Abstract
Ovarian cancer is one of the most common cancers in women. Its early stages may be asymptomatic, and as a result diagnosis frequently occurrs at an advanced, often incurable, stage. The high mortality and low survival rates associated with ovarian cancer can in part be attributed to the lack of diagnostic methods allowing for early detection, yet a methodology to identify patients with early-stage ovarian cancer remains to be established. In order to investigate the frequency of antibodies against a panel of multiple carefully-selected tumor-associated antigens (TAAs) in sera from patients with ovarian cancer, and to determine the possibility and usefulness of such a panel of TAAs in the immunodiagnosis of ovarian cancer, sera from 32 ovarian cancer patients and 82 normal individuals were tested using an enzyme-linked immunosorbent assay (ELISA) for the presence of autoantibodies to a panel of 13 TAAs. ELISA results were also confirmed by immunoblotting analysis. The sensitivity and specificity of the multiple anti-TAA antibodies in the detection of ovarian cancer was 62.5 and 85.4%, respectively. With the successive addition of TAAs to a total of 7 antigens (survivin, p53, p16, cyclin B1, cyclin D1, cyclin A and cyclin E), there was a stepwise increase in sensitivity of up to 62.5%, and in specificity of 90.2%. With the addition of more antigens to the panel, no further increase in sensitivity was detected. This study further supports our previous hypothesis that a combination of antibodies might acquire higher sensitivity for the diagnosis of cancer, and also indicates that, in the selection of ovarian cancer-associated TAAs, some may be specific to ovarian cancer while others may not be. This emphasizes the importance of a comprehensive analysis of antibody response to selected TAAs in various disease conditions, such as ovarian cancer, in benign ovarian diseases, and in normal individuals, before conclusions can be drawn regarding their contribution to ovarian cancer.
Keywords: ovarian cancer, autoantibodies, tumor-associated antigens, immunodiagnosis, enzyme-linked immunosorbent assay, immunoblotting analysis, sensitivity, specificity, antigen mini-array
Introduction
The highly specific autoantibody response to autoimmune diseases generally predicts the biologic phenotype of the disease, making autoantibodies clinically valuable and diagnostically useful. Whether a similar mechanism operates in the humoral immune response to cancer remains to be established, but it appears to be a possibility (1). Previous studies by our group and by others have demonstrated that cancer sera contain autoantibodies that react with a unique group of autologous cellular antigens, called tumor-associated antigens (TAAs) (1–3). Cancer has long been recognized as a multi-step process that involves not only genetic changes conferring growth advantage, but also factors which disrupt the regulation of growth and differentiation (4,5). It is possible that some of these factors could be identified and their functions evaluated with the aid of autoantibodies arising during tumorigenesis. Our recent studies have indicated that the detection of autoantibodies in cancer can be enhanced by the use of a panel of multiple TAAs, such as p53, c-myc, IMP1, p62, Koc, cyclin B1 and survivin, as target antigens (6–9). Ovarian cancer is the sixth most common cancer in women, and accounts for 5% of all cancers in females. It is diagnosed in about 23,000 women in the United States each year, and almost 14,000 women die of the disease annually. The overall 5-year survival rate for ovarian cancer is 42% (10–12). It is clear that this low survival rate can in part be attributed to the lack of diagnostic methods allowing for early detection. A methodology to identify patients with early-stage ovarian cancer remains to be established. This study investigated the frequency of antibodies against a panel of multiple carefully-selected TAAs in sera from patients with ovarian cancer, and determined the possibility and usefulness of such a panel in the immunodiagnosis of ovarian cancer.
Materials and methods
Serum samples
Sera from 32 patients with ovarian cancer and 82 normal human sera were obtained from the serum bank of the Tumor Cell Engineering Laboratory of Xia’men University (Fujian Province, P.R. China). All ovarian cancer sera were collected at the initial time of cancer diagnosis, prior to patients being treated with chemotherapy or radio-therapy. Normal human sera were collected during annual health examinations from adults with no obvious evidence of malignancy. Due to regulations concerning studies on human subjects, patient name and identification number were not disclosed to investigators, and some clinical information for sera used in the study was not available. The study was approved by the Institutional Review Board of Xia’men University and collaborating institutions.
