Abstract
Schizophrenia is associated with cognitive processing deficits, including deficits in executive processing, that represent a core component of the disorder. In the Task Switching Test, subjects view ambiguous stimuli and must alternate between competing rules to generate correct responses. Subjects show worse performance (prolonged response time and/or increased error rates) on the first response after a switch than on subsequent responses (“switch costs”), as well as performing worse when stimuli are incongruent as opposed to congruent (“congruence costs”). Finally, subjects show worse performance in the dual vs single task condition (“mixing costs”). In monkeys, the N-methyl-D-aspartate (NMDA) antagonist ketamine has been shown to increase congruence but not switch costs. Here, subjects viewed colored letters and had to respond alternately based upon letter (X vs O) or color (red vs blue). Switch, congruence and mixing costs were calculated. Patients with schizophrenia (n = 16) and controls (n = 17) showed similar switch costs, consistent with prior literature. Patients nevertheless showed increased congruence and mixing costs. In addition, relative to controls, patients showed worse performance across conditions in the letter vs color tasks, suggesting deficits in form vs color processing. Overall, while confirming executive dysfunction in schizophrenia, this study indicates that not all aspects of executive control are impaired and that the task switching paradigm may be useful for evaluating neurochemical vs neuroanatomic hypotheses of schizophrenia.
Keywords: executive control, task switching, schizophrenia, N-methyl-D-aspartate
In order to function effectively in an environment such as ours—an environment that is often unpredictable—it is important to adjust one's behavior dynamically to varying environmental contingencies. For instance, when attending a basketball game, it is acceptable (indeed, all but necessary) to stand and cheer at important points in the game. When attending an opera, such behavior is not acceptable. Thus, despite the many (gross) similarities in these 2 contexts (being seated in a crowd, viewing performers moving about in a well-lit space, etc), very different behavior is required.
The ability to instantiate a new behavioral set—ie, to switch from one behavior (task) to another—has been taken to be one of the core functions of executive processes, eg,1–3. This, in turn, has led many researchers to study executive processes by employing a paradigm in which subjects must switch between two tasks e.g., 1–16. On some trials of such a ‘task-switching’ paradigm, subjects must repeatedly perform each task, while on others, they must switch between the tasks. When task-switch trials are compared to task-repeat trials, large performance costs are found, suggesting that this paradigm might allow one to study the time taken by executive control processes to function.
Executive processing in general and task switching in particular is postulated to reflect a distributed network incorporating both prefrontal and parietal cortices. Brain lesion literature shows impairments in executive function following either frontal or parietal lesions.17 Similarly, functional magnetic resonance imaging (fMRI) investigations of the executive processes involved in task switching show activity in a frontoparietal network of areas when switch trials are compared to repeat trials e.g., 18–26, while event-related potential (ERP) studies show voltage modulations on switch trials relative to repeat trials over both prefrontal and posterior scalp,27 with posterior modulations preceding prefrontal (see 28 and 29 for more on this issue).
Schizophrenia is known to affect executive processes. For example, patients show large effect size deficits in performing the Wisconsin Card Sorting Test (WCST) and in particular show large increases in rates of perseverative responding. Patients also show impaired Stroop performance, which is also considered a classic test of executive processing,30 along with impaired performance in letter number sequencing31 in which stimuli must be stored and manipulated simultaneously. Finally, patients show reduced performance in tasks such as the AX continuous performance task, where inhibition of response is required based upon trial-by-trial cueing.32 Altogether disturbances in these processes provide strong support for the concept of executive dysfunction and prefrontal/parietal processing disturbances in schizophrenia.
Against this backdrop of literature showing highly significant executive processing deficits, task-switching paradigms stand out. In as much as the task-switching paradigm is thought to index one important aspect of executive processes and in as much as patients suffering from schizophrenia are thought to have deficits in executive function, it seems natural to ask whether patients suffering from schizophrenia show deficits in a task-switching paradigm. Indeed, this question has been addressed, and a surprising result has been found consistently: patients with schizophrenia show comparable behavioral costs when switching (switch costs) compared to control subjects.33–35 In only one case, have larger switch costs been found for patients, relative to controls,6 and in that experiment this was found unambiguously only when patients were given very little time (132 milliseconds) to prepare for a forthcoming switch of task. Thus, while patients show evidence of executive processing deficits on many tasks, the literature suggests that such deficits may not be evident in all tasks that engage prefrontal/parietal executive processing networks. As opposed to increased switch costs, patients have been shown to have increased costs, measured in terms of both error rate and reaction time, when stimuli are incongruent—ie, when response for a stimulus would differ in the 2 tasks of the switching paradigm—compared with when they are congruent.36 Similar effects are seen in monkeys treated with the N-methyl-D-aspartate (NMDA) antagonist Phencyclidine (PCP),37 suggesting that the pattern of set-shifting deficit observed in schizophrenia, like other aspects of neurocognitive dysfunction in schizophrenia, may be attributable to underlying NMDA receptor dysfunction.38
In the present study, we implemented a paradigm in patients similar to that used in the used in the monkey NMDA paradigm, in which subjects switched between performing a color vs form (letter) categorization task, and both error rates and reaction times were analyzed not only as a function both of switch vs nonswitch trials but also as a function of congruence/incongruence between the 2 competing tasks. Three, rather than 2, repeats of each task were employed to permit better assessment of switch vs repeat costs. As with the primate study, relative deficits were assessed for switch costs, defined as difference in performance between switch and repeat trials, and congruence costs, defined as the difference in performance between congruent and incongruent trials within task. One primary aim of the study was to replicate prior reports of increased congruence costs but unchanged switch costs in schizophrenia using a color/form task. We hypothesized that the pattern of task switching deficit in schizophrenia would correspond closely to the pattern observed in ketamine-treated primates, similar to that observed in prior studies in schizophrenia.39
Finally, in order to better understand task switching deficits, we explored mixing costs, reflecting costs of performing switch tasks vs repetitive trials of a single task.
