Abstract
The present functional magnetic resonance imaging study investigated the neural correlates of practice-associated activation changes in patients with schizophrenia and their association with symptom severity. A group of patients (n = 24) were divided into more successful and less successful learners and were asked to perform a verbal overlearning task in the scanner. We found that both patient groups profited from practice, showing significant decreases in mean response times as well as significant learning-related decreases in cerebral activation. Direct comparison between groups yielded a relative hyperactivation in the group of the less successful learners at the beginning of practice, which showed a reduction with increasing practice. This was reflected by relatively stronger signal decreases in a predominantly fronto-parieto-cerebellar network. In the group of less successful learners, there was a negative correlation between general symptom scores and learning-related signal decreases in a task-relevant network involving cerebellar, inferior and middle frontal (BA 45/47, 46), superior parietal (BA 31), and superior temporal (BA 39) regions. Present data indicate that hyperactivity under high task demands might serve to identify those patients with less potential to profit from practice. However, at least in the context of moderate– to low–working memory demands, this activation abnormality seems to constitute a state rather than a trait characteristic, which patients manage to reduce by successful short-term learning. The findings also suggest that successful learners can better compensate potentially interfering effects exerted by disorder-related psychopathology.
Keywords: overlearning, psychopathology, working memory
Introduction
Among the cognitive impairments going along with the disorder of schizophrenia, working memory (WM) deficits belong to the most prevalent. They have been shown to be strongly predictive of long-term outcomes in most patients and to be largely inapproachable by antipsychotic treatment.1 A deeper understanding of the mechanisms underlying these deficits and their most relevant influencing factors is therefore of major clinical importance. Surprisingly, the possibilities to positively modify these impairments by practice have barely been investigated. In healthy subjects, practice has been shown to exert significant effects both on performance and on underlying cerebral activation. Thus, practice by repeated presentation of verbal or nonverbal stimulus material has been demonstrated to be associated with performance improvements and activation decreases in task-relevant brain areas.2–4 These decreases have been interpreted in light of a reduced recruitment of task-relevant regions due to decreasing demands on cognitive processes like performance monitoring or maintenance effort going along with an increasingly automated processing. First evidence from studies with schizophrenia patients indicates that the potential to profit from short-term practice under stable learning conditions (ie, when the same stimulus material is repeatedly being processed) is largely preserved. van Raalten and colleagues5 found that, although the patients’ learning ability was impaired when frequent information updating was required, their capability to profit from practice under stable learning conditions was unimpaired. Here, patients showed significant performance improvements and significantly reduced WM activity with increasingly successful processing. A recent study of our own group6 yielded similar results. In this study, practice of WM retrieval on the basis of repeated presentation of (the same) verbal stimulus material led to significant performance improvements and exponential signal decreases in a task-relevant network in healthy volunteers and patients with schizophrenia. Patients showed significantly stronger signal decreases in the dorsal part of the cingulate as well as superior frontal and parietal regions, which are known to be critically involved in processes like executive control and short-term maintenance.7 It is important to note that in this preceding study not all patients showed the same capability to profit from practice. Due to small sample size, we were unable to examine potential reasons or factors influencing these interindividual differences in short-term learning abilities within the patient sample.
There is strong reason to assume that symptom severity plays a major role in this context. A great number of studies investigated the effect of symptomatology on different cognitive processes necessary for successful short-term learning like attention, short-term maintenance, and executive processing. Some of these studies found no relation8; some reported a negative relation between severity of negative symptoms and visuospatial WM performance,9 executive processing,9 attention,10,11 or verbal memory10; some revealed a negative correlation between severity of positive symptoms and recognition memory,12 verbal fluency,12 or psychomotor speed10; and even others reported a negative correlation between negative and disorganized symptom severity and sustained attention, WM, and psychomotor speed.13 Thus, most of those studies that post hoc investigated a potential relation between psychopathology and cognition suggest that the psychopathological status influences different cognitive domains and their neural correlates.14,15
As none of these studies explored the relation between psychopathology and practice-related changes in performance and neural activity, the present study had 2 major aims. First, to more closely investigate the neural dynamics underlying more and less successful WM learning in patients with schizophrenia, irrespective of psychopathological status. Second, to specifically examine the potential influence of psychopathological status on learning-related temporal dynamics in neural activation.
We applied an already published Sternberg task6 demanding repeated learning of the same stimulus material and divided the patient sample into more and less successful learners. Based on our previous study, we expected the less successful learners to show stronger learning-related signal decreases in a task-relevant network. We moreover anticipated symptom severity to critically impair less successful learners’ capability to equally profit from practice and show associated decreases in recruitment of neural WM resources.