Expression and purification of recombinant proteins
Thirteen antigens, Imp1, p62, Koc, p53, c-myc, cyclin B1, survivin, p16, cyclin D1, cyclin A, cyclin E, CDK2 and p90, were selected for the expression of recombinant proteins. Imp1, p62, Koc, p53, c-myc, cyclin B1, survivin, p16, p90, cyclin D1 and cyclin A had been prepared and used in our previous studies (6–9). Cyclin E and CDK2 were isolated from pGEX constructs expressing these proteins with glutathione S transferase fusion partner. These two constructs were originally provided by Dr M. Eng Tan’s lab at the Scripps Research Institute (La Jolla, CA). Expression of adequate amounts of recombinant protein was examined by SDS-PAGE, and Coomasie Blue staining was used to determine whether expression products of expected molecular sizes were produced. In addition, Western blot analysis was used to confirm that the bands seen in SDS-PAGE were reactive with reference antibodies. The antibodies used in this study were rabbit polyclonal anti-Imp1 against a C-terminal peptide, kindly supplied by F.C. Nielsen (13). Anti-Koc/Imp3 was raised against specific C-terminal peptides of the respective proteins (14), and anti-p62 (15) and anti-p90 (16) were raised against the full-length proteins. Reactivities of p53, c-myc, cyclin B1, p16, cyclin D1, cyclin A, cyclin E and CDK2 were determined with monoclonal antibodies obtained from Oncogene Research Products, Boston, MA. Rabbit polyclonal anti-survivin antibody raised against the C-terminal peptide was obtained from ProSci Inc., Poway, CA.
Enzyme-linked immunosorbent assay
Purified recombinant proteins were individually diluted in PBS to a final concentration of 0.5 μg/ml for coating Immunolon2 microtiter plates (Fisher Scientific, Houston, TX) overnight at 4°C. Human serum samples diluted 1:200 were incubated with the antigen-coated wells for 90 min, then incubated with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Caltag Laboratories, Burlingame, CA) diluted 1:5,000 for 90 min as a secondary antibody, followed by washing with PBS containing 0.05% Tween-20. The substrate 2,2′-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt (Sigma, St. Louis, MO) was used as the detecting agent. The O.D. of each well was read at 405 nm, and the cut-off value for determining a positive reaction was designated as the mean absorbance of the 82 normal Chinese sera plus 3 standard deviations (mean ± 3 SD). Each sample was tested in duplicate. As previously described, 8 normal human sera representing a range of 3 SD above and below the mean of the 82 Chinese normal sera was used in each experiment, and the average value of the 8 normal sera was used in each run to normalize all absorbance values to the standard mean of the entire sample of 82 normal sera. In addition, all positive sera were confirmed with repeat testing, as were some negative sera. The detailed protocol of enzyme-linked immunosorbent assay (ELISA) was used as described by Rubin (17).
Western blotting and Slot blot
Serum samples that were determined, using the ELISA method, to contain autoantibodies were further tested by immunoblotting to confirm the immunoreactivity of the sera. Western blotting was essentially performed as described by Chan and Pollard (18). In brief, purified recombinant proteins were electrophoresed by SDS-PAGE and subsequently transferred to a nitrocellulose membrane. Nitrocellulose membranes were cut into strips, and the individual strips were pre-blocked in PBS containing 0.05% Tween-20 (PBST) with 5% non-fat milk for 30 min at room temperature, incubated for 90 min with patient sera diluted 1:200, and finally incubated with HRP-conjugated goat anti-human IgG diluted 1:3,000 for 90 min followed by washing with PBST solution. Positive signals were captured by autoradiography using chemiluminescence (Pierce Bio-technology, Rockford, IL) according to the manufacturer’s instructions. The method for Slot blot was identical to Western blotting, with the exception that the purified recombinant protein (100 ng/well) was applied directly to the nitrocellulose membrane using a vacuum source. Membranes were not cut into strips; therefore, the detection of autoantibodies in individual patient serum to eleven TAAs, with the exception of Cyclin A and p90, could be performed simultaneously on one blot.