Whereas switch costs have been extensively studied in schizophrenia, mixing costs have not and the absence of such studies has been noted.40 A second primary aim of the study, therefore, was to explore mixing costs deficits. It has been proposed that mixing cost and switching cost reflect somewhat different control processes, with mixing cost but not switching cost reflecting global control mechanisms or sustained control processes, whereas switching cost appears to be exclusively related to specific or transient control mechanisms.4,18,41,42 Mixing and switch costs may also be differentiated photogenically, in that switch costs are relatively unaffected in old age but mixing costs are increased, whereas in attention deficit/hyperactivity disorder, mixing costs are unaffected but switch costs are increased.42 Mixing costs and switch costs may also have distinct functional neuroanatomy, with right anterior prefrontal cortex (PFC) playing a predominant role in the processes related to mixed task environments and mixing costs, left lateral PFC playing a predominant role in maintenance of task-set information and superior parietal cortex involved in processes associated with the online reconfiguration and updating of task-set information immediately following a switch in task, and thus contributing to genesis of switch costs.18 Complementary activity between prefrontal and parietal activity is also seen in ERP paradigms, with increased activity in frontoparietal networks on switch vs repeat trials.27–29
Methods
Subjects
A total of 16 patients (14 males) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria for schizophrenia and/or schizoaffective disorders (SZ) at the Nathan Kline Institute (NKI) for Psychiatric Research provided written informed consent after the procedures had been fully explained. Diagnoses were obtained by means of chart review, consultation with the treating psychiatrists, and the Structured Clinical Interview for DSM-IV (SCID). Patients were excluded if they had any neurological or ophthalmologic disorders that might affect performance, significant current drug or alcohol abuse, or dependence within past 6 months. Eleven of the patients were taking atypical antipsychotics, and the remainder were receiving typical antipsychotic medications. The mean chlorpromazine-equivalent dose was 1334 ± 600 mg/day (range = 500–2500).
Seventeen healthy comparison (HC) volunteers (4 women), age matched to the patients, obtained from the normal volunteer pool at NKI, provided written informed consent after the procedures had been fully explained. Comparison volunteers with a history of psychiatric, neurological, and/or ophthalmologic disorders were excluded. Sample sizes were sufficient a priori to detect large effect size (d = 0.8) between groups.
The SZ and HC groups did not differ significantly in age (patient: 35.4 ± 11 years; comparison: 30.9 ± 9.6 years, t = −1.2, df = 28, P = .2). As expected, socioeconomic status, as measured with the 4-factor Hollingshead Scale43 was significantly lower for the SZ (24 ± 9.6) than for the HC subjects (49.2 ± 12.9) (t = 5.3, df = 21, P = .0001). However, patients and controls did not differ on parental education level (patient: 14.8 ± 12.3 years; control: 17.1 ± 4.3 years; t = 1.40, P = .15). Patients’ scores on the Brief Psychiatric Rating scale (BPRS) were 30.7 ± 11.1, 42.8 ± 8.3, and 10.7 ± 4.1, for positive symptoms, negative symptoms, and general psychopathology, respectively.
Tasks and Stimuli
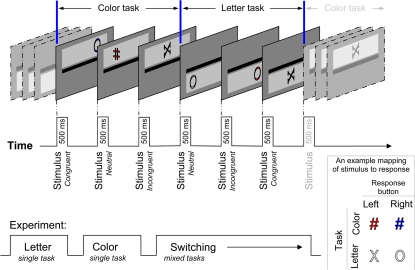
On each trial, a colored letter was presented either above or below a thick, horizontal line that bisected the display (figure 1). For three trials in a row, the letters were presented above the line, for the next 3 trials, they were presented below the line, and so on. Subjects were instructed that when the letter was presented above the line, they were to perform one task (eg, a color categorization task), and when it was presented below the line, they were to perform the other task (a letter categorization task). The task to location mapping was counterbalanced across participants. For the letter task, subjects were instructed to press one key (with their index finger) if the letter was an X and another (with their middle finger) if the letter was an O. All subjects used their dominant hand to respond. For the color task, subjects were instructed to press one key (index finger) if the letter was colored red and another key (middle finger) if it was colored blue.
Fig. 1.
A Graphical Depiction of the Sequence of Stimuli and Tasks Across Trials. In the upper left, an example of the task sequence is shown. In the lower left, the organization of blocks (“pure,” single-task blocks, and mixed-task, switching blocks) is shown. In the lower right, an example of stimulus-response mapping is shown. For clarity, neutral stimuli are used.
