Abstract
The calgranulins are a subgroup of proteins in the S100 family (calgranulin A, S100A8; calgranulin B, S100A9 and calgranulin C, S100A12) that provide protective anti-infective and anti-inflammatory functions for the mammalian host. In this review, we discuss the structure-function relationships whereby S100A8 and S100A9, and for comparison, S100A12, provide intra- and extracellular protection during the complex interplay between infection and inflammation and how the calgranulins are regulated to optimally protect the host. Ideally located to support epithelial barrier function, calprotectin, a complex of S100A8/S100A9, is expressed in squamous mucosal keratinocytes and innate immune cells present at mucosal surfaces. The calgranulins are also abundantly produced in neutrophils and monocytes, whereas expression is induced in epidermal keratinocytes, gastrointestinal epithelial cells and fibroblasts during inflammation. The calgranulins show species-specific expression and function. For example, S100A8 is chemotactic in rodents but not in humans. In humans, S100A12 appears to serve as a functional chemotactic homolog to murine S100A8. Transition metal-binding and oxidation sites within calgranulins are able to create structural changes that may orchestrate new protective functions or binding targets. The calgranulins thus appear to adopt a variety of roles to protect the host. In addition to serving as a leukocyte chemoattractant, protective functions include oxidant scavenging, antimicrobial activity, and chemokine-like activities. Each function may reflect the concentration of the calgranulin, post-transcriptional modifications, oligomeric forms, and the proximal intracellular or extracellular environments. Calprotectin and the calgranulins are remarkable as multifunctional proteins dedicated to protecting the intra- and extracellular environments during infection and inflammation.
Keywords: Calgranulins, anti-infection, anti-inflammation, structure, induction, macrophages, keratinocytes
INTRODUCTION
Inflammation is central to the acute responses to infection and calgranulins are important antimicrobial proteins that also mediate innate and acquired immune responses. The calgranulins play important roles in resistance of the host to infection and protection against adverse affects of inflammation. These remarkable proteins are multifunctional, operating intra- and extracellularly and there are characteristics that differ between humans and rodents. The calgranulins are a subgroup of proteins in the S100 family (calgranulin A, S100A8; calgranulin B, S100A9 and calgranulin C, S100A12) associated with acute/chronic inflammatory disorders 1 and more recently, with various cancers 2.
The S100A8/A9 (MRP-8/MRP-14) complex was named calprotectin to reflect its protective, anti-microbial properties, 1 although termed L1 antigen in early studies 3. The antimicrobial mechanisms are suggested to involve chelation of divalent cations such as manganese and zinc, which depletes these essential transition metals from the microenvironment. This mechanism appears to contribute to intra- and extracellular growth inhibition of a broad range of bacteria and fungi. Through mechanisms independent of direct antimicrobial activity, S100A8/A9 also contributes to the resistance of epithelial cells to bacterial invasion and the intra- and extracellular effects support the barrier function of mucosal epithelia.
In concert with antimicrobial activity, the calgranulins may also modulate inflammatory responses. Successful resolution of infection involves neutrophil and/or monocyte recruitment. At sites of infection, activated leukocytes release anti-microbial peptides (AMPs) and generate anti-infective reactive oxygen (ROS) and anti-inflammatory (NO-removing) nitrogen (RNS) species. To protect the host against the collateral tissue-injurious effects of NO and ROS, some calgranulins may scavenge and neutralize these compounds. This review will focus on the intra- and extracellular anti-infective and anti-inflammatory properties of the calgranulins. To explain the molecular basis of protection of the host, the structure and functions of these proteins will be discussed, to better understand the contributions of key cellular players including neutrophils, macrophages and keratinocytes.
STRUCTURAL FEATURES OF S100 CALGRANULINS ASSOCIATED WITH HOST PROTECTIVE FUNCTIONS
We will briefly detail some structural and functional features of the calgranulins that contribute to their manifold roles in immunity. Although amino acid sequence structure is correlated with function, changes of even a single amino acid can dramatically change function without significantly altering overall structure (e.g. normal vs. sickle cell hemoglobin). Therefore, we will attempt to point out some structural features that may be of functional importance.
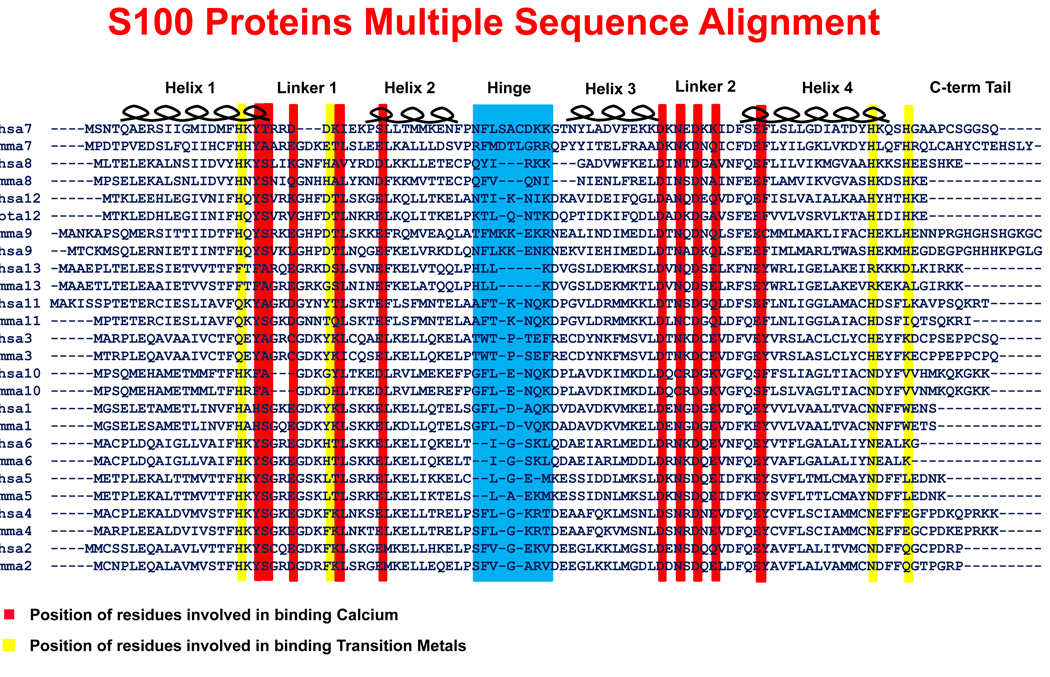
Although most S100 proteins contain only approximately 100 amino acids, these proteins can assume multiple functional forms because of their numerous functional motifs. Each S100 protein typically contains two EF hand calcium-binding loop motifs separated by a “hinge” region, which is distinct for each family member and in some cases followed by a family unique carboxy-terminal tail (see Figure 1) 4. The S100 proteins primarily form homodimers or higher order oligomers, but S100A8 and S100A9 uniquely form heterodimeric complexes, known as calprotectin.
Figure 1. Multiple sequence alignment of S100 protein family.
A pair of sequences, mouse and human if available, for the S100A1 thru S100A13 proteins was used to generate the alignment. The common features of the S100 proteins are the first EF Hand (containing helices 1 & 2 as well as the linker 1 region), the hinge region, the second EF Hand (containing helices 3 & 4 as well as the linker 2 region), and the carboxy-terminal tail. A red background indicates the locations of the residues responsible for binding calcium. Although no S100 monomer is able to bind a transition metal, a pair of yellow lines indicates the location of the residues that create each half of the transition metal binding site within a dimer. The hinge region is highlighted in blue. The hinge region and helix 3 in S100A8 are shortened relative to other S100 proteins.
EF Hand: Calcium-free and calcium-bound conformations of calgranulins
When calcium is bound by an EF-hand motif, the topology (conformational change) and charge of the protein are altered. Topology is key for characterizing electrostatic potential, since charge potential is amplified at peaks (^) or in the inverse case of valleys/clefts (V) 5. A macroscopic example of the effect of topology and charge potential relates to why we have lightning rods instead of lightning sheets. To understand the effect of calcium-binding on the functional forms of calgranulins, therefore, the calcium-free and calcium-bound conformations must be compared.
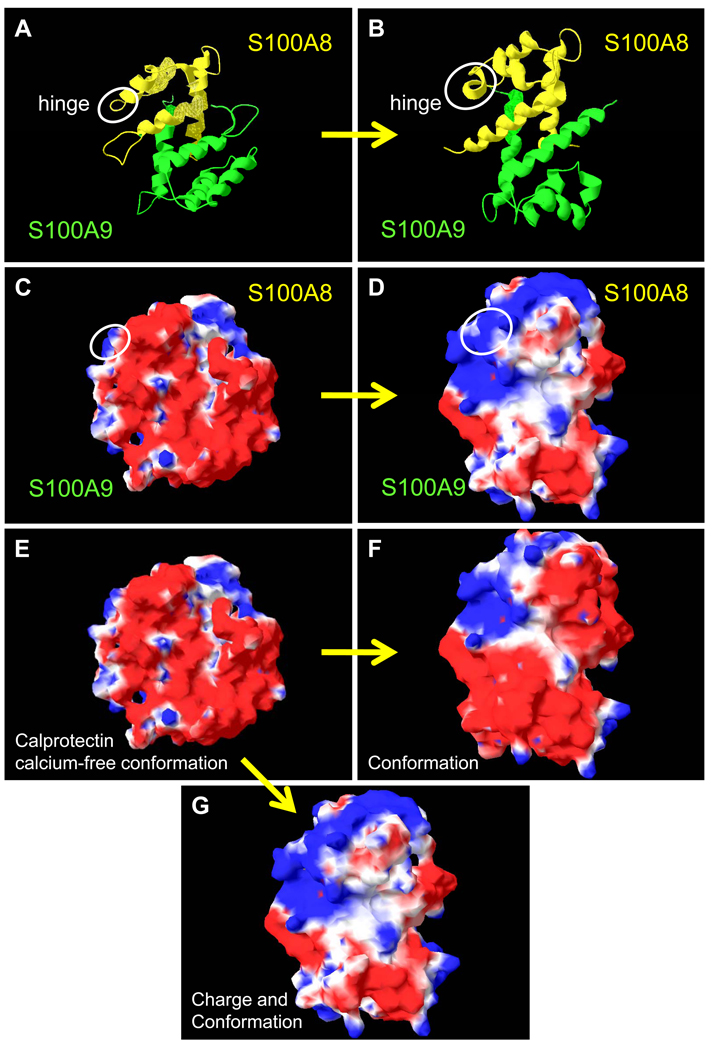
Although the structure of calcium-free calprotectin has not been experimentally determined, we used the program MODELLER 6 to generate the homology model (Figure 2) 7. The calcium-bound form of calprotectin has been experimentally determined (pdb entry 1XK4) 8. The ribbon diagrams, molecular surface, and calculation of electrostatic potential were developed using Swiss-PdbViewer (from http://www.expasy.org/spdbv/) (Figure 2) 9.
Figure 2. Predicted surface structures of S100A8 and S100A9 in the presence and absence of calcium.
The ribbon diagrams (S100A8, yellow and S100A9, green) of (A) calcium-free and (B) calcium-bound human calprotectin. The white circle indicates the hinge region of S100A8 and highlights the random coil to alpha helix transition upon calcium-binding. The solvent accessible surfaces of (C) calcium-free and (D) calcium-bound calprotectin: blue represents positive potential, red is negative potential, and white indicates a hydrophobic region. (E) The conformation and charge of the solvent accessible surface of the calprotectin calcium-free model structure (as in C). (F) The changes in the molecular surface of calprotectin upon binding calcium without taking into account the positive charge associated with the calcium ion. This electrostatic component is added to the calculation for panel G. (G) Calcium-binding induces a conformation change and neutralizes a large area of negatively-charged surface of calprotectin.
Calcium binding changes the secondary structure of the hinge region and converts a portion of the extended disordered region following the carboxy-terminal helix 4 in S100A8 and S100A9 into additional turns of alpha helix. Upon calcium-binding, helices 2 and 3 rotate relative to helices 1 and 4. These changes in the size and orientation of secondary structural features alters the solvent accessible surface of calprotectin and may create potentially new functional interactions from a single protein complex. On the other hand, complexes of S100 proteins or divalent cations may not be required for some functions such as the chemotactic activity of the α-helical hinge domains of mS100A8 and S100A12 hydrophobic peptides 10.