Statistical analysis
To determine whether the frequency of autoantibodies to thirteen TAAs in sera from patients with ovarian cancer was significantly higher than in sera from normal individuals, data were analyzed using the χ2 test with Yates’ correction. Two significant levels (0.05 and 0.01) were used. Methods for calculating the sensitivity/specificity, positive or negative predictive values (positive PV/negative PV), and false positives/false negatives were based on the methodology provided (19).
Results
Frequency and titer of autoantibodies to a panel of 13 TAAs in ovarian cancer
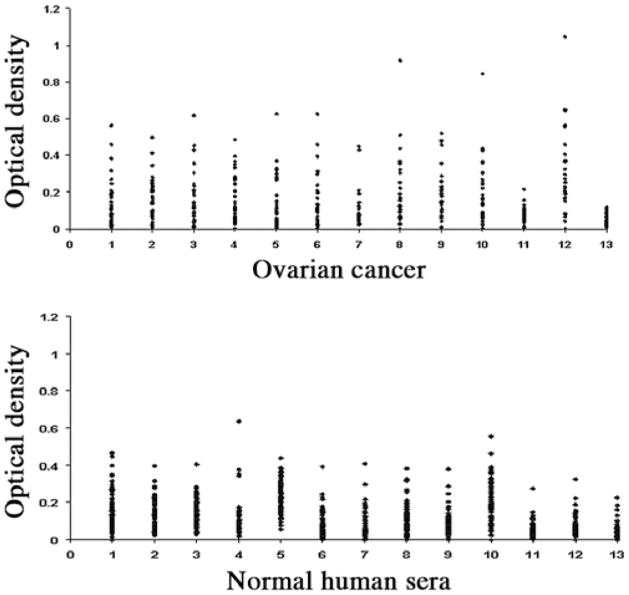
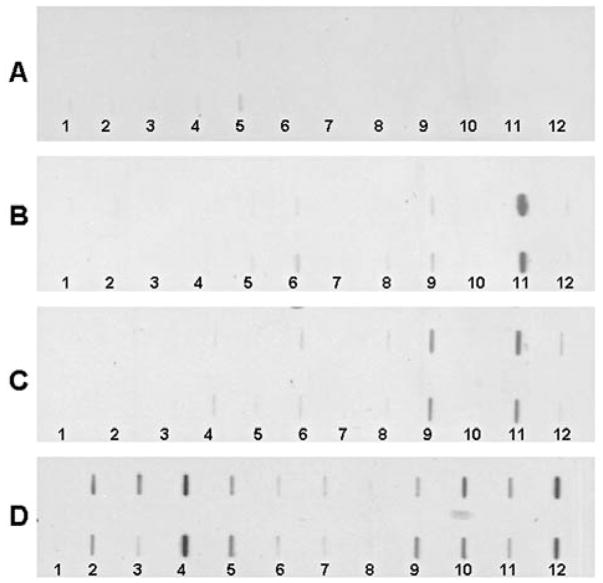
In the present study, 13 purified recombinant TAAs were used as coating antigens in ELISA to detect antibodies against these 13 TAAs in ovarian cancer. The panel of 13 TAAs included the recombinant full-length proteins survivin, p53, p16, cyclin B1, cyclin D1, cyclin A, cyclin E, koc, IMP1, p62, CDK2, p90 and c-myc. Eleven of the TAAs, not including cyclin E and CDK2, had been used in our previous studies (6–9). Cell cycle deregulation is one of the hallmarks of cancer. Progression through the cell cycle is governed by cyclin-dependent kinases (CDKs), which are regulated by phosphorylation, activated by binding to cyclins, and inhibited by CDK inhibitors (20,21). The cyclin E and CDK2 complex is one of the main regulators of the G1/S transition. Several studies have demonstrated that cyclin E is associated with disease progression in various malignancies, as well as with poor prognosis in patients with breast, ovarian, bladder and colorectal cancers (22–25). Autoantibodies against cyclin E and CDK2 in cancer were not reported. Sera from 32 patients with ovarian cancer and 82 normal human sera were available for study. As shown in Table I, autoantibodies against 13 TAAs in ovarian cancer were tested by ELISA. The cumulative antibody frequency to 13 TAAs was 62.5% (20/32), significantly higher than the frequency in sera from normal individuals (14.6%). The ELISA results were also confirmed by immunoblotting analysis. The highest frequencies of antibodies to an individual TAA in ovarian cancer were against p53 (25.0%), p16 (25.0%), cyclin B1 (25.0%) and cyclin D1 (25%), followed by survivin (21.9%), cyclin E (21.9%), cyclin A (18.8%) and koc (18.8%). The range of antibody titers to these 13 TAAs is shown in Fig. 1. The high titer of reactivities in certain cancer sera and the distinct difference between ovarian cancer and normal human sera are also demonstrated in this figure. Some ovarian cancer sera showed optical density values over 2-folds above the cut-off values (mean ± 3 SD of 82 normal human sera), indicating that antibody responses to these 13 TAAs in certain cancer patients were quite robust and not just mildly elevated. Fig. 2 shows a mini-array analysis of 11 antigens with 4 representative sera using Slot blot. Three ovarian cancer sera revealed different antibody profiles with the 11 TAAs. For example, one serum shows strong reactivity to only CDK2, the second shows reactivity with both IMP1 and CDK2, and the third shows reactivity to multiple TAAs, such as survivin, p53, p16, cyclin B1, IMP1, p62 CDK2 and c-MYC. However, a normal human serum shows no reactivity to any of the 11 TAAs.
Table I.
Frequency of autoantibodies to thirteen TAAs in ovarian cancer.
| No. (%) of autoantibodies to |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sera | No. tested |
Survivin | p53 | p16 | Cyclin B1 | Cyclin D1 | Cyclin A | Cyclin E | koc | IMP 1 | p62 | CDK2 | p90 | c-myc | Cumulative to 13 antigens |
| OCa | 32 | 7b (21.9) | 8b (25.0) | 8b (25.0) | 8b (25.0) | 8b (25.0) | 6b (18.8) | 7b (21.9) | 6b (18.8) | 3 (9.4) | 3 (9.4) | 3a (9.4) | 3a (9.4) | 1 (3.1) | 20b (62.5) |
| NHS | 82 | 2 (2.4) | 2 (2.4) | 1 (1.2) | 2 (2.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 2 (2.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (14.6) |
OCa, ovarian cancer; NHS, normal human sera. Cut-off value: mean ± 3 SD of NSH; p-values between cancer and NHS:
p<0.05,
p<0.01.
Figure 1.
Titer of autoantibodies to the panel of 13 TAAs in ovarian cancer and normal individuals. The range of antibody titers to each of the 13 TAAs is expressed as optical density (OD) obtained from ELISA. The high titer reactivity of some ovarian cancer sera and the distinct difference between cancer and normal individuals are demonstrated. Y-axis, OD values. X-axis, the panel of 13 TAAs. 1, survivin; 2, p53; 3, p16; 4, cyclin B1; 5, cyclin D1; 6, cyclin E; 7, cyclin E; 8, Koc; 9, Imp1; 10, p62; 11, CDK2; 12, p90; 13, c-Myc.
Figure 2.
