Abstract
The issue of neurodegeneration in schizophrenia is controversial. Although most studies indicate that neurocognitive deficits are relatively stable over the course of the illness, conclusions are limited by relatively short follow-up periods and absence of age-matched control groups. Furthermore, nearly all studies deal with adult-onset schizophrenia, and few studies have considered the possible effect of age of onset. The current study represents the first attempt to compare groups of adolescents with schizophrenia, attention deficit/hyperactivity disorder (ADHD), and normal controls on a comprehensive neurocognitive test battery in a longitudinal design over 13 years. In the baseline study, adolescents with schizophrenia were examined with a broad battery of neurocognitive tests. The comparison groups consisted of adolescents with ADHD and adolescents without a psychiatric diagnosis, between 12 and 18 years of age. In the follow-up study, the schizophrenia group consisted of 15 of the initial 19 individuals, the ADHD group of 19 of the 20 individuals, and the normal comparison group of all 30 individuals. They were reevaluated with the neurocognitive test battery and clinical measures. Subjects with schizophrenia showed a significant decline or arrest in neurocognitive functioning compared with the other 2 groups, particularly in verbal memory, attention, and processing speed. The impairments may be specific to early-onset schizophrenia due to interaction between ongoing brain maturation during adolescence and disease-related mechanisms and/or secondary to neuroleptic treatment in young age and/or social isolation.
Keywords: neurocognition, early-onset schizophrenia, longitudinal
Introduction
Schizophrenia has primarily been viewed as a neurodevelopmental disorder. Recent neurobiological studies suggest that there are both neurodevelopmental and neurodegenerative components to the illness. Longitudinal neurobiological studies have found progressive ventricular enlargement and cortical gray matter loss in frontal and temporal areas during adolescence in patients with childhood-onset schizophrenia compared with normal controls.1 These studies indicate a neurobiological degenerative process in the pathophysiology of schizophrenia.
Neurocognitive studies have reported deficits to be present before onset and early in the course of schizophrenia.2–6 However, longitudinal neurocognitive studies have reported first-episode schizophrenia do not indicate a progression of neurocognitive dysfunction over the first year of illness (see Rund et al7). One study concluded that first-episode patients have relatively stable neurocognitive deficits through at least 10 years of illness.8 Thus, findings from longitudinal neurocognitive studies support a view of schizophrenia as a static encephalopathy. One study of first-episode patients, however, found decline in visuospatial functioning over a 10- to 12-year period.9 Neurocognitive decline has also been described in geriatric, chronically institutionalized patients.10 Further, a recent 4-year follow-up study of adolescents with early-onset schizophrenia reported that most aspects of neurocognitive function remained relatively stable, but there was evidence for deterioration in immediate verbal memory and attention.11 Thus, there has been some conflicting evidence concerning whether the progression of neurostructural abnormalities observed after the onset of symptoms is associated with worsening of neurocognitive functioning.
Most longitudinal neurocognitive studies of schizophrenia patients are limited by relatively short follow-up periods ranging from 8 months to 5 years. Only two studies have a longer follow-up time, ie, 10 years.8,9 Further, most studies are restricted to adult patient samples and confounded by varying durations of illness within the groups. A third critique of most studies is the lack of normal comparison groups and the limited range of tests used. The strongest evidence for progression of neurobiological events comes from studies of adolescents.1 The brains of patients with early-onset schizophrenia may follow different pathological growth patterns as evidenced by slowed, arrested, or reversed maturational development.12 In behavioral terms, the consequence will be smaller improvements in performance on neurocognitive tests compared with controls, stable raw scores leading to decline in age-scaled scores, or a genuine decrease in actual performance. Thus, longitudinal studies of neurocognitive functioning in adolescents with schizophrenia have the potential of detecting which of these cognitive patterns of changes are associated with developing neurobiological degenerative processes.
In order to investigate to which degree changes in neurocognition specifically affect subjects with schizophrenia, the current study includes 2 comparison groups, ie, healthy controls and subjects with attention /hyperactivity disorder (ADHD). Studies of neurocognition in children with ADHD have shown that they improve their performance as they reach late adolescence, although they remain impaired in executive dysfunctions, attention, and memory compared with healthy controls.13 Only 2 neurocognitive studies have followed children with ADHD into adulthood (see Seidman13). These studies found that neurocognitive deficits remain present in young adult. Another study found that growth curves of total cerebral volume and cerebellar volume were lower, but parallel for ADHD patients, compared with healthy controls, ie, no progressive deficit.14
The current study seeks to meet all the above mentioned demands. It is the first study to investigate the course of neurocognitive status after a follow-up time of 13 years in subjects with early-onset schizophrenia and subjects with ADHD using a broad neurocognitive test battery. On the basis of studies indicating progression of frontotemporal cortical gray loss in schizophrenia, we hypothesize that if neurocognitive arrest or decline is present, the change should be detectable on measures of attention, memory, and executive functioning. We also predict that the ADHD subjects and the healthy controls will improve their neurocognitive performance as they get older but that the ADHD group will perform significantly below the healthy control group also at follow-up.