Transition metal binding sites
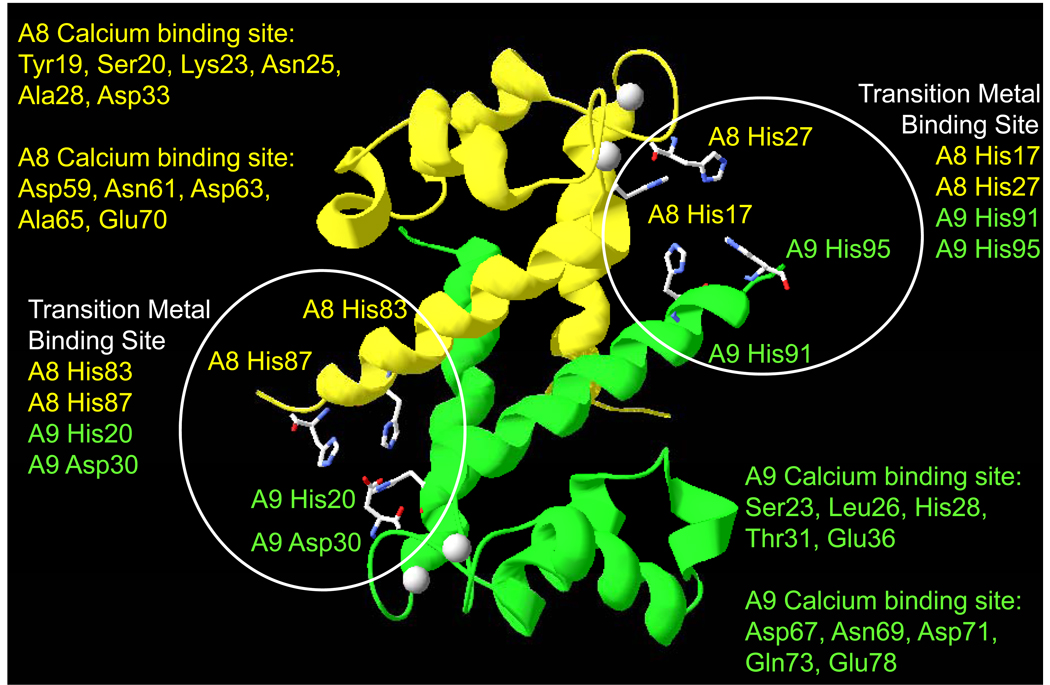
Like several S100 protein family members (e.g. S100A2, S100A5, S100A12, S100A13, and S100B), calprotectin contains two additional tetrahedrally-coordinated transition metal-binding sites created by the dimer interface with two of the four ligands donated by each monomeric subunit (Figure 3). One site is created by His83 and His87 residues in S100A8, in conjunction with His20 and Asp30 residues in S100A9. The second site involves residues His17 and His27 in S100A8 and His91 and His95 in S100A9. The calprotectin metal-binding sites are brought into proximity upon calcium-binding, which orders secondary structure elements. In S100A12, zinc/copper binding to the calcium-free protein also partially orders the EF hands and promotes calcium-binding 11, 12. Transition metal binding is predicted to further shift the surface of calprotectin to contain positively-charged features and create an additional set of functional conformations with new functions. For example, Zn2+ is implicated in the apoptotic activity of S100A8/A9 13, 14, suggesting structural changes that regulate new functions. Similarly, chelation of Zn2+ and Mn2+ by extracellular S100A8/A9 are proposed mechanisms of antimicrobial activity 15–17.
Figure 3. Ribbon diagram of calcium-bound calprotectin showing likely transition metal binding sites.
S100A8 (yellow ribbon) and S100A9 (green) residues coordinating calcium are listed, and coloured according to their location binding sites (highlighted with white circles) in S100A8 or S100A9. Coordination is created by the dimer interface and the ligands are coloured accordingly.
Additional functional forms are created in S100A12 upon binding of transition metals. S100A12 binds bivalent ions such as copper, manganese and zinc 18. Zn2+ binding increases calcium binding affinity by 1500-fold and raises the affinity of binding to a putative extracellular receptor, the receptor for advanced glycation end product (RAGE) 11, 12. For example, S100A12 shows increased binding to human gastric carcinoma MKN74 cells in the presence of Zn2+ 12 and calcium and zinc are required for the interaction of S100A12 with paramyosin 18, 19. Even though copper and zinc have the same binding site, the ions promote non-identical configurations of the S100A12 complex 12. The function of the S100A12-copper complex is currently unknown although alternative zinc binding appears to reverse redox function 18, 19.
Which metal is bound by calgranulins in the physiological context is generally unknown, since zinc, copper or manganese availability may be limited in the surrounding milieu. The flexibility of proteins also enables the same site to bind different metals. As the protein folds, the availability of specific metals within the intra- or extracellular environments may be important determinants of binding and function 20.
Oligomers
Formation of dimers, trimers, or tetramers involves burial of portions of the protein surface and creation of new clefts and peaks. Compared to monomeric forms, the topology of protein complexes becomes significantly altered and additional functional forms can be created (also reduced). The conformation of the complex will depend upon its constituents, and homodimers of S100A8 or S100A9 will differ significantly in form and function from the heterodimeric calprotectin (S100A8/S100A9). Formation of heterodimers is calcium-independent 21, 22, and calcium binding by calprotectin can induce a conformational change that is also required for tetramer formation 22, 23. Tetramers are the suggested biologically-active form 21, 22. S100A12 also forms tetramers and hexamers in the presence of zinc and calcium ions 12, 24–26, potentially expanding its functional roles.
C-terminal extension
Unlike S100A8 and most other members of the S100 family, S100A9 has an extended carboxy-terminal region, containing two additional functional motifs. The penultimate residue in human calprotectin (Thr-113) can be phosphorylated by protein kinase C 27. The second motif contains histidine residues (103–105 in humans) involved in binding arachidonic acid 28.
Murine vs. human calprotectin
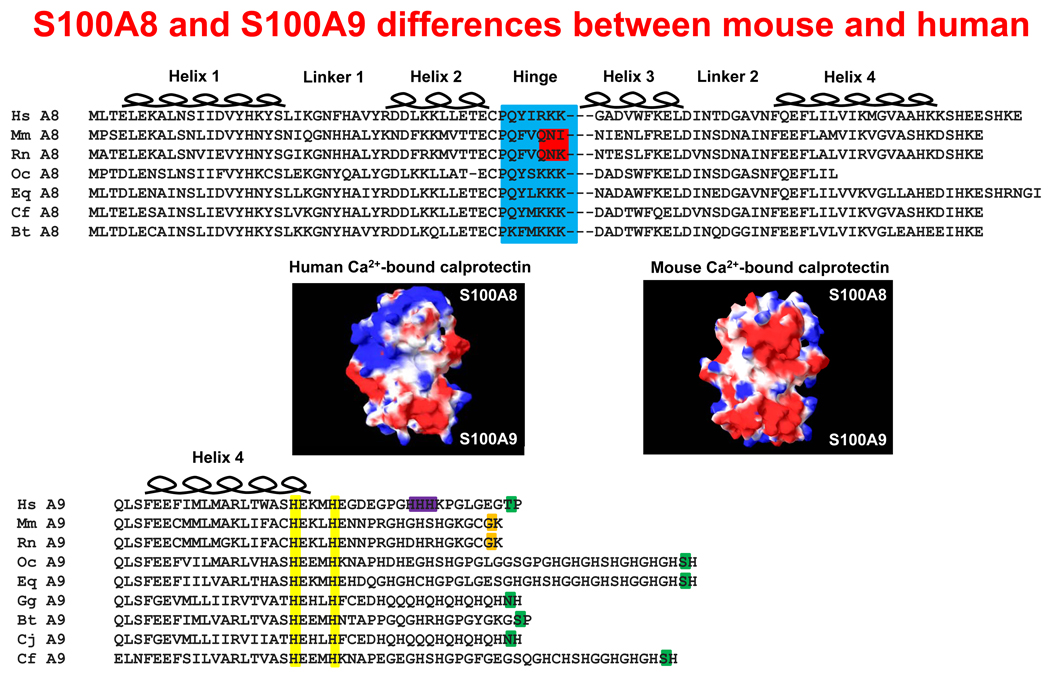
In addition to the absence of S100A12 in mice, murine and human S100A8 differ in the hinge region, S100A9 in the carboxy-terminal tail, and murine and human calprotectin have different electrostatic potentials (Figure 4).
Figure 4. Structural comparision of murine and human calprotectin.
Alignment of seven species of S100A8 sequences (top panel). The hinge region (highlighted in blue) contains three positively-charged residues, except in the case of mouse and rat, which have polar glutamine and asparagine residues (highlighted in red). In the calcium-bound conformation, the electrostatic potential differences for human and mouse calprotectin are given (middle panel). The solvent-accessible surfaces are represented: blue indicates positive potential, red shows negative potential, and white indicates a hydrophobic region. The multiple sequence alignment shows the penultimate C-terminal phosphorylatable residues (highlighted in green) and the non-phosphorylatable equivalent residues in murine and rat S100A9 (orange) (bottom panel). Residues involved in binding transition metals (yellow) and possible arachidonic acid binding (purple) are indicated.
Intriguingly, the hinge region contributes to the chemotactic activity of murine, but not human S100A8. Similar residues in the hinge region of human S100A12 are also implicated in chemotaxis 10. Since the surface features of homo- and heterodimers will vary with the environmental conditions, reported functions of the hinge and other regions of the calgranulins can vary in different experimental protocols.
INTRACELLULAR FUNCTIONS OF CALPROTECTIN
Cellular differentiation and inflammatory response
The intracellular S100A8/A9 complex, calprotectin, may function in a cell type-specific manner. In hematopoietic progenitor cells, proliferation and maturation may be regulated by S100A8/A9-mediated inhibition of casein kinase I and II, enzymes that phosphorylate topoisomerase I and RNA polymerase I and II 29. S100A8/A9 expression also suppresses differentiation of murine myeloid-derived suppressor cells (MDSC), potentially through S100A9-dependent induction of NADPH oxidase and generation of reactive oxygen species (ROS). In immature myeloid cells, S100A9 up-regulation inhibits differentiation into dendritic cells and macrophages 30. The response to S100A9 appears to be STAT3-dependent, leading to enhanced production of MDSCs and immune tolerance 30. MDSCs promote immune tolerance by suppressing CD4+ and CD8+ T cell activation, resulting in polarized immunity to a type-2 phenotype 31. MDSC inhibit T cell function by regulating metabolism of the essential amino acid L-arginine by arginase I and NOS2 32. Expression of these enzymes is immunosuppressive 33 and may synergize to produce peroxynitrites, which cause T cell apoptosis 34. Similarly, S100A8 and S100A9 in neutrophils can readily be S-nitrosylated 35 and their intracellular functions in MDSC warrant further studies. Since L-arginine is metabolized by NOS2 to produce NO, scavenging of NO by S100A8 and S100A9 35 could influence MDSC/regulatory macrophage functions.
S100A8/A9 in neutrophils also binds fatty acids in a calcium-dependent manner 36, 37. Polyunsaturated fatty acids, including arachidonic acid (AA) have a higher affinity for S100A8/A9 than saturated or monounsaturated fatty acids and may contribute to their uptake and release 38. The released AA-calprotectin complex could be internalized and metabolized by nearby cells to generate eicosanoids, which contribute to the initiation and regulation of inflammatory responses 39. S100A8/A9 may also serve as a scaffold for AA in the cytosol of neutrophils and epithelial cells to facilitate activation of NADPH oxidase to yield ROS 28, 40, which are potent microbicides against invading pathogens.
Antimicrobial functions
In addition to its extracellular antimicrobial activity, calprotectin-expressing cells also resist bacterial adherence and invasion 41, 42. Calprotectin-expressing keratinocytes are found to internalize fewer invasive pathogens, like Listeria 43. Approximately three-fold fewer bacteria are found to bind to calprotectin-expressing cells, but there are 10-fold fewer intracellular microorganisms. The reduction in apparent invasion can be due to direct intracellular antimicrobial effects of calprotectin and the ability of the calprotectin-expressing cell to resist the invasion process. Whereas calprotectin-expressing cells generally contain fewer invaded pathogens, Listeria, an invasive pathogen that can grow in the cytoplasm, appears to subvert intracellular antimicrobial activity by inducing translocation of intracellular S100A8/A9 to microtubules 43. When calprotectin co-localizes with tubulin, keratinocytes contain more intracellular Listeria than in cells without co-localization. In the presence of polymerized microtubules, the antimicrobial activity of calprotectin against Listeria is neutralized in vitro 43. As we reported recently, however, S100A8/A9 expressing cells are also resistant to bacterial invasion independently of intracellular antimicrobial activity. Induction of keratinocyte resistance to bacterial invasion is dependent on the calcium-binding domains within S100A9 in the intracellular heteromeric complex, and does not necessarily correlate with changes in binding of bacteria to the cell surface 7. Hence, intracellular calprotectin appears to thwart internalization of invasive pathogens by calcium-dependent intracellular protein-protein interactions and may also inhibit the cytoplasmic growth of those bacteria that manage to invade.
