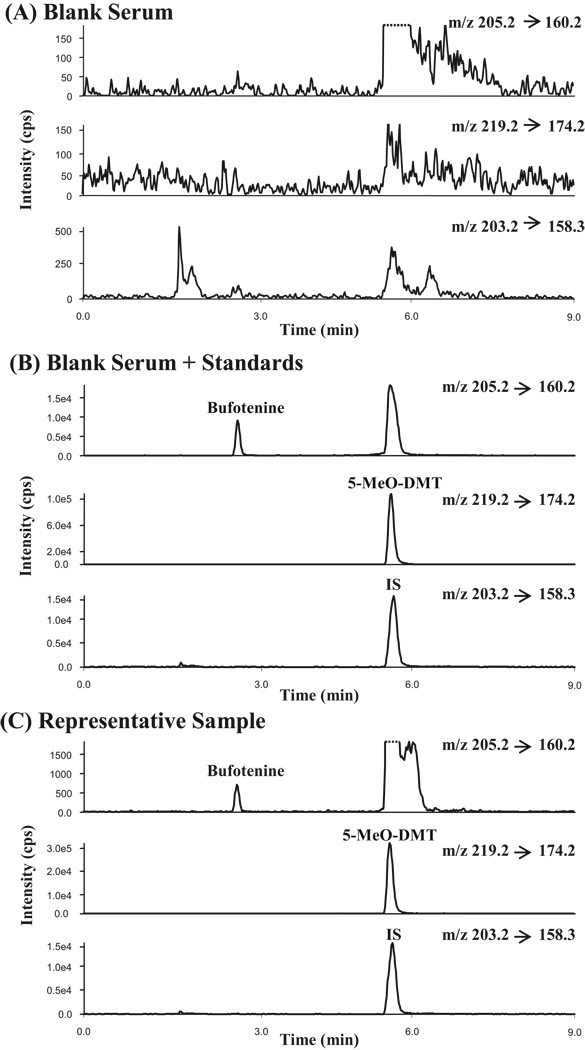
In the positive ion mode and under turbo ion spray ionization conditions, 5-MeO-DMT and bufotenine gave precursor ions [M+H]+ of m/z 219.2 and 205.2 as the base ions, respectively. Product ion of m/z 174.2 was found to be predominant for 5-MeO-DMT and m/z 160.2 for bufotenine due to the loss of dimethylamino group under the collision energy of 30 V. This fragmentation was thought to be characteristics for tryptamine derivatives [20]. Likewise, the IS, 5-Me-DMT, gave a protonated ion of m/z 203.2 and a major product ion of m/z 158.3 under the same ionization and collision conditions. Therefore, the MRM transitions m/z 219.2→174.2, 205.2→160.2 and 203.2→158.3 were chosen to analyze 5-MeO-DMT, bufotenine and 5-Me-DMT, respectively (Fig. 2), which offered the strongest signals for corresponding compounds when compared to other MRM transitions.
Figure 2.
Chromatograms of 5 µL injection of blank mouse serum (A), blank mouse serum spiked with 5-MeO-DMT (655 ng/mL), bufotenine (613 ng/mL) and IS (10 ng/mL) (B), and a representative serum sample from mouse administered with 5-MeO-DMT (C).
A simple and fast protein precipitation, coupled with an optimal gradient elution, allowed efficient assay of complex serum samples and accomplished baseline separation of bufotenine (2.8 min) from 5-MeO-DMT (5.6 min). The retention time for 5-Me-DMT (IS) was revealed as 5.7 min under this condition. In contrast to the standards, blank serum samples (n = 12) from individual mice did not show any MRM responses for any analytes at their corresponding retention times (Fig. 2), demonstrating that this assay is high selective and specific for the analysis of 5-MeO-DMT and bufotenine in the biofluid.
