Abstract
High-intensity and/or prolonged exposure to noise causes temporary or permanent threshold shifts in auditory perception. Occupational exposure to solvents or administration of clinically important drugs, such as aminoglycoside antibiotics and cisplatin, also can induce permanent hearing loss. The mechanisms by which these ototoxic insults cause auditory dysfunction are still being unraveled, yet they share common sequelae, particularly generation of reactive oxygen species, that ultimately lead to hearing loss and deafness. Individuals are frequently exposed to ototoxic chemical contaminants (e.g., fuel) and noise simultaneously in a variety of work and recreational environments. Does simultaneous exposure to chemical ototoxins and noise potentiate auditory dysfunction? Exposure to solvent vapor in noisy environments potentiates the permanent threshold shifts induced by noise alone. Moderate noise levels potentiate both aminoglycoside- and cisplatin-induced ototoxicity in both rate of onset and in severity of auditory dysfunction. Thus, simultaneous exposure to chemical ototoxins and moderate levels of noise can potentiate auditory dysfunction. Preventing the ototoxic synergy of noise and chemical ototoxins requires removing exposure to ototoxins and/or attenuating noise exposure levels when chemical ototoxins are present.
Keywords: Noise, ototoxins, ototoxic drugs, synergistic effects, deafness
Congenital hearing loss occurs in ~3 of every 1000 live births1–3 and is the most common sensory deficit in newborns.4 In the general population, 1 in 10 individuals experiences varying degrees of hearing loss, rising to more than 1 in 3 individuals over the age 65 years.5,6 The acquisition of hearing loss and deafness over our lifetimes comes from a variety of sources, including congenital and genetic deafness, noise trauma, exposure to ototoxins (including organic solvents), infections, and aging.
Toxic noise is present in a variety of environments, including occupational (e.g., construction and military), or recreational (rock concerts, personal stereo-players), and comes in two major forms. First, blast/impulse noise from explosions or gunshots that produce high-intensity sound that physically damages hair cell stereocilia and produces discrete lesions in the sensory epithelia of the cochlea. Second, chronic exposure to noise generated at rock concerts, on commercial and military jet planes, and at heavy industrial plants generates toxic levels of reactive oxygen species (ROS) and physiologic changes in the blood-labyrinth barrier that culminate in temporary auditory dysfunction and often permanent hearing loss.7–11
Chemical ototoxins are present in a variety of situations, including occupational (e.g., paint solvents, gas stations), recreational abuse of organic solvent-based propellants (e.g., spray adhesives), and as essential lifesaving drugs (e.g., antineoplastic cisplatin and bactericidal aminoglycoside antibiotics). These chemical ototoxins also generate ROS and may interfere with numerous physiologic and biochemical processes; for example, dysfunction of the blood-labyrinth barrier, ion regulation (loop diuretics) blockade of ion channels (aminoglycosides), and DNA damage (cisplatin and derivatives).12–14
Exposure to any of these insults is rarely a singular event, and several may occur simultaneously; for example, loud noise and solvent inhalation by dockyard workers.15 Thus, it is important to understand whether these insults can synergistically enhance their ototoxic impact. A review of the published literature not only reveals that chemical ototoxicity is potentiated by noise exposure but also that there appear to be two types of synergism between noise and chemical ototoxicity.
SYNERGISM BETWEEN NOISE AND ORGANIC SOLVENTS
Organic solvents are ubiquitously present in our environment, as alcohol, in paints and adhesives, and perhaps most significantly as heating (propane, kerosene) and automotive (diesel, gasoline) fuels, although they generally do not impair auditory perception by themselves. Toxic exposure to many of these organic solvents leads to severe physiologic dysfunction before directly affecting hearing, including pulmonary edema after exposure to solvent vapor, dysfunction of peripheral and central neural systems, cell lysis, carcinogenesis, and renal and hepatic failure.
However, exposure to organic solvents in noisy environments, particularly for personnel in military and chemical plant environments, carries significant risks for auditory dysfunction. Jet fuel (JP-8) is the primary contaminant of U.S. and NATO military personnel and is closely related to Jet A fuel used for U.S. domestic aviation.16 Jet fuel contains a variety of ototoxic aromatic hydrocarbons including toluene, styrene, ethyl benzene, and a variety of xylenes, which when present in other work-places, including pathology laboratories, also have been shown to be ototoxic (see Fechter et al16 for complete list of references).