Cellular protection against stressors and wounding
Both pro- and anti-inflammatory mediators regulate the S100A8 and S100A9 genes. The intracellular functions of S100A8 and S100A9 may depend on cell type-specific receptors that signal in response to particular extracellular stressors. Oxidation and zinc binding can each signal calprotectin-dependent cellular responses. In some circumstances, intracellular calgranulins mediate host protection. For example, elevated S100A8 and S100A9 expression levels in epidermal incision wounds may enable wound healing by suppressing adverse effects of inflammation 44. As we describe above, S100A8/A9 in epithelia may be crucial for protection against bacterial infections, whereas the ability to scavenge free radicals may protect the skin against UVA-mediated oxidative damage 45. Given that S100A8 is an efficient scavenger of ROS 46, 47, induction in murine keratinocytes and release during UVA irradiation may regulate intracellular redox-mediated pathways involved in cell growth and/or differentiation. Therefore, intracellular calprotectin may play critical protective roles in maintaining epithelial barrier functions by supporting repair of damaged tissues, in addition to limiting microbial invasion and enhancing transepithelial migration of leukocytes 48 into infected sites.
Calgranulins and the cytoskeleton
Calgranulins translocate to the plasma membrane in a calcium-dependent manner and interact with cytoskeletal proteins, including tubulins, keratin intermediate filaments and microfilaments in activated monocytes and epithelial cells 49–52. S100A8/A9 microtubule-binding has been shown to alter microtubule polymerization kinetics 53, 54. Binding to cytoskeletal filaments and microtubules may control cell morphology, motility, and organization of the cytoskeleton of epithelial cells during migration and wound healing, and granulocytes during chemotaxis 55. Structurally, calcium-induced S100A8/A9 tetramers appear important for microtubule polymerization and stabilization and may facilitate transendothelial migration of phagocytes 21. In keratinocytes, mutation of calcium binding domains in S100A9 in keratinocytes abrogates calprotectin MT interactions (Champaiboon et al, 2009 unpublished data). S100A12 also binds the cytoskeleton in a calcium-dependent manner 1, 23, 56 although the function implications of this interaction are unknown.
EXTRACELLULAR PROTECTION
Release of calgranulins
Release of hS100A8/S100A9 is suggested to follow cell disruption or death 57. In monocytes, however, hS100A8/S100A9 release depends on PMA induced-protein kinase C activation and requires an intact tubulin network 27. Since, hS100A8/S100A9, but not homodimers, bind AA, binding to plasma membrane lipid could regulate translocation to the surface and release from the cell 36. Some proinflammatory cytokines and bacterial products like lipopolysaccharide (LPS) promote hS100A8/S100A9 release from neutrophils and monocytes 58, 59. During inflammation and infection, hS100A8/S100A9 and S100A12 are released and detected in body fluids such as serum, saliva, sputum and amniotic fluid 60–63. It is assumed that calgranulins in mucosal fluids are released from intact keratinocytes but formal study is needed.
Calgranulins as chemokine-like mediators in response to infection
Microbial or parasitic infections are generally averted or resolved by cellular mechanisms that prevent pathogen invasion. Factors produced by mast cells, neutrophils, keratinocytes, macrophages and dendritic cells (DCs) regulate responses to infection. For example, the calgranulins are released from the cytoplasm of dead and dying neutrophils into abscess fluid 64. In S. aureus-induced abscesses in mice, the high concentrations of extracellular antimicrobial calprotectin depends on the density of the neutrophil population 15 and correlates with loss of neutrophil viability 57. The calgranulins can be released from cells, but are not secreted via the endoplasmic reticular/Golgi-mediated pathway as they lack signal peptides.
Calprotectin release may be signaled by pathways such as activation of PKC by pro-inflammatory stimuli, or elevation of intracellular [Ca2+] by contact with activated endothelium 65. In particular, lipopolysaccharide (LPS) from several bacterial species 58 and C5a 66 cause rapid release of S100A8 and S100A9 from neutrophils and monocytes in an active, tubulin-dependent process 27. Stimulated monocytes and macrophages also release the calgranulins 67 although activated dermal keratinocytes 45 and fibroblasts 68 do not.
Calgranulins and chemotaxis
Among the antimicrobial agents in neutrophils, the cytoplasmic calgranulins include S100A8 and S100A9, which comprise about 45% of the cytosolic proteins 69, and S100A12, which represents about ~5% of the total proteins 70. Neutrophils are rapidly recruited to sites of acute infection in response to bacterial chemoattractants and/or activated complement components such as C5a. Acute response modifiers such as C5a may also trigger degranulation of mast cells, releasing histamine and other preformed mediators such as tumour necrosis factor (TNF) and the neutrophil chemokine IL-8 71. IL-8 rapidly recruits additional neutrophils, whereas microbial products such as LPS activate mast cell cytokine production via TLR ligation 72. Once activated, neutrophils release cytosolic components, degranulate and generate a robust oxidative burst via the NADPH oxidase and myeloperoxidase systems.
Our group made the initial observation that S100 proteins have “chemokine-like” properties; murine (m) S100A8 has potent chemotactic activity for neutrophils and monocytes in vitro and in vivo 73. Injection of mS100A8 promotes leukocyte recruitment 74 with neutrophils appearing at 4–6 h followed by monocytes over 24 hours 75, 76. Given the functional similarities in leukocyte trafficking, we later identified S100A12 as the probable human functional homologue of chemotactic mS100A8, with optimal activity for monocytes at picomolar levels 77, 78, 79. In our hands, human (h) S100A8 has only weak leukocyte chemotactic activity in vitro 74 and in vivo 74. Indeed, it is unclear whether human S100A8 and S100A9 are chemoattractants, although there is 57% amino acid identity with mS100A8, and we questioned whether the proteins were orthologs 74. We showed that human S100A12 is chemotactic 77, 78, although no homologs were encoded in rodent genomes 79. The functional and sequence divergence suggested complex evolution of the S100 family in mammals. Absent in the human S100A8 hinge 10, we identified amino acid residues essential for chemotaxis in the hinge region of S100A12 and conserved in the active mS100A8 hinge region 74. The hinge regions of mS100A8 and S100A12 are structurally similar and electro-negatively charged, whereas this region in hS100A8 is positively charged. Some functional differences in the hS100A8 and mS100A8 reported by several groups 80, 81 may reflect concomitant activity as efficient oxidant scavengers and the oxidation state of the proteins 80. In contrast to classical chemoattractants (eg., fMLP, IL-8), mS100A8, like TGF-β, does not cause Ca2+ flux or changes in integrin or L-selectin expression, yet may mediate initial diapedesis 82. Murine S100A8 and S100A12 may signal through pertussis toxin-sensitive, G-protein coupled receptors 82, 10 and although mS100A8 does not activate mast cells, its chemokine-like characteristics are similar to S100A12.
S100A12 also upregulates expression of adhesion molecules such as CD11b and increases shedding of L-selectin from neutrophils, enhancing adhesion to endothelial cells (ECs) 80. S100A12 may also induce ICAM-1 and VCAM-1 expression on ECs, contributing to the strong adhesion required for extravasation 83. Thus, like other neutrophil-derived proteins such as the defensins 84, 85, 86 and cathepsin G 84, 87, 88, calgranulins are chemotactic and inhibit microbial growth 89.
During inflammation, hS100A8 and hS100A9 may contribute to migration of leukocytes via localization of the calprotectin complex released from transmigrating neutrophils to endothelial cells in vivo 90. Recombinant hS100A8 and hS100A9 increases β2 integrin CD11b expression and affinity on phagocytes 81 80. Adhesion of leukocytes to activated endothelium may depend primarily on hS100A9 in the calprotectin complex since hS100A9 alone facilitates adhesion of neutrophils to fibrinogen 80 and fibronectin 91 and monocytes to the endothelium in vitro 92. Furthermore, mS100A9−/− neutrophils have reduced capacity to migrate through endothelial cell monolayers in vitro in response to chemoattractants such as IL-8 or leukotriene B4 and, in contrast to wild-type cells, IL-8 fails to increase surface expression of CD11b 93. mS100A9−/− neutrophils show reduced Ca2+ transients and chemotaxis in response to certain chemokines when compared to wild-type cells94, suggesting that altered chemotactic activity may reflect dysregulated cytoskeletal dynamics in the absence of mS100A9 53.
In human chronic rhinosinusitis, expression of hS100A8/A9 by the nasal mucosal epithelium appears to correlate with resistance whereas reduced expression is linked to increased susceptibility (reviewed in 95). During the course of Streptococcus pneumoniae infection in murine lung, mS100A8 and mS100A9 are released differentially 89. In the early phase, pneumocytes produce mS100A8 and mS100A9, followed by neutrophil and macrophage migration. Importantly, anti-mS100A8 and anti-mS100A9 antibody blockade strongly inhibited transepithelial leukocyte recruitment to the alveoli, without affecting the transendothelial cell migration or sequestration of neutrophils within the vasculature 89. In this model, antibody blockade had no influence on bacterial load or survival of the mice. In S100A9-null mice, accumulation and the time-course of leukocytes recruited in thioglycollate-induced peritonitis or into the air pouch using LPS 96 were similar, although neutrophil infiltration was reduced into skin wounds 53 and into pancreas in a model of caerulein-induced pancreatitis 48. mS100A9−/− mice are less responsive to LPS stimulation and resistant to LPS-induced septic shock 97. Neutrophils from S100A9−/− mice do not contain mS100A8 protein although they express the mRNA 93, 96. In S100A9−/− cells, however, responses to ATP or platelet activating factor are reduced 98. The S100A9 scaffold for cytosolic phox proteins interacts with membrane-bound cytochrome b and may be important for activation of dormant NADPH oxidase activity 40. When stimulated with phorbol esters, however, the oxidative burst generated by S100A9−/− neutrophils and wild-type cells is similar 96. S100A9 may therefore specifically regulate diacylglycerol signalling in response to some agonists 94, and this may explain why leukocyte influx is disrupted in some in vivo models but appears normal in others. Although studies to date suggest that calgranulins in murine models reflect the proinflammatory effects of the human proteins, the anti-infective mechanisms seen in humans may not be completely represented in mice.
S100A12-dependent mast cell regulation and leukocyte recruitment
Mast cell products such as TNF and histamine are critical in innate and adaptive immunity 71, 99. Our recent studies show that S100A12 is a mast cell chemoattractant in vitro and sequesters these cells when injected intraperitoneally or into the skin 10. S100A12 also stimulates degranulation of murine and human mucosal and skin mast cells 100, which could influence the resolution of infection. Mast-cell-derived TNF is stored in granules and secreted upon degranulation. In mast cell-deficient mice, caecal ligation and puncture is lethal, because of a lack of TNF 101, 102. Mast cell-derived TNF signals the TNF receptor 1 expressed on endothelium, contributing to neutrophil recruitment to sites of inflammation 103 and clearance of bacteria or mycobacteria 104, 105. Histamine triggers immediate increases in vascular permeability, mobilizes E-selectin to the endothelial surface and promotes neutrophil transmigration 106,99, 107, 108. S100A12 rapidly induces mast cell-dependent neutrophil adhesion and extravasation 100, oedema and leukocyte (neutrophil and monocyte) recruitment in mice 78. S100A12 also induced IL-6, IL-8, MCP-1, and MIP-1β production in human cord blood-derived mast cells 100. Hence, mast cells are likely to be a key target of S100A12, generating localized histamine, chemokines, and cytokines (e.g. TNF), activation of the vasculature and sustained leukocyte recruitment.