The post-column infusion experiment demonstrated that bufotenine had ion-suppression by the co-eluted matrix, whereas neither 5-MeO-DMT nor 5-Me-DMT experienced any ion-suppression or enhancement. The absolute matrix effect value for bufotenine was 84.0% when the peak areas were compared between the samples prepared in blank-matrix treatment supernatant and the samples prepared in acetonitrile-water solution. The bufotenine/IS peak area ratios in nine blank serum samples from different mice were approximately equal (relative standard deviation RSD = 5.20%). These data suggest that the matrix effect would have limited influence on the quantification of bufotenine.
The recovery of 5-MeO-DMT and bufotenine was evaluated by spiking blank mouse serum with the analytes at different concentrations. It was revealed that the recovery of 5-MeO-DMT from 20 µL of serum containing 8.08, 72.7 and 655 ng/mL of the analyte was 75.8 ± 2.7%, 77.6 ± 1.6% and 79.6 ± 0.2%, respectively (n = 3). The recovery of bufotenine at concentrations of 7.56, 68.1 and 613 ng/mL was 75.8 ± 2.7%, 77.6 ± 1.6% and 80.0 ± 0.2%, respectively (n = 3).
Calibration plot was linear from 0.90 to 5,890 ng/mL for 5-MeO-DMT and from 2.52 to 5,510 ng/mL for bufotenine. Excellent correlation (R2 > 0.99) was achieved over the range by weighted (1/X2) linear regression. Then the intra-day accuracy and precision were determined with quality control samples containing 5-MeO-DMT and bufotenine at five different concentrations (n = 3). The results showed that intra-day accuracy of the assay was 91.6–106% for 5-MeO-DMT and 90.9–108% for bufotenine, and intra-day precision was less than 10% for both 5-MeO-DMT and bufotenine (Table 1). Inter-day accuracy and precision were further assessed in 3 different days (n = 9), which was shown to be 96.6–104% and 3.13%-8.30% for 5-MeO-DMT, respectively, and 91.7–102% and 4.13%-12.3% for bufotenine, respectively (Table 1). Overall, this method provided a lower limit of quantification (LLOQ) of 1.12 pg (or 224 pg/mL) on column for 5-MeO-DMT and 3.14 pg (628 pg/mL) for bufotenine with signal-to-noise ratios of 28.2 and 12.2, respectively, indicating excellent sensitivity for the analysis of 5-MeO-DMT and bufotenine.