On aircraft carriers, exposure to jet fuel occurs in extremely noisy environments, where fighter jet take-off reaches noise levels of 140 dBA (E. Berger, personal Communication, 2008). Fechter and colleagues16 investigated the possible synergism between jet fuel and noise. Jet fuel alone (1000 mg/m3 for 4 hours) generally had little or no effect on DPOAEs and hair cell survival. If animals received a single exposure to 4 hours of 105 dB SPL broadband noise (centered at 8 kHz), a 10 dB permanent threshold shift in DPOAEs was observed 4 weeks after treatment (Fig. 1A). However, after serial exposure to jet fuel vapor and then noise (4 hours each), 20 dB permanent threshold shifts in DPOAE amplitudes were observed 4 weeks after treatment (Fig. 1A, B).
Figure 1.
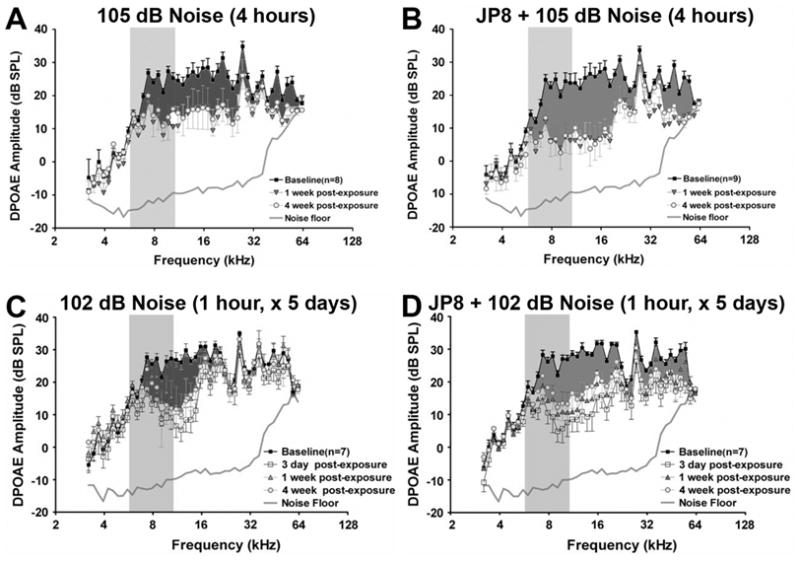
DPOAE amplitude shifts from a baseline (preexposure) in animals treated with noise or JP-8 and noise. (A) Rats exposed to 4 hours of 105 dB broadband noise displayed a 10 dB permanent threshold shift in DPOAEs across a wide range of frequencies 4 weeks after treatment (darker shaded area). (B) After serial exposure to jet fuel vapor (4 hours, 1000 mg/m3) and then noise (105 dB for 4 hours), 20 dB permanent threshold shifts in the DPOAEs were observed over a similar, wide frequency range 4 weeks after treatment. (C) Rats that received repeated exposure to noise (102 dB broadband noise for 1 hour/day) for 5 days had a narrow range of temporary threshold shifts (between 6 and 16 kHz), with a maximum shift of ~10 to 15 dB 1 week after exposure. After 4 weeks, DPOAE thresholds recovered slightly at some frequencies, resulting in a permanent threshold shift of ~10 dB (darker shaded area). (D) Rats exposed to both jet fuel (1000 mg/m3 for 4 hours) and noise (102 dB for 1 hour/day) for 5 days had temporary threshold shifts in DPOAE amplitudes over a wide range of frequencies (between 6 and 60 kHz) of ~20 dB. After 4 weeks, some recovery in thresholds can be observed, but permanent threshold shifts (darker shaded areas) of ~15 dB over a wide frequency range occurred compared with noise-only treated animals. In all experiments, untreated animals and animals that received jet fuel vapor alone showed little or no change in DPOAE thresholds (data not shown). (Adapted from Fechter LD, Gearhart C, Fulton S, et al. JP-8 jet fuel can promote auditory impairment resulting from subsequent noise exposure in rats. Toxicol Sci 2007;98:510–525.)