Extracellular oxidant scavengers
Oxidation is a post-translational modification of calgranulins that may alter crucial functions. To kill invasive microbes, phagocytes produce high levels of intracellular ROS and nitrogen species (RNS). ROS release into the surrounding tissue, however, can cause injury and contribute to inflammation by modifying lipids and proteins, causing oxidative stress 109. Our group has explored the possibility that calgranulins may scavenge ROS within the inflammatory milieu thus reducing oxidative damage to surrounding tissue (reviewed extensively in 110).
The mS100A8 Cys41 residue oxidizes in the presence of Cu2+, low levels of hypochlorite (OCL−) or PMA stimulated neutrophils 46. Methionine residues in the calgranulins can be oxidized to methionine sulfoxides 46. Sulfinamide bonds, covalent links between Cys-thiol groups and the ε-amine group of Lys residues, are also generated. Unlike disulfide bonds, these bonds cannot be reduced by normal cellular reduction systems or the chemical reductant, dithiothreitol (DTT) 47. Murine S100A8 intra- (between Lys34/35 and Cys41) and intermolecular (between Lys6, Lys76, Lys83 Lys87 and Cys41) sulfinamide bonds are generated with as little as 1:1 molar equivalents of HOCl− in vitro 111, 112, or the presence of PMA-stimulated neutrophils 47. Several hundred-fold more sensitive to HOCl− oxidation, S100A8 and to a lesser extent S100A9 appear to oxidize proportionally more effectively than low-density lipoproteins 113 or bovine serum albumin 112. Oxidized monomers and disulfide-linked complexes are detected in vivo in LPS-treated lung lavage fluid from mice 46, and DTT-stable cross-linked complexes (dimeric S100A82, S100A92, S100A8/A9 and S100A8/A92) are found in human atherosclerotic lesions 112.
The redox-sensitive Cys residue in mS100A8 resides immediately before the hinge domain. Chemotactic activity in vitro or in vivo appears unaffected by mutation of Cys to Ala although the H2O2-generated disulfide-bonded dimer lost activity 46. On the other hand, sulfinamide-containing HOCl-oxidized S100A8 monomers (between Cys41 and Lys34/35) remain active, whereas intramolecular sulfinamide-linked mS100A8 oligomers are not chemotactic 47. Thus, oxidative modifications that generate covalent oligomerization of Cys41, which flanks the hinge domain, may mask the chemotactic site of mS100A8 by sterically hindering access to the cellular receptor without directly altering activity. Mild HOCl oxidation would stabilise mS100A8 chemotactic activity and leukocyte recruitment, whereas cross-linking by high levels of oxidants may dampen the response.
In contrast, S100A12 is relatively resistant to covalent modification by oxidants since it has no Cys or Met residues. Since numerous classical chemoattractants are susceptible to oxidative inactivation 46, S100A12 may be important in propagating monocyte mast cell/monocyte recruitment in a ROS-rich environment. We suggest that S100A8 duplication and divergence in humans, compared to rodents, permits dichotomous chemotactic activities. In mice, the abundant S100A8 acts an anti-oxidant and chemotactic agent within the picomolar range, but chemotactic activity is ROS-sensitive 46. In addition to functions attributed to the S100A8/A9 complex, hS100A8 may function as an anti-oxidant, whereas S100A12 may mediate monocyte and mast cell recruitment and mast cell activation 10, 78, 100.
Human S100A8 and hS100A9 may actually show fugetactic (cell repulsion) activity for human neutrophils 114, 115. In S100A9, the fugetactic activity was inhibited by oxidation of Met63 and Met83 115. Clearly, oxidation can alter S100A8 and S100A9 functions and could modulate leukocyte recruitment.
Other functions of the calgranulins can be affected by oxidation. For example, the anti-fungal activity of hS100A8/A9 is abolished when Cys42 of S100A8, or S100A9 Met63 or Met83 (but not S100A9 Met81) are mutated to Ala. Hence, oxidation inhibits anti-fungal activity, suggesting that activity can persist only in conditions of limited oxidative stress 116.
Resolution by S100A8-SNO
By acting as a nitric oxide (NO) shuttle, S100A8 may regulate mast cell activation and modulation of vessel tone 35. NO binds covalently to cysteine residues in proteins. Through S-nitrosylation (the covalent coupling of NO to Cys residues), NO mediates vascular homeostasis and anti-microbial defence, and regulates transcription and apoptosis 117. Few proteins are targets for S-nitrosylation and the specificity requires a local hydrophobic environment with acidic and basic residues proximal to the target Cys to facilitate reduction of the thiol pKa 118, 119. We showed that S100A8 is readily S-nitrosylated on Cys41 35. In the S100A8/A9 complex, S100A8 is preferentially nitrosylated. S100A8-SNO is detected in normal human neutrophils and levels increase in neutrophils treated with NO donors 35. The S100A8-SNO bond is relatively stable enabling transnitrosylation of hemoglobin, for example, thereby shuttling NO that may regulate blood vessel tone 35. Triggered by mast cell activation in the mesenteric circulation of live rats, mast cell degranulation, leukocyte adhesion and transmigration are inhibited by S100A8-SNO 35. As we have shown, microvascular endothelial cells activated with IL-1β or LPS induce S100A8 120. When S-nitrosylated by endothelial cell-derived NO, S100A8-SNO may restore blood flow in congested vessels and maintain patency of neovessels.
During infection, the inflammatory response maximizes the production of anti-microbial ROS by phagocytes. When activated macrophages produce S100A8, microbial killing would appear to be compromised. Although LPS or IFN-γ induces S100A8 in murine macrophages 121, when combined, LPS and IFN-γ induce high amounts of iNOS, but not S100A8 35. Thus, when ROS/RNS generated by macrophages activation is required for microbial killing, S100A8 is not produced. In the extracellular milieu, however, ROS can injure the host and S100A8 may serve as a protective scavenger when generated by the appropriate stimulants.
Extracellular calgranulins as antimicrobial proteins
Cell-free calgranulins may be directly antimicrobial. Pure hS100A8/A9 has broad spectrum antimicrobial activities against microorganisms including Capnocytophaga sputigena 122, 123, Candida albicans 124, Escherichia coli, Staphylococcus aureus, S. epidermis 17, Borrelia burgdorferi 125, and Listeria monocytogenes 43 in vitro.
S100A12 has antimicrobial activity against filarial parasites 126, possibly mediated by direct binding to paramyosin, a target protein on the parasites 11, 12. Calcitermin, homologous to the 15 C-terminal residues of S100A12, has antimicrobial activity against E. coli, Pseudomonas aeruginosa and C. albicans, particularly in the presence of zinc and low pH 127. Hence, the C-terminal region of S100A12 may be crucial for its antimicrobial function.
Since Zn2+ is required for bacterial growth, S100A8/A9 sequestration of zinc is suggested as a mechanism of inhibiting bacterial growth 128. Recently, Mn2+ in tissue abscesses was shown to contribute to S100A8/A9 antimicrobial activity against S. aureus 15, 16. As described above (see STRUCTURAL FEATURES OF S100 CALGRANULINS), the transition metal content within the local environment may modulate the ability of the calgranulins to interact with other protein targets, including bacteria. Thus the antimicrobial potential of the calgranulins must be established within a physiological context.
Mucosal fluids are replete with antimicrobial proteins and peptides and the calgranulins may contribute to limiting growth of commensal organisms and preventing intrusion of pathogens. Calprotectin, for example, is found in human airway secretions 127, gingival crevicular fluid and periodontal pockets 129. Furthermore, calprotectin is prominent in mucosal abscesses in association with candidiasis 130. S100A8, S100A9 and S100A12 are also expressed in inflamed gastric mucosa of H. pylori-infected children, but not normal mucosa 131. Hence, the calgranulins may serve as innate antimicrobial proteins to limit infections at mucosal surfaces.
Extracellular calgranulins and signal transduction
Calprotectin functions in the extracellular milieu, but specific surface receptors and signaling pathways are not fully characterized. S100A8/A9 interacts with heparin and heparan sulfate glycoaminoglycans 90 and carboxylated glycans 132 on endothelial cells in vitro. On endothelial cells, S100A8/A9 also binds to the scavenger receptor FAT/CD36 and may facilitate AA uptake by acting as a fatty acid transporter protein 133. In murine macrophages, mS100A8/A9 may induce inducible NO synthase, and NO production by signalling through the TLR4 pathway 134. In mice, mS100A8 and the mS100A8/A9 complex apparently interacts with TLR4 to promote lethal septic shock 97. RAGE may serve as a common receptor for S100 proteins/calgranulins. Indeed, S100A1, S100A4, S100A7, S100A11, S100A12 (EN-RAGE), S100A13, S100B and S100P are all ligands for RAGE 55, 83, 135–137. S100A8/A9 also binds RAGE and activates the MAP kinase pathway 138–140. Moreover, N-glycans on RAGE may be critical for S100A8/A9 binding 141. Since some cell types may express multiple receptors for S100A8/A9, the downstream signaling pathways are not well understood. In the local environment, RAGE interaction may depend on the availability of calcium and zinc, which are also required for oligomerization of calgranulins 12. Binding to RAGE is proposed to trigger MAP kinase and NF-κB downstream signaling pathways 138, 140, 142, and the complexity of downstream responses may depend on the types of ligands and the cell-type specific repertoire of receptors. Although TLR and RAGE ligation by calgranulins are likely to contribute to activation 143, it is clear that additional receptors are important. For example, mast cell activation by S100A12 is RAGE-independent 100 and not mediated by TLR4 (J. Goyette, unpublished data) and chemotaxis stimulated by mS100A8 and S100A12 is likely mediated by a G-protein-coupled mechanism as discussed above.
CALGRANULIN EXPRESSION AND REGULATION: OF MICE AND MEN
Expression
To optimize protective functions, the calgranulins are either constitutively expressed and tightly regulated, as in the squamous mucosal epithelia, or strictly inducible as in the skin, and intestinal mucosa. How the calgranulins are regulated depends on the type of cell, anatomic locale and environmental stimuli. Murine and human S100A8 and/or S100A9 are constitutively expressed in platelets, osteoclasts and hypertrophic chondrocytes, and trophoblasts in developing embryos. Calprotectin is also expressed in keratinocytes and cells of the myeloid linage, in which expression is correlated with the stage of differentiation 144, 145. Human S100A9 is first apparent during maturation of promyelocytes to myelocytes and maintained in high abundance in mature neutrophils 146. Circulating monocytes constitutively express S100A8 and S100A9 147, 148 and calprotectin expression decreases during extravasation from blood and differentiation in tissues, given that normal macrophages do not express S100A8 and S100A9 149, 150. Expression of S100A12 151 mirrors S100A8 and S100A9, also suggesting an association with myeloid cell differentiation 152. In the following section, we will focus on the regulation of calgranulins in keratinocytes, fibroblasts and macrophages in response to proinflammatory and anti-inflammatory signaling. In an environmental agent-specific manner, calgranulin expression (S100A8, S100A9 and/or S100A12) may be modulated by physiological and traumatic stressors 45, 153,78.
Epithelial cells
Keratinocytes form the outermost layer of epithelial cells in the epidermis and mucosal surfaces and are in continual contact with invasive microorganisms. Alerting neighbouring cells to the presence of the pathogen, epithelial cells recognize microbial endotoxins, including LPS, and produce and release proinflammatory cytokines, including the interleukin-1 (IL-1) family 154. The IL-1 family of cytokines generally contribute to host resistance to pathogens in the oral cavity. The IL-1 family cytokine produced by oral epithelial cells that signals proximal cells is typically IL-1α since oral keratinocytes lack functional caspase-1 and therefore express, but do not produce mature IL-1β 155. Similarly, basally-expressed in human gingival keratinocytes, S100A8 and S100A9 expression can be stimulated by PMA (24 hr), but not by LPS or IL-1β (2–4 hr) 156, strongly suggesting a role for IL-1α, but not IL-1β, in the calprotectin-dependent protection of oral epithelial cells. When epithelial cells are exposed to pathogenic microorganisms, IL-1α, a cytokine produced by keratinocytes, and leukocyte IL-22 appear to induce upregulation of S100A8/A9 and other antimicrobial peptides (AMPs) 157–160. S100A12 upregulation was not reported. IL-1α induces S100A8/S100A9 expression whereas transforming growth factor (TGF-β) reduces expression in human gingival keratinocytes 159. IL-22, a member of the IL-10 family of cytokines, appears to induce expression of several AMPs including human beta-defensin 2 (HBD2), HBD3, S100A7 and S100A8/S100A9 and regulates keratinocyte migration and differentiation 157, 158, 161. Bronchial mucosal epithelial cells increase S100A8/S100A9 expression by 24 hours in response to LPS through TLR4 stimulation 162. In addition, IL-1α and TNFα increase S100A12 expression in bovine keratocytes 126. That oral epithelial cells are unable to increase S100A8/S100A9 expression in response to LPS or IL-1β within 2–4 hr suggests that intracellular S100A8/S100A9 functions as a secondary responder to invasive microbes (discussed later). Epithelial cells, therefore, appear to initiate calprotectin-dependent innate immunity in an autocrine/paracrine manner.