Table 1.
Intra- and inter-day precision and accuracy for 5-MeO-DMT and bufotenine in mouse serum.
| Intra-day (n = 3) | Inter-day (n = 9) | |||||
|---|---|---|---|---|---|---|
| Nominal Concentration (ng/mL) |
Measured Concentration (Mean ± SD; ng/mL) |
RSD (%) |
Accuracy (%) |
Measured Concentration (Mean ± SD; ng/mL) |
RSD (%) |
Accuracy (%) |
| 5-MeO-DMT | ||||||
| 8.08 | 8.58 ± 0.34 | 3.94 | 106 | 8.39 ± 0.26 | 3.13 | 104 |
| 24.2 | 25.4 ± 0.9 | 3.58 | 105 | 25.3 ± 0.8 | 3.18 | 104 |
| 72.7 | 76.2 ± 0.6 | 0.72 | 105 | 74.6 ± 3.5 | 4.72 | 103 |
| 218 | 213 ± 6 | 2.88 | 97.8 | 214 ± 7 | 3.48 | 98.3 |
| 655 | 599 ± 7 | 1.11 | 91.6 | 633 ± 53 | 8.30 | 96.6 |
| Bufotenine | ||||||
| 7.56 | 8.14 ± 0.09 | 1.16 | 108 | 7.71 ± 0.62 | 8.06 | 102 |
| 22.7 | 23.3 ± 1.0 | 4.13 | 103 | 22.2 ± 1.8 | 8.09 | 97.8 |
| 68.1 | 68.3 ± 6.3 | 9.24 | 100 | 64.8 ± 5.2 | 8.04 | 95.2 |
| 204 | 186 ± 3 | 1.59 | 90.9 | 193 ± 24 | 12.3 | 94.4 |
| 613 | 560 ± 9 | 1.59 | 91.3 | 562 ± 23 | 4.13 | 91.7 |
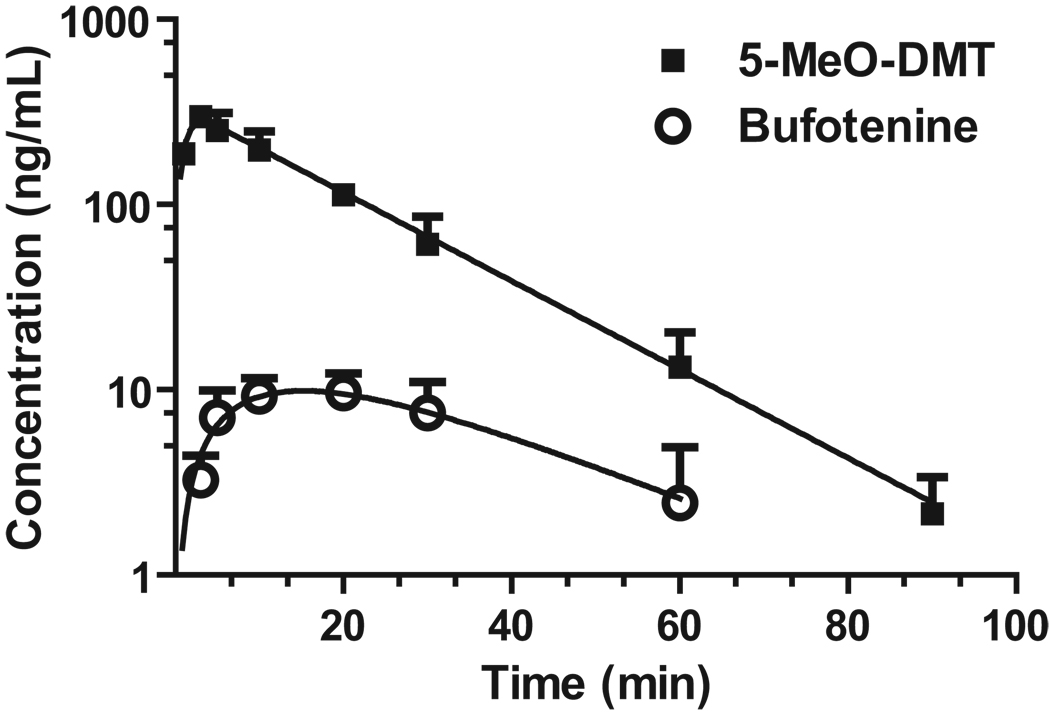
The validated assay was employed to measure 5-MeO-DMT and bufotenine concentrations in serum samples collected from mice at different time points after the administration of 5-MeO-DMT (2 mg/kg, i.p.). As expected, not only 5-MeO-DMT but also its active metabolite, bufotenine, was readily determined in serum (Fig. 2) although the latter was present at relatively lower concentrations. As indicated by serum drug and metabolite concentrations vs. time curves (Fig. 3), 5-MeO-DMT was well absorbed with the maximum drug concentrations (Cmax) at 3 min (Table 2). After absorption, 5-MeO-DMT was extensively eliminated in mice as its serum concentration decreased from the Cmax to 4.4% at 60 min after drug administration. The terminal elimination half-life (T1/2) was estimated to be around 12 min for 5-MeO-DMT. Additionally, the metabolite bufotenine reached the Cmax around 13 min, and decreased rapidly with an apparent T1/2 about 22 min. When compared to the exposure to 5-MeO-DMT (AUC, 6,000 µg·min/L), the exposure to bufotenine metabolite was much lower (388 µg·min/L).
Figure 3.