The synergistic effect of noise and organic solvents is more compelling after repeated exposure at lower exposure levels. Control animals and animals that received jet fuel vapor alone for 5 days (4 hours each day) showed little or no change in DPOAE thresholds. Animals that received 102 dB SPL broadband noise for 1 hour a day (one-half sound intensity of 105 dB SPL for one-quarter of the time used in the above experiments) for 5 days showed a narrow range of temporary threshold shifts (between 6 and 16 kHz), with a maximum shift of 10 to 15 dB 1 week after exposure. After 4 weeks, DPOAE thresholds recovered slightly at some frequencies, resulting in a permanent threshold shift of 8 to 10 dB (Fig. 1C). When animals were serially exposed to both jet fuel (for 4 hours) and noise (102 dB for 1 hour/day) for 5 days, animals experienced a 20 dB loss of DPOAE sensitivity across a broad frequency range (6 and 60 kHz) 3 days after treatment (Fig. 1D). After 4 weeks, some recovery of DPOAE thresholds occurred at the lower frequencies, but permanent threshold shifts of 10 to 12 dB over a wide frequency range was still present.16 Thus, although repeated noise exposure can induce permanent threshold shifts, exposure to JP-8 jet fuel and noise potentiates the breadth and severity of these permanent threshold shifts, particularly as JP-8 alone did not induce hair cell loss or temporary threshold shifts.
Because jet fuel is composed of a complex mix of aromatic hydrocarbons, this same research group went on to investigate two known ototoxins, toluene and ethyl benzene. When animals were exposed to the vapor of these solvents (4000 mg/m3) alone for 5 days, no change in DPOAEs from control, nonexposed animals were observed. When animals were exposed to broadband noise exposure at 93 dB SPL (lower than that for experiments described above) for 5 days, only temporary threshold shifts of ~20 dB were observed between 8 and 20 kHz 3 days after cessation of treatment. After 4 weeks, the DPOAE threshold had recovered to baseline values. However, when vapor inhalation was followed by broadband noise exposure at 93 dB SPL (for 5 days), greater temporary threshold shifts (~25 to 30 dB) were observed 3 days after treatment, with only partial recovery of DPOAE thresholds, resulting in permanent threshold shifts of 10 to 15 dB 4 weeks after cessation of treatment.17 The permanency of these threshold shifts was confirmed by the greater number and extent of missing outer hair cells in the solvent/noise group compared with that in the noise alone, solvent alone, or control groups.
Solvent-induced ototoxicity display a preferential loss of outer hair cells in the outermost rows.18,19 Exposure to jet fuel or toluene/ethyl benzene together with noise, however, enhanced the loss of the innermost (first) row of outer hair cells, suggesting that these solvents potentiate the mechanisms involved in noise-induced hearing loss, rather than initiate additional cytotoxic mechanisms. Similar solvent-potentiation of permanent threshold shifts and outer hair cells loss that characterize noise-induced hearing loss (NIHL) occurs during exposure to acrylonitrile (vinyl cyanide), an important component of the plastic, butyl rubber, and textile industries,20,21 and during exposure to styrenes, an important component in the plastics industry.22–24 These data demonstrate the importance of reducing noise exposure levels in solvent-rich environments to prevent solvent-enhanced potentiation of NIHL. This can be achieved by removing sources of noise generation, or adding efficient external ear hearing protection (e.g., ear plugs or muffs), and preferentially with respiratory protection as well.
SYNERGISM BETWEEN NOISE AND CLINICAL DRUGS
A variety of clinical drugs, including aminoglycoside antibiotics, cisplatin, quinines, and loop diuretics, are ototoxic and cause varying degrees of temporary and permanent hearing loss. Loop diuretics are well known for inducing transient hearing loss, due to blockade of potassium recycling, loss of endolymphatic potential (EP), and edema in the stria vascularis.25–27 However, noise has not been shown to potentiate this effect.28,29
Potentiation of aminoglycoside-induced ototoxicity by simultaneous exposure to noise has been occasionally investigated since it was first described by Gannon and Tso in 1969 and confirmed in subsequent animal studies.30–38 Interestingly, noise exposure followed by aminoglycoside treatment also induced enhanced auditory dysfunction, but this was not observed for aminoglycoside treatment followed by noise exposure.31,32,35 These studies are clinically relevant because aminoglycosides are systemically administered for prophylaxis to casualties with blast and gunshot wounds during medical evacuation from battlefields and relocation to major medical centers. Medical evacuation typically occurs in armored personnel carriers or aircraft, where noise levels frequently exceed 90 dBA (E. Berger, personal Communication, 2008), and systemic aminoglycoside administration often occurs during transportation or shortly afterwards. Exposure to moderate or intense noise during battle followed by injury and subsequent aminoglycoside treatment increases the risk of sustaining noise-enhanced aminoglycoside-induced hearing loss. The impact of acquired hearing loss in adults has been estimated to economic costs of approximately $297,000 (in year 2000 dollars), due to loss of economic earning power, reduced work productivity, decreased career advancement prospects, combined with increased expenditure for rehabilitation, education, and accessibility. 39
Aminoglycoside treatment is also frequently mandated in the neonatal intensive care unit (NICU), where the infant is placed in a mechanical ventilator, surrounded by various monitors, each of which generate mechanical noise, or have high-intensity alarms. At least 600,000 infants pass through the NICU in the United States each year, and a large number of them receive aminoglycosides for suspected bacterial sepsis for at least 48 hours, or until a negative bacteriologic assay is reported.40,41 The impact of congenital or prelingually acquired severe to profound deafness has been estimated to have economic costs greater than $1 million (in year 2000 dollars), due to loss of economic earning power, reduced work productivity, decreased career advancement prospects, combined with increased expenditure for rehabilitation, education, and accessibility.39 Thus there is considerable socioeconomic pressure to determine if noise, or other potential insults, can synergistically enhance the ototoxicity of aminoglycosides.