Keratinocytes actively participate in immunological events and upregulation of cytokines such as IL-1, IL-6 and TNF-α reflect inflammatory activation in dermatological disorders 163, 164, resulting in S100A8/S100A9 upregulation in keratinocytes 165. In psoriasis for example, S100A8/S100A9 complexes are highly expressed in abnormally-differentiated keratinocytes 166, and activated human epidermal keratinocytes (HaCaT cells) release the S100A8/S100A9 heterodimer 167. In psoriasis 168 and wound healing 167, hyperproliferative responses appear to be S100A8/S100A9-dependent. Not expressed in normal skin, S100A12 is also present in human keratinocytes in psoriatic lesions. 169.
Epidermal keratinocytes contain high levels of anti-oxidants and enzymes involved in anti-oxidant defense. In addition, high levels of ROS are generated by ionizing radiation, including UVA-induced sunburn. In the tissues, excess ROS can overwhelm normal cellular defenses to oxidants and require increased antioxidant protection. Confirming ROS-mediated induction in a murine UVA irradiation model, mS100A8, but not mS100A9, is induced and released by keratinocytes and may contribute to antioxidant defense 45. Similar to oxidative stress, mice with LPS-induced pulmonary injury released a chemotactically inactive form of S100A8 dimer into lavage fluid reflecting that disulfide-dependent dimerization may be a regulatory mechanism controlling leukocyte recruitment 46. As an efficient scavenger of ROS 46, 47, mS100A8 may regulate intra- and extracellular redox-mediated pathways.
Fibroblasts
Underlying the epithelial and Langerhans cells forming the mucosal surface, the submucosa contains fibroblasts, DCs, macrophage and mononuclear cells. Pathogens subvert the epithelial barrier, either by escaping from intracellular confinement of epithelial cells or through interaction with intra-epithelial dendritic (Langerhans) cells, which may process and present the pathogen to adaptive or other innate immune cells. Fibroblasts, on the other hand, do not appear to be involved in the S100-dependent response to invasive microbes, but are essential for wound healing. FGF-2 and IL-1β, but not LPS, induce mS100A8 expression in murine fibroblasts, whereas TGF-β suppresses mS100A8 expression 68. In non-inflamed skin, S100A8/A9 is not detectable, but increased S100A8 expression in fibroblasts in rat dermal wounds suggests a role in repair/remodelling. During the initiation of wound repair, TGF-β expression is inversely related to the increase in fibroblast S100A8, suggesting its involvement in the fibroblast-directed response to inflammation and wound repair 68.
Macrophages
Increases in S100A8 and S100A9 expression, particularly S100A9, is regulated by the transcription factor CAAT enhancing binding protein (C/EBPα) 170. Pro-inflammatory cytokines and LPS may induce binding of C/EBPα to the promoter region to activate S100A8 and S100A9 gene expression 58. In addition to C/EBPα, the myeloid-related protein regulatory element (MRE)-binding complex A (MbcA) and MRE-binding complex B also regulate expression of S100A9 by binding the S100A9 promoter 146. C/EBPβ is a transcriptional enhancer of the human S100A8 gene, the activity of which is antagonized by the retinoic acid receptor (RAR) in a ligand-dependent manner 171.
Macrophages contribute to host defence, wound healing, and immune regulation. Macrophage polarization into proinflammatory and anti-inflammatory activities occurs in response to encountered mediators in specific environments 172. The preferential production of proinflammatory IL-12 or anti-inflammatory IL-10 is the basis of the M1/M2 polarization paradigm, and M2 polarization is further subdivided into M2a, M2b, and M2c 173–176. The M2c or regulatory macrophage paradigm (as proposed by Mosser and Edwards 176) can be modelled when macrophages are stimulated with IL-10, TGF-β, or glucocorticoids (GCs) resulting in down-regulation of pro-inflammatory cytokines, increased oxidant scavenging capacity, and a pro-healing functional program 176.
Regulatory macrophages normally emerge in response to two stimuli inducing anti-inflammatory activity 176. The first signals include IL-10, immune complexes, prostaglandins, glucocorticoids, and apoptotic cells and generally has little stimulatory function. A second stimulus is needed, normally TLR ligand. Characterized by the requirement of IL-10, the S100A8 induction profile in murine and human macrophages appears consistent with the M2c or regulatory type macrophage. We proposed in this review that unlike constitutively expressed S100A8 in neutrophils, macrophage induction in the late stage of inflammation could modulate the immune response, promoting resolution.
Murine and human macrophages differ in their responses to LPS and IL-10. Unstimulated murine primary macrophages express little detectable S100, but activated macrophages express mS100A8 in the absence of mS100A9 121, 177. Similarly, S100A8, but not S100A9, is upregulated in murine monocyte/macrophage-like cell lines in response to LPS 178. Stability of mS100A8 protein is proposed to be dependent on mS100A9 as mS100A9 −/− mice express S100A8 mRNA, but not protein 93, 96. However mS100A9 is not induced in macrophages by any of these stimuli, although human S100A8 and S100A9 are generally co-induced in monocytes and macrophages 148, 179. mS100A8 mRNA is induced in macrophages by TLR ligands such as LPS 178, dsRNA 179, CpG (K. Hsu, in preparation), TNFα, transforming growth factor β (TGF-β), interferon-γ (IFNγ) 121 and IFNβ 179. In macrophages, therefore, S100A8 induction: occurs late, and mRNA is first observed after 6 h, peaking around 16–24 hours post-stimulation; requires de novo protein synthesis; and depends on anti-inflammatory factors such as IL-10 and/or PGE2.
In contrast to the specific S100A8 response in murine macrophages, S100A8 and S100A9 are both IL-10-dependent response genes in human macrophages; IL-10 alone has little direct effect, but is essential to potentiate these responses 177, 179. Induction of mS100A8 by a dsRNA mimetic or LPS is IL-10 and protein kinase R (PKR) dependent 177, 179 and signaling occurs through the MAP kinase ERK1/2 and p38 pathways 148, 179. When signaled through TLR4 but not TLR3, mS100A8 mRNA induction is also cyclooxygenase 2 (COX-2)-dependent 121, 148. The COX-2 metabolites PGE2 and cAMP also contribute to S100A8 induction by LPS, but not by Poly (I:C). Gene induction is suppressed by IL-4 and IL-13 121, 148. Since S100A8 is not induced in stimulated macrophages from IL-10-null mice, mS100A8 in macrophage is likely expressed in response to local IL-10 during pathogen infection. Our results strongly suggest that S100A8 has a critical role in the anti-inflammatory responses of macrophages.
Consistent with our hypothesis, we found that glucocorticoids (GCs) strongly enhance LPS-mediated induction of S100A8 and S100A9 in human monocytes, mS100A8 in murine macrophages, primary fibroblasts, microvascular endothelial cells, and S100A8-positive macrophage numbers increase in rheumatoid synovium from RA patients treated with high dose GC 68, 148. After intranasal administration of LPS, mS100A8 levels increased in lungs of GC-treated mice 180. GCs also induced S100A8 in human dendritic cells 181 and potentiated induction by IL-17 in a human keratinocyte cell line 182. In wild-type mice, however, GCs suppressed mS100A8 and mS100A9 expression following phorbol ester-induced skin inflammation 183. In contrast, suppression of mS100A8 and mS100A9 was not seen in c-fos−/− mice, suggesting that these are negatively-regulated c-fos/AP-1-target genes 183. Since IL-10 and GC are anti-inflammatory and immunosuppressive (reviewed in 184, 185), this pattern of gene expression indicates that S100A8 may be involved in the resolution phase of inflammation perhaps in an oxidant-scavenging role 110. The anti-inflammatory function proposed for S100A8 is independent of heterocomplex formation with S100A9. Anti-inflammatory S100A8 may represent a paracrine mechanism of control of inflammation when considered in contrast to the pro-inflammatory role recently proposed for S100A8 ligation of TLR4 97. As described above, the structure and function of the calgranulins can be markedly affected by the content of transition metals in different microenvironments; alternative and seemingly opposing functions are possible.
In protection of the host, calgranulins can also be pro-inflammatory mediators in response to infection. Inflammatory stimuli activate resident tissue macrophages to produce inflammatory and effector mediators, the pattern of which is differentially regulated by the microenvironment and stage of inflammation. Early immunohistochemical studies found S100A8/S100A9 in macrophages in human tissues such as rheumatoid arthritis 186. More recently, calgranulins expression and release from activated macrophages are considered as markers of inflammation (reviewed in 1. Monocytes and macrophages activated in vitro express and release calgranulins, including S100A12, in response to proinflammatory ligands, including polyI:C, LPS and TNFα 78, 179. When produced at pico-molar levels, the calgranulins may be important in initiating leukocyte recruitment, such as described for TGF-β67, whereas higher concentrations may contribute to resolution. In differentiated THP-1 macrophages, IL-6 induces S100A12 through the JAK-STAT pathway and de novo protein synthesis is required 67. In contrast, peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists inhibit S100A12 mRNA induction in these cells 67. Ligand-bound PPAR-γ represses transcription of many inflammatory cytokines through interactions with promoter-bound co-regulatory proteins 187. Similarly, and in contrast to S100A8 and S100A9, we found that GC does not upregulate the S100A12 gene in activated human monocytes (K. Hsu, C. Geczy, unpublished data). Although circulating eosinophils do not express S100A12, eosinophils from asthmatic lungs express S100A12 100, indicating that S100A12 is inducible and may contribute to allergic, anti-viral and anti-parasitic responses. The rapid upregulation of S100A12 by pro-inflammatory stimuli, and repression by PPAR-γ agonists is more consistent with a pro-inflammatory role for S100A12, although its zinc-binding function may limit leukocyte recruitment and tissue damage by inhibiting matrix metalloproteinases 26.
CONCLUDING REMARKS
Calprotectin and the calgranulins are remarkable multifunctional proteins dedicated to protecting the intra- and extracellular environments during infection and inflammation. In affected cells and tissues, calgranulin binding of transition metals and oxidation conditions are likely to alter function. The calgranulins, therefore, are proposed to tailor their functions to the microenvironmental conditions and the need to protect the host. For several functions, the mouse may represent a good model of human calgranulin given structural similarities in crucial functional domains and regions. For each function to be studied in vivo, careful consideration must be given to the comparability of the murine and human proteins. We must learn how calprotectin and S100A12 become functionally active proteins in health and when the host is stressed. Consideration must be given to the microenvironmental-specific release mechanisms that translocate calgranulins to the extracellular environment and the bodily fluids and how transitional metal binding and oxidation state cooperatively modulate function intra- and extracellularly. To more fully understand the structure and functions of the calgranulins, we must resolve fully how oxidation state and availability of transition metals affect function in terms of formation of higher order oligomers and receptor binding in significant microenvironments.
Acknowledgements
We are grateful to the National Health & Medical Research Council of Australia for research funding to KH and CLG, NIH/NIDCR R01DE11831 and R01DE08590 to MCH and KFR, T32DE07288 for BSS, and to all members of our groups who have contributed to these studies.
Abbreviations
- RA
rheumatoid arthritis
- TLR
Toll-like receptor
- mS100A8
murine S100A8
- mS100A9
murine S100A9
- TGFβ
transforming growth factor β
- IFN
interferon
- MDSC
myeloid-derived suppressor cells
- EC
endothelial cells
- FGF
fibroblast growth factor
- GC
glucocorticoids
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- C/EBP
CAAT enhancing binding protein
- RAGE
receptor for advanced glycation end-products
- NADPH
nicotinamide adenine dinucleotide phosphate
- MMP
matrix metalloproteinase.