Serum drug and metabolite concentration versus time profile in mice (n = 3 at each time point) dosed intraperitoneally with 2.0 mg/kg of 5-MeO-DMT. Values represent Mean ± SD. Bufotenine concentration at 90 min was below the LLOQ.
Table 2.
Pharmacokinetic parameters estimated for 5-MeO-DMT (2 mg/kg, i.p.) and its active metabolite, bufotenine, in mice.
| 5-MeO-DMT | Bufotenine | |
|---|---|---|
| Cmax (ng/mL) | 298 ± 29 | 10.6 ± 2.3 |
| Tmax (min) | 3.0 | 13.3 ± 5.8 |
| a AUC0→t (µg·min/L) | 6,000 ± 1,240 | 388 ± 82 |
| T1/2 (min) | 12.1 ± 1.0 | 21.6 ± 17.3 |
| MRT (min) | 17.6 ± 2.1 | 24.3 ± 2.2 |
AUC0→90 min for 5-MeO-DMT and AUC0→60 min for bufotenine.
Discussion
A LC-MS/MS method for simultaneous quantitation of blood 5-MeO-DMT and its active metabolite bufotenine has been developed and validated toward pharmacokinetic analysis. This method is simple, rapid and highly sensitive and specific. Straightforward protein precipitation with acetonitrile offers satisfactory recovery of individual analytes from limited quantity of serum sample thus eliminates the need for any complicated extraction procedures. 5-MeO-DMT (retention time 2.8 min) and bufotenine (5.6 min) are assayed by MRM of m/z 219.2→174.2 and 205.2→160.2, respectively, in the positive ion mode in 9 min, without any interfering peak. The assay has wide calibration range for both 5-MeO-DMT and bufotenine, which facilitates the pharmacokinetic study in mouse model.
The use of LC-MS provides extremely high sensitivity and selectivity when compared to earlier methods such as gas chromatography mass spectrometry and HPLC [12,16,21]. Among the LC-MS assays, MRM [12,17–19] could be more specific than single ion monitoring [6]. Existing LC-MS/MS methods mainly aimed at the measurement of endogenous IAAs, which involved the processing of relatively large quantity of sample (e.g. 1 mL of urine) [17]. This assay was developed for pharmacokinetic study with limited quantity (e.g. 20 µL) of serum sample. The LC-MS/MS method reported by Karkkainen et al. [17] seems to be the most sensitive for bufotenine, with a limit of detection (LOD) of 0.5 pg on column after using preparative HPLC following initial extraction of 1 mL of urine sample. The assay here offers a LLOQ of 3.14 pg for bufotenine on column and represents comparable sensitivity. Nevertheless, bufotenine (metabolite) produced from 5-MeO-DMT (2 mg/kg) was below the LLOQ at 90 min (Fig. 3), suggesting that sensitivity should be improved for bufotenine pharmacokinetic study when low dose of 5-MeO-DMT is administered. In contrast, the sensitivity for 5-MeO-DMT (substrate) is relatively higher. With a LLOQ of 1.12 pg (224 pg/mL) on column, the assay suffices pharmacokinetic study of 5-MeO-DMT (2 mg/kg, i.p.) itself. Additionally, the assay described has much wider linear calibration range, 0.90–5,890 ng/mL for 5-MeO-DMT and 2.52–5,510 ng/mL for bufotenine. Together, easier sample preparation, increased sensitivity and wider linear range make this method more suitable for pharmacokinetic study.