Although many pediatricians (mistakenly) consider aminoglycosides administered during the neonatal period to be less ototoxic compared with administration in adults, Bernard demonstrated that the auditory brain-stem responses (ABRs) of neonates (mean gestational age = 34.6 weeks) treated with a minoglycosides had significantly altered auditory responses.42 This hypothesis has been reexamined recently by Rees43 in a study that also documented noise levels generated by monitors within the NICU.
The rate of hearing loss among full-term babies is between 0.1% and 0.3% and is usually of congenital origin.1–3 Among the NICU population, the rate of hearing loss increases to between 2 % and 15%, that is, up to 90,000 individuals in the United States per year, the majority with no known etiology.44–46 The mean noise levels of ventilators and monitors in the room with these preterm infants is ~65 dBA, and for the alarms it is greater than 80 dBA. Infants who did not receive aminoglycosides passed their hearing screenings (automated auditory brain-stem response) at the same rate as the baseline, regardless of gestational age or noise exposure. However, infants who received aminoglycosides for 7 days (without mechanical ventilation) were 15% more likely to fail their hearing screenings. Infants who received aminoglycosides for 7 days, with more than 4 days of mechanical ventilation and experienced noise above 80 dB, were 30% more likely to fail their hearing tests. Significantly, infants who received aminoglycosides and mechanical ventilation with occasional noise exposure above 80 dB for just 4 days had nearly the same rate of failed hearing screening as did the infants that received aminoglycosides without noise for 7 days. Thus, short-term exposure to moderate levels of noise (i.e., >80 dBA) potentiates the ototoxic effects of aminoglycosides, and this potentiation is greater than additive,43 corroborating conclusions derived from a variety of animal studies that noise potentiates aminoglycoside-induced ototoxicity.30–38 In contrast, exposure to noise in the absence of aminoglycosides did not generally cause hearing loss, unless noise levels themselves were ototoxic.
Cisplatin ototoxicity also is potentiated by moderate levels of noise exposure in animal studies.47,48 Interestingly, animals with previous noise exposure also show potentiation of subsequent cisplatin ototoxicity, as for aminoglycosides.31,32,35,49 Potentiation of cisplatin ototoxicity by previous noise exposure also has been reported in clinical cases.50 Thus, noise potentiates drug-induced auditory dysfunction, in contrast with solvent enhancement of NIHL.
PREVENTING SYNERGISTICALLY ACQUIRED HEARING LOSS
Because subtoxic levels of organic solvents potentiate NIHL during simultaneous exposure, it is necessary to remove exposure to one or both noxious phenomena. This is dependent on the environmental context, and it is important that refueling personnel on aircraft carriers and paint sprayers use hearing (and respiratory) protection that reduces noise levels to a greater extent than that required for individuals exposed to the same noise levels in the absence of solvent vapors. Once organic solvents have entered the body, their lipophilic nature enables them to diffuse across cellular membranes and therefore cross epithelial blood-brain and blood-labyrinth barriers with ease. Therefore, preventing both inhalation of solvent vapors and exposure to low-level noise is critical to prevent solvent-potentiation of NIHL.