References
- 1.Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344(1–2):37–51. doi: 10.1016/j.cccn.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34(4):357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Berntzen HB, Fagerhol MK. L1, a major granulocyte protein: antigenic properties of its subunits. Scand J Clin Lab Invest. 1988;48(7):647–652. [PubMed] [Google Scholar]
- 4.Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9) Biochim Biophys Acta. 1998;1448(2):200–211. doi: 10.1016/s0167-4889(98)00144-x. [DOI] [PubMed] [Google Scholar]
- 5.Honig B, Nicholls A. Classical electrostatics in biology and chemistry. Science. 1995;268(5214):1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
- 6.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 7.Champaiboon C, Sappington KJ, Guenther BD, Ross KF, Herzberg MC. Calprotectin S100A9 calcium-binding loops I and II are essential for keratinocyte resistance to bacterial invasion. J Biol Chem. 2009;284(11):7078–7090. doi: 10.1074/jbc.M806605200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korndorfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370(5):887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 9.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 10.Yan WX, Armishaw C, Goyette J, Yang Z, Cai H, Alewood P, Geczy CL. Mast cell and monocyte recruitment by S100A12 and its hinge domain. J Biol Chem. 2008;283(19):13035–13043. doi: 10.1074/jbc.M710388200. [DOI] [PubMed] [Google Scholar]
- 11.Moroz OV, Blagova EV, Wilkinson AJ, Wilson KS, Bronstein IB. The crystal structures of human S100A12 in apo form and in complex with zinc: new insights into S100A12 oligomerisation. J Mol Biol. 2009;391(3):536–551. doi: 10.1016/j.jmb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Moroz OV, Burkitt W, Wittkowski H, He W, Ianoul A, Novitskaya V, Xie J, Polyakova O, Lednev IK, Shekhtman A, Derrick PJ, Bjoerk P, Foell D, Bronstein IB. Both Ca2+ and Zn2+ are essential for S100A12 protein oligomerization and function. BMC Biochem. 2009;10:11. doi: 10.1186/1471-2091-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yui S, Mikami M, Tsurumaki K, Yamazaki M. Growth-inhibitory and apoptosis-inducing activities of calprotectin derived from inflammatory exudate cells on normal fibroblasts: regulation by metal ions. J Leukoc Biol. 1997;61(1):50–57. [PubMed] [Google Scholar]
- 14.Yui S, Nakatani Y, Hunter MJ, Chazin WJ, Yamazaki M. Implication of extracellular zinc exclusion by recombinant human calprotectin (MRP8 and MRP14) from target cells in its apoptosis-inducing activity. Mediators Inflamm. 2002;11(3):165–172. doi: 10.1080/09622935020138208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319(5865):962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 16.Russell DG. Staphylococcus and the healing power of pus. Cell Host Microbe. 2008;3(3):115–116. doi: 10.1016/j.chom.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Sohnle PG, Collins-Lech C, Wiessner JH. The zinc-reversible antimicrobial activity of neutrophil lysates and abscess fluid supernatants. J Infect Dis. 1991;164(1):137–142. doi: 10.1093/infdis/164.1.137. [DOI] [PubMed] [Google Scholar]
- 18.Moroz OV, Antson AA, Grist SJ, Maitland NJ, Dodson GG, Wilson KS, Lukanidin E, Bronstein IB. Structure of the human S100A12-copper complex: implications for host-parasite defence. Acta Crystallogr D Biol Crystallogr. 2003;59(Pt 5):859–867. doi: 10.1107/s0907444903004700. [DOI] [PubMed] [Google Scholar]
- 19.Moroz OV, Dodson GG, Wilson KS, Lukanidin E, Bronstein IB. Multiple structural states of S100A12: A key to its functional diversity. Microsc Res Tech. 2003;60(6):581–592. doi: 10.1002/jemt.10300. [DOI] [PubMed] [Google Scholar]
- 20.Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, Sato K, Cheek TR, Gray J, Banfield MJ, Dennison C, Robinson NJ. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature. 2008;455(7216):1138–1142. doi: 10.1038/nature07340. [DOI] [PubMed] [Google Scholar]
- 21.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359(4):961–972. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Strupat K, Rogniaux H, Van Dorsselaer A, Roth J, Vogl T. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J Am Soc Mass Spectrom. 2000;11(9):780–788. doi: 10.1016/S1044-0305(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 23.Vogl T, Roth J, Sorg C, Hillenkamp F, Strupat K. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 1999;10(11):1124–1130. doi: 10.1016/s1044-0305(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 24.Pietzsch J, Hoppmann S. Human S100A12: a novel key player in inflammation? Amino Acids. 2009;36(3):381–389. doi: 10.1007/s00726-008-0097-7. [DOI] [PubMed] [Google Scholar]
- 25.Moroz OV, Antson AA, Murshudov GN, Maitland NJ, Dodson GG, Wilson KS, Skibshoj I, Lukanidin EM, Bronstein IB. The three-dimensional structure of human S100A12. Acta Crystallogr D Biol Crystallogr. 2001;57(Pt 1):20–29. doi: 10.1107/s090744490001458x. [DOI] [PubMed] [Google Scholar]
- 26.Goyette J, Yan WX, Yamen E, Chung YM, Lim SY, Hsu K, Rahimi F, Di Girolamo N, Song C, Jessup W, Kockx M, Bobryshev YV, Freedman SB, Geczy CL. Pleiotropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol. 2009;183(1):593–603. doi: 10.4049/jimmunol.0900373. [DOI] [PubMed] [Google Scholar]
- 27.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272(14):9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 28.Benedyk M, Sopalla C, Nacken W, Bode G, Melkonyan H, Banfi B, Kerkhoff C. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J Invest Dermatol. 2007;127(8):2001–2011. doi: 10.1038/sj.jid.5700820. [DOI] [PubMed] [Google Scholar]
- 29.Murao S, Collart FR, Huberman E. A protein containing the cystic fibrosis antigen is an inhibitor of protein kinases. J Biol Chem. 1989;264(14):8356–8360. [PubMed] [Google Scholar]
- 30.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135(3):871–881. doi: 10.1053/j.gastro.2008.06.032. 881 e1–5. [DOI] [PubMed] [Google Scholar]
- 34.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, Thebault P, Renaudin K, Vanhove B. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180(12):7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 35.Lim SY, Raftery M, Cai H, Hsu K, Yan WX, Hseih HL, Watts RN, Richardson D, Thomas S, Perry M, Geczy CL. S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol. 2008;181(8):5627–5636. doi: 10.4049/jimmunol.181.8.5627. [DOI] [PubMed] [Google Scholar]
- 36.Klempt M, Melkonyan H, Nacken W, Wiesmann D, Holtkemper U, Sorg C. The heterodimer of the Ca2+-binding proteins MRP8 and MRP14 binds to arachidonic acid. FEBS Lett. 1997;408(1):81–84. doi: 10.1016/s0014-5793(97)00394-3. [DOI] [PubMed] [Google Scholar]
- 37.Siegenthaler G, Roulin K, Chatellard-Gruaz D, Hotz R, Saurat JH, Hellman U, Hagens G. A heterocomplex formed by the calcium-binding proteins MRP8 (S100A8) and MRP14 (S100A9) binds unsaturated fatty acids with high affinity. J Biol Chem. 1997;272(14):9371–9377. doi: 10.1074/jbc.272.14.9371. [DOI] [PubMed] [Google Scholar]
- 38.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274(46):32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 39.Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60(6):569–580. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- 40.Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. Faseb J. 2005;19(3):467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- 41.Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect Immun. 2001;69(7):4242–4247. doi: 10.1128/IAI.69.7.4242-4247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression inhibits bacterial binding to mucosal epithelial cells. Infect Immun. 2001;69(6):3692–3696. doi: 10.1128/IAI.69.6.3692-3696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaia AA, Sappington KJ, Nisapakultorn K, Chazin WJ, Dietrich EA, Ross KF, Herzberg MC. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. 2009;2(1):43–53. doi: 10.1038/mi.2008.63. [DOI] [PubMed] [Google Scholar]
- 44.Wu N, Davidson JM. Migration inhibitory factor-related protein (MRP)8 and MRP14 are differentially expressed in free-electron laser and scalpel incisions. Wound Repair Regen. 2004;12(3):327–336. doi: 10.1111/j.1067-1927.2004.012313.x. [DOI] [PubMed] [Google Scholar]
- 45.Grimbaldeston MA, Geczy CL, Tedla N, Finlay-Jones JJ, Hart PH. S100A8 induction in keratinocytes by ultraviolet A irradiation is dependent on reactive oxygen intermediates. J Invest Dermatol. 2003;121(5):1168–1174. doi: 10.1046/j.1523-1747.2003.12561.x. [DOI] [PubMed] [Google Scholar]
- 46.Harrison CA, Raftery MJ, Walsh J, Alewood P, Iismaa SE, Thliveris S, Geczy CL. Oxidation regulates the inflammatory properties of the murine S100 protein S100A8. J Biol Chem. 1999;274(13):8561–8569. doi: 10.1074/jbc.274.13.8561. [DOI] [PubMed] [Google Scholar]
- 47.Raftery MJ, Yang Z, Valenzuela SM, Geczy CL. Novel intra- and inter-molecular sulfinamide bonds in S100A8 produced by hypochlorite oxidation. J Biol Chem. 2001;276(36):33393–33401. doi: 10.1074/jbc.M101566200. [DOI] [PubMed] [Google Scholar]
- 48.Schnekenburger J, Schick V, Kruger B, Manitz MP, Sorg C, Nacken W, Kerkhoff C, Kahlert A, Mayerle J, Domschke W, Lerch MM. The calcium binding protein S100A9 is essential for pancreatic leukocyte infiltration and induces disruption of cell-cell contacts. J Cell Physiol. 2008;216(2):558–567. doi: 10.1002/jcp.21433. [DOI] [PubMed] [Google Scholar]
- 49.Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood. 1993;82(6):1875–1883. [PubMed] [Google Scholar]
- 50.van den Bos C, Roth J, Koch HG, Hartmann M, Sorg C. Phosphorylation of MRP14, an S100 protein expressed during monocytic differentiation, modulates Ca(2+)-dependent translocation from cytoplasm to membranes and cytoskeleton. J Immunol. 1996;156(3):1247–1254. [PubMed] [Google Scholar]
- 51.Goebeler M, Roth J, van den Bos C, Ader G, Sorg C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem J. 1995;309(Pt 2):419–424. doi: 10.1042/bj3090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burwinkel F, Roth J, Goebeler M, Bitter U, Wrocklage V, Vollmer E, Roessner A, Sorg C, Bocker W. Ultrastructural localization of the S-100-like proteins MRP8 and MRP14 in monocytes is calcium-dependent. Histochemistry. 1994;101(2):113–120. doi: 10.1007/BF00269357. [DOI] [PubMed] [Google Scholar]
- 53.Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, Roth J. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104(13):4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 54.Leukert N, Sorg C, Roth J. Molecular basis of the complex formation between the two calcium-binding proteins S100A8 (MRP8) and S100A9 (MRP14) Biol Chem. 2005;386(5):429–434. doi: 10.1515/BC.2005.051. [DOI] [PubMed] [Google Scholar]
- 55.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33(7):637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 56.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81(1):28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 57.Voganatsi A, Panyutich A, Miyasaki KT, Murthy RK. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol. 2001;70(1):130–134. [PubMed] [Google Scholar]
- 58.Kido J, Hayashi N, Kataoka M, Nagata T. Calprotectin expression in human monocytes: induction by porphyromonas gingivalis lipopolysaccharide, tumor necrosis factor-alpha, and interleukin-1beta. J Periodontol. 2005;76(3):437–442. doi: 10.1902/jop.2005.76.3.437. [DOI] [PubMed] [Google Scholar]
- 59.Kido J, Kido R, Suryono, Kataoka M, Fagerhol MK, Nagata T. Calprotectin release from human neutrophils is induced by Porphyromonas gingivalis lipopolysaccharide via the CD-14-Toll-like receptor-nuclear factor kappaB pathway. J Periodontal Res. 2003;38(6):557–563. doi: 10.1034/j.1600-0765.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 60.Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G, Wang E, Bhandari V. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. 2007;61(3):318–324. doi: 10.1203/01.pdr.0000252439.48564.37. [DOI] [PubMed] [Google Scholar]