5-MeO-DMT has been known as a nonselective 5-HT receptor agonist, and both 5-HT1A and 5-HT2 receptors are involved in its drug effects [8,22,23]. Given the fact that 5-MeO-DMT is more potent 5-HT1A agonist and bufotenine is more potent 5-HT2A agonist, the formation of bufotenine from 5-MeO-DMT in vivo may provide a good explanation for its apparent nonselectivity. This is supported by our pharmacokinetic study, showing that mice treated with 5-MeO-DMT are inevitably exposed to bufotenine in vivo, a metabolite produced from 5-MeO-DMT. Although the systemic exposure (AUC) to bufotenine is only about 1/14 of that to 5-MeO-DMT, bufotenine may be equally important for the overall pharmacological and toxicological effects because bufotenine acts on 5-HT2A receptor with an affinity more than 10-fold higher than 5-MeO-DMT [14]. Additionally, mice are known for impaired CYP2D6 activity [24], which catalyzes the biotransformation of 5-MeO-DMT to bufotenine more efficiently [12,13]. It is of interest to investigate the exposure to bufotenine in CYP2D6-humanized mice [25] after the treatment of 5-MeO-DMT. Nevertheless, delineation of cerebral exposure to the drug and metabolite is warranted since IAA agents act on the central nervous system. In addition, the clinical significance of bufotenine production from 5-MeO-DMT is amenable to prospective studies in humans.
Conclusions
In conclusion, the assay described is a sensitive and reliable LC-MS/MS method for determination of 5-MeO-DMT and bufotenine concentrations in blood using 5-Me-DMT as the internal standard. It has been validated as accurate and reproducible. Sample preparation via protein precipitation is straightforward and needs only a small volume of serum sample (20 µL). This assay has a wide linear calibration range for both 5-MeO-DMT and bufotenine. Utilization of this method has facilitated the delineation of 5-MeO-DMT and bufotenine pharmacokinetics in mice, which suggests that exposure to the active metabolite bufotenine should be considered when studying 5-MeO-DMT pharmacology and toxicology.
Future Perspective
Accurate quantitation of substrate drug and its active metabolite is essential for understanding the resultant pharmacological and toxicological effects of the drug. Our LC-MS/MS method represents a reliable and sensitive assay for the analysis of 5-MeO-DMT and bufotenine, and our pharmacokinetic data may provide novel insight into understanding 5-MeO-DMT drug effects, in which both 5-HT1A and 5-HT2 receptors are involved. Future pharmaco/toxicokinetic and dynamic studies would help evaluate the role of bufotenine (active metabolite) in 5-MeO-DMT pharmacology and toxicology, and potential interactions with other drugs. Likewise, preclinical findings may help disclose the risk factors in abuse of these IAA drugs and offer helpful clues for any clinical investigations.
Executive Summary
A simple and fast protein precipitation, coupled with an optimal gradient elution, allowed efficient assay of complex serum samples and accomplished baseline separation of bufotenine (2.8 min) from 5-MeO-DMT (5.6 min).
Specific and sensitive quantitation of the analytes was accomplished by multiple reaction monitoring (m/z 219.2→174.2 for 5-MeO-DMT and 205.2→160.2 for bufotenine) in positive ion mode.
The assay was linear over the range 0.90–5,890 ng/mL (1.12–7,360 pg on-column) for 5-MeO-DMT and 2.52–5,510 ng/mL (3.14–6,890 pg) for bufotenine. Intra- and inter-day precision and accuracy were within 15% for both analytes.
Pharmacokinetic study revealed that the systemic exposure (area under the curve) to the active metabolite bufotenine (5-HT2A agonist) was about 1/14 of that to 5-MeO-DMT (5-HT1A agonist), suggesting that bufotenine may significantly contribute to the apparent pharmacological and toxicological effects of 5-MeO-DMT.
Acknowledgments
Acknowledgements and disclosure: The project described was supported by Award Number R01DA021172 from the National Institute On Drug Abuse, National Institutes of Health (NIH).
Key Words and Definitions
- Methoxydimethyltryptamines
Compounds that contain the biogenic monoamine tryptamine and are substituted with one methoxy group and two methyl groups. Compounds in this group include several potent hallucinogens and migraine medications.
- Bufotenine
A hallucinogenic serotonin analog found in frog or toad skins, mushrooms, higher plants, and mammals, especially in the brains, plasma, and urine of schizophrenics. Bufotenine has been used as a tool in CNS studies and misused as a psychedelic.