The ototoxic effect of aminoglycosides is potentiated by simultaneous exposure to noise at dose levels that induce little or no auditory dysfunction when either insult is given separately. Prior noise exposure also potentiates the ototoxic effect of aminoglycosides given weeks later.32,51 Therefore, it is important to remove sources of toxic noise prior to and during critical, lifesaving systemic aminoglycoside administration for treating bacterial sepsis, meningitis, and during prophylaxis. Because it is not possible to remove prior exposure to noise in many cases requiring aminoglycoside treatment (e.g., military casualties), it becomes increasingly necessary to understand how aminoglycosides cross the blood-labyrinth barrier (similar to the blood-brain barrier) into the cochlear fluids where they are taken up by hair cells (i) via apical endocytosis in vivo and (ii) through their mechanosensitive transduction channels at the tips of their stereocilia 52,53 (i.e., from endolymph bathing the apical surfaces of hair cells).
Unlike organic solvents, which can diffuse across lipid membranes, aminoglycosides are highly hydrophilic cationic molecules, and their trafficking from the vasculature into the cochlea fluids during systemic administration remains poorly understood. The preferential loading of marginal cells in the stria vascularis by aminoglycosides suggests that systemically administered aminoglycosides cross the strial blood-labyrinth barrier and are trafficked into marginal cells and from there into endolymph directly.54–56 Once in endolymph, the cationic aminoglycosides would be electrophoretically driven into negatively polarized hair cells to exert their cytotoxic effect, disrupting auditory function.
Although it is not yet known how noise can potentiate aminoglycoside ototoxicity, several mechanisms have been proposed: (i) by increasing strial blood-labyrinth barrier permeability,9–11 which could enable greater cochlear fluid and hair cell uptake of a minoglycosides; (ii) by increasing the open probability of aminoglycoside-permissive mechanosensitive transduction channel (from 0.1 to 0.5) during sound stimulation, which could enhance inner hair cell uptake of aminoglycosides and subsequent toxicity52,57,58; (iii) increased aminoglycoside entry via a noise-induced, ATP-activated short circuit through P2X2channels, which are permissive to large organic molecules59–62; or (iv) summation of noise-induced generation of ROS and that generated by aminoglycosides. 12,14 Because removing noise to prevent potentiation of aminoglycoside ototoxicity is not always possible, the most effective strategies will be dependent on identifying and then preventing the mechanisms of aminoglycoside uptake and trafficking within the stria vascularis, as well a preventing aminoglycoside toxicity within cochlear hair cells using antioxidant therapy. 63–65
The mechanisms by which cisplatin (and its derivatives) enter the cochlea and hair cells remain unknown. Noise potentiation of cisplatin ototoxicity47,48 could theoretically follow similar trafficking mechanisms as for aminoglycosides described above, because cisplatin is also a cationic molecule, albeit of a different chemical structure, and generates toxic ROS.14
CONCLUSION
Moderate levels of noise (>80 dBA) potentiate drug-induced ototoxicity, including a greater degree of permanent threshold shifts. In contrast, subtoxic levels of solvents potentiate NIHL, including solvent-induced permanent threshold shifts at noise levels that induce little or no auditory dysfunction when given alone. Prevention synergism between noise and potential ototoxins is best and most easily achieved by removing exposure to chemical ototoxins, noise sources, and/or damping the levels of noise perceived. Further research is required to understand (1) the mechanisms of how solvents potentiate NIHL; (2) how noise enhances drug-induced auditory dysfunction, and (3) the underlying mechanisms of ototoxicity induced by solvents and clinical drugs themselves.
Acknowledgments
Supported by the National Institute of Deafness and other Communication Disorders (DC 04555).
Footnotes
Learning Outcomes: As a result of this activity, the participant will be able to gain new insights into noise potentiation of chemical ototoxicity and the potential routes by which ototoxic drugs cross the blood-labyrinth barrier to exert their cytotoxic effects in hair cells.
Noise Damage and Traumatic Brain Injury: Emerging Therapies and Evidence-Based Practices: Proceedings from the National Center for Rehabilitative Auditory Research (NCRAR), 2008; Guest Editor, Dawn Konrad-Martin, Ph.D.