Methods
Participants
Fifteen subjects from a baseline (T1) sample of 19 subjects with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), diagnosis of schizophrenia (see Øie and Rund2 and Øie et al3) and 19 subjects from a baseline sample of 20 subjects with ADHD were available for reassessment (T2) with psychiatric and neurocognitive measures after 13 years (T2). The same sample of 30 healthy comparison subjects was included at T1 and T2. None of the healthy comparison subjects had a history of neurological, somatic, or psychiatric illness known to influence neurocognitive function. At T1, 15 of the subjects with schizophrenia were inpatients, while the 4 outpatients had never been hospitalized. The schizophrenia diagnosis and subtype determination was assigned based on clinical interviews by senior clinicians and patient case records. Consensus regarding diagnosis was investigated on a subsample of 13 patients. Two senior psychologists agreed on the schizophrenia diagnosis in 12 (92%) of the cases. All 19 patients were reassessed 1 year later, and the diagnosis was confirmed in each case. At T1, all ADHD subjects were outpatients. Diagnostic evaluations at T1 for the ADHD sample were conducted using semistructured clinical interviews and standardized rating scales. The adolescents fulfilled at least 8 of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition Revised, criteria for the condition. In addition, all had shown significant hyperactivity, impulsivity, and inattention between ages 6 and 10 years as assessed by the Parent's Rating Scale.15 None of the patients had a history of psychosis. The subjects in the normal group attended regular school classes at normal grade levels.
Of the 19 subjects with schizophrenia included at T1, 2 were deceased and 2 declined to be tested at T2. Among the 15 who volunteered to participate at T2, 3 were recovered. Of the 20 subjects with ADHD at T1, one was deceased. Among the 19 who were tested at T2, 4 were recovered. All subjects in the study were screened for mental retardation (Wechsler Abbreviated Scale of Intelligence [WASI]18 IQ > 70). Of the 30 healthy comparison subjects, all still fulfilled the criteria to serve as healthy controls.
At T2, diagnoses in the schizophrenia group were based on the Structured Clinical Interview for the DSM-IV and information from patients’ parents and/or their psychiatrists, nurses, and/or social workers. One psychologist and one psychiatrist reviewed all available information for agreement on the DSM-IV diagnosis. They agreed on the diagnosis in 94% of the cases. Demographics are presented in table 1 and specific schizophrenia diagnoses in table 2. Ten of the patients in the schizophrenia group had been hospitalized during the follow-up period (mean weeks 193.3, SD 261.4). The mean duration of illness for patients with a schizophrenia diagnoses at T2 was 12.7 years (SD 1.5). At T2, 8 were outpatients, 4 were inpatients, and 3 were recovered. One patient was using anticholinergic medication; 2 were using clozapine. One patient sporadically used cannabis. The patients were tested when they were judged by the examiner and/or by their clinician to be clinically stabilized on their antipsychotic medication and not experiencing an acute episode of illness.
Table 1.
Demographics
| ANOVA (df = 2, 61) |
||||||
| Variable | Schizophrenia (n = 15) | ADHD (n = 19) | Healthy Controls (n = 30) | F | P | Scheffé |
| Sex (male/female) | 10/5 | 19/0 | 16/14 | .001 | (Fisher) | |
| Hand dominance (R/L) | 15/0 | 16/3 | 29/1 | .183 | (Fisher) | |
| Age (y) | 27.7 (1.4) | 25.8 (1.5) | 27.6 (1.5) | 10.8 | <.001 | A < S, C |
| Time since T1 (y) | 11.6 (0.9) | 11.7 (0.6) | 12.1 (0.6) | 2.7 | .075 | — |
| Education (y) | 10.3 (1.5) | 11.5 (2.1) | 15.4 (1.7) | 50.4 | <.001 | S, A < C |
| Mother's education (y) | 12.2 (2.8) | 12.6 (2.5) | 14.7 (2.3) | 6.9 | .002 | S, A < C |
| Mean age at onset (y) | 14.8 (1.4) | — | — | |||
| Time from diagnoses to T1 (mo) | 14.0 (10.0) | — | — | |||
| FSIQ (WASI)a | 92.6 (14.4) | 104.2 (8.8) | 112.6 (8.6) | 19.2 | <.001 | S < A < C |
| GASb | ||||||
| Symptom | 4.9 (1.6) | 6.5 (1.5) | 9.0 (0.2) | 71.1 | <.001 | S < A < C |
| Function | 47.7 (17.0) | 66.4 (15.8) | 88.7 (2.6) | 62.2 | <.001 | S < A < C |
| BPRSc | ||||||
| Positive | 13.3 (8.3) | — | — | |||
| Negative | 6.3 (3.1) | — | — | |||
| Total | 45.0 (17.5) | — | — | |||
| Medication | ||||||
| DDDd | 2.7 (1.8) | 1.2 (0.7) | — | 2.3 | .152 | |
| Typical antipsychotic | n = 2 | — | ||||
| Atypical –‘’ – | n = 4 | n = 1 | ||||
| Both –‘’ – | n = 4 | — | ||||
| Stimulants | — | n = 2 | ||||
| Atomoksetin | — | n = 1 | ||||
| Lamotrigin | — | n = 1 |
Note: ADHD, attention deficit/hyperactivity disorder; ANOVA, analysis of variance.
Full-Scale IQ from the Wechsler Abbreviated Scale of Intelligence.
Global Assessment Scale.
Brief Psychiatric Rating Scale (Positive Scale = 7 items, Negative Scale = 3 items).
Defined daily doses (Norwegian Medial Depot), schizophrenia: n = 10, ADHD: n = 4.
Table 2.