- 5-HT
Serotonin, a biochemical messenger and regulator, synthesized from the essential amino acid L-tryptophan.
- Chromatography, High Pressure Liquid
Liquid chromatographic techniques which feature high inlet pressures, high sensitivity, and high speed.
- Mass Spectrometry
An analytical method used in determining the identity of a chemical based on its mass using mass analyzers/mass spectrometers.
- Pharmacokinetics
Dynamic and kinetic mechanisms of exogenous chemical and drug absorption, biological transport, tissue distribution, biotransformation, elimination and toxicology as a function of dosage, and rate of metabolism.
- Biotransformation
The chemical alteration of an exogenous substance by or in a biological system. The alteration may inactivate the compound or it may result in the production of an active metabolite of an inactive parent compound.
- CYP2D6
A cytochrome P450 enzyme that catalyzes the hydroxylation of many drugs and environmental chemicals, such as debrisoquine and tricyclic antidepressants. This enzyme is deficient in up to 10 percent of the Caucasian population.
- Monoamine Oxidase
An enzyme that catalyzes the oxidative deamination of naturally occurring monoamines. It is a flavin-containing enzyme that is localized in mitochondrial membranes, whether in nerve terminals, the liver, or other organs.
- Mice
The common name for the genus Mus.
References
- 1. Yu AM. Indolealkylamines: biotransformations and potential drug-drug interactions. Aaps J. 2008;10(2):242–253. doi: 10.1208/s12248-008-9028-5. Reviews the metabolism and potential drug-drug interactions of some important indolealkylamine drugs that have been used therapeutically or recreationally.
- 2.Brush DE, Bird SB, Boyer EW. Monoamine oxidase inhibitor poisoning resulting from Internet misinformation on illicit substances. J Toxicol Clin Toxicol. 2004;42(2):191–195. doi: 10.1081/clt-120030949. [DOI] [PubMed] [Google Scholar]
- 3.Long H, Nelson LS, Hoffman RS. Alpha-methyltryptamine revisited via easy Internet access. Vet Hum Toxicol. 2003;45(3):149. [PubMed] [Google Scholar]
- 4.Muller AA. New drugs of abuse update: Foxy Methoxy. J Emerg Nurs. 2004;30(5):507–508. doi: 10.1016/j.jen.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Alatrash G, Majhail NS, Pile JC. Rhabdomyolysis after ingestion of "foxy," a hallucinogenic tryptamine derivative. Mayo Clin Proc. 2006;81(4):550–551. doi: 10.4065/81.4.550. [DOI] [PubMed] [Google Scholar]
- 6. Sklerov J, Levine B, Moore KA, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J Anal Toxicol. 2005;29(8):838–841. doi: 10.1093/jat/29.8.838. Describes a case of a 25-year-old white male who was found dead and whose heart blood was found to contain 1.88 mg/L of 5-methoxy-N,N-dimethyltryptamine after LC-MS analysis.
- 7.Ott J. Pharmanopo-psychonautics: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine. J Psychoactive Drugs. 2001;33(3):273–281. doi: 10.1080/02791072.2001.10400574. [DOI] [PubMed] [Google Scholar]
- 8.Ott J. Pharmepena-Psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs. 2001;33(4):403–407. doi: 10.1080/02791072.2001.10399925. [DOI] [PubMed] [Google Scholar]
- 9.McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther. 2004;102(2):111–129. doi: 10.1016/j.pharmthera.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Callaway JC, Grob CS, McKenna DJ, et al. A demand for clarity regarding a case report on the ingestion of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) in an Ayahuasca preparation. J Anal Toxicol. 2006;30(6):406–407. doi: 10.1093/jat/30.6.406. author reply 407. [DOI] [PubMed] [Google Scholar]
- 11.Agurell S, Holmstedt B, Lindgren JE. Metabolism of 5-methoxy-N,-N dimethyltryptamine- 14 C in the rat. Biochem Pharmacol. 1969;18(10):2771–2781. doi: 10.1016/0006-2952(69)90185-3. [DOI] [PubMed] [Google Scholar]
- 12. Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003;13(6):307–319. doi: 10.1097/01.fpc.0000054094.48725.b7. Shows the importance of CYP2D6 in O-demethylation biotransformations of indolealkylamine drugs that include the production of bufotenine from 5-methoxy-N,N-dimethyltryptamine. The reported LC-MS/MS methods were not fully validated.