Slot blot analysis of three representative ovarian cancer sera. Each blot represents a duplicate test for autoantibodies against a panel of eleven recombinant cancer antigens, with phosphate-buffered saline (PBS) as a negative control. Lane 1, PBS; lane 2, survivin; lane 3, p53; lane 4, p16; lane 5, cyclin B1; lane 6, cyclin D1; lane 7, cyclin E; lane 8, Koc; lane 9, Imp1; lane 10, p62; lane 11, CDK2; lane 12, c-Myc. (A) Normal human serum shows no reactivity to any of the eleven TAAs. (B) One ovarian cancer serum shows strong reactivity to just CDK2. (C) Another ovarian cancer serum shows reactivity to Imp1 and CDK2. (D) A third ovarian cancer serum shows strong reactivity to p16, p62 and c-myc, and weak reactivity to survivin, p53, cyclin B1, Imp1 and CDK2.
Evaluation of the diagnostic values of a panel of 13 TAAs in the immunodiagnosis of ovarian cancer
As described above, antibody frequency to any individual TAA in ovarian cancer varied between 3.1 and 25.0%. The results from Table II demonstrate that, with the successive addition of TAAs to a total of 7 antigens, there was a stepwise increase in positive antibody reactions of up to 62.5%. The validity of a test is defined as its ability to distinguish between who has a disease and who does not. In order to address the question of how valuable the approach of antibody detection to a mini-array of multiple TAAs is in separating people with and without ovarian cancer, the sensitivity/specificity, false negative/false positive and the positive predictive value/negative predictive value were calculated. The results are summarized in Table III. With the successive addition of TAAs to a total of 7 antigens, there was a stepwise increase in sensitivity of up to 62.5%, while the specificity remained over 90%. With the addition of more antigens (koc, Imp1, p62, CDK2, p90 and c-myc) to the panel, no further increase in sensitivity was observed, consistent with the results of the other two components (false negative and false positive). The positive and negative predictive values were also variable in different combinations of TAAs. In the panel of a total of 7 TAAs, the positive predictive value was 71.4% and the negative predictive value 86.0%. These results indicate that a panel of 7 TAAs, including survivin, p53, p16, cyclin B1, cyclin D1, cyclin A and cyclin E, might be sufficient to distinguish ovarian cancer from normal individuals. However, it remains to be determined whether this TAA combination can distinguish ovarian cancer from other cancers.
Table II.
Sequential addition of antigens to the panel of 13 TAAs.
| Percentage (no.) of autoantibodies |
||
|---|---|---|
| No. of different TAA panels | Ovarian cancer (32) | NHS (82) |
| 1. Survivin | 21.9 (7/32)b | 2.4 (2/82) |
| 2. Survivin + p53 | 37.5 (12/32)b | 4.9 (4/82) |
| 3. Survivin + p53 + p16 | 46.9 (15/32)b | 6.1 (5/82) |
| 4. Survivin + p53 + p16 + cyclin B1 | 50.0 (16/32)b | 8.5 (7/82) |
| 5. Survivin + p53 + p16 + cyclin B1 + cyclin D1 | 56.3 (18/32)b | 9.8 (8/82) |
| 6. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A | 59.4 (19/32)b | 9.8 (8/82) |
| 7. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A + cyclin E | 62.5 (20/32)b | 9.8 (8/82) |
| 8. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A + cyclin E + Koc | 62.5 (20/32)b | 11.0 (9/82) |
| 9. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A + cyclin E + Koc + IMP1 | 62.5 (20/32)b | 13.4 (11/82) |
| 10. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A + cyclin E + Koc + IMP1+p62 | 62.5 (20/32)b | 14.6 (12/82) |
| 11. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A + cyclin E + Koc + IMP1+p62+CDK2 | 62.5 (20/32)b | 14.6 (12/82) |
| 12. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A + cyclin E + Koc + IMP1+p62+CDK2+p90 | 62.5 (20/32)b | 14.6 (12/82) |
| 13. Survivin + p53 + p16 + cyclin B1 + cyclin D1 + cyclin A + cyclin E + Koc + IMP1+p62+CDK2+p90+c-myc | 62.5 (20/32)b | 14.6 (12/82) |
Cut-off value: mean ± 3 SD of NHS; p-values relative to NHS:
p<0.05,
p<0.01.