Methods
Participants
Participants were 24 (16 male and 8 female) right-handed patients with the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition; DSM-IV) diagnosis of schizophrenia. They were all inpatients recruited from the Department of Psychiatry and Psychotherapy, University of Jena. All patients were in remission from an acute psychotic episode. Diagnosis was established using a symptom checklist on the basis of DSM-IV criteria (abbreviated Structured Clinical Interview for DSM) and confirmed by 2 clinical psychiatrists (R.G.M.S. and C.S.). Patients with comorbid axis I disorder were not included in the study.
The patients were 27.6 ± 7.3 (mean ± SD) years old and had an education of 11.7 ± 1.8 years. Psychopathological status of the patients was assessed by the Positive and Negative Syndrome Scale (PANSS).16 Ratings were 16.3 ± 6.7 on the Positive Symptom Scale, 17.8 ± 7.1 on the Negative Symptom Scale, and 35.9 ± 10.0 on the General Psychopathology Scale. The protocol was approved by the local ethical committee, and all participants gave written informed consent prior to the study. Post hoc, we compared both patient groups with an age- and gender-matched healthy control group (for details regarding sociodemographic and performance data, refer to the online supplementary material [Supplement 1]).
Task Design
Using the presentation software package (Neurobehavioral Systems Inc, San Francisco, CA), participants were presented a modified delayed match-to-sample task.
Four pairs of consonants (target sets) were simultaneously presented on the screen for 5340 ms (encoding phase). This was immediately followed by a retrieval phase, which started with the presentation of an asterisk in the center of the screen. After 500 ms, the asterisk was replaced by a pair of 2 consonants simultaneously presented for 1670 ms. Subjects were instructed to memorize the target set and to subsequently decide as quickly as possible for each presented pair of consonants whether it belonged to the target set or not. Ten consonant pairs were displayed within these blocks, each preceded by an asterisk for 500 ms, yielding a total retrieval block length of 21 700 ms. This duration guaranteed optimal sensitivity and maximal orthogonality between the single regressors (ie, encoding and retrieval).
Each block was followed by a 10 000-ms break during which participants were instructed not to memorize the target set. This was controlled by a short debriefing after the measurement. The whole design consisted of 3 different target set sessions comprising 15 blocks each across which the target sets stayed constant. Thus, all examined learning effects relate to the change in behavioral performance or blood oxygen level dependent (BOLD) signal across sessions, ie, the signal change across the 15 retrieval blocks (each consisting of 10 consonant pairs) averaged across the 3 sessions. All participants performed a practice task with different stimuli 1 day before measurement. Hereby, task-specific skill learning effects should be controlled.
Data Acquisition
Imaging was performed on a 1.5-T Magnetom Vision whole-body system (Siemens Medical Solutions, Erlangen, Germany) equipped with a fast gradient system for echo-planar imaging. A custom head holder was used to restrict movement. T2*-weighted images sensitive to BOLD contrast were obtained using a gradient-echo echo-planar sequence (repetition time [TR] = 2700 ms, echo time [TE] = 60 ms, flip angle = 90°, and gap 0.5 mm) with 24 contiguous transverse slices of 5 mm thickness. Matrix size was 64 × 64 pixels with an in-plane resolution of 3.75 × 3.75 mm corresponding to a field of view of 220 mm. Additionally, high-resolution anatomical T1-weighted volume scans with isotropic voxel resolution were obtained in sagittal orientation (TR = 15 ms, TE = 5 ms, flip angle = 30°, and field of view of 256 mm).
Data Analysis
Behavioral Data.
As the present study aimed at specifically investigating the neural correlates of more and less successful learning in patients with schizophrenia, the whole patient group was divided into poor and good learners. For this, we first calculated the “mean response time improvement” for the whole group, which is the difference between mean response times in the first and the last quarter of the learning process. Then, the group was divided into good and poor learners by median split of the distribution of the mean response time improvement. (In a previous study, we characterized response time improvements by fitting response time decreases to an exponential model [for further details, see Koch et al6]. Because in the present study some data sets did not converge with this model, we could not resort to this method.) Two-tailed independent-sample t tests were used to investigate potential differences between the patient groups regarding age, education, and symptom severity as assessed by the PANSS. A χ2 test was applied to test for potential differences in gender distribution between the groups. Behavioral performance was analyzed with 1-way repeated-measures analysis of variance (ANOVA), with group (poor vs good learners) as between-subject factor and learning process (ie, response time changes across the 15 retrieval blocks averaged across the 3 sessions) as within-subject factor. In addition, exponential regression analyses were used to examine exponential response time decreases (ie, response time changes across the 15 retrieval blocks averaged across the 3 sessions) in each group.
Functional Magnetic Resonance Imaging Data.