Because subjects used the same 2 keys/fingers to respond to both tasks, some of the stimuli were response congruent and others were response incongruent. For example, if subjects were instructed to respond with the left key (index finger) when the letter was an X (for the letter task) and with the same key when the letter was red (color task), then a red X (and a blue O) was response congruent. This is because, regardless of the currently relevant task, the same response was required. For response-incongruent stimuli, different responses were required for the 2 tasks. To continue with the above example, if a blue X was presented, then the correct response would depend on which task the subject was performing (letter: left key; color: right key). Here, 3 categories of stimulus were presented: congruent, incongruent, and neutral. For the neutral stimuli, the irrelevant attribute was absent. For the letter task, this meant that an “uncolored” stimulus was presented. For the color task, a # character was presented in 1 of the 2 colors.
The display consisted of a black, horizontal line (650 × 30 pixels) that was continuously present on a gray background (45% red, 45% green, 45% blue). The screen resolution was set at 640 × 480 pixels. Each run of 3 trials began with the presentation of a light gray (78% red, 78% green, 78% blue) box (620 × 130 pixels) either above or below the line (85 pixels, on center). This light gray box served as a cue for the relevant task and was displayed continuously for the 3-trial run. On the first trial of the run, the light gray box appeared coincidently with the imperative stimulus (ie, the X, O, or neutral stimulus). The imperative stimulus was presented for 500 milliseconds, and the next trial was presented after immediately after a response or after 3000 milliseconds. This resulted in a response-to-stimulus interval (RSI) of 0 millisecond. We chose this RSI because the only study to find differences in performance between patients and controls40 did so when the RSI was short: 132 milliseconds.
The imperative stimulus was presented inside the light gray box and was one of 3 characters (X [73 × 69 pixels], O [77 × 73 pixels], # [55 × 73 pixels]) and was colored red (100% red, 0% green, 0% blue), blue (0% red, 0% green, 100% blue), or light gray (the same color as the background for these stimuli). The location of the imperative stimulus was such that it was always centered in the vertical dimension within the light gray box (cue). In the horizontal dimension, its location varied randomly and was one of 5 values: centered horizontally, ±90 pixels from the center, or ±180 pixels from the center.
The sequence of tasks was completely regular and changed every 3 trials (letter, letter, letter, color, color, color, letter, letter, letter, etc). Therefore, the location cue served as a redundant reminder of the task that was relevant on each trial. An alternating runs paradigm was adopted because it was felt that a regularly alternating sequence would be easier for patients to follow than one in which the tasks were randomly cued. While others have used randomly cued paradigms to good effect,36,40 we opted for a more conservative approach. No error feedback was provided.
Apparatus
The experiment was carried out in a dark room. Visual stimuli were displayed on a 19” Iiyama Vision Master Pro 514 CRT monitor. The stimuli were generated using Presentation (V0.6, Neurobehavioral systems, http://nbs.neuro-bs.com/). All subjects responded with a 2-alternative, forced choice key press, using a USB response pad.
Procedure
The experiment was run in 2 discrete stages. In the first stage, subjects practiced both the letter task and the color task (whether the letter task or color task was practiced first was counterbalanced across subjects). Each task was performed in a 90-trial block that was divided into three, 30-trial sessions, with breaks provided between sessions. In the second stage, subjects switched from one task to the other on every third trial. This switching block lasted for 540 trials and was also divided into 30-trial sessions. Subjects were encouraged to wait for as long as necessary during the breaks before proceeding with the experiment.
All subjects worked though the 2 stages in the order described above (single task [letter and color] then switching). Subjects were seated at a comfortable viewing distance from the monitor, and the requirement of the first block of the first stage was explained to them. At the end of the first block of the first stage, the task requirements of the second block of the first stage were explained. No mention of switching was made until the beginning of the switching stage.
Statistics
RT values were derived from correct trials only and were log transformed to improve normality. Between-group performance was analyzed using 1-way or repeated-measures multivariate analysis of variance (MANOVA), with between-subject factor of group (patient/control) and within-subject factor as appropriate. Statistics were performed using SPSS 15.0. In order to evaluate relative discriminating power for the letter and number tasks, true score variance (TSV) was computed by dividing observed score variance by reliability as determined by Cronbach α statistic assessed using split half data. Data in text are mean ± SD unless otherwise specified. Preset α level for statistical significance is P < .05 throughout.
Results
Results were analyzed separately for error rate and reaction time. Two levels of analysis were conducted. First, data were analyzed separately for pure task and switch conditions using repeated-measures analysis of variance (ANOVA) to isolate main and interaction effects. Second, specific task-related indices, including mixing costs, switch costs, and congruence costs, were calculated and compared across groups.
Pure Task
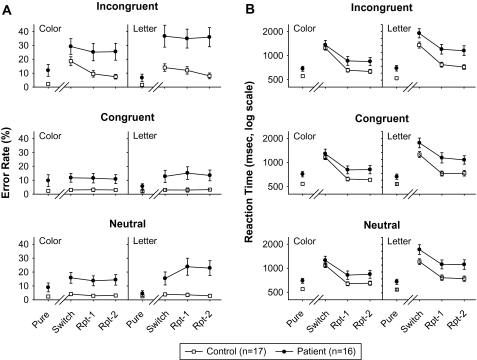
Data from the pure task were analyzed with a mixed, between- and within- subjects ANOVA, with between-subjects factor of group (patient vs control) and within-subjects factor of task (letter vs color). Patients showed increased error rate compared with controls as reflected in a significant main effect of Group (F1,30 = 5.89, P = .021). (figure 2A, upper). In RT analyses, patients showed increased RTs compared with controls, as reflected in significant main effect of group (F1,30 = 8.67, P = .006) (figure 2A, lower). No significant main effect of task or group × task interaction was observed for either error rate or RT.
