Abstract
Since its first description in 1990, sentinel node (SN) biopsy has become the standard for accurate staging of a melanoma-draining regional lymphatic basin. This minimally invasive, multidisciplinary technique can detect occult metastases by selective sampling and focused pathologic analysis of the first nodes on the afferent lymphatic pathway from a primary cutaneous melanoma. An understanding of preoperative lymphoscintigraphy, intraoperative lymphatic mapping, and the definition of SN are critical for surgical expertise with SN biopsy.
Keywords: Sentinel node biopsy, lymphoscintigraphy
INTRODUCTION
Regional nodal status is the most powerful prognostic indicator for cutaneous melanoma [1]. Survival for early-stage melanoma is excellent; however, 5-year survival for patients with clinically palpable regional nodal metastasis is only about 45% [2]. Controversy has surrounded the surgical management of regional lymph nodes since Herbert Snow gave his seminal lecture on “Melanotic Cancerous Disease” in 1892 [3]. Snow believed that melanoma spreads first through the lymphatics to the regional nodes and subsequently to distant sites. He therefore advocated elective lymph node dissection (ELND) as a surgical technique to control lymphatic spread by interrupting the metastatic cascade from a primary melanoma. ELND became a cornerstone in the management of early melanoma and was routinely performed in patients with clinically normal regional lymph nodes. Several retrospective studies reported a survival advantage of ELND in these patients [4,5]; however, prospective randomized trials failed to show an overall survival benefit for wide local excision (WLE) plus ELND versus WLE plus observation [6–9] (although Balch et al [10] reported that certain subsets of patients appeared to benefit). The utility of ELND was questioned because only 20% of patients with clinically localized melanoma have occult nodal disease and therefore might benefit from regional lymphadenectomy; the remaining 80% do not need nodal surgery because they have no nodal metastasis [2]. Since there was no reliable noninvasive means to predict which patients would have nodal disease, the substantial morbidity of ELND (lymphedema, nerve injury, seroma) had to be balanced against its potential staging value and uncertain survival advantage.
Nearly a century later, the controversy surrounding ELND was addressed when Morton and colleagues introduced a minimally invasive alternative based on lymphatic mapping of the path for tumor metastasis to regional nodes [11,12]. Lymphatic mapping assumes that tumors drain via afferent lymphatics to a limited number of sentry or sentinel nodes (SNs) that are at high risk for metastasis because they represent the first nodal sites of tumor cells migrating to the regional drainage basin. The tumor status of the SN therefore reflects the tumor status of the entire nodal basin: a tumor-negative SN predicts that other nodes in the same drainage basin (nonsentinel nodes) will also be tumor free, and a tumor-positive SN predicts that nonsentinel nodes might also harbor metastasis. Selective biopsy of SNs can identify the 20% of patients who have occult regional metastasis and therefore may benefit from complete lymph node dissection (CLND); it also can identify the remaining 80% of patients who have no nodal metastases. Because these patients have a better prognosis, they can avoid the potential morbidity of CLND [13]. Moreover, SN biopsy allows for more intensive staging (ultrastaging) of the regional tumor-draining nodes: the relatively limited SN specimen can be scrutinized by immunohistochemical staining (IHC) of multiple sections from each node, which would not be practical for the much larger CLND specimen. IHC of serial nodal sections reportedly detects occult micrometastases in 14% of lymph nodes that are negative by routine sectioning and hematoxylin and eosin staining (H&E) [14].
SN biopsy is a multidisciplinary procedure that requires close cooperation between nuclear medicine physicians, surgeons and pathologists for accurate lymphatic mapping, dissection, and histopathologic analysis of the tumor-draining nodes. All three disciplines must master their respective techniques for success. Preferably, SN biopsy is performed at the time of WLE. Before surgery, a radiotracer is injected intradermally at the site of the primary tumor and lymphoscintigraphy is performed by the nuclear medicine physician, who marks the skin directly over the site of the regional SN. At the time of surgery, blue dye is injected at the site of the primary melanoma. Using the lymphoscintigram and the marked skin site as a road map, the surgeon moves a hand-held gamma probe over the regional nodal basin to confirm elevated radioactivity (a “hot spot”). After an incision is made over this hot spot, the hand-held gamma probe guides the surgeon in identifying radioactive node(s). The SN is identified by a combination of radioactivity and visual identification of blue staining. All SNs are excised and evaluated for metastases by permanent sectioning and staining with H&E, and with IHC using antibodies to MART-1, HMB-45 and S-100. If metastases are found, CLND is routinely performed as a second operation.
Successful SN biopsy requires the removal of all SNs with minimal morbidity. The surgical procedure is critical because technical variations can influence SN identification rate and morbidity. Surprisingly, despite wide enthusiasm for SN biopsy, there are few published guidelines on its application in melanoma [15,16]. This review describes the technical aspects of SN biopsy in melanoma based on our experience at the John Wayne Cancer Institute (JWCI).