Functional magnetic resonance imaging (fMRI) data analysis was done with SPM5 (http://www.fil.ion.ucl.ac.uk/spm). The first 3 functional scans were discarded in order to allow for signal saturation. Scans were corrected for motion effects and for differences in slice time acquisition by sinc interpolation. The anatomical high-resolution images were linearly and nonlinearly transformed to the reference brain of the Montreal Neurological Institute, corresponding to the Talairach and Tournoux coordinate system.17 An 8-mm full-width-at-half-maximum Gaussian smoothing kernel was applied to the data to optimize the signal-to-noise ratio and compensate for intersubject anatomical variation. Analysis was based on the first order autoregressive model.
A fixed-effects model was used for first-level analysis. Phases of encoding, retrieval, and resting state were assigned to the respective scans. The trials for each condition and participant were modeled using a boxcar model convolved with a canonical hemodynamic response function to form covariates in a general linear model. In our previous study, we found the learning process to be associated with exponential BOLD signal decreases in task-relevant regions in both patients and healthy volunteers. In the present study, we followed the same analysis strategies as reported previously (for a detailed illustration, see Koch et al6). Accordingly, we modeled learning-related signal changes across the 4 quarters as well as the exponential signal decrease across the whole learning process on the first level.
All analyses at the second level were based on the contrast retrieval vs resting state. We started out with correlating improvement in performance (in terms of a change in mean response times between first and last quarter of the learning process) and exponential signal decrease in the whole group of patients.
With regard to the comparison between good and poor learners, we first aimed at investigating activation differences between the groups independently from psychopathological status. In a second step, we explored potential relations between psychopathology and activation to find out more about the potential influence of psychopathological status on learning-related activation changes in good and poor learners. As poor learners turned out to be significantly more affected in the general psychopathology domain (see Results section), scores on this scale were used either as a covariate-of-no-interest or as a covariate-of-interest in all analyses. Thus, we performed 1 analysis of covariance (ANCOVA) with group (poor vs good learners) as between-subject factor and PANSS general score as covariate to compare the exponential signal decreases between both groups controlling for psychopathological status. Another 2 ANCOVAs with group (poor vs good learners) as between-subject factor and PANSS general score as covariate were applied to compare activation between both groups at the beginning (ie, activation during the first quarters of the retrieval periods averaged across the 3 sessions) and at the end (ie, activation during the last quarters of the retrieval periods averaged across the 3 sessions) of learning, controlling for psychopathological status. Regression analyses as implemented in SPM5 (flexible factorial design) with group (poor vs good learners) as between-subject factor and PANSS general score as covariate-of-interest were used to investigate the direct relation between learning-related signal decrease and symptom severity. Post hoc, we compared exponential signal decreases of both patient groups to exponential signal decreases of an age- and gender-matched healthy control group performing an ANOVA with group (poor learners, good learners, and healthy controls) as between-subject factor. All group comparisons were based on a threshold of P < .001 (uncorrected for multiple comparisons) with number of expected voxels as a spatial extent threshold. MNI coordinates were converted to Talairach coordinates using the mni2tal algorithm.18
Results
Sample Characteristics and Performance
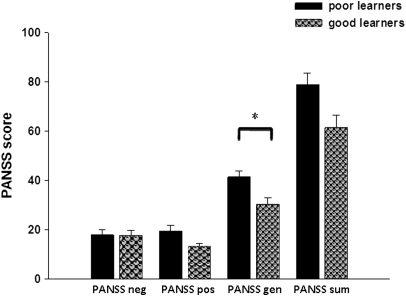
After dividing the whole patient group into good and poor learners by median split, the group of the poor learners consisted of 6 female and 6 male patients and the group of the good learners consisted of 10 male and 2 female patients. There were no significant differences between good and poor learners with respect to gender (χ12 = 3, not significant [NS]), age (t22 = −0.8, NS), and education (t22 = −1.1, NS). In the group of the poor learners, 9 patients were clinically stabilized for at least 14 days with atypical antipsychotic medication, 2 patients were clinically stabilized for at least 14 days with typical antipsychotic medication, and 1 patient was unmedicated. In the group of the good learners, all patients were clinically stabilized for at least 14 days with atypical antipsychotic medication and 2 were additionally receiving antidepressants (1 serotonin–norepinephrine reuptake inhibitors and 1 selective serotonin reuptake inhibitors). Mean chlorpromazine equivalent dosage did not differ between the groups (poor learners: 445.5, good learners: 459.7, t22 = −0.2, NS). Regarding symptom severity, there were no significant differences between the groups with respect to positive (t17 = 2.6, NS) or negative (t22 = 0.09, NS) symptoms or with respect to PANSS sum score (t22 = 2.5, NS), but poor learners had a significantly higher PANSS general psychopathology score (t22 = 3.2, P < .005; corrected for multiple comparisons) (figure 1).
Fig. 1.