References
- 1.Barsky-Firkser L, Sun S. Universal newborn hearing screenings: a three-year experience. Pediatrics. 1997;99:E4. doi: 10.1542/peds.99.6.e4. [DOI] [PubMed] [Google Scholar]
- 2.Finitzo T, Albright K, O’Neal J. The newborn with hearing loss: detection in the nursery. Pediatrics. 1998;102:1452–1460. doi: 10.1542/peds.102.6.1452. [DOI] [PubMed] [Google Scholar]
- 3.Mehl AL, Thomson V. Newborn hearing screening: the great omission. Pediatrics. 1998;101:E4. doi: 10.1542/peds.101.1.e4. [DOI] [PubMed] [Google Scholar]
- 4.Steel KP. Science, medicine, and the future: new interventions in hearing impairment. BMJ. 2000;320:622–625. doi: 10.1136/bmj.320.7235.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochkin S. Hearing loss population tops 31 million people. Hearing Rev. 2005;12:16–29. [Google Scholar]
- 6.Davis AC. Hearing in Adults: The Prevalence and Distribution of Hearing Impairment and Reported Hearing Disability in the MRC Institute of Hearing Research’s National Study of Hearing. London, United Kingdom: Whurr; 1995. p. xv. [Google Scholar]
- 7.Ohlemiller KK, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;45:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- 8.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Yamasoba T, Ishibashi T, Miller JM, Kaga K. Effect of noise exposure on blood-labyrinth barrier in guinea pigs. Hear Res. 2002;164:12–18. doi: 10.1016/s0378-5955(01)00397-5. [DOI] [PubMed] [Google Scholar]
- 10.Goldwyn BG, Quirk WS. Calcium channel blockade reduces noise-induced vascular permeability in cochlear stria vascularis. Laryngoscope. 1997;107:1112–1116. doi: 10.1097/00005537-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Hukee MJ, Duvall AJ., III Cochlear vessel permeability to horseradish peroxidase in the normal and acoustically traumatized chinchilla: a reevaluation. Ann Otol Rhinol Laryngol. 1985;94:297–303. [PubMed] [Google Scholar]
- 12.Kopke R, Allen KA, Henderson D, et al. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann NY Acad Sci. 1999;884:171–191. doi: 10.1111/j.1749-6632.1999.tb08641.x. [DOI] [PubMed] [Google Scholar]
- 13.Revilla AS, Pestana CR, Pardo-Andreu GL, et al. Potential toxicity of toluene and xylene evoked by mitochondrial uncoupling. Toxicol In Vitro. 2007;21:782–788. doi: 10.1016/j.tiv.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Clerici WJ, Hensley K, DiMartino DL, Butter-field DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 15.Sliwinska-Kowalska M, Zamyslowska-Szmytke E, Szymczak W, et al. Effects of coexposure to noise and mixture of organic solvents on hearing in dockyard workers. J Occup Environ Med. 2004;46:30–38. doi: 10.1097/01.jom.0000105912.29242.5b. [DOI] [PubMed] [Google Scholar]
- 16.Fechter LD, Gearhart C, Fulton S, et al. JP-8 jet fuel can promote auditory impairment resulting from subsequent noise exposure in rats. Toxicol Sci. 2007;98:510–525. doi: 10.1093/toxsci/kfm101. [DOI] [PubMed] [Google Scholar]
- 17.Fechter LD, Gearhart C, Fulton S, et al. Promotion of noise-induced cochlear injury by toluene and ethylbenzene in the rat. Toxicol Sci. 2007;98:542–551. doi: 10.1093/toxsci/kfm109. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MJ, Rarey KE, Conolly RB. Ototoxicity of toluene in rats. Neurotoxicol Teratol. 1988;10:525–530. doi: 10.1016/0892-0362(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 19.Lataye R, Campo P. Combined effects of a simultaneous exposure to noise and toluene on hearing function. Neurotoxicol Teratol. 1997;19:373–382. doi: 10.1016/s0892-0362(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 20.Pouyatos B, Gearhart C, Nelson-Miller A, Fulton S, Fechter L. Oxidative stress pathways in the potentiation of noise-induced hearing loss by acrylonitrile. Hear Res. 2007;224:61–74. doi: 10.1016/j.heares.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Pouyatos B, Gearhart CA, Fechter LD. Acrylonitrile potentiates hearing loss and cochlear damage induced by moderate noise exposure in rats. Toxicol Appl Pharmacol. 2005;204:46–56. doi: 10.1016/j.taap.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Sliwinska-Kowalska M, Bilski B, Zamyslowska-Szmytke E, et al. Hearing impairment in the plastics industry workers exposed to styrene and noise. Med Pr. 2001;52:297–303. [PubMed] [Google Scholar]