Fig. 2.
The Error Rates (ERs) and log Response Times (RTs) for Pure and Mixed Blocks. In all cases, error bars depict the SE. Panel A shows the ERs from the pure task block (pure), followed by switch, first repeat (Rpt-1), and second repeat (Rpt-2) trials from the switch block for patients (filled symbols) and controls (open symbols). Data are plotted separately for incongruent (top), congruent (middle), and neutral (bottom) trials. Panel B shows RTs for the same conditions. RTs were analyzed using log values to evaluate proportionate costs and are plotted on log axes.
Mixed Task
Data from the set-shifting task were analyzed using repeated-measures MANOVA with a between-subject factor of group (patient/control) and within-group factors of congruity (neutral, incongruent, congruent), trial (switch, first repeat, second repeat), and task (letter, number).
Error Rates.
For ER, there was a highly significant main effect of group (F1,31 = 13.5, P = .001), which was due to patients making more errors than controls across trials and tasks (figure 2B, upper). Across all levels of congruity (congruent, incongruent, neutral), the main effect of trial was significant at trend level only (F2,30 = 3.02, P = .064), reflecting small switch costs in the task overall. However, the main effect of congruity was highly reliable (F2,30 = 12.7, P < .0001) because all subjects made substantially more errors when the stimuli were incongruent than when they were congruent or neutral. Congruity also significantly modified the trial effect (F4,28 = 4.68, P = .005), reflecting much stronger switch costs for incongruent than congruent or neutral trials. The trial × group (F2,30 = 3.82, P = .033) and congruity × group (F2,30 = 3.75, P = .035) interactions were both significant, reflecting lesser switch costs for patients than controls and greater incongruence costs, respectively (see “Switch Costs” and “Congruence Costs” sections, below). The congruity × trial interaction was not significantly modified by group (F4,28 = 1.53, P = .25).
The main effect of task was not significant (F1,31 = 2.77, P = .11). There was a trend toward a significant task × group interaction (F1,31 = 2.97, P = .095) reflecting worse performance of patients in the letter vs color task relative to controls. The task effect was significantly modified by trial (F2,30 = 5.94, P = .007), reflecting reduced switch costs in the letter vs color tasks across groups. The task × trial effect was not significantly modified by group (F2,30 = 0.87, P = .4) or congruence (F4,28 = 1.42, P = .25). All other 3- and 4-way interactions were also nonsignificant (all P's > .2).
Reaction Time.
For RT, the main effect of group was also reliable (F1,31 = 6.31, P = .02) and reflected the fact that patients responded with longer latencies than controls (figure 2B, lower). The main effect of trial was reliable (F2,30 = 42.5, P < .0001) because RTs were much longer on the first trial (the switch trial) than on subsequent, repeated-task trials. However, the trial × group interaction was not significant (F1,30 = 0.6, P = .6), reflecting similar switch costs across groups.
There was a significant main effect of congruity (F2,30 = 3.90, P = .03) that was due to subjects responding with longer latencies to incongruent stimuli than to either neutral or congruent stimuli (RTs were very similar for neutral and congruent stimuli). This effect was modulated by trial, as shown by an interaction between trial and congruity (F4,28 = 0.6, P = .008). This was because when the stimuli were neutral or congruent the only difference between the three trials was between the switch trial and the first repeat trial. However, when the stimuli were incongruent, there was also a reliable difference between the 2 repeat trials because subjects continued to accelerate across the three trials. There was no significant group × congruity (F2,30 = 0.1, P = .8) or group × congruity × trial (F4,28 = 0.6, P = .7) interaction.
There was a significant main effect of task (F1,31 = 45.6, P < .0001), reflecting longer RTs in the letter than color task across groups. This effect was larger in patients than controls, leading to a significant task × group interaction (F1,31 = 7.3, P = .011). Task effects were not significantly modified by either trial (F2,30 = 0.8, P = .4) or congruity (F2,30 = 0.1, P = .9). Task × trial × group (F2,30 = 1.1, P = .3) and task × congruity × group (F4,28 = 0.2, P = .95) were also nonsignificant, as was the 4-way interaction (F4,28 = 0.3, P = .9).
Cost Components
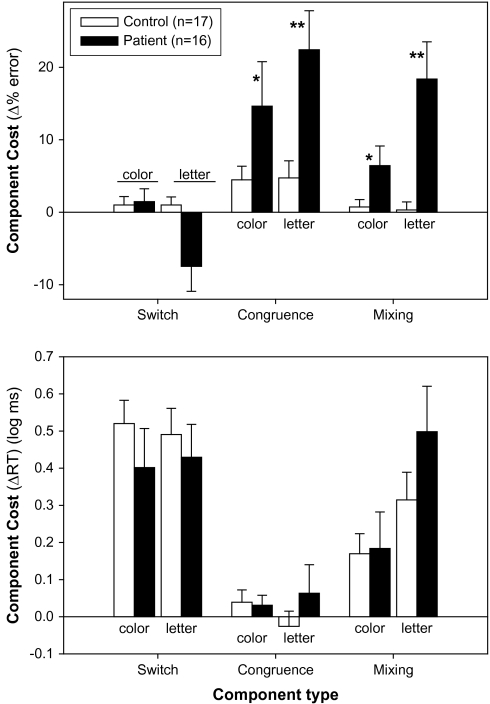
In order to illustrate specific cost components of the task, specific preplanned contrasts were performed across conditions and trials.42 Switch costs were calculated by subtracting ER and RT on trial 3 from trial 1, averaged across conditions (figure 3, left). Congruence costs were calculated by subtracting performance on incongruent trials from congruent trials for repeat trials (figure 3, middle). Mixing costs were calculated by subtracting trial performance in the pure task condition from performance in the mixed condition (figure 3, right). All costs were calculated separately for color and letter tasks and compared across groups using repeated-measures ANOVA with within-subject factor of task and between-subject factor of diagnostic group.