Symptom Severity as Assessed by the PANSS in the Group of the Good and the Poor Learners (PANSS, Positive and Negative Syndrome Scale; PANSS neg, PANSS negative subscale; PANSS pos, PANSS positive subscale; PANSS gen, PANSS general psychopathology subscale; PANSS sum, PANSS sum score).
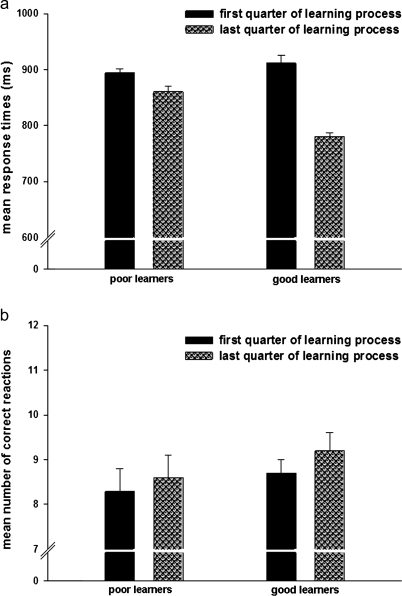
Regarding mean response times, poor learners showed 893.9 ± 25.2 ms in the first quarter and 860.8 ± 32.0 ms in the last quarter of the learning process. Good learners exhibited 911.9 ± 46.3 ms and 780.8 ± 19.1 ms in the first and the last quarter, respectively (figure 2a). Regarding the mean number of correct responses, poor learners showed 8.3 ± 1.7 in the first quarter and 8.6 ± 1.7 in the last quarter of the learning process. Good learners exhibited 8.7 ± 1.1 and 9.2 ± 1.2 in the first and the last quarter, respectively (figure 2b).
Fig. 2.
Mean Response Times (a) and Mean Number of Correct Responses (b) in the Group of the Good and the Poor Learners for the First and the Last Quarter of the Learning Process.
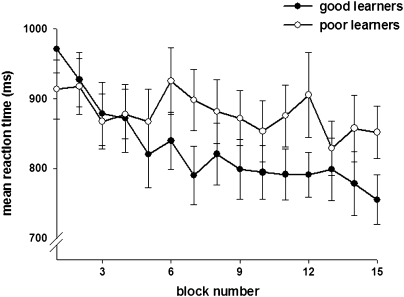
Group comparisons checking for potential differences between good and poor learners at baseline level (ie, mean response times in first quarter of the learning process) yielded no significant effect (t22 = 0.4, NS). The 1-way repeated-measures ANOVA with group (poor vs good learners) as between-subject factor and learning process (ie, response time changes across the 15 retrieval blocks averaged across the 3 sessions) as within-subject factor yielded no significant main effect of group (F1,22 = 0.9, NS), a significant main effect of learning process (F14,22 = 9.6, P < .001), and a significant interaction between group and learning process (F14,22 = 3.9, P < .001), indicating significantly slower learning-related response time improvements in the group of the poor learners (figure 3). The exponential regression analysis on the mean response times (ie, change in response time across the 15 retrieval blocks averaged across the 3 sessions) revealed significant decreases across time both for the group of the good learners (F1,13 = 50.6, P < .001, R2 = .8) and for the group of the poor learners (F1,13 = 7.1, P = .02, R2 = .4). Moreover, while response time improvement in good learners was comparable with that in healthy controls, poor learners showed significantly slower learning-related response time improvements compared with healthy controls (for details, refer to the online supplementary material [Supplement 1]).
Fig. 3.
Change in Mean Response Times Across the Learning Process in Poor and Good Learners.
fMRI Data
The regression analysis testing for positive and negative correlation between response time improvement and exponential signal decrease yielded significant effects in superior parietal regions bilaterally and the left frontal cortex including the middle frontal gyrus (BA 9) and the cingulate (BA 24) for the negative correlation. This indicates stronger activation decreases in these areas in association with poorer learning performance in the whole group of patients (table 1). The opposite contrast testing for positive correlations yielded no significant effects.
Table 1.
Talairach Coordinates of Activation Maxima (SPM [T] Value) for the Correlation Between Exponential Signal Decrease and Performance Improvement (ie, Change in Mean Response Times Between First and Last Quarter of the Learning Process) at P < .001
| Region | Side | x | y | z | k | T |
| Parietal lobe, precuneus, BA 19 | Right | 30 | −72 | 35 | 75 | 4.76 |
| Parietal lobe, precuneus, BA 31 | Left | −24 | −70 | 27 | 143 | 4.54 |
| Middle frontal gyrus, BA 9 | Left | −28 | 34 | 22 | 50 | 4.23 |
| Cingulate gyrus, BA 24 | Left | −6 | −5 | 26 | 39 | 4.15 |
| Parietal lobe, BA 40 | Left | 55 | −24 | 16 | 60 | 3.88 |
The ANCOVA on the exponential signal decreases with group (poor vs good learners) as between-subject factor and PANSS general psychopathology score as covariate yielded significantly stronger learning-related exponential signal decreases in the poor as compared with the good learners in a network comprising mainly superior parietal, cerebellar, and lateral frontal regions (table 2; figure 4). The opposite contrast yielded no significant effects. (Figures illustrating group-specific results are provided as online supplementary material [Supplement figure 1].)