Diagnoses at T1 and T2 in the Schizophrenia Group
| Case | T1 | T2 |
| 1 | Schizophrenia disorganized | Schizophrenia disorganized |
| 2 | Schizophrenia disorganized | Schizophrenia disorganized |
| 3 | Schizophrenia disorganized | Schizophrenia disorganized |
| 4 | Schizophrenia disorganized | Schizophrenia disorganized |
| 5 | Schizophrenia disorganized | Schizophrenia disorganized |
| 6 | Schizophrenia disorganized | Schizophrenia disorganized |
| 7 | Schizophrenia paranoid | Schizophrenia paranoid |
| 8 | Schizophrenia paranoid | Schizophrenia paranoid |
| 9 | Schizophrenia disorganized | Schizoaffective disorder |
| 10 | Schizophrenia disorganized | Schizophrenia paranoid |
| 11 | Schizophrenia undifferentiated | Schizoaffective disorder |
| 12 | Schizophrenia disorganized | Schizophrenia paranoid |
| 13 | Schizophrenia disorganized | Recovered |
| 14 | Schizophreniform disorder | Recovered |
| 15 | Schizophrenia paranoid | Recovered |
| 16 | Schizophrenia disorganized | Deceased |
| 17 | Schizophrenia paranoid | Deceased |
| 18 | Delusional disorder | Declined to be tested |
| 19 | Schizoaffective disorder | Declined to be tested |
At T2, the ADHD group consisted of 15 subjects with a DSM-IV diagnosis of current ADHD (inattentive or combined subtypes). The remaining 4 subjects were symptom free and received no diagnoses. Four of the ADHD subjects had been hospitalized in the follow-up period due to substance abuse (mean 62.0 weeks, SD 107.5). None were inpatients at T2. Medication was discontinued at least 15 hours before testing. One of the ADHD patients, treated with methylphenidate, was also prescribed Risperidon. None were excluded due to a reported a history of neurological or somatic illness known to influence neurocognitive function. The study was approved by the Regional Committee for Medical Research Ethics in Eastern Norway and was conducted in accordance with the Helsinki Declaration of the World Medical Association Assembly. After a complete description of the study, written informed consent was obtained.
In line with diagnostic group characteristics, male subjects were more frequently represented in both clinical groups compared with healthy controls. The ADHD group was slightly younger than the other 2 groups. This age difference is not considered large enough to represent a serious confounder to the research question and will not be corrected for in the analyses. The healthy comparison group had significantly longer education, significantly higher IQ scores, and mothers with significantly longer education than the 2 clinical groups. Because lower education and IQ may be considered side effects of having schizophrenia or ADHD, only mother's education was used as covariate in follow-up analyses of group differences in neurocognitive function.
Neurocognitive Test Battery
All subjects were assessed at T2 using the same comprehensive neurocognitive test battery as used at T1 except for replacing age-appropriate versions of the digit span and digit symbol subtests from Wechsler Intelligence Scale for Children - Revised16 with those of Wechsler Adult Intelligence Scale - Third Edition17 and for using WASI18 to screen for IQ level. All subjects were tested individually and received the tests in the same fixed order. Total time for assessment was about 3 hours, including breaks. The test battery included (in order of administration): Wisconsin Card Sorting Test (WCST),19 matrix reasoning from WASI,18 digit span from WAIS-III,17 digit symbol from WAIS-III,17 California Verbal Learning Test (CVLT),20 Kimura Recurring Figures Test,21 Seashore Rhythm Test,22 Digit Repetition Test,23 CVLT delayed recall and recognition,20 Trail Making Test, A and B (TMT A, B),22 Similarities from WASI,18 Block Design from WASI,18 Backward Masking Test (BMT),24 Grooved Pegboard Test,22 Vocabulary from WASI.18 The 4 WASI subtests were used to calculate full-scale IQ (see table 1). The test battery also included a dichotic listening test; the data are reported in a separate study.25
Psychiatric Ratings
At T1 and T2, the subjects in the schizophrenia group were assessed with the expanded Brief Psychiatric Rating Scale (BPRS)26 within 1 week of testing. The interrater reliability for the BPRS total score was 0.99 (Intraclass Correlation Coefficient = 1.2) at baseline. A “positive symptom” score and a “negative symptom” score based on a factor analysis conducted by Ph.d. Joseph Ventura, Ph.d. Keith H. Nuechterlein, and Ph.D. Kenneth Subotnik at UCLA (1995; unpublished manuscript) were extracted from the BPRS. The 2 patient groups were also assessed using the Global Assessment Scale (GAS).27 The schizophrenia patients scored significantly lower (P < .001) on the GAS than the ADHD patients, both at T1 and at T2.
Data Analyses
Analyses were conducted using the statistical package SPSS for Windows, version 12.0 (SPSS, Inc., Chicago, IL). Differences in demographic characteristics were investigated by the Fisher exact probability test (nominal variables) and analysis of variance (ANOVA) (continuous variables) followed up by Scheffés post hoc tests for group comparisons. Changes in test results from T1 to T2 were first analyzed using repeated-measure multiple ANOVA with time of testing (T1, T2) and neuropsychological test measures (20 variables) as within-subject factors and diagnostic group (schizophrenia, ADHD, healthy controls) as the between-group factor. In case of significant effects, particularly significant interactions between time and group, as well as between time, test, and group, follow-up repeated-measure ANOVAs with time (T1, T2) and group (schizophrenia, ADHD, healthy controls) as factors were conducted. Effect sizes (η2) for the interaction effect between time and group were computed. On tests with large interaction effects, paired-sample t tests were performed for each group comparing T1 and T2 results. A second set of a multiple analysis of covariance (MANCOVA) and follow-up analyses of covariance (ANCOVAs) were performed with mother's education as a covariate, to control for possible artifacts due to differences in neurocognitive inheritance.