Fig. 3.
Switching Cost, Congruence Cost, and Mixing Cost. The Error rates (ERs; upper) and log response times (RTs; lower) are shown for switching costs (left), congruence cost (middle), and mixing costs (right). Filled bars depict data from patients; open bars depict data from controls. Switching costs were calculated by subtracting the relevant data (ER or RT) on Repeat2 trials from the data on switch trials. Congruence costs were calculated by subtracting the data on congruent trials from incongruent trials. Mixing costs were calculated by subtracting the data on pure-task trials from mixed-task trials.
Switch Costs.
Rather than showing increased switch costs for error rate, patients showed somewhat of a tendency toward decreased costs across tasks (F1,31 = 3.67, P = .065). There was also a significant group × task interaction (F1,31 = 4.95, P = .034), reflecting significantly lower switch costs in the patient group compared with the healthy control (HC) group in the letter (t = 2.40, df = 31, P = .023) but not color (t = 0.2, df = 31, P = .8) conditions. Patients did not show change in RT accompanying the increase in error relative to controls (F1,31 = 0.66, P = .42), suggesting that both groups applied similar speed/accuracy trade-offs.
Congruence Costs.
Congruence costs were most apparent in error rate. Across tasks, there was a significant main effect of group (F1,31 = 6.87, P = .013), reflecting larger congruence costs for patients than controls. The task × group interaction was nonsignificant (F1,31 = 1.80, P = .19). Patients did not show change in RT accompanying the increase in error rate relative to controls, suggesting that both groups applied similar speed/accuracy trade-offs.
Mixing Costs.
As with congruence costs, mixing costs were also most apparent in error rate. Patients had larger mixing costs for error rate than controls across color and letter tasks (F1,30 = 12.1, P = .002). There was also a significant main effect of task (F1,30 = 10.0, P = .004), reflecting greater mixing costs across groups for the letter vs the color task. Finally, task interacted reliably with group (F1,30 = 11.3, P = .002), reflecting that for patients (t14 = 3.23, P = .006), but not HC (t16 = 0.3, P = .7), mixing costs were significantly greater for the letter, as compared with color, task. Differential deficits are unlikely to reflect differential discriminating power of the tests because SDs for the 2 tasks were extremely similar in and mean values, if anything were higher for the color, than letter, task. Patients did not show change in RT accompanying the increase in error rate relative to controls, suggesting that both groups applied similar speed/accuracy trade-offs.
Discriminating Power
In order to evaluate relative discriminating power for color and letter tasks, true score variance (TSV) was computed for the letter and color tasks based upon reliability and observed variance scores in controls. Split-half reliability for error rate measurements for the color and letter tasks was 0.86 and 0.83, respectively, yielding TSV values of 5.95 and 6.64 percentage points. Split-half reliability measures for the RT measures were 0.94 and 0.97, respectively, yielding TSV values of 229.5 and 278.2 milliseconds. TSV values thus appear similar across tasks.
Discussion
Schizophrenia is associated with widespread neurocognitive dysfunction that represents a core feature of the disorder. The present study evaluated switch costs relative to underlying task parameters (ie, congruity, trial, task type) in order to examine bases of normal and abnormal performance in schizophrenia. The paradigm involved competing letter and color tasks as in prior studies from our group. In addition, task-switching performance was assessed relative to stimulus congruence or incongruence and mixing costs. The primary findings are that patients showed no increase in switch costs under any condition in this paradigm, consistent with prior clinical literature.36,40,44,45 In contrast to normal switch performance, patients showed increased error rate and RT overall as well as increased effects of stimulus incongruence and increased costs of performing a divided vs pure task (“mixing” costs). Patients also showed differential deficits based upon whether letter or color cues were to be attended, suggesting potential contributions of sensory processing to the overall pattern of task-switching deficits.
In task-switching paradigms, subjects must respond differentially to a stimulus based upon task instructions. A classic finding in these experiments is that even though subjects are explicitly aware of the task on each trial, nevertheless they perform more slowly and usually less accurately on the first trial after a switch than on subsequent trials. The difference in RT between the switch and repeat trial, referred to as switch cost, is thought to reflect competition between competing response networks. fMRI investigations of task switching show increased activity in a frontoparietal network of areas when switch trials are compared to repeat trials, eg, 18,19–26,36,44, suggesting that this network mediates the competition between tasks.26
Although frontoparietal processing deficits are widely discussed in schizophrenia, the present set shifting findings, along with other findings with this task, would argue against global dysfunction of frontoparietal function as an explanatory construct in schizophrenia. In particular, while an increase in switch costs is seen following brain damage, particularly to the left hemisphere,17,46 it is not observed in the present study. The present findings of preserved switch costs adds to a growing body of literature of paradoxically preserved frontoparietal functions in schizophrenia, such as intact resistance to cross-modal distraction in tone matching paradigms47 and preserved ability to allocate visual attention.48
An alternative approach to conceptualization of cognitive dysfunction in schizophrenia is that rather than involving specific brain regions, it involves specific neurochemical processes across brain regions. In particular, it has been proposed that deficits in NMDA functioning may be widespread through the brain in schizophrenia and may underlie the pattern of cognitive dysfunction seen in schizophrenia.38 Administration of NMDA antagonists to normal volunteers induces deficits in a variety of cognitive tasks such as Stroop,49 WCST,50 or AX-CPT51 that closely resemble the pattern observed in schizophrenia.