Table 2.
Talairach Coordinates of Activation Maxima (SPM [T] Value) for the Exponential Signal Decrease (Poor Learners > Good Learners) at P < .001 With Positive and Negative Syndrome Scale General Score as Covariate-of-No-Interest
| Region | Side | x | y | z | k | T |
| Parietal lobe, precuneus, BA 19 | Left | −26 | −70 | 31 | 634 | 4.38 |
| Superior temporal gyrus, BA 39 | Left | −38 | −49 | 28 | 540 | 5.77 |
| Precentral gyrus, BA 6 | Left | −48 | 1 | 48 | 44 | 5.61 |
| Parietal lobe, precuneus, BA 7 | Right | 18 | −69 | 31 | 232 | 5.37 |
| Cerebellum | Left | −8 | −77 | −20 | 66 | 4.81 |
| Cerebellum | Right | 14 | −78 | −13 | 44 | 4.50 |
| Cingulate gyrus, BA 31 | Right | 4 | −25 | 40 | 50 | 4.16 |
| Parietal lobe, BA 40 | Right | 34 | −45 | 32 | 77 | 4.06 |
| Inferior frontal gyrus, BA 45 | Left | −46 | 22 | 17 | 75 | 3.90 |
| Middle temporal gyrus, BA 38 | Left | −48 | 5 | −22 | 32 | 3.90 |
Fig. 4.
Group Comparison (Poor Learners > Good Learners) for the Learning-Related Exponential Signal Decrease at P < .001.
The ANCOVA with group (poor vs good learners) as between-subject factor and PANSS general score as covariate comparing activation between both groups in the first quarter of the learning process yielded significantly stronger signal in poor as compared with good learners in a mainly left-lateralized fronto-parieto-temporal network including the superior temporal gyrus (BA 39), the inferior frontal gyrus (BA 45), the superior parietal lobe (BA 7), and the cingulate (BA 24) (table 3). There were no relative hyperactivations in good as compared with poor learners.
Table 3.
Talairach Coordinates of Activation Maxima (SPM [T] Value) for Activation Differences in the First Quarter of the Learning Process (Poor Learners > Good Learners) at P < .001 With Positive and Negative Syndrome Scale General Score as Covariate-of-No-Interest
| Region | Side | x | y | z | k | T |
| Superior temporal gyrus, BA 39 | Left | −44 | −53 | 28 | 54 | 4.92 |
| Inferior frontal gyrus, BA 45 | Left | −54 | 24 | 19 | 50 | 4.82 |
| Middle temporal gyrus, BA 21 | Left | −50 | −1 | −19 | 57 | 4.43 |
| Superior parietal lobe, BA 7 | Right | 20 | −60 | 62 | 27 | 4.42 |
| Cingulate gyrus, BA 24 | Left | −10 | −15 | 41 | 64 | 4.36 |
ANCOVA of activation differences in the last quarter of the learning process yielded a small hyperactivation in the occipital lobe in poor vs good learners (x = 22, y = −82, z = 28, k = 38, T = 4.7) and no relative hyperactivations in good vs poor learners.
The regression analysis testing for group differences in the positive correlation between symptom severity (PANSS general score) and learning-related signal decrease yielded no significant effects. The regression analysis testing for group differences in the negative correlation between symptom severity (PANSS general score) and learning-related signal decrease yielded significantly stronger activations in the group of the poor as compared with the good learners in a predominantly fronto-parieto-cerebellar network (table 4).
Table 4.