Results
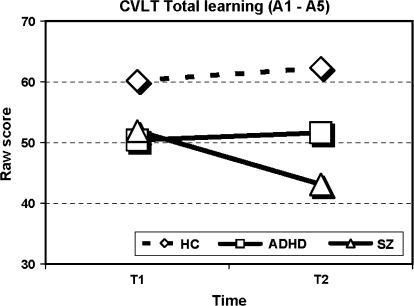
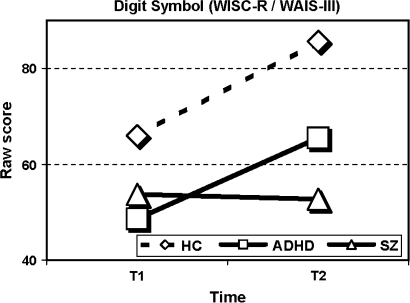
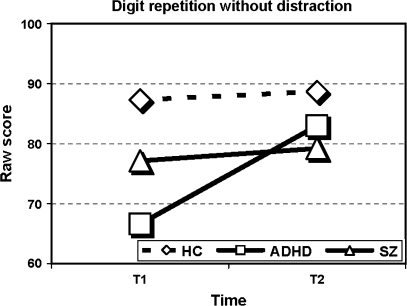
Results are shown in table 3. Significant interaction effects between time and group (F2,56 = 4.0, P = .024) and between time, group, and neurocognitive measure (F38,78 = 3.2, P < .001) were found thus inviting to follow-up analyses for each measure separately across group and time. Significant effects of group across the 2 assessment times were found for all measures except digit span forward and the 2 masking conditions of the BMT. Significant changes in performance over time, irrespective of group, were found on the Digit Repetition tasks (without and with distraction), on the TMT B, the digit symbol subtest, and the no-mask and the 33-millisecond masking condition of the BMT. Most important for the research question was the finding of multiple time × group interaction effects. On the CVLT, the WCST (perseverative responses), the Digit Repetition (without and with distraction), the Seashore Rhythm Test, the digit symbol subtest, and all the conditions of the BMT, the schizophrenia group did not show the same improvement in performance from T1 to T2 as did the ADHD group and the healthy controls. On the CVLT, total learning trials (see figure 1), the schizophrenia group showed a significant decline (t14 = 3.3, P = .005) in performance while the other 2 groups showed similar results at follow-up compared with baseline. This interaction effect is both significant and large (η2 = 0.21). On other tests, the schizophrenia group obtained similar scores, whereas the other 2 groups showed improved performance. This effect was most evident on the processing speed demanding digit symbol subtest (η2 = 0.27) where both ADHD (t18 = 5.3, P < .001) and healthy controls (t29 = 8.7, P < .001) improve their performance compared with the nonsignificant change in scores for the schizophrenia group (see figure 2). The significant interaction effect of group and time seen on the attention demanding Digit Repetition task (see figure 3) was explained by the ADHD group's significantly improved scores from T1 to T2 compared with controls (without distraction: F1,47 = 12.4, P = .001, with distraction: F1,47 = 4.4, P = .04). The schizophrenia group showed the same parallel development as the healthy comparison group (without distraction: F1,43 = 0.1, P = .864, with distraction: F1,43 = 1.7, P = .193).
Table 3.
Neurocognitive Test Results at T1 and T2
| Schizophrenia (n = 15) |
ADHD (n = 19) |
Healthy (n = 30) |
Group (df = 2, 61) |
Time (df = 1, 61) |
Time × Group (df = 2, 61) |
||||||||
| T1 | T2 | T1 | T2 | T1 | T2 | F | P | F | P | F | P | η2 | |
| California Verbal Learning Test | |||||||||||||
| Total A1–A5 | 51.9 (10.4) | 43.1 (11.9) | 50.4 (9.0) | 51.6 ( 6.9) | 60.2 (8.2) | 62.3 (8.7) | 19.1 | <.001 | 2.9 | .096 | 8.0 | <.001 | .21 |
| Delayed free recall | 11.4 (2.6) | 9.4 (3.4) | 10.6 (2.9) | 12.1 (2.2) | 13.1 (2.2) | 13.7 (1.8) | 12.3 | <.001 | 0.1 | .872 | 8.7 | <.001 | .22 |
| Recognition (hits-FA) | 14.3 (1.7) | 12.4 (4.0) | 14.0 (2.4) | 13.9 (2.8) | 14.8 (1.7) | 15.0 (1.1) | 3.6 | .034 | 3.5 | .067 | 3.7 | .031 | .11 |
| Kimura Recurring Figure Test (Kimura) | |||||||||||||
| Recognition (hits-FA) | 26.3 (9.7) | 25.1 (8.6) | 34.7 (10.3) | 31.7 (9.1) | 39.0 (6.4) | 36.7 (7.1) | 15.2 | <.001 | 3.4 | .069 | 0.2 | .850 | .01 |