- 23.Sliwinska-Kowalska M, Zamyslowska-Szmytke E, Szymczak W, et al. Ototoxic effects of occupational exposure to styrene and co-exposure to styrene and noise. J Occup Environ Med. 2003;45:15–24. doi: 10.1097/00043764-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Morata TC, Dunn DE, Sieber WK. Occupational exposure to noise and ototoxic organic solvents. Arch Environ Health. 1994;49:359–365. doi: 10.1080/00039896.1994.9954988. [DOI] [PubMed] [Google Scholar]
- 25.Santi PA, Duvall AJ., III Morphological alteration of the stria vascularis after administration of the diuretic bumetanide. Acta Otolaryngol. 1979;88:1–12. doi: 10.3109/00016487909137133. [DOI] [PubMed] [Google Scholar]
- 26.Higashiyama K, Takeuchi S, Azuma H, et al. Bumetanide-induced enlargement of the intercellular space in the stria vascularis critically depends on Na+ transport. Hear Res. 2003;186:1–9. doi: 10.1016/s0378-5955(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 27.Kusakari J, Ise I, Comegys TH, Thalmann I, Thalmann R. Effect of ethacrynic acid, furosemide, and ouabain upon the endolymphatic potential and upon high energy phosphates of the stria vascularis. Laryngoscope. 1978;88(1 Pt 1):12–37. doi: 10.1002/lary.1978.88.1.12. [DOI] [PubMed] [Google Scholar]
- 28.Rybak LP. Pathophysiology of furosemide ototoxicity. J Otolaryngol. 1982;11:127–133. [PubMed] [Google Scholar]
- 29.Vernon J, Brummett RE. Noise trauma in the presence of loop-inhibiting drugs. Trans Am Acad Ophthalmol Otolaryngol. 1977;84:407–413. [PubMed] [Google Scholar]
- 30.Gannon RP, Tso SS, Chung DY. Interaction of kanamycin and noise exposure. J Laryngol Otol. 1979;93:341–347. doi: 10.1017/s0022215100087119. [DOI] [PubMed] [Google Scholar]
- 31.Ryan AF, Bone RC. Potentiation of kanamycin ototoxicity by a history of noise exposure. Otolaryngology. 1978;86:ORL-125–ORL-128. doi: 10.1177/019459987808600130. [DOI] [PubMed] [Google Scholar]
- 32.Ryan AF, Bone RC. Non-simultaneous interaction of exposure to noise and kanamycin intoxication in the chinchilla. Am J Otolaryngol. 1982;3:264–272. doi: 10.1016/s0196-0709(82)80065-3. [DOI] [PubMed] [Google Scholar]
- 33.Vernon J, Brown J, Meikle M, Brummett RE. The potentiation of noise-induced hearing loss by neomycin. Otolaryngology. 1978;86:ORL-123–ORL-124. doi: 10.1177/019459987808600129. [DOI] [PubMed] [Google Scholar]
- 34.Dayal VS, Kokshanian A, Mitchell DP. Combined effects of noise and kanamycin. Ann Otol Rhinol Laryngol. 1971;80:897–902. doi: 10.1177/000348947108000616. [DOI] [PubMed] [Google Scholar]
- 35.Collins PW. Synergistic interactions of gentamicin and pure tones causing cochlear hair cell loss in pigmented guinea pigs. Hear Res. 1988;36:249–259. doi: 10.1016/0378-5955(88)90066-4. [DOI] [PubMed] [Google Scholar]
- 36.Tan CT, Hsu CJ, Lee SY, Liu SH, Lin-Shiau SY. Potentiation of noise-induced hearing loss by amikacin in guinea pigs. Hear Res. 2001;161:72–80. doi: 10.1016/s0378-5955(01)00359-8. [DOI] [PubMed] [Google Scholar]
- 37.Pye A, Collins P. Interaction between sound and gentamicin: immediate threshold and stereociliary changes. Br J Audiol. 1991;25:381–390. doi: 10.3109/03005369109076613. [DOI] [PubMed] [Google Scholar]
- 38.Dodson HC, Bannister LH, Douek EE. The effects of combined gentamicin and white noise on the spiral organ of young guinea pigs. A structural study. Acta Otolaryngol. 1982;94:193–202. doi: 10.3109/00016488209128905. [DOI] [PubMed] [Google Scholar]
- 39.Mohr PE, Feldman JJ, Dunbar JL, et al. The societal costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care. 2000;16:1120–1135. doi: 10.1017/s0266462300103162. [DOI] [PubMed] [Google Scholar]
- 40.Escobar GJ. The neonatal “sepsis work-up”: personal reflections on the development of an evidence-based approach toward newborn infections in a managed care organization. Pediatrics. 1999;103(1, Suppl E):360–373. [PubMed] [Google Scholar]
- 41.Pillers DM, Schleiss MR. Gentamicin in the clinical setting. Volta Review. 2005;105:205–210. [Google Scholar]