Talairach Coordinates of Activation Maxima (SPM [T] Value) for the Negative Correlation Between Symptom Severity (Positive and Negative Syndrome Scale General Score) and Learning-Related Signal Decrease (Poor Learners > Good Learners) at P < .001
| Region | Side | x | y | z | k | T |
| Posterior cingulate, BA 30 | Right | 20 | −60 | 12 | 238 | 6.04 |
| Parietal lobe, BA 39 | Right | 54 | −59 | 18 | 327 | 5.64 |
| Middle temporal gyrus, BA 39 | Left | −40 | −67 | 14 | 319 | 4.91 |
| Cerebellum | Left | −8 | −37 | −3 | 55 | 4.80 |
| Cerebellum | Right | 16 | −47 | −11 | 179 | 4.70 |
| Cerebellum | Left | −18 | −45 | −13 | 125 | 4.51 |
| Inferior frontal gyrus, BA 47 | Right | 38 | 29 | −10 | 124 | 4.47 |
| Cerebellum | Right | 4 | −68 | −7 | 133 | 4.31 |
| Middle temporal gyrus, BA 21 | Right | 52 | −31 | −2 | 53 | 4.06 |
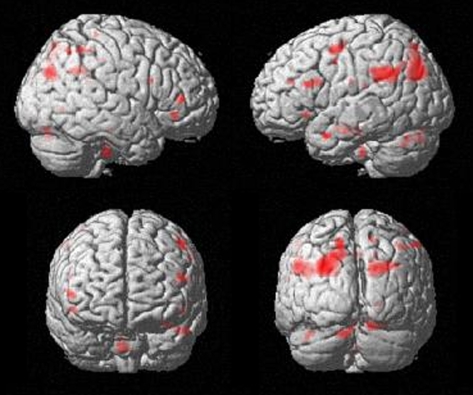
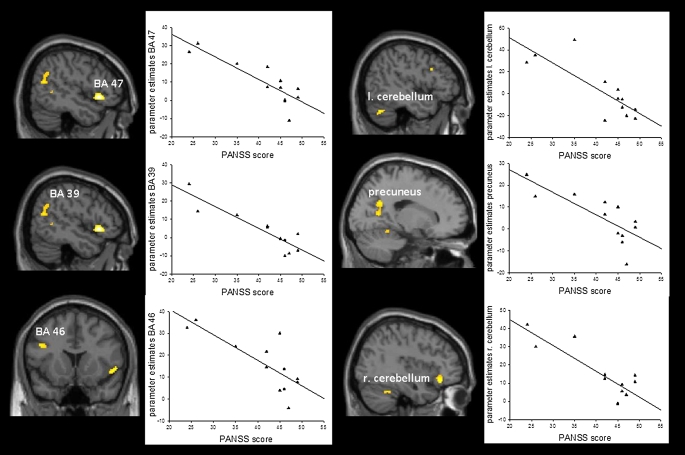
Analysis of the positive correlation between symptom severity (PANSS general score) and learning-related signal decrease in the group of the poor learners revealed no significant results, whereas the negative correlation yielded significant activations in a predominantly fronto-parieto-cerebellar network containing, among others, the inferior frontal gyrus (BA 45/47), the left middle frontal gyrus (BA 46), and the right precuneus (BA 31) (table 5; figure 5).
Table 5.
Talairach Coordinates of Activation Maxima (SPM [T] Value) for the Negative Correlation Between Symptom Severity (Positive and Negative Syndrome Scale General Score) and Learning-Related Signal Decrease in the Group of the Poor Learners at P < .001
| Region | Side | x | y | z | k | T |
| Inferior frontal gyrus, BA 45/47 | Right | 50 | 19 | −4 | 231 | 6.39 |
| Cerebellum | Left | −8 | −37 | −3 | 137 | 4.65 |
| Cerebellum | Left | −46 | −62 | −26 | 68 | 4.47 |
| Superior temporal gyrus, BA 39 | Right | 52 | −59 | 18 | 143 | 4.45 |
| Precuneus, BA 31 | Right | 18 | −55 | 27 | 134 | 4.38 |
| Middle frontal gyrus, BA 46 | Left | −42 | 15 | 25 | 31 | 4.28 |
| Cerebellum | Right | 38 | −48 | −23 | 39 | 4.12 |
| Cerebellum | Right | 6 | −77 | −15 | 58 | 4.82 |
Fig. 5.
Regression Analysis Testing for Negative Correlations Between Symptom Severity (Positive and Negative Syndrome Scale General Score) and Learning-Related Signal Decreases in the Group of the Poor Learners. Highly significant negative correlations between symptom severity and learning-related signal decrease were detectable in right inferior frontal gyrus (BA 45/47), left and right cerebellum, superior temporal gyrus (BA 39), precuneus (BA 31), and middle frontal gyrus (BA 46).
In the group of the good learners, analysis of the positive correlation between symptom severity (PANSS general score) and signal decrease revealed a significant signal in the left middle temporal gyrus (x = −42, y = −67, z = 13, k = 271, T = 5.41), while the negative correlation showed no significant results.
A direct comparison of the exponential signal decreases between patients and an age- and gender-matched group of healthy controls yielded no significantly increased activation in controls (neither as compared with poor nor as compared with good learners). The opposite contrast yielded no activation differences between healthy controls and good learners, but significantly stronger exponential signal decreases in the patients with the poor learning performance compared with controls in a network comprising predominantly frontal and superior parietal regions (for details, refer to the online supplementary material [Supplement table 1]).