- 61.Cuida M, Halse AK, Johannessen AC, Tynning T, Jonsson R. Indicators of salivary gland inflammation in primary Sjogren's syndrome. Eur J Oral Sci. 1997;105(3):228–233. doi: 10.1111/j.1600-0722.1997.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 62.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, Blackwell S, Whitty J, Berman S, Redman M, Yoon BH, Sorokin Y. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13(1):2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 63.Hammer HB, Kvien TK, Glennas A, Melby K. A longitudinal study of calprotectin as an inflammatory marker in patients with reactive arthritis. Clin Exp Rheumatol. 1995;13(1):59–64. [PubMed] [Google Scholar]
- 64.Kocher M, Kenny PA, Farram E, Abdul Majid KB, Finlay-Jones JJ, Geczy CL. Functional chemotactic factor CP-10 and MRP-14 are abundant in murine abscesses. Infect Immun. 1996;64(4):1342–1350. doi: 10.1128/iai.64.4.1342-1350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frosch M, Strey A, Vogl T, Wulffraat NM, Kuis W, Sunderkotter C, Harms E, Sorg C, Roth J. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43(3):628–637. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 66.Hetland G, Talgo GJ, Fagerhol MK. Chemotaxins C5a and fMLP induce release of calprotectin (leucocyte L1 protein) from polymorphonuclear cells in vitro. Mol Pathol. 1998;51(3):143–148. doi: 10.1136/mp.51.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasegawa T, Kosaki A, Kimura T, Matsubara H, Mori Y, Okigaki M, Masaki H, Toyoda N, Inoue-Shibata M, Kimura Y, Nishikawa M, Iwasaka T. The regulation of EN-RAGE (S100A12) gene expression in human THP-1 macrophages. Atherosclerosis. 2003;171(2):211–218. doi: 10.1016/j.atherosclerosis.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 68.Rahimi F, Hsu K, Endoh Y, Geczy CL. FGF-2, IL-1beta and TGF-beta regulate fibroblast expression of S100A8. Febs J. 2005;272(11):2811–2827. doi: 10.1111/j.1742-4658.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- 69.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266(12):7706–7713. [PubMed] [Google Scholar]
- 70.Guignard F, Mauel J, Markert M. Identification and characterization of a novel human neutrophil protein related to the S100 family. Biochem J. 1995;309(Pt 2):395–401. doi: 10.1042/bj3090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 72.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 73.Lackmann M, Cornish CJ, Simpson RJ, Moritz RL, Geczy CL. Purification and structural analysis of a murine chemotactic cytokine (CP-10) with sequence homology to S100 proteins. J Biol Chem. 1992;267(11):7499–7504. [PubMed] [Google Scholar]
- 74.Lackmann M, Rajasekariah P, Iismaa SE, Jones G, Cornish CJ, Hu S, Simpson RJ, Moritz RL, Geczy CL. Identification of a chemotactic domain of the pro-inflammatory S100 protein CP-10. J Immunol. 1993;150(7):2981–2991. [PubMed] [Google Scholar]
- 75.Devery JM, King NJ, Geczy CL. Acute inflammatory activity of the S100 protein CP-10. Activation of neutrophils in vivo and in vitro. J Immunol. 1994;152(4):1888–1897. [PubMed] [Google Scholar]
- 76.Lau W, Devery JM, Geczy CL. A chemotactic S100 peptide enhances scavenger receptor and Mac-1 expression and cholesteryl ester accumulation in murine peritoneal macrophages in vivo. J Clin Invest. 1995;95(5):1957–1965. doi: 10.1172/JCI117879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miranda LP, Tao T, Jones A, Chernushevich I, Standing KG, Geczy CL, Alewood PF. Total chemical synthesis and chemotactic activity of human S100A12 (EN-RAGE) FEBS Lett. 2001;488(1–2):85–90. doi: 10.1016/s0014-5793(00)02392-9. [DOI] [PubMed] [Google Scholar]
- 78.Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69(6):986–994. [PubMed] [Google Scholar]
- 79.Ravasi T, Hsu K, Goyette J, Schroder K, Yang Z, Rahimi F, Miranda LP, Alewood PF, Hume DA, Geczy C. Probing the S100 protein family through genomic and functional analysis. Genomics. 2004;84(1):10–22. doi: 10.1016/j.ygeno.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170(6):3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 81.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J Immunol. 1998;160(3):1427–1435. [PubMed] [Google Scholar]
- 82.Cornish CJ, Devery JM, Poronnik P, Lackmann M, Cook DI, Geczy CL. S100 protein CP-10 stimulates myeloid cell chemotaxis without activation. J Cell Physiol. 1996;166(2):427–437. doi: 10.1002/(SICI)1097-4652(199602)166:2<427::AID-JCP21>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 83.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 84.Chertov O, Yang D, Howard OM, Oppenheim JJ. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev. 2000;177:68–78. doi: 10.1034/j.1600-065x.2000.17702.x. [DOI] [PubMed] [Google Scholar]
- 85.Mahida YR, Cunliffe RN. Defensins and mucosal protection. Novartis Found Symp. 2004;263:71–77. doi: 10.1002/0470090480.ch6. discussion 77–84, 211-8. [DOI] [PubMed] [Google Scholar]
- 86.Grigat J, Soruri A, Forssmann U, Riggert J, Zwirner J. Chemoattraction of macrophages, Tlymphocytes, and mast cells is evolutionarily conserved within the human alpha-defensin family. J Immunol. 2007;179(6):3958–3965. doi: 10.4049/jimmunol.179.6.3958. [DOI] [PubMed] [Google Scholar]
- 87.Meyer-Hoffert U. Neutrophil-derived serine proteases modulate innate immune responses. Front Biosci. 2009;14:3409–3418. doi: 10.2741/3462. [DOI] [PubMed] [Google Scholar]
- 88.Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90(2):227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Raquil MA, Anceriz N, Rouleau P, Tessier PA. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J Immunol. 2008;180(5):3366–3374. doi: 10.4049/jimmunol.180.5.3366. [DOI] [PubMed] [Google Scholar]
- 90.Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem. 2002;277(5):3658–3665. doi: 10.1074/jbc.M102950200. [DOI] [PubMed] [Google Scholar]
- 91.Anceriz N, Vandal K, Tessier PA. S100A9 mediates neutrophil adhesion to fibronectin through activation of beta2 integrins. Biochem Biophys Res Commun. 2007;354(1):84–89. doi: 10.1016/j.bbrc.2006.12.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eue I, Pietz B, Storck J, Klempt M, Sorg C. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 2000;12(11):1593–1604. doi: 10.1093/intimm/12.11.1593. [DOI] [PubMed] [Google Scholar]
- 93.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, Nacken W. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23(3):1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McNeill E, Conway SJ, Roderick HL, Bootman MD, Hogg N. Defective chemoattractant-induced calcium signalling in S100A9 null neutrophils. Cell Calcium. 2007;41(2):107–121. doi: 10.1016/j.ceca.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124(1):37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hobbs JA, May R, Tanousis K, McNeill E, Mathies M, Gebhardt C, Henderson R, Robinson MJ, Hogg N. Myeloid cell function in MRP-14 (S100A9) null mice. Mol Cell Biol. 2003;23(7):2564–2576. doi: 10.1128/MCB.23.7.2564-2576.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 98.Nacken W, Mooren FC, Manitz MP, Bode G, Sorg C, Kerkhoff C. S100A9 deficiency alters adenosine-5'-triphosphate induced calcium signalling but does not generally interfere with calcium and zinc homeostasis in murine neutrophils. Int J Biochem Cell Biol. 2005;37(6):1241–1253. doi: 10.1016/j.biocel.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7(2):93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 100.Yang Z, Yan WX, Cai H, Tedla N, Armishaw C, Di Girolamo N, Wang HW, Hampartzoumian T, Simpson JL, Gibson PG, Hunt J, Hart P, Hughes JM, Perry MA, Alewood PF, Geczy CL. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol. 2007;119(1):106–114. doi: 10.1016/j.jaci.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 101.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381(6577):75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 102.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8(6):478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kneilling M, Mailhammer R, Hultner L, Schonberger T, Fuchs K, Schaller M, Bukala D, Massberg S, Sander CA, Braumuller H, Eichner M, Maier KL, Hallmann R, Pichler BJ, Haubner R, Gawaz M, Pfeffer K, Biedermann T, Rocken M. Direct crosstalk between mast cell-TNF and TNFR1-expressing endothelia mediates local tissue inflammation. Blood. 2009 doi: 10.1182/blood-2008-11-187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 105.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 106.Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. J Leukoc Biol. 1999;65(3):299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- 107.Rattmann YD, Pereira CR, Cury Y, Gremski W, Marques MC, da Silva-Santos JE. Vascular permeability and vasodilation induced by the Loxosceles intermedia venom in rats: involvement of mast cell degranulation, histamine and 5-HT receptors. Toxicon. 2008;51(3):363–372. doi: 10.1016/j.toxicon.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 108.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9(6):263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 109.Halliwell B. Phagocyte-derived reactive species: salvation or suicide? Trends Biochem Sci. 2006;31(9):509–515. doi: 10.1016/j.tibs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Lim SY, Raftery MJ, Goyette J, Hsu K, Geczy CL. Oxidative modifications of S100 proteins: functional regulation by redox. J Leukoc Biol. 2009 doi: 10.1189/jlb.1008608. [DOI] [PubMed] [Google Scholar]
- 111.Raftery MJ, Geczy CL. Electrospray low energy CID and MALDI PSD fragmentations of protonated sulfinamide cross-linked peptides. J Am Soc Mass Spectrom. 2002;13(6):709–718. doi: 10.1016/S1044-0305(02)00359-8. [DOI] [PubMed] [Google Scholar]
- 112.McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280(50):41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 113.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97(6):1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. S100A8 triggers oxidation-sensitive repulsion of neutrophils. J Dent Res. 2006;85(9):829–833. doi: 10.1177/154405910608500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81(3):818–824. doi: 10.1189/jlb.0706433. [DOI] [PubMed] [Google Scholar]
- 116.Sroussi HY, Kohler GA, Agabian N, Villines D, Palefsky JM. Substitution of methionine 63 or 83 in S100A9 and cysteine 42 in S100A8 abrogate the antifungal activities of S100A8/A9: potential role for oxidative regulation. FEMS Immunol Med Microbiol. 2009;55(1):55–61. doi: 10.1111/j.1574-695X.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3(2):193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 118.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 119.Perez-Mato I, Castro C, Ruiz FA, Corrales FJ, Mato JM. Methionine adenosyltransferase S-nitrosylation is regulated by the basic and acidic amino acids surrounding the target thiol. J Biol Chem. 1999;274(24):17075–17079. doi: 10.1074/jbc.274.24.17075. [DOI] [PubMed] [Google Scholar]
- 120.Yen T, Harrison CA, Devery JM, Leong S, Iismaa SE, Yoshimura T, Geczy CL. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood. 1997;90(12):4812–4821. [PubMed] [Google Scholar]
- 121.Xu K, Geczy CL. IFN-gamma and TNF regulate macrophage expression of the chemotactic S100 protein S100A8. J Immunol. 2000;164(9):4916–4923. doi: 10.4049/jimmunol.164.9.4916. [DOI] [PubMed] [Google Scholar]
- 122.Miyasaki KT, Bodeau AL, Murthy AR, Lehrer RI. In vitro antimicrobial activity of the human neutrophil cytosolic S-100 protein complex, calprotectin, against Capnocytophaga sputigena. J Dent Res. 1993;72(2):517–523. doi: 10.1177/00220345930720020801. [DOI] [PubMed] [Google Scholar]
- 123.Miyasaki KT, Bodeau AL, Pohl J, Shafer WM. Bactericidal activities of synthetic human leukocyte cathepsin G-derived antibiotic peptides and congeners against Actinobacillus actinomycetemcomitans and Capnocytophaga sputigena. Antimicrob Agents Chemother. 1993;37(12):2710–2715. doi: 10.1128/aac.37.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murthy AR, Lehrer RI, Harwig SS, Miyasaki KT. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151(11):6291–6301. [PubMed] [Google Scholar]
- 125.Lusitani D, Malawista SE, Montgomery RR. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect Immun. 2003;71(8):4711–4716. doi: 10.1128/IAI.71.8.4711-4716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gottsch JD, Li Q, Ashraf F, O'Brien TP, Stark WJ, Liu SH. Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol. 1999;91(1):34–40. doi: 10.1006/clim.1998.4681. [DOI] [PubMed] [Google Scholar]