Table III.
Evaluation of diagnostic values of different TAA panels in the diagnosis of ovarian cancer.
| No. of different TAA panelsa | Positive no. and (%) of ovarian cancer | Positive no. and (%) of NHS | Sensitivity/specificity | Positive PV/negative PV | False positive/false negative |
|---|---|---|---|---|---|
| 1 | 7 (21.9) | 2 (2.4) | 21.9/97.6 | 77.8/76.2 | 2.4/78.1 |
| 2 | 12 (37.5) | 4 (4.9) | 37.5/95.1 | 75.0/79.6 | 4.9/62.5 |
| 3 | 15 (46.9) | 5 (6.1) | 46.9/93.9 | 75.0/81.9 | 6.1/53.1 |
| 4 | 16 (50.0) | 7 (8.5) | 50.0/91.5 | 69.6/82.4 | 8.5/50.0 |
| 5 | 18 (56.3) | 8 (9.8) | 56.3/90.2 | 69.2/84.1 | 9.8/43.8 |
| 6 | 19 (59.4) | 8 (9.8) | 59.4/90.2 | 70.4/85.1 | 9.8/40.6 |
| 7 | 20 (62.5) | 8 (9.8) | 62.5/90.2 | 71.4/86.0 | 9.8/37.5 |
| 8 | 20 (62.5) | 9 (11.0) | 62.5/89.0 | 69.0/85.9 | 11.0/37.5 |
| 9 | 20 (62.5) | 11 (13.4) | 62.5/86.6 | 64.5/85.5 | 13.4/37.5 |
| 10 | 20 (62.5) | 12 (14.6) | 62.5/85.4 | 62.5/85.4 | 14.6/37.5 |
| 11 | 20 (62.5) | 12 (14.6) | 62.5/85.4 | 62.5/85.4 | 14.6/37.5 |
| 12 | 20 (62.5) | 12 (14.6) | 62.5/85.4 | 62.5/85.4 | 14.6/37.5 |
| 13 | 20 (62.5) | 12 (14.6) | 62.5/85.4 | 62.5/85.4 | 14.6/37.5 |
No. of different TAA panels, corresponding to the no. shown in Table II. Positive PV, positive predictive value; negative PV, negative predictive value.
Discussion
Ovarian cancer is characterized by few early symptoms, presentation at an advanced stage and poor survival. Although advances in surgery and treatment modalities have slightly improved 5-year survival rates, the prognosis for most patients continues to be poor (10–12). Retrovaginal examination, transvaginal ultrasonography and the CA-125 blood test are three modalities currently used to screen for ovarian cancer. Studies have shown CA-125 to be elevated in over 90% of women with advanced ovarian cancer and in 40% of patients with any primary cancer with extensive intra-abdominal disease. CA-125 is also associated with the inflammatory cells of the pleura, pericardium and peritoneum, and is therefore often elevated in the context of benign conditions, such as peritonitis, endometriosis and liver cirrhosis, causing ascites (26–28). CA-125 would appear to not be specific; therefore, it is an inadequate screening tool. In cancer, prognosis is improved by earlier diagnosis. This fact is particularly pertinent to ovarian cancer, where early stages may be asymptomatic and result in diagnosis frequently occurring at an advanced, often incurable stage. Women who present with stage I or II ovarian cancer have a 5-year survival rate of 90 and 70%, respectively, whereas those with advanced stage III or IV ovarian cancer have a 5-year survival rate of just 20%. These statistics stress the importance of investigating the early diagnosis of ovarian cancer, with the emphasis on studies on the efficacy of the use of serial measurements of a number of serum markers.