| Wisconsin Card Sorting Test | |||||||||||||
| Categories | 4.7 (1.6) | 4.3 (2.1) | 5.1 (1.4) | 5.6 (0.9) | 5.6 (0.7) | 5.9 (0.4) | 7.5 | .001 | 0.6 | .458 | 3.1 | .054 | .10 |
| Perseverative responses | 21.9 (12.4) | 25.6 (20.9) | 19.0 (8.4) | 12.4 (5.0) | 15.6 (6.9) | 10.2 (5.1) | 8.0 | .001 | 3.7 | .061 | 4.3 | .018 | .13 |
| Failure to maintain set | 0.8 (0.6) | 1.3 (0.9) | 1.4 (1.4) | 1.6 (2.0) | 1.0 (1.4) | 0.5 (0.8) | 3.5 | .038 | 0.1 | .827 | 1.7 | .195 | .06 |
| Digit span (WISC-R/WAIS-III) | |||||||||||||
| Forward | 5.9 (1.2) | 5.9 (1.1) | 5.8 (1.2) | 6.1 (1.4) | 6.3 (1.2) | 6.6 (1.2) | 2.2 | .120 | 0.8 | .381 | 0.4 | .701 | .01 |
| Backward | 4.1 (1.7) | 3.9 (1.2) | 3.9 (1.0) | 4.3 (1.1) | 4.9 (1.4) | 4.7 (1.3) | 3.8 | .027 | 0.1 | .944 | 1.1 | .354 | .03 |
| Digit Repetition task | |||||||||||||
| Without distraction | 77.1 (20.9) | 79.2 (18.2) | 66.6 (20.0) | 83.0 (8.9) | 87.3 (10.6) | 88.7 (7.9) | 8.0 | .001 | 11.5 | .001 | 6.4 | .003 | .17 |
| With distraction | 73.1 (22.9) | 76.3 (23.9) | 63.3 (22.0) | 83.9 (13.6) | 83.6 (14.2) | 94.0 (7.2) | 8.3 | .001 | 25.2 | <.001 | 4.3 | .018 | .12 |
| Seashore Rhythm Test | |||||||||||||
| Correct | 24.9 (3.0) | 23.1 (5.3) | 25.3 (3.0) | 26.0 (2.1) | 27.1 (3.0) | 27.2 (2.7) | 6.2 | .003 | 1.0 | .325 | 4.1 | .022 | .12 |
| Grooved Pegboard Test | |||||||||||||
| Dominant hand | 76.1 (28.4) | 77.5 (25.4) | 66.6 (11.6) | 65.6 (11.3) | 61.2 (9.6) | 55.3 (7.5) | 9.5 | <.001 | 0.8 | .365 | 1.3 | .269 | .04 |
| Nondominant hand | 89.1 (23.0) | 94.0 (47.1) | 78.2 (14.4) | 74.4 (18.9) | 69.8 (8.9) | 64.6 (8.7) | 9.6 | <.001 | 0.2 | .647 | 1.1 | .344 | .03 |
| Trail Making Test | |||||||||||||
| Part A | 31.8 (15.6) | 32.3 (11.0) | 27.0 (5.2) | 26.8 (7.7) | 24.1 (6.4) | 20.2 (5.2) | 10.9 | <.001 | 0.9 | .357 | 1.3 | .273 | .04 |
| Part B | 81.7 (18.2) | 74.8 (35.6) | 80.0 (31.9) | 62.1 (21.7) | 59.8 (19.2) | 46.2 (13.6) | 9.1 | <.001 | 20.9 | <.001 | 1.1 | .338 | .04 |
| Digit Symbol (WISC-R/WAIS-III) | |||||||||||||
| Correct | 53.7 (14.8) | 52.7 (19.2) | 48.6 (12.9) | 65.5 (16.0) | 65.9 (12.2) | 85.6 (11.4) | 23.1 | <.001 | 40.9 | <.001 | 11.2 | <.001 | .27 |
| Backward Masking task | |||||||||||||
| No mask | 19.5 (1.3) | 14.7 (5.7) | 19.7 (0.7) | 17.9 (2.0) | 19.6 (0.7) | 18.1 (1.5) | 6.6 | .003 | 42.0 | <.001 | 6.0 | .004 | .17 |
| 33 ms | 4.0 (3.0) | 5.7 (4.8) | 3.9 (3.1) | 9.1 (5.3) | 5.6 (4.1) | 7.6 (3.2) | 1.4 | .250 | 31.6 | <.000 | 4.5 | .014 | .13 |
| 49 ms | 7.1 (4.4) | 7.7 (4.2) | 7.2 (5.3) | 10.9 (5.0) | 10.3 (5.1) | 9.3 (4.9) | 1.6 | .208 | 3.1 | .085 | 5.6 | .006 | .16 |
Note: ADHD, attention deficit/hyperactivity disorder; WISC-R, Wecshler Intelligence Scale for Children - Revised; WAIS-III, Wechsler Adult Intelligence Scale - Third Edition; FA, false alarms. Boldface represents the significance of value.
Fig. 1.
Performance on the California Verbal Learning Test (CVLT), Total Learning Trials (A1–A5), for the 3 Groups at T1 and T2.
Fig. 2.
Performance on the digit symbol subtest (Wechsler Intelligence Scale for Children - Revised [WISC-R] at T1/Wechsler Adult Intelligence Scale - Third Edition [WAIS-III] at T2) for the 3 Groups at T1 and T2.
Fig. 3.
Performance on the Digit Repetition Without Distraction Test for the 3 Groups at T1 and T2.