- 127.Cole AM, Kim YH, Tahk S, Hong T, Weis P, Waring AJ, Ganz T. Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett. 2001;504(1–2):5–10. doi: 10.1016/s0014-5793(01)02731-4. [DOI] [PubMed] [Google Scholar]
- 128.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182(4):1272–1275. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- 129.Kido J, Nakamura T, Kido R, Ohishi K, Yamauchi N, Kataoka M, Nagata T. Calprotectin in gingival crevicular fluid correlates with clinical and biochemical markers of periodontal disease. J Clin Periodontol. 1999;26(10):653–657. doi: 10.1034/j.1600-051x.1999.261004.x. [DOI] [PubMed] [Google Scholar]
- 130.Eversole LR, Miyasaki KT, Christensen RE. Keratinocyte expression of calprotectin in oral inflammatory mucosal diseases. J Oral Pathol Med. 1993;22(7):303–307. doi: 10.1111/j.1600-0714.1993.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 131.Leach ST, Mitchell HM, Geczy CL, Sherman PM, Day AS. S100 calgranulin proteins S100A8, S100A9 and S100A12 are expressed in the inflamed gastric mucosa of Helicobacter pylori-infected children. Can J Gastroenterol. 2008;22(5):461–464. doi: 10.1155/2008/308942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Srikrishna G, Panneerselvam K, Westphal V, Abraham V, Varki A, Freeze HH. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol. 2001;166(7):4678–4688. doi: 10.4049/jimmunol.166.7.4678. [DOI] [PubMed] [Google Scholar]
- 133.Kerkhoff C, Sorg C, Tandon NN, Nacken W. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry. 2001;40(1):241–248. doi: 10.1021/bi001791k. [DOI] [PubMed] [Google Scholar]
- 134.Pouliot P, Plante I, Raquil MA, Tessier PA, Olivier M. Myeloid-related proteins rapidly modulate macrophage nitric oxide production during innate immune response. J Immunol. 2008;181(5):3595–3601. doi: 10.4049/jimmunol.181.5.3595. [DOI] [PubMed] [Google Scholar]
- 135.Arumugam T, Simeone DM, Van Golen K, Logsdon CD. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res. 2005;11(15):5356–5364. doi: 10.1158/1078-0432.CCR-05-0092. [DOI] [PubMed] [Google Scholar]
- 136.Wolf R, Howard OM, Dong HF, Voscopoulos C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B, Chavakis T, Oppenheim JJ, Yuspa SH. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol. 2008;181(2):1499–1506. doi: 10.4049/jimmunol.181.2.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hsieh HL, Schafer BW, Weigle B, Heizmann CW. S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun. 2004;316(3):949–959. doi: 10.1016/j.bbrc.2004.02.135. [DOI] [PubMed] [Google Scholar]
- 138.Ghavami S, Rashedi I, Dattilo BM, Eshraghi M, Chazin WJ, Hashemi M, Wesselborg S, Kerkhoff C, Los M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol. 2008;83(6):1484–1492. doi: 10.1189/jlb.0607397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res. 2008;102(10):1239–1246. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 140.Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312(2):184–197. doi: 10.1016/j.yexcr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 141.Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, Varki N, Kronenberg M, Freeze HH, Srikrishna G. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29(10):2035–2043. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009 doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 144.Koike T, Harada N, Yoshida T, Morikawa M. Regulation of myeloid-specific calcium binding protein synthesis by cytosolic protein kinase C. J Biochem. 1992;112(5):624–630. doi: 10.1093/oxfordjournals.jbchem.a123950. [DOI] [PubMed] [Google Scholar]
- 145.Sellmayer A, Krane SM, Ouellette AJ, Bonventre JV. 1 alpha,25-(OH)2 vitamin D3 enhances expression of the genes encoding Ca(2+)-binding proteins MRP-8 and MRP-14. Am J Physiol. 1992;262(1 Pt 1):C235–C242. doi: 10.1152/ajpcell.1992.262.1.C235. [DOI] [PubMed] [Google Scholar]
- 146.Kerkhoff C, Hofmann HA, Vormoor J, Melkonyan H, Roth J, Sorg C, Klempt M. Binding of two nuclear complexes to a novel regulatory element within the human S100A9 promoter drives the S100A9 gene expression. J Biol Chem. 2002;277(44):41879–41887. doi: 10.1074/jbc.M207990200. [DOI] [PubMed] [Google Scholar]
- 147.Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993;53(2):197–204. [PubMed] [Google Scholar]
- 148.Hsu K, Passey RJ, Endoh Y, Rahimi F, Youssef P, Yen T, Geczy CL. Regulation of S100A8 by glucocorticoids. J Immunol. 2005;174(4):2318–2326. doi: 10.4049/jimmunol.174.4.2318. [DOI] [PubMed] [Google Scholar]
- 149.Lagasse E, Clerc RG. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol. 1988;8(6):2402–2410. doi: 10.1128/mcb.8.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zwadlo G, Bruggen J, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988;72(3):510–515. [PMC free article] [PubMed] [Google Scholar]
- 151.Kerkhoff C, Vogl T, Nacken W, Sopalla C, Sorg C. Zinc binding reverses the calcium-induced arachidonic acid-binding capacity of the S100A8/A9 protein complex. FEBS Lett. 1999;460(1):134–138. doi: 10.1016/s0014-5793(99)01322-8. [DOI] [PubMed] [Google Scholar]
- 152.Robinson MJ, Hogg N. A comparison of human S100A12 with MRP-14 (S100A9) Biochem Biophys Res Commun. 2000;275(3):865–870. doi: 10.1006/bbrc.2000.3407. [DOI] [PubMed] [Google Scholar]
- 153.Suryono, Kido J, Hayashi N, Kataoka M, Shinohara Y, Nagata T. Norepinephrine stimulates calprotectin expression in human monocytic cells. J Periodontal Res. 2006;41(3):159–164. doi: 10.1111/j.1600-0765.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 154.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193(10):1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 155.Mizutani H, Black R, Kupper TS. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J Clin Invest. 1991;87(3):1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ross KF, Herzberg MC. Calprotectin expression by gingival epithelial cells. Infect Immun. 2001;69(5):3248–3254. doi: 10.1128/IAI.69.5.3248-3254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36(5):1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 158.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21(2):241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 159.Hayashi N, Kido J, Kido R, Wada C, Kataoka M, Shinohara Y, Nagata T. Regulation of calprotectin expression by interleukin-1alpha and transforming growth factor-beta in human gingival keratinocytes. J Periodontal Res. 2007;42(1):1–7. doi: 10.1111/j.1600-0765.2005.00857.x. [DOI] [PubMed] [Google Scholar]
- 160.Bando M, Hiroshima Y, Kataoka M, Shinohara Y, Herzberg MC, Ross KF, Nagata T, Kido J. Interleukin-1alpha regulates antimicrobial peptide expression in human keratinocytes. Immunol Cell Biol. 2007;85(7):532–537. doi: 10.1038/sj.icb.7100078. [DOI] [PubMed] [Google Scholar]
- 161.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174(6):3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 162.Henke MO, Renner A, Rubin BK, Gyves JI, Lorenz E, Koo JS. Up-regulation of S100A8 and S100A9 protein in bronchial epithelial cells by lipopolysaccharide. Exp Lung Res. 2006;32(8):331–347. doi: 10.1080/01902140600959580. [DOI] [PubMed] [Google Scholar]
- 163.Kelly SE, Jones DB, Fleming S. Calgranulin expression in inflammatory dermatoses. J Pathol. 1989;159(1):17–21. doi: 10.1002/path.1711590107. [DOI] [PubMed] [Google Scholar]
- 164.Nickoloff BJ, Xin H, Nestle FO, Qin JZ. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25(6):568–573. doi: 10.1016/j.clindermatol.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 165.Brandtzaeg P, Dale I, Fagerhol MK. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. II. Normal and aberrant occurrence in various epithelia. Am J Clin Pathol. 1987;87(6):700–707. doi: 10.1093/ajcp/87.6.700. [DOI] [PubMed] [Google Scholar]
- 166.Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99(3):299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- 167.Thorey IS, Roth J, Regenbogen J, Halle JP, Bittner M, Vogl T, Kaesler S, Bugnon P, Reitmaier B, Durka S, Graf A, Wockner M, Rieger N, Konstantinow A, Wolf E, Goppelt A, Werner S. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem. 2001;276(38):35818–35825. doi: 10.1074/jbc.M104871200. [DOI] [PubMed] [Google Scholar]
- 168.Gabrielsen TO, Dale I, Brandtzaeg P, Hoel PS, Fagerhol MK, Larsen TE, Thune PO. Epidermal and dermal distribution of a myelomonocytic antigen (L1) shared by epithelial cells in various inflammatory skin diseases. J Am Acad Dermatol. 1986;15(2 Pt 1):173–179. doi: 10.1016/s0190-9622(86)70152-7. [DOI] [PubMed] [Google Scholar]
- 169.Mirmohammadsadegh A, Tschakarjan E, Ljoljic A, Bohner K, Michel G, Ruzicka T, Goos M, Hengge UR. Calgranulin C is overexpressed in lesional psoriasis. J Invest Dermatol. 2000;114(6):1207–1208. doi: 10.1046/j.1523-1747.2000.00005-2.x. [DOI] [PubMed] [Google Scholar]
- 170.Klempt M, Melkonyan H, Hofmann HA, Eue I, Sorg C. The transcription factors c-myb and C/EBP alpha regulate the monocytic/myeloic gene MRP14. Immunobiology. 1998;199(1):148–151. doi: 10.1016/s0171-2985(98)80070-3. [DOI] [PubMed] [Google Scholar]
- 171.DiSepio D, Malhotra M, Chandraratna RA, Nagpal S. Retinoic acid receptor-nuclear factor-interleukin 6 antagonism. A novel mechanism of retinoid-dependent inhibition of a keratinocyte hyperproliferative differentiation marker. J Biol Chem. 1997;272(41):25555–25559. doi: 10.1074/jbc.272.41.25555. [DOI] [PubMed] [Google Scholar]
- 172.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10(2):137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 173.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 174.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 175.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 176.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu K, Yen T, Geczy CL. IL-10 up-regulates macrophage expression of the S100 protein S100A8. J Immunol. 2001;166(10):6358–6366. doi: 10.4049/jimmunol.166.10.6358. [DOI] [PubMed] [Google Scholar]
- 178.Hu SP, Harrison C, Xu K, Cornish CJ, Geczy CL. Induction of the chemotactic S100 protein, CP-10, in monocyte/macrophages by lipopolysaccharide. Blood. 1996;87(9):3919–3928. [PubMed] [Google Scholar]
- 179.Endoh Y, Chung YM, Clark IA, Geczy CL, Hsu K. IL-10-dependent S100A8 gene induction in monocytes/macrophages by double-stranded RNA. J Immunol. 2009;182(4):2258–2268. doi: 10.4049/jimmunol.0802683. [DOI] [PubMed] [Google Scholar]
- 180.Bozinovski S, Cross M, Vlahos R, Jones JE, Hsuu K, Tessier PA, Reynolds EC, Hume DA, Hamilton JA, Geczy CL, Anderson GP. S100A8 chemotactic protein is abundantly increased, but only a minor contributor to LPS-induced steroid resistant neutrophilic lung inflammation in vivo. J Proteome Res. 2005;4(1):136–145. doi: 10.1021/pr049829t. [DOI] [PubMed] [Google Scholar]
- 181.Kumar A, Steinkasserer A, Berchtold S. Interleukin-10 influences the expression of MRP8 and MRP14 in human dendritic cells. Int Arch Allergy Immunol. 2003;132(1):40–47. doi: 10.1159/000073263. [DOI] [PubMed] [Google Scholar]
- 182.Bai B, Yamamoto K, Sato H, Sugiura H, Tanaka T. Complex regulation of S100A8 by IL-17, dexamethasone, IL-4 and IL-13 in HaCat cells (human keratinocyte cell line) J Dermatol Sci. 2007;47(3):259–262. doi: 10.1016/j.jdermsci.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 183.Gebhardt C, Breitenbach U, Tuckermann JP, Dittrich BT, Richter KH, Angel P. Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene. 2002;21(27):4266–4276. doi: 10.1038/sj.onc.1205521. [DOI] [PubMed] [Google Scholar]
- 184.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 185.Wilckens T. Glucocorticoids and immune function: physiological relevance and pathogenic potential of hormonal dysfunction. Trends Pharmacol Sci. 1995;16(6):193–197. doi: 10.1016/s0165-6147(00)89021-5. [DOI] [PubMed] [Google Scholar]
- 186.Odink K, Cerletti N, Bruggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330(6143):80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 187.Pascual G, Glass CK. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab. 2006;17(8):321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]