In monkeys, ketamine treatment induces a pattern of prolongation of RT and increased incongruence costs but no increase in switch costs37 similar to that observed here. Interpretability of that study is somewhat reduced by the absence of significant switch and congruence costs under nonketamine conditions, suggesting the need for further ketamine challenge studies in monkeys with shorter preparation time or in humans. Nevertheless, our findings are consistent with the hypothesis that deficits in NMDA transmission, rather than anatomic disturbances, may give rise to the pattern of task-switching deficit observed in schizophrenia.
Although the present study found the predicted absence of elevated switch costs in schizophrenia, 2 differences were observed between performance patterns in patients and that of controls. First, in addition to showing increased incongruity costs, patients also showed increased mixing costs. Mixing costs have not been previously evaluated in schizophrenia, with the absence of such studies having been noted previously.40 In general, 2 theories have been proposed to account for mixing costs. First, it has been proposed that such costs are related to sustained processes related to maintenance of working memory load required to maintain additional stimulus-response relationships, particularly involving left prefrontal regions.18 Alternatively, mixing costs have been proposed to reflect increased transient cost of resolving task ambiguity even when, as for neutral stimuli, the competing task is not relevant to the instant stimulus.26,42,52 As such, mixing costs are expected to increase along with congruence costs. The fact that patients with schizophrenia show increased mixing costs along with increased congruence costs, but no increase in switch costs, supports the concept that mixing costs, in general, are related to task competition and dissociated from processes involved in generating switch costs as proposed by Rubin and Meiran.42
The increase in congruence and mixing costs observed in this study were manifest only in error rate, not RT, raising issues of whether patients were using different speed/accuracy trade-offs. However, RT costs were similar across groups, suggesting that the increased error rates were not related to differential trade-offs vs speed, and reflected true increased costs for patients vs controls. The fact that both mixing and congruence costs increase in parallel in schizophrenia supports the concept that both may be manifestations of similar task competition mechanisms. We have recently observed that mixing costs are associated with a change in ERP generator configuration, reflecting competition between networks, whereas switch costs are associated with increased global field power but no change in generator configuration, reflecting only increased degree of activation within an already selected network.29 Thus, increased congruity and mixing costs in the face of preserved switch costs may relate to mechanisms underlying competition across, rather than within, specific neural networks.
A secondary finding with respect to mixing costs is that patients showed increased mixing costs for letter vs color stimuli whereas controls performed the task similarly whether they attended to letter or color, leading to a significant group × task interactions (P = .002). This result may relate to recent findings of impaired sensory processing in schizophrenia and, particularly, reading.53 We have also previously reported that performance in AX-CPT tasks in schizophrenia is relatively improved when color, rather than letter, stimuli are used as cues.54 Previous task-switching studies in schizophrenia have employed a variety of dimensions such as direction (up/down vs right/left),40 size/shape,36 or picture/line naming45 and have in general found prolonged RT but no increase in switch costs. In contrast, Cools et al35 used color vs shape stimuli and did not observe either increased switch costs or increased RT, suggesting that specific stimulus features may be critical for interpreting these tasks.
In our study, it is unlikely that differential discriminating power was responsible for the finding of differential deficit between performance on letter and color task because controls performed somewhat better on the color task (in which no deficit was found) relative to the letter task (in which deficit was found), so differences in discriminating power, if anything, should work against finding a between-group differential deficit.55 Further, variance in controls on the 2 tasks was virtually identical, suggesting relatively similar discriminating power. As with congruence costs, it has been found in monkeys that ketamine treatment impairs ability to perform a delayed match to sample task involving shapes but not colors,56 suggesting that impaired relative performance on the letter vs color task in patients may, like increased congruence costs, reflect underlying NMDA dysfunction.
A final interesting observation in our dataset is that switch costs measured using error rate showed a somewhat different pattern than switch costs measured using RT. For error rate, switch costs were observed across groups only when the switch stimulus was incongruent, leading to a highly significant congruity × trial interaction (P = .005). For congruent and neutral trials, in contrast, no significant switch costs were observed. This effect was similar across groups, as reflected in a nonsignificant 3-way congruity × trial × group interaction (P = .25). For RT analyses, a significant congruity × trial interaction was also observed (P = .008), again reflecting greater switch costs for incongruent than congruent or neutral trials. In this case, however, significant switch costs were observed even for the congruent and neutral stimuli (figure 3). As with error rate, the 3-way congruity × trial × group interaction was not significant (P = .7), suggesting similar effect in both groups. The lack of switch cost on error rate for congruent and neutral stimuli may reflect a floor effect because controls showed extremely low error rates even for switch trials when stimuli were congruent or neutral. An alternative explanation, however, is that both patients and controls employ similar speed/accuracy trade-off such that they “defend” the RT of the congruent switch stimulus to a greater extent than they defend the error rate. Thus, both groups make more errors in the incongruent, than congruent, switch condition, limiting the degree to which this condition leads to a disproportionate increase in RT. Although exact neurophysiological mechanisms underlying this effect cannot be determined in the present dataset, the lack of congruency × trial × group interactions for either error rate or RT suggests that both groups employ a similar strategy despite the disparate absolute performance levels.