When controlling for mothers educational level by MANCOVA, significant time × group (F2,53 = 3.5, P = .036) and time × group × test (F38,70 = 2.7, P < .001) interactions remained significant. The follow-up ANCOVAs for each measure maintain all significant interaction effects except for 2, ie, WCST (perseverative responses) (P = .59) and Seashore Rhythm Test (P = .88). The overall trend showing a decline or nonimprovement in performance on the CVLT and digit symbol subtest among the schizophrenia subjects cannot be attributed to a lower inherited premorbid neurocognitive reserve as estimated by their mother's level of education.
Discussion
The current study is the longest follow-up study to date of early-onset schizophrenia compared with both a healthy and a neuropsychiatric (ADHD) control group using an extended neurocognitive test battery. The main finding is a significant decline in verbal memory and learning and a neurocognitive arrest (ie, lack of improvement with age) in attention and processing speed, after 13 years in subjects with early-onset schizophrenia. The results imply that impaired neurocognition is present early in the illness process (nevrodevelopmental), but certain later maturational processes may also be dysfunctional. Elsewhere we have reported findings on the dichotic listening (DL) procedure showing the same trend.25 Normal DL performance characterized the schizophrenia group at baseline, while the group showed significantly impaired executive attentional control at follow-up. The findings support the hypothesis of neurocognitive decline during postillness neurodevelopment in early-onset schizophrenia.
These results stand in contrast to stability of neurocognitive functioning reported in the majority of longitudinal neurocognitive studies in adults with schizophrenia. However, the results support the findings from the recent follow-up study of adolescents with early-onset schizophrenia, which found deterioration in immediate verbal memory and attention over a 4-year period.11
What could explain the results of the current study? Most of the other previous studies have included only adult patients. One hypothesis is that decline in verbal learning and memory and arrest in attention and processing speed may be secondary to a progressive pathological process in frontal and temporal areas taking place during adolescence.1 The pathological process may intersect with normal neuromaturational process of the brain during adolescence and affect the brain before it is fully maturated. Thus, if this pathological process occurs during adolescence, it may not be detected in longitudinal studies of adults with schizophrenia.
There is a broad consensus that there is underlying heterogeneity in etiology and pathophysiology in patients with schizophrenia. There is increasing evidence that early onset is associated with a more striking abnormal pattern of selective, severe frontal gray matter loss after the onset of psychosis compared with later onset schizophrenia.1 Further, some studies indicate that early-onset schizophrenia is associated with more neurocognitive impairments and more negative symptoms than adult-onset schizophrenia.28 Thus, another possible explanation of the current results is that the neurocognitive decline or arrest may be specific to early-onset schizophrenia and other subgroups of very ill patients.
Most longitudinal studies of neurocognition in schizophrenia have a relatively short follow-up time ranging from 8 months to 5 years of illness. Another possible explanation of our results is that the brain may have the capacity to partially maintain its function in the face of neurostructural progression in the first few years of illness. However, after a longer duration of illness, the brain does not continue its compensation for the neurostructural deficits. There is some evidence that younger age of onset and longer duration of illness are associated with greater impairment in executive functioning,29,30 while other studies dispute this.31,32
The present study was initiated prior to the introduction of novel antipsychotic medication, and most of the patients in the study had used both typical and atypical neuroleptics. Typical neuroleptics have been shown to reduce performance on some neurocognitive tasks.33 Most of the subjects in the schizophrenia group used atypical medication at T2. Studies have reported that atypical neuroleptics may improve some aspects of neurocognitive functioning.34 However, the effect of neuroleptic medication might not be the same for patients with early-onset as opposed to later onset schizophrenia due to ongoing development of neurotransmitter receptor system during childhood and adolescence.35 Thus, we cannot rule out that medication could have affected the present results.
The subjects in the schizophrenia group became ill at a young age and had limited social contact with other adolescents and little experience with school or work. Longitudinal studies of brain development suggest that substantial brain maturation takes place between adolescence and adulthood. Moreover, a study showed that there was a region-specific increase in gray matter volume during preadolescence, followed by a postadolescent decrease.36 The decline in verbal memory and learning with increasing illness duration could also be a reflection of “losing it” because of “not using it.”37 It is also possible that the impairments in the schizophrenia group could be what caused the social isolation in the first place because intact neurocognitive functions are important for social and occupational functioning.38
As predicted, subjects in the schizophrenia group showed a significant decline in verbal memory and learning when controlling for premorbid neurocognitive capacity. Verbal memory deficits have been found to be the greatest impairment in relatives of patients with schizophrenia, suggesting a genetic component to this deficit.39 Impaired speed of information processing has also been identified as a central neurocognitive deficit in schizophrenia.40 Our results confirm this finding in that change in performance on the digit symbol subtest shows the largest effect size of time by group for all measures, followed by verbal memory (CVLT) and speeded visual attention (BMT). Other neurocognitive functions were already compromised at T1 and showed little change over time.
There were no significant changes over time in either positive or negative symptom scores for 12 of the subjects with schizophrenia. Three subjects had recovered at T2. Thus, the differences in clinical state between T1and T2 cannot explain the significant neurocognitive decline or arrest at T2.