Numerous studies indicate that no single marker can completely distinguish cancer groups from healthy controls. However, a combination of multiple markers may provide a promising tool for the early detection of cancer. On the other hand, the multi-factorial and multi-step nature of the molecular pathogenesis of human cancer can also be considered in the design and interpretation of studies to identify biomarkers useful for the early detection of cancer. Many investigators have been interested in the use of autoantibodies as serological markers for cancer diagnosis, especially because of the general absence of these autoantibodies in normal individuals and in non-cancer conditions. Enthusiasm for this approach has been tempered by low sensitivity. We have observed that this drawback can be overcome by using a panel of carefully-selected TAAs, and that different types of cancer may require different panels of TAAs to achieve the sensitivity and specificity required to make immunodiagnosis a feasible adjunct to tumor diagnosis (2,29). This is an innovative feature of this study, which tested a group of ovarian cancer sera for the presence of autoantibodies to a mini-array of 13 TAAs, and found the sensitivity and specificity of antibodies in the detection of ovarian cancer to be 62.5 and 85.4%, respectively. With the successive addition of TAAs to a total of 7 antigens (survivin, p53, p16, cyclin B1, cyclin D1, cyclin A and cyclin E), there was a stepwise increase in sensitivity of up to 62.5%, and in specificity of 90.2%. With the addition of more antigens to the panel, no further increase in sensitivity was observed. These results indicate that, in the selection of ovarian cancer-associated TAAs, some may be specific to ovarian cancer while others may not be. They also emphasize the importance of a comprehensive analysis of antibody response to selected TAAs in different disease conditions, such as ovarian cancer, in benign ovarian diseases, and in normal individuals, before conclusions can be drawn regarding their contribution to ovarian cancer.
In conclusion, this preliminary study further supports our previous hypothesis that a combination of antibodies might acquire higher sensitivity for the diagnosis of cancer. The data also suggest that additional ovarian cancer-specific TAAs need to be added to a panel of multiple TAAs in order to enhance autoantibody detection in the immunodiagnosis of ovarian cancer. It is conceivable that autoantibody profiles involving different panels or arrays of TAAs might be developed in the future, with results that could be useful in the diagnosis of different types of cancer.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China, the China Scholarship Council and the Henan Outstanding Scientist Award, and in part by NIH Grants 5G12RR08124 and 2S06GM008012-37.
References
- 1.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411–1415. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang JY. Tumor-associated antigen array to enhance antibody detection for cancer diagnosis. Cancer Detect Prev. 2004;28:114–118. doi: 10.1016/j.cdp.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Old LJ, Chen YT. New paths in human cancer serology. J Exp Med. 1998;187:1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 5.Sarasin A. An overview of the mechanisms of mutagenesis and carcinogenesis. Mutat Res. 2003;544:99–106. doi: 10.1016/j.mrrev.2003.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- 7.Koziol JA, Zhang JY, Casiano CA, Peng XX, Shi FD, Feng AC, Chan EK, Tan EM. Recursive partitioning as an approach to selection of immune markers for tumor diagnosis. Clin Cancer Res. 2003;9:5120–5126. [PubMed] [Google Scholar]
- 8.Shi FD, Zhang JY, Liu D, Rearden A, Elliot M, Nachtsheim D, Daniels T, Casiano CA, Heeb MJ, Chan EK, Tan EM. Preferential humoral immune response in prostate cancer to cellular proteins p90 and p62 in a panel of tumor-associated antigens. Prostate. 2005;63:252–258. doi: 10.1002/pros.20181. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EK. Antibody detection using tumor-associated antigen mini-array in diagnosing human hepatocellular carcinoma. J Hepatol. 2007;46:107–114. doi: 10.1016/j.jhep.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoskins WJ. Prospective on ovarian cancer: why prevent? J Cell Biochem Suppl. 1995;23:189–199. doi: 10.1002/jcb.240590926. [DOI] [PubMed] [Google Scholar]