Discussion
The present study explored the neural mechanisms of short-term learning in patients with schizophrenia by differentiating between good and poor learners and investigating the learning-related signal changes with respect to more and less successful learning performance. In addition, the study intended to specifically examine the relation between symptom severity and learning-related activation changes because psychopathological status can be assumed to exert a major influence on patients’ capability to profit from short-term practice.
Analyses of mean response times in both groups revealed no significant group effect, indicating that response times in the poor and the good learners were comparable irrespective of learning-related changes. Moreover, performance analyses showed a significant effect for learning process (ie, change in response times across the 15 blocks), indicating response time improvements with increasing practice. While both patient groups showed comparable response times at the beginning of the learning process, good learners exhibited a significantly steeper improvement slope across the learning process compared with poor learners (figure 3). This finding was also reflected by a significant interaction between group and learning process (ie, change in response times across the 15 blocks). The present task predominantly involves declarative memory components as far as the stored stimuli are concerned. However, several subprocesses (like stimulus retrieval or response execution) can be regarded as skills that become increasingly automated with continuous practice throughout the task. The relatively stronger performance improvement in the good as compared with the poor learners, therefore, is most probably attributable to increased declarative learning capability and, to a small extent, to procedural learning capabilities.
As a main finding with regard to cerebral activation, patients with a poor learning performance exhibited significant hyperactivations at the beginning of the learning task, which decreased with proceeding practice. Importantly, this relative hyperactivation was detectable irrespective of symptom severity. Thus, the practice-associated response time improvements in poor learners were associated with abnormal hyperactivation under higher task demands (ie, at the beginning of learning). This hyperactivation seemed to constitute the basis for the stronger subsequent signal decreases in poor as compared with good learners. Hence, our data indicate that it is predominantly the increased activation under higher task demands that differentiates patients with a small learning potential from patients with a high learning potential. Accordingly, the learning-associated signal decreases were mainly detectable in a network of those regions that exhibited relative hyperactivation in poor learners at the beginning of the learning process but no longer in the last phase of the learning process. This network contained several regions known to be critically involved in WM, performance monitoring, and cognitive control: The cingulate cortex, which receives strong afferents from the thalamic nuclei and is known to be relevant in the context of performance monitoring and cognitive control processes;19,20 the ventrolateral prefrontal cortex, which becomes activated predominantly during WM maintenance;21 as well as superior parietal and cerebellar areas, which have been shown to be critically involved in vigilance and attention.7,22 These relative hyperactivations indicate that under increased task demands, patients with a decreased capability to promptly profit from short-term practice are forced to involve regions relevant for WM, performance monitoring, and cognitive control to a significantly greater degree than patients with a higher learning capability. Honey et al23 investigated functional connectivity in patients with schizophrenia using a WM task with different task difficulties. They found patients to show a disrupted connectivity between the superior frontal gyrus and both the anterior cingulate and the cerebellum in association with higher task demands and impaired task performance. Of note, disturbed connectivity within task-relevant networks, which has repeatedly been reported in patients24–27 has mostly been detected in the presence of impaired cognitive performance. Hence, altered interregional brain integration may likewise underlie the fronto-cingulo-cerebellar hyperactivation that the poorly performing patients showed under increased task demands in the present study. Evidence pointing to disruptions in whiter matter structures connecting frontal, cingulate, and cerebellar areas28 furthermore suggests that structural abnormalities may also play a role in this context.
Interestingly, the present findings display striking analogies to the findings of our preceding study6 in which we compared learning-related activation changes between healthy volunteers and a smaller sample of patients with schizophrenia. Here, we also found relative hyperactivations in patients under increased task demands and significantly stronger exponential signal decreases in association with relatively reduced but generally preserved learning capabilities in patients. In the present study, learning performance of good learners did not significantly differ from learning performance in healthy controls. Of note, the comparison between these patients with good learning performance and healthy controls yielded no significant effects, indicating that learning-related activation in patients does not significantly differ from that in healthy subjects when patients learn successfully. Hence, present and earlier findings suggest that average-performing patients as compared with healthy controls as well as poor as compared with good learners are characterized by relative activation abnormalities under increased task demands that they, however, manage to reduce with proceeding practice. This hyperactivation has been formalized by the model of “cortical inefficiency,” which assumes that these activation abnormalities do not constitute a stable marker of the disorder but change in dependence on task demands and associated performance.29,30 Present results are in line with this concept and to some degree extend the model of cortical inefficiency by illustrating that this hyperactivation under increased task demands identifies less successful learners and seems to normalize with increasingly successful processing.