- 13.Yu AM, Idle JR, Gonzalez FJ. Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates. Drug Metab Rev. 2004;36(2):243–277. doi: 10.1081/dmr-120034000. [DOI] [PubMed] [Google Scholar]
- 14.Roth BL, Choudhary MS, Khan N, Uluer AZ. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J Pharmacol Exp Ther. 1997;280(2):576–583. [PubMed] [Google Scholar]
- 15.McBride MC. Bufotenine: toward an understanding of possible psychoactive mechanisms. J Psychoactive Drugs. 2000;32(3):321–331. doi: 10.1080/02791072.2000.10400456. [DOI] [PubMed] [Google Scholar]
- 16.Fuller RW, Snoddy HD, Perry KW. Tissue distribution, metabolism and effects of bufotenine administered to rats. Neuropharmacology. 1995;34(7):799–804. doi: 10.1016/0028-3908(95)00049-c. [DOI] [PubMed] [Google Scholar]
- 17. Karkkainen J, Forsstrom T, Tornaeus J, et al. Potentially hallucinogenic 5-hydroxytryptamine receptor ligands bufotenine and dimethyltryptamine in blood and tissues. Scand J Clin Lab Invest. 2005;65(3):189–199. doi: 10.1080/00365510510013604. Describes a LC-MS/MS method that is the most sensitive for bufotenine among existing methods. It was employed to measure the endogenous bufotenine and other dimethyltryptamine agents in blood and tissues.
- 18.Forsstrom T, Tuominen J, Karkkainen J. Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS. Scand J Clin Lab Invest. 2001;61(7):547–556. doi: 10.1080/003655101753218319. [DOI] [PubMed] [Google Scholar]
- 19.Barker SA, Littlefield-Chabaud MA, David C. Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: application of a new solid-phase extraction LC-APcI-MS-MS-isotope dilution method. J Chromatogr B Biomed Sci Appl. 2001;751(1):37–47. doi: 10.1016/s0378-4347(00)00442-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen BH, Liu JT, Chen WX, Chen HM, Lin CH. A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation. Talanta. 2008;74(4):512–517. doi: 10.1016/j.talanta.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Karkkainen J, Raisanen M, Huttunen MO, et al. Urinary excretion of bufotenin (N,N-dimethyl-5-hydroxytryptamine) is increased in suspicious violent offenders: a confirmatory study. Psychiatry Res. 1995;58(2):145–152. doi: 10.1016/0165-1781(95)02747-k. [DOI] [PubMed] [Google Scholar]
- 22. Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65(1):75–82. doi: 10.1016/s0091-3057(99)00178-1. Illustrates the complexity of 5-methoxy-N,N-dimethyltryptamine drug actions and shows the role of 5-HT2 receptors in stimulus control effects of 5-methoxy-N,N-dimethyltryptamine in rats.
- 23. Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology (Berl) 2006;189(3):319–329. doi: 10.1007/s00213-006-0566-1. Demonstrates both 5-HT1A and 5-HT2 receptors are involved in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats.
- 24.Yu AM, Haining RL. Expression, purification, and characterization of mouse CYP2d22. Drug Metab Dispos. 2006;34(7):1167–1174. doi: 10.1124/dmd.105.008870. [DOI] [PubMed] [Google Scholar]
- 25.Corchero J, Granvil CP, Akiyama TE, et al. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol. 2001;60(6):1260–1267. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]