At T1,2,3 patients with ADHD showed deficits in the ability to control attention/distractibility and assumed to reflect a frontal dysfunction. Although they still performed significantly below the healthy comparison group on the attention demanding Digit Repetition task at T2, they evidenced a significant improvement in attention in contrast to the other 2 groups. These findings support results from a recent study, reporting neuroanatomic evidence supportive of delayed cortical maturation in ADHD.41 In this study, the delay was most prominent in prefrontal regions important for control of cognitive processes including attention and motor planning. Together, the neurocognitive results from the current follow-up study and results from the recent neuroanatomic study41 support a maturational lag hypothesis of the pathogenesis of ADHD. The results imply that the decline in neurocognitive function at T2 was specific for the schizophrenia group compared with another group with attention deficits in adolescence (ADHD). However, because the patients with schizophrenia represent an especially severe and disabled group with long-term hospitalization, changes in neurocognitive functioning are not fully comparable between the 2 patient groups.
Strengths of the present study include assessment of subjects early in the illness process, inclusion of subjects with early-onset schizophrenia, long follow-up time, inclusion of both a healthy control group and a neuropsychiatric control group, the same control groups at both time points, a high retention rate (93%), and use of a comprehensive neurocognitive test battery. However, the results should be interpreted with several limitations in mind. The relatively small sample size presents a methodological shortcoming. Further, some patients were assessed without medication at T1 and with medication at T2, thereby confounding the course of cognition with potential medication effects. Patients had also received different psychosocial treatments, which may have affected neurocognition in different manners. Further, some studies have also shown that neurocognitive impairment may be more severe in males than in females with schizophrenia.42 Thus, theoretically the observed decline or arrest in neurocognitive functioning at T2 could be confounded by gender.
Subjects with ADHD frequently have comorbid substance abuse. Many of the subjects in the ADHD group (ca 70%) had a history of substance abuse, which may independently be associated with neurocognitive deficits. Further, the ADHD group consisted of only males, which may also be considered a limitation.
Conclusions
The results from the present study show significant deterioration or arrest in neurocognitive performance in early-onset schizophrenia over 13 years. These findings were not present in a neuropsychiatric comparison group with ADHD. One hypothesis is that the neurocognitive decline or arrest may be specific to early-onset schizophrenia due to interaction between ongoing brain maturation during adolescence and disease-related mechanisms. The decline or arrest may also be secondary to neuroleptic treatment in young adolescent's years and/or to limited social stimulation or some interaction of these factors.
The results emphasize the importance of studying neurocognition in schizophrenia within the context of brain development to identify possible interactions between developmental and disease-related mechanisms on cognition. Larger samples using several repeated neurocognitive measures at different time intervals would be important to investigate the exact timing of neurocognitive changes and to disentangle the potential effects of psychosis from the possible effects of treatment. Verbal memory deficits and attention deficits are known to be highly associated with functional outcome in schizophrenia.38 The present results underline the importance of early intervention targeting specific neurocognitive functions in an attempt to preserve functioning prior to decline.
Funding
The Eastern Norway Regional Health Authority (150039); The Centre for Child and Adolescent Mental Health, Eastern and Southern Norway (R.BUP).
Acknowledgments
The authors would like to thank Unni Bratlien, MD, at Innlandet Hospital Trust Lillehammer and Ole Andreassen, MD, PhD, at the University of Oslo and Ullevaal University Hospital for valuable comments on an early draft of the manuscript.
References
- 1.Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34:30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Øie M, Rund BR. Neuropsychological deficits in adolescent-onset schizophrenia compared with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1216–1222. doi: 10.1176/ajp.156.8.1216. [DOI] [PubMed] [Google Scholar]
- 3.Øie M, Sundet K, Rund BR. Contrasts in memory functions between adolescents with schizophrenia or ADHD. Neuropsychologia. 1999;37:1351–1358. doi: 10.1016/s0028-3932(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 4.Rund BR, Melle I, Friis S, et al. Neurocognitive dysfunction in first-episode psychosis: correlates with symptoms, premorbid adjustment and duration of untreated psychosis. Am J Psychiatry. 2004;161:266–472. doi: 10.1176/appi.ajp.161.3.466. [DOI] [PubMed] [Google Scholar]
- 5.Ueland T, Øie M, Landrø NI, Rund BR. Cognitive functioning in adolescents with schizophrenia spectrum disorders. Psychiatry Res. 2004;126:229–239. doi: 10.1016/j.psychres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6.White T, Ho BC, Ward J, O'Leary D, Andreasen NC. Neuropsychological performance in first-episode adolescents with schizophrenia: a comparison with first episode adults and adolescent control subjects. Biol Psychiatry. 2006;60:463–471. doi: 10.1016/j.biopsych.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Rund BR, Melle I, Friis S, et al. The course of neurocognitive functioning in first-episode psychosis and its relation to premorbid adjustment, duration of untreated psychosis, and relapse. Schizophr Res. 2007;91:132–140. doi: 10.1016/j.schres.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78:27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Stirling J, White C, Lewis S, et al. Neurocognitive function and outcome in first-episode schizophrenia: a 10-year follow-up of an epidemiological cohort. Schizophr Res. 2003;65:75–86. doi: 10.1016/s0920-9964(03)00014-8. [DOI] [PubMed] [Google Scholar]