The second aim of the study was to investigate a potential influence of psychopathological status on learning capabilities and their neural correlates in patients with schizophrenia. The fact that the group of less successful learners, as determined by median split based on the decrease in mean response times across the learning process, turned out to be significantly more affected with regard to their general psychopathology, might be recognized as an a priori indication of the influence that the severity of general symptoms exerts on learning proficiency. Accordingly, the systematic investigation of the relationship between general symptom severity and neuronal activation yielded a negative correlation between symptom severity and learning-related signal decreases in a fronto-parieto-cerebellar network only in the group of the less successful learners. Thus, whereas in this group higher symptom severity was associated with smaller learning-related activation normalization, this relation was not detectable in the group of the good learners. Hence, there is reason to assume that cognitive capacities of the more successful learners allow them to effectively compensate the putatively debilitating effect that psychopathological symptoms exert on cognition and to reduce processing resources in the course of increasingly automatic retrieval. In less successful learners, as opposed, higher symptom severity inhibits learning-related activation normalization to a certain degree.
Longitudinal studies might be helpful to determine whether the practice-related activation characteristics detectable in more and less successful learners in association with tasks as the present one possess some predictive value regarding functional outcome and long-term cognitive changes in these patients.
Supplementary Material
Supplementary material Supplement 1, figure 1, and table 1 are available at http://schizophreniabulletin.oxfordjournals.org.
Funding
German Research Foundation (Deutsche Forschungsgemeinschaft [KO 3744/1-1 to K.K.]); BMBF (FKZ01ZZ0405); IZKF and TMWFK (B307-04004).
Supplementary Material
References
- 1.Sharma T, Antonova L. Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 2.Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Koch K, Wagner G, von Consbruch K, et al. Temporal changes in neural activation during practice of information retrieval from short-term memory: an fMRI study. Brain Res. 2006;1107:140–150. doi: 10.1016/j.brainres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 5.van Raalten TR, Ramsey NF, Jansma JM, Jager G, Kahn RS. Automatization and working memory capacity in schizophrenia. Schizophr Res. 2008;100:161–171. doi: 10.1016/j.schres.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Koch K, Wagner G, Nenadic I, et al. Temporal modeling demonstrates preserved overlearning processes in schizophrenia: an fMRI study. Neuroscience. 2007;146:1474–1483. doi: 10.1016/j.neuroscience.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Morrison-Stewart SL, Williamson PC, Corning WC, et al. Frontal and non-frontal lobe neuropsychological test performance and clinical symptomatology in schizophrenia. Psychol Med. 1992;22:353–359. doi: 10.1017/s0033291700030294. [DOI] [PubMed] [Google Scholar]
- 9.Cameron AM, Oram J, Geffen GM, et al. Working memory correlates of three symptom clusters in schizophrenia. Psychiatry Res. 2002;110:49–61. doi: 10.1016/s0165-1781(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 10.Bozikas VP, Kosmidis MH, Kioperlidou K, Karavatos A. Relationship between psychopathology and cognitive functioning in schizophrenia. Compr Psychiatry. 2004;45:392–400. doi: 10.1016/j.comppsych.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan RW, Strauss ME, Breier A, Kirkpatrick B, Carpenter WT., Jr Attentional impairments in deficit and nondeficit forms of schizophrenia. Am J Psychiatry. 1997;154:363–370. doi: 10.1176/ajp.154.3.363. [DOI] [PubMed] [Google Scholar]
- 12.Caligiuri MP, Hellige JB, Cherry BJ, et al. Lateralized cognitive dysfunction and psychotic symptoms in schizophrenia. Schizophr Res. 2005;80:151–161. doi: 10.1016/j.schres.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 13.O'Grada C, Barry S, McGlade N, et al. Does the ability to sustain attention underlie symptom severity in schizophrenia? Schizophr Res. 2009;107:319–323. doi: 10.1016/j.schres.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Honey GD, Sharma T, Suckling J, et al. The functional neuroanatomy of schizophrenic subsyndromes. Psychol Med. 2003;33:1007–1018. doi: 10.1017/s0033291703007864. [DOI] [PubMed] [Google Scholar]
- 15.Wolf RC, Vasic N, Hose A, Spitzer M, Walter H. Changes over time in frontotemporal activation during a working memory task in patients with schizophrenia. Schizophr Res. 2007;91:141–150. doi: 10.1016/j.schres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Talairach J, Tournoux P, editors. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- 18.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 19.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 20.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 21.Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon N. The cerebellum and cognition. Eur J Paediatr Neurol. 2007;11:232–234. doi: 10.1016/j.ejpn.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Honey GD, Pomarol-Clotet E, Corlett PR, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benetti S, Mechelli A, Picchioni M, et al. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–2436. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- 25.Schlosser R, Gesierich T, Kaufmann B, et al. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- 26.Schlosser R, Koch K, Wagner G, et al. Intensive practice of a cognitive task is associated with enhanced functional integration in schizophrenia. Psychol Med. 2009;39:1809–1819. doi: 10.1017/S0033291709005820. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Minzenberg MJ, Ursu S, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23:255–273. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Callicott JH, Mattay VS, Verchinski BA, et al. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 30.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.