In summary, the present findings are consistent with prior literature showing a paradoxical lack of task switching difficulty in patients with schizophrenia, given widespread deficits in other aspects of executive functioning. Nevertheless, increased RTs were observed, as well as increased incongruency and mixing costs, and suggestion of greater deficits for letter, than color, stimuli. Similar to the pattern observed here, recent primate studies in monkeys treated with the NMDA antagonist ketamine have found increased incongruency costs and RT prolongation but no increase in switch costs. Similarly, studies in monkeys have found form processing to be more affected by ketamine administration than color. The present findings suggest that schizophrenia is associated with a specific pattern of dysfunction in the task switching paradigm and that this pattern may be useful for evaluating specific neurochemical, as well as neuroanatomical, models of schizophrenia.
References
- 1.Monsell S. Control of mental processes. In: Bruce V, editor. Unsolved Mysteries of the Mind: Tutorial Essays in Cognition. Hove, UK: Erlbaum; 1996. pp. 93–148. [Google Scholar]
- 2.Rogers RD, Monsell S. The cost of a predictable switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124:207–231. [Google Scholar]
- 3.Meiran N. Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn. 1996;22:1–20. [Google Scholar]
- 4.Logan GD, Bundesen C. Clever homunculus: is there an endogenous act of control in the explicit task-cuing procedure? J Exp Psychol Hum Percept Perform. 2003;29:575–599. doi: 10.1037/0096-1523.29.3.575. [DOI] [PubMed] [Google Scholar]
- 5.Mayr U, Keele SW. Changing internal constraints on action: the role of backward inhibition. J Exp Psychol Gen. 2000;129:4–26. doi: 10.1037//0096-3445.129.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Meiran N, Levine J, Henik A. Task set switching in schizophrenia. Neuropsychology. 2000;14:471–482. doi: 10.1037//0894-4105.14.3.471. [DOI] [PubMed] [Google Scholar]
- 7.Wylie GR, Allport A. Task switching and the measurement of “switch costs”. Psychol Res. 2000;63:212–233. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]
- 8.Waszak F, Hommel B, Allport A. Task-switching and long-term priming: role of episodic stimulus-task bindings in task-shift costs. Cognit Psychol. 2003;46:361–413. doi: 10.1016/s0010-0285(02)00520-0. [DOI] [PubMed] [Google Scholar]
- 9.Spector A, Biederman I. Mental set and shift revisited. Am J Psychology. 1976;89:669–679. [Google Scholar]
- 10.Rubinstein JS, Meyer DE, Evans JE. Executive control of cognitive processes in task switching. J Exp Psychol Hum Percept Perform. 2001;27:763–797. doi: 10.1037//0096-1523.27.4.763. [DOI] [PubMed] [Google Scholar]
- 11.Rushworth MF, Passingham RE, Nobre AC. Components of switching intentional set. J Cogn Neurosci. 2002;14:1139–1150. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- 12.Monsell S, Yeung N, Azuma R. Reconfiguration of task-set: is it easier to switch to the weaker task? Psychol Res. 2000;63:250–264. doi: 10.1007/s004269900005. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh S, Allport A. Shifting attention in a rapid visual search paradigm. Percept Mot Skills. 1994;79(pt 1):315–335. doi: 10.2466/pms.1994.79.1.315. [DOI] [PubMed] [Google Scholar]
- 14.DeJong R. An intention-activation account of residual switch costs. In: Monsell S, Driver JS, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, Mass: MIT Press; 2001. pp. 357–376. [Google Scholar]
- 15.Allport DA, Styles EA, Hsieh S. Shifting intentional set: exploring the dynamic control of tasks. In: Umilta C, Moscovitch, editors. Attention and Performance XI. Cambridge, Mass: MIT Press; 1994. pp. 107–132. [Google Scholar]
- 16.Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- 17.Mecklinger AD, von Cramon DY, Springer A, Matthes-von Cramon G. Executive control functions in task switching: evidence from brain injured patients. J Clin Exp Neuropsychol. 1999;21:606–619. doi: 10.1076/jcen.21.5.606.873. [DOI] [PubMed] [Google Scholar]
- 18.Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 19.Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- 20.Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- 21.Dreher JC, Berman KF. Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci USA. 2002;99:14595–14600. doi: 10.1073/pnas.222193299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 23.Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 24.Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sylvester CY, Wager TD, Lacey SC, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 26.Wylie GR, Javitt DC, Foxe JJ. Don't think of a white bear: an fMRI investigation of the effects of sequential instructional sets on cortical activity in a task-switching paradigm. Hum Brain Mapp. 2004;21:279–297. doi: 10.1002/hbm.20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wylie GR, Javitt DC, Foxe JJ. Task switching: a high-density electrical mapping study. Neuroimage. 2003;20:2322–2342. doi: 10.1016/j.neuroimage.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci. 2005;17:1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- 29.Wylie GR, Murray MM, Javitt DC, Foxe JJ. Distinct neurophysiological mechanisms mediate mixing costs and switch costs [Epub ahead of print May 13, 2008] J Cogn Neurosci. 2008 doi: 10.1162/jocn.2009.21009. [DOI] [PubMed] [Google Scholar]