HISTORY OF SENTINEL NODE BIOPSY
Mapping the lymphatic path of tumor metastasis to the SN is the product of several centuries of observations and experiments. The term lymphatic was first introduced in 1653 by Thomas Bartholin of Copenhagen [17]. Subsequent studies on lymphatic mapping with mercury by William Cruikshank led to the publication of The Anatomy of the Absorbing Vessels of the Human Body in 1786. Marie Philibert Constant Sappey, a professor of anatomy in Paris, further refined lymphatic injection and dissection techniques and published several lymphatic atlases during the late 19th century [17].
The concept of a gatekeeper or sentinel node evolved over several decades. In 1960, Gould and colleagues [18] identified a lymph node adjacent to the inferior aspect of the parotid gland, which contained metastases in 3 of 5 patients with tumors of the parotid gland. In 1977, Cabanas [19] reported that tumor cells from primary penile cancer drained to nodes adjacent to the pubis. However, both studies defined SNs by a fixed anatomic location, and their results were not consistently reproduced by other investigators. Meanwhile, in the mid-1970s our group independently performed mapping studies of lymphatic drainage patterns from primary cutaneous melanoma [20,21], and in 1977 we reported successful use of cutaneous lymphoscintigraphy to determine the nodal basins at risk for metastasis in 32 patients with primary truncal melanomas [21]. Our findings contradicted the notion of an anatomically fixed SN; instead, these lymphoscintigraphic studies showed that the location of a melanoma-draining lymphatic basin depended on the pattern of lymphatic drainage, which can be quite variable among patients. These early lymphoscintigraphy studies also set the stage for development of an intraoperative mapping technique to accurately identify the first tumor-draining node (SN) within the drainage basin.
Our preclinical [22]and clinical development of intraoperative mapping relied solely on vital dyes to identify the SNs. Clinical trials of intraoperative lymphatic mapping and SN biopsy began in 1985, and we presented our technique and results at the 1990 annual meeting of the Society of Surgical Oncology [11]. In this study, 223 patients with cutaneous melanoma underwent 237 dye-directed mapping procedures. At the time of surgery, blue dye was injected intradermally at the primary site, an incision was made over the regional nodal basin identified by lymphoscintigraphy, and skin flaps were raised toward the primary site to identify the blue-stained lymphatic channel. The blue lymphatic channel was then traced by meticulous dissection to the blue-stained SN, which was excised. In this initial study, all patients underwent CLND of the melanoma-draining basin, regardless of the status of the SN. Our rate of SN identification was 82% and our rate of false-negative procedures (tumor-negative SN biopsy but tumor-positive CLND) was less than 1%. Despite these results, the technique was met with skepticism and the initial report was rejected by several prestigious medical journals. However, after its 1992 publication in Archives of Surgery [12], SN biopsy was confirmed by other groups and soon gained widespread acceptance.
To facilitate identification of the SN, we incorporated a second mapping agent into the intraoperative procedure. We first reported the clinical use of dual-agent SN mapping at the 1994 annual meeting of the Society of Surgical Oncology [23]. SNs were identified by blue staining and/or by radioactive counts measured by a hand-held gamma probe. Because the gamma probe could be used to guide dissection, dual-agent mapping improved the SN identification rate. It also eliminated the need for dissection of subcutaneous flaps. In our initial study, the radiotracer was injected in the operating room, at the same time as the blue dye; we subsequently simplified the procedure by injecting the radiotracer in the nuclear medicine department a few hours before the operative procedure.
The benefit of SN biopsy was addressed by the Multicenter Selective Lymphadenectomy Trial (MSLT) group in a phase III international multicenter trial [24]. The first MSLT study (MSLT-I) randomized 1,269 patients with intermediate-thickness melanoma (1.2–3.5 mm) to WLE plus observation or to WLE plus SN biopsy. Patients in the observation group underwent CLND only if they developed clinical evidence of nodal disease (delayed CLND), whereas patients in the SN group underwent CLND if the SN specimen was tumor-positive (immediate CLND). In the subgroup of patients with nodal metastasis, interim results showed a significant overall survival advantage for SN biopsy followed by immediate CLND versus nodal observation followed by delayed CLND. In the entire group, interim results showed a significant disease-free survival advantage for SN biopsy [24]. Lymphatic mapping plus SN biopsy for melanoma has been shown to be safe, accurate and reproducible by multiple centers. It has gained widespread acceptance for staging of melanoma, and its appealing simplicity has led to its successful adaptation for other solid tumors that metastasize by lymphatics, such as breast cancer, for which it has become the standard of care.
Several initial reports suggested that SN biopsy might increase the risk of in-transit metastasis [25,26]. These single-center retrospective studies reported a higher incidence of intransit metastasis in relatively small series of patients who underwent WLE plus SN biopsy versus WLE alone. However, three larger single-center retrospective studies did not support this claim [27–29], and a prospective randomized trial confirmed no increase in the rate of in-transit metastasis with SN biopsy [24].