- 11.Tortolero-Luna G, Mitchell MF. The epidemiology of ovarian cancer. J Cell Biochem Suppl. 1995;23:200–207. doi: 10.1002/jcb.240590927. [DOI] [PubMed] [Google Scholar]
- 12.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Molec Cell Biol. 1999;19:1262–1270. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller-Pillasch F, Lacher U, Wallrapp C, et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14:2729–2733. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JY, Chan EKL, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–1110. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soo Hoo L, Zhang JY, Chan EKL. Cloning and characterization of a novel 90-kDa ‘companion’ auto-antigen of p62 over-expressed in cancer. Oncogene. 2002;21:5006–5015. doi: 10.1038/sj.onc.1205625. [DOI] [PubMed] [Google Scholar]
- 17.Rubin RL. Enzyme-linked immunosorbent assays for antibodies to native DNA, histones and (H2A-H2B)-DNA. In: Rose NR, Conway De Macario E, Folds JD, Lane HC, Nakamura RM, editors. Manual of Clinical Laboratory Immunology. 1. American Society for Microbiology; Washington DC: 1997. pp. 935–941. [Google Scholar]
- 18.Chan EKL, Pollard KM. Detection of autoantibodies to ribonucleoprotein particles by immunoblotting. In: Rose NR, Conway De Macario E, Folds JD, Lane HC, Nakamura RM, editors. Manual of Clinical Laboratory Immunology. 1. American Society for Microbiology; Washington DC: 1997. pp. 928–934. [Google Scholar]
- 19.Gordis L. Assessing the validity and reliability of diagnostic and screening tests. In: Gordis L, editor. Epidemiology. 3. Elsevier Saunders; Philadelphia PA: 2004. pp. 71–94. [Google Scholar]
- 20.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 21.MacLachlan TK, Sang N, Giordano A. Cyclins, cyclin-dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Crit Rev Eukaryot Gene Exp. 1995;5:127–156. doi: 10.1615/critreveukargeneexpr.v5.i2.20. [DOI] [PubMed] [Google Scholar]
- 22.Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 23.Rosen DG, Yang G, Deavers MT, Malpica A, Kavanagh JJ, Mills GB, Liu J. Cyclin E expression is correlated with tumor progression and predicts a poor prognosis in patients with ovarian carcinoma. Cancer. 2006;106:1925–1932. doi: 10.1002/cncr.21767. [DOI] [PubMed] [Google Scholar]
- 24.Del Pizzo JJ, Borkowski A, Jacobs SC, Kyprianou N. Loss of cell cycle regulators p27(Kip1) and cyclin E in transitional cell carcinoma of the bladder correlates with tumor grade and patient survival. Am J Pathol. 1999;155:1129–1136. doi: 10.1016/S0002-9440(10)65216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JQ, Miki H, Ohmori M, Wu F, Funamoto Y. Expression of cyclin E and cyclin-dependent kinase 2 correlates with metastasis and prognosis in colorectal carcinoma. Hum Pathol. 2001;32:945–953. doi: 10.1053/hupa.2001.27116. [DOI] [PubMed] [Google Scholar]
- 26.Whitehouse C, Solomon E. Current status of the molecular characterization of the ovarian cancer antigen CA125 and implications for its use in clinical screening. Gynecol Oncol. 2003;88:S152–S157. doi: 10.1006/gyno.2002.6708. [DOI] [PubMed] [Google Scholar]
- 27.Menon U, Jacobs IJ. Recent developments in ovarian cancer screening. Curr Opin Obstet Gynecol. 2000;12:39–42. doi: 10.1097/00001703-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Guppy AE, Rustin GJ. CA125 response: can it replace the traditional response criteria in ovarian cancer? Oncologist. 2002;7:437–443. doi: 10.1634/theoncologist.7-5-437. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JY. Mini-array of tumor-associated antigens to enhance autoantibody detection for immunodiagnosis of hepatocellular carcinoma. Autoimmun Rev. 2007;6:143–148. doi: 10.1016/j.autrev.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]