- 42.Bernard PA. Freedom from ototoxicity in aminoglycoside treated neonates: a mistaken notion. Laryngoscope. 1981;91:1985–1994. doi: 10.1288/00005537-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Rees KC. Public Health. Baltimore, MD: The Johns Hopkins University; 2007. The combined effect of noise and aminoglycoside antibiotic exposure on the auditory system of the pre-term infant. [Google Scholar]
- 44.Erenberg A, Lemons J, Sia C, Trunkel D, Ziring P. Newborn and infant hearing loss: detection and intervention. American Academy of Pediatrics. Task Force on Newborn and Infant Hearing, 1998–1999. Pediatrics. 1999;103:527–530. doi: 10.1542/peds.103.2.527. [DOI] [PubMed] [Google Scholar]
- 45.Behrman RE Butler ASInstitute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. (U.S.). Committee on Understanding Premature Birth and Assuring Healthy Outcomes. [PubMed] [Google Scholar]
- 46.Ertl T, Hadzsiev K, Vincze O, et al. Hyponatremia and sensorineural hearing loss in preterm infants. Biol Neonate. 2001;79:109–112. doi: 10.1159/000047076. [DOI] [PubMed] [Google Scholar]
- 47.Gratton MA, Salvi RJ, Kamen BA, Saunders SS. Interaction of cisplatin and noise on the peripheral auditory system. Hear Res. 1990;50:211–223. doi: 10.1016/0378-5955(90)90046-r. [DOI] [PubMed] [Google Scholar]
- 48.Gratton MA, Kamen BA. Potentiation of cisplatin ototoxicity by noise. J Clin Oncol. 1990;8:2091–2092. doi: 10.1200/JCO.1990.8.12.2091. [DOI] [PubMed] [Google Scholar]
- 49.Laurell GF. Combined effects of noise and cisplatin: short- and long-term follow-up. Ann Otol Rhinol Laryngol. 1992;101:969–976. doi: 10.1177/000348949210101202. [DOI] [PubMed] [Google Scholar]
- 50.Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan AF, Bone RC. Potentiation of kanamycin ototoxicity by a history of noise exposure. Otolaryngology. 1978;86:ORL-125–ORL-128. doi: 10.1177/019459987808600130. [DOI] [PubMed] [Google Scholar]
- 52.Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567(Pt 2):505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashino E, Shero M. Endocytosis of aminoglycoside antibiotics in sensory hair cells. Brain Res. 1995;704:135–140. doi: 10.1016/0006-8993(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 54.Steyger PS. Cellular uptake of aminoglycosides. Volta Review. 2005;105:299–324. [Google Scholar]
- 55.Dai CF, Steyger PS. A systemic gentamicin pathway across the stria vascularis. Hear Res. 2008;235:114–124. doi: 10.1016/j.heares.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, Balaban C, Salt AN, Steyger PS. Aminoglycosides preferentially load the blood-endolymph barrier over the blood-perilymph barrier: implications for drug induced ototoxicity. Paper presented at: 13th Annual Blood-Brain Barrier Consortium Meeting; 2007. [Google Scholar]
- 57.Jia S, Dallos P, He DZ. Mechanoelectric transduction of adult inner hair cells. J Neurosci. 2007;27:1006–1014. doi: 10.1523/JNEUROSCI.5452-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell IJ. Origin of the receptor potential in inner hair cells of the mammalian cochlea—evidence for Davis’ theory. Nature. 1983;301:334–336. doi: 10.1038/301334a0. [DOI] [PubMed] [Google Scholar]
- 59.Munoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 60.Thorne PR, Munoz DJ, Housley GD. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the guinea pig. J Assoc Res Otolaryngol. 2004;5:58–65. doi: 10.1007/s10162-003-4003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Housley GD, Kanjhan R, Raybould NP, et al. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neuro-transmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyers JR, MacDonald RB, Duggan A, et al. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McFadden SL, Ding D, Salvemini D, Salvi RJ. M40403, a superoxide dismutase mimetic, protects cochlear hair cells from gentamicin, but not cisplatin toxicity. Toxicol Appl Pharmacol. 2003;186:46–54. doi: 10.1016/s0041-008x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 64.Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 65.Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoxicity. Lab Invest. 1999;79:807–813. [PubMed] [Google Scholar]