CLINICAL INDICATIONS
Patients with primary lesions at least 1 mm thick are candidates for SN biopsy [30]. Reported rates of SN metastasis are 12% to 20% for 1- to 2-mm melanomas, 28% to 33% for 2-to 4-mm melanomas, and 28% to 44% for melanomas thicker than 4 mm [30,31]. SN biopsy for thin melanoma (<1 mm) remains controversial. Factors that may increase the risk of nodal metastasis in patients with thin melanoma include ulceration, Breslow thickness >0.75 mm, Clark level IV, mitotic rate >0 [32–34], young age and male sex [35]. Contraindications to SN biopsy include histologically confirmed metastatic lymph nodes and prior extensive reconstructive flap surgery that disrupts the skin lymphatics and would make SN biopsy inaccurate. Special precautions need to be considered for pregnant patients. Though radiation to the fetus is thought to be negligible, SN biopsy should be modified to minimize radiation exposure. Because the safety of blue dye and radiotracer in pregnant patients has not been determined, we generally perform WLE during pregnancy and delay SN biopsy until after birth.
PREOPERATIVE LYMPHOSCINTIGRAPHY
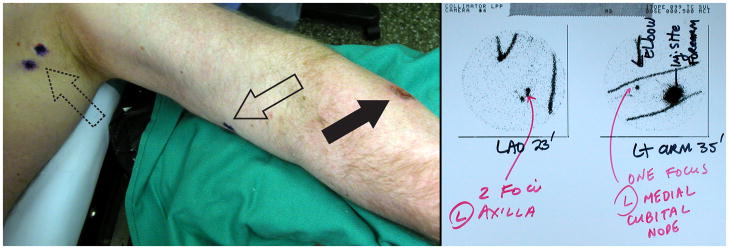
Preoperative lymphoscintigraphy is the first step in lymphatic mapping of the SN. The images provide a road map to guide the surgeon in identifying the regional nodal basin(s) at risk and estimating the location of SNs within that basin [36]. Preoperative lymphoscintigraphy can distinguish nodes on the direct afferent lymphatic channel (SNs) from nodes that may be adjacent to SNs but do not receive direct drainage; these second-tier nodes are not SNs [37]. Preoperative lymphoscintigraphy also enables the surgeon to identify SNs located in unpredictable regional basins, such as contralateral sites in patients with melanoma of the trunk or head and neck. In addition, lymphoscintigrams can reveal ectopic basins or nodes (Figure 1). These may include popliteal and epitrochlear basins in patients with melanoma of the extremity, nodes in the triangular intermuscular space of patients with melanoma of the back and flank, or intercostal nodes in patients with melanoma of the chest and abdomen. Finally, the lymphoscintigram can identify intransit lymph nodes located between the drainage basin and primary tumor site [32,38–42].
Figure 1.
Preoperative lymphoscintigram (right panel) reveals drainage of a forearm melanoma (left panel, solid arrow) to an ectopic cubital node in the upper arm (left panel, open arrow), in addition to expected foci in the axillary basin (left panel, dotted arrow).
Multiple radiotracers are available and can be used alone or with blue dye [40]. The most widely used radiotracers are 99mTc-labeled sulfur colloid (United States), 99mTc-antimony trisulphide colloid (Australia), and 99mTc-labeled albumin (Europe) [16]. The specific radiotracer used is determined largely by availability and approval by the national governing agency. Radiotracer injections are primarily performed by nuclear medicine physicians. Four wheals should be produced by intradermal injections of 0.1 to 0.2 ml of 10mBq (0.5mCi) radiocolloid around the primary tumor/scar at a distance of approximately 1 to 5 mm[16]. Lower doses may be useful in the head and neck region to avoid excess radioactivity from the injection site, which might obscure adjacent SNs. Care should be taken to avoid skin contamination with the radiotracer.
Scintillation cameras are used to obtain dynamic images that allow for 1) identification of SNs within the regional nodal basin and 2) discrimination of second-tier nodes which on delayed imaging may be falsely interpreted as SNs. Dynamic images can identify the SN 1 to 30 minutes after the injection of the tracer; by 4 hours after injection, the radiotracer dissipates and SNs may no longer be discriminated from non-SNs (Figure 2) [43]. The surface location of the SNs is marked on the skin by the nuclear medicine physician to assist the surgeon in localizing the SN intraoperatively. Hence, it is important the skin be marked in the same position as will be used in the operating room. Not all hot nodes seen on delayed images should be marked because these nodes may represent second-tier nodes and therefore their removal can lead to unnecessary dissection and increased morbidity [44]. Also, the number of direct lymphatic channels should equal the number of SNs. Images must be available to the surgeon at the time of operation and should contain information on the number and location of lymphatic channels draining to SNs, and comments on unusual draining patterns.
Figure 2.