- 10.Harvey PD, Reichenberg A, Bowie CR. Cognition and ageing in psychopathology: focus on schizophrenia and depression. Annu Rev Clin Psychol. 2006;2:389–409. doi: 10.1146/annurev.clinpsy.2.022305.095206. [DOI] [PubMed] [Google Scholar]
- 11.Frangou S, Hadjulis M, Vourdas A. The Maudsley early onset schizophrenia study. Cognitive function over a 4-year follow-up period. Schizophr Bull. 2008;34:52–59. doi: 10.1093/schbul/sbm124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood SJ, De Luca CR, Anderson V, Pantelis C. Cognitive development in adolescence: cerebral underpinnings, neural trajectories, and the impact of aberrations. In: Keshavan MS, Kennedy JL, Murray RM, editors. Neurodevelopment and Schizophrenia. Cambridge, UK: Cambridge University Press; 2004. pp. 69–88. [Google Scholar]
- 13.Seidman LJ. Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev. 2006;26:466–485. doi: 10.1016/j.cpr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Wender PH, Wood DR, Reimherr FW. Pharmacological treatment of Attention Deficit Disorder, Residual Type (ADD, RT, “Minimal Brain Dysfunction”,”Hyperactivity”) in adults. Psychopharmacol Bull. 1985;21:222–231. [PubMed] [Google Scholar]
- 16.Wechsler D. WISC-R Manual: Wechsler Intelligence Scale for Children—Revised. New York, NY: Psychological Corperation; 1974. [Google Scholar]
- 17.Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed. San Antonio, Tex: Psychological Corporation; 1997. [Google Scholar]
- 18.Wechsler D. Abbreviated Scale of Intelligence (WASI), Norwegian Manual Supplement. Stockholm, Sweden: Harcourt Assessment, Inc; 2007. [Google Scholar]
- 19.Heaton RK. Wisconsin Card Sorting Test, Manual. Odessa, Fla: Psychological Assessment Resources; 1981. [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test (CVLT) Manual. New York, NY: Psychological Corporation; 1987. [Google Scholar]
- 21.Kimura D. Functional asymmetry of the brain in dichotic listening. Cortex. 1967;3:163–168. [Google Scholar]
- 22.Lezak MD. Neuropsychological Assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 23.Oltmanns TF, Neale JM. Schizophrenic performance when distractors are present: attention deficit or differential task difficulty? J Abnorm Psychol. 1975;84:205–209. doi: 10.1037/h0076721. [DOI] [PubMed] [Google Scholar]
- 24.Rund BR, Øie M, Sundet K. Backward-masking in adolescents with schizophrenia disorders or attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153:1154–1157. doi: 10.1176/ajp.153.9.1154. [DOI] [PubMed] [Google Scholar]
- 25.Øie M, Hugdahl K. A 10-13 year follow-up of changes in perception and executive attention in patients with early-onset schizophrenia: a dichotic listening study [published online ahead of print January 17, 2008] Schizophr Res. 2008 doi: 10.1016/j.schres.2007.11.036. 10.1016/j.schres.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Lukoff D, Nuechterlein KH, Ventura J. Appendix A. Manual for Expanded Brief Psychiatric Rating Scale (BPRS) Schizophr Bull. 1986;12:594–602. [Google Scholar]
- 27.Endicott J, Spitzer RL, Fleiss J, Cohen J. The Global Assessment Scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 28.Kumra S, Schulz SC. Editorial: research progress in early-onset schizophrenia. Schizophr Bull. 2008;34:15–17. doi: 10.1093/schbul/sbm123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A. A deficit profile of executive, memory, and motor functions in schizophrenia. Biol Psychiatry. 1994;36:641–653. doi: 10.1016/0006-3223(94)91173-8. [DOI] [PubMed] [Google Scholar]
- 30.Albus M, Hubmann W, Ehrenberg C, et al. Neuropsychological impairment in first-episode and chronic schizophrenia patients. Eur Arch Psychiatr Clin Neurosci. 1996;246:249–255. doi: 10.1007/BF02190276. [DOI] [PubMed] [Google Scholar]
- 31.Heaton R, Paulsen JS, McAdams LA, et al. Neuropsychological deficits in schizophrenics: relationship to age, chronicity and dementia. Arch Gen Psychiatry. 1994;51:469–476. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- 32.Chen EYH, Lam LCW, Chen RYL, Nguyen DGH, Chan CKY. Prefrontal neuropsychological impairment and illness duration in schizophrenia: a study of 204 patients in Hong Kong. Acta Psychiatr Scand. 1996;93:144–150. doi: 10.1111/j.1600-0447.1996.tb09816.x. [DOI] [PubMed] [Google Scholar]
- 33.Moritz S, Woodward TS, Krausz M, Naber D. PERSIST Study Group. Relationship between neuroleptic dosage and subjective cognitive dysfunction in schizophrenic patients treated with either conventional or atypical neuroleptic medication. Int Clin Psychopharmacol. 2002;17:41–44. doi: 10.1097/00004850-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Keefe RS, Bilder RM, Davis SM, et al. CATIE Investigators; Neurocognitive Working group. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 35.Kumra S, Oberstar JV, Sikich L, et al. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:60–71. doi: 10.1093/schbul/sbm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Giedd JN, Jeffries NO, Blumenthal J, et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 38.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Toulopoulou T, Rabe-Hesketh S, King H, Murray RM, Morris RG. Episodic memory in schizophrenic patients and their relatives. Schizophr Res. 2003;63:261–271. doi: 10.1016/s0920-9964(02)00324-9. [DOI] [PubMed] [Google Scholar]
- 40.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious. A meta-analytic comparison of Digit Symbol Coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 41.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60:229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]