- 30.Barch DM, Carter CS, Cohen JD. Factors influencing Stroop performance in schizophrenia. Neuropsychology. 2004;18:477–484. doi: 10.1037/0894-4105.18.3.477. [DOI] [PubMed] [Google Scholar]
- 31.Twamley EW, Palmer BW, Jeste DV, Taylor MJ, Heaton RK. Transient and executive function working memory in schizophrenia. Schizophr Res. 2006;87:185–190. doi: 10.1016/j.schres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- 33.Barton JJ, Cherkasova MV, Lindgren K, Goff DC, Intriligator JM, Manoach DS. Antisaccades and task switching: studies of control processes in saccadic function in normal subjects and schizophrenic patients. Ann N Y Acad Sci. 2002;956:250–263. doi: 10.1111/j.1749-6632.2002.tb02824.x. [DOI] [PubMed] [Google Scholar]
- 34.Manoach DS, Lindgren KA, Cherkasova MV, et al. Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biol Psychiatry. 2002;51:816–826. doi: 10.1016/s0006-3223(01)01356-7. [DOI] [PubMed] [Google Scholar]
- 35.Cools R, Brouwer WH, de Jong R, Slooff C. Flexibility, inhibition, and planning: frontal dysfunctioning in schizophrenia. Brain Cogn. 2000;43:108–112. [PubMed] [Google Scholar]
- 36.Kieffaber PD, Kappenman ES, Bodkins M, Shekhar A, O'Donnell BF, Hetrick WP. Switch and maintenance of task set in schizophrenia. Schizophr Res. 2006;84:345–358. doi: 10.1016/j.schres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Stoet G, Snyder LH. Effects of the NMDA antagonist ketamine on task-switching performance: evidence for specific impairments of executive control. Neuropsychopharmacology. 2006;31:1675–1681. doi: 10.1038/sj.npp.1300930. [DOI] [PubMed] [Google Scholar]
- 38.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 39.Kieffaber PD, Hetrick WP. Event-related potential correlates of task switching and switch costs. Psychophysiology. 2005;42:56–71. doi: 10.1111/j.1469-8986.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 40.Meiran N, Levine J, Meiran N, Henik A. Task set switching in schizophrenia. Neuropsychology. 2000;14:471–482. doi: 10.1037//0894-4105.14.3.471. [DOI] [PubMed] [Google Scholar]
- 41.Mayr U, Kliegl R. Differential effects of cue changes and task changes on task-set selection costs. J Exp Psychol. 2003;29:362–372. doi: 10.1037/0278-7393.29.3.362. [DOI] [PubMed] [Google Scholar]
- 42.Rubin O, Meiran N. On the origins of the task mixing cost in the cuing task-switching paradigm. J Exp Psychology. 2005;31:1477–1491. doi: 10.1037/0278-7393.31.6.1477. [DOI] [PubMed] [Google Scholar]
- 43.Hollingshead AB, Redlich FC. Schizophrenia and social structure. Am J Psychiatry. 1954;110:695–701. doi: 10.1176/ajp.110.9.695. [DOI] [PubMed] [Google Scholar]
- 44.Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP. Event related brain potential evidence for preserved attentional set switching in schizophrenia. Schizophr Res. 2007;93:355–365. doi: 10.1016/j.schres.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karayanidis F, Nicholson R, Schall U, Meem L, Fulham R, Michie PT. Switching between univalent task-sets in schizophrenia: ERP evidence of an anticipatory task-set reconfiguration deficit. Clin Neurophysiol. 2006;117:2172–2190. doi: 10.1016/j.clinph.2006.06.716. [DOI] [PubMed] [Google Scholar]
- 46.Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain. 1998;121(pt 5):815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 47.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Archives of general psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 48.Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. 2006;115:266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- 49.Cohen BD, Rosenbaum G, Luby ED, Gottlieb JS. Comparison of phencyclidine hydrochloride (sernyl) with other drugs. simulation of schizophrenic performance with phencyclidine hydrochloride (sernyl), lysergic acid diethylamide (LSD-25) and amobarbital (Amytal) sodium, II: symbolic and sequential thinking. Arch Gen Psychiatry. 1962;6:79–85. doi: 10.1001/archpsyc.1962.01710230063007. [DOI] [PubMed] [Google Scholar]
- 50.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 51.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 52.Wylie GR, Javitt DC, Foxe JJ. Cognitive control processes during an anticipated switch of task. Eur J Neuroscience. 2003;17:667–672. doi: 10.1046/j.1460-9568.2003.02474.x. [DOI] [PubMed] [Google Scholar]
- 53.Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87:238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javitt DC, Rabinowicz E, Silipo G, Dias EC. Encoding vs. retention: differential effects of cue manipulation on working memory performance in schizophrenia. Schizophr Res. 2007;91:159–168. doi: 10.1016/j.schres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strauss ME. Demonstrating specific cognitive deficits: a psychometric perspective. J Abnorm Psychol. 2001;110:6–14. doi: 10.1037//0021-843x.110.1.6. [DOI] [PubMed] [Google Scholar]
- 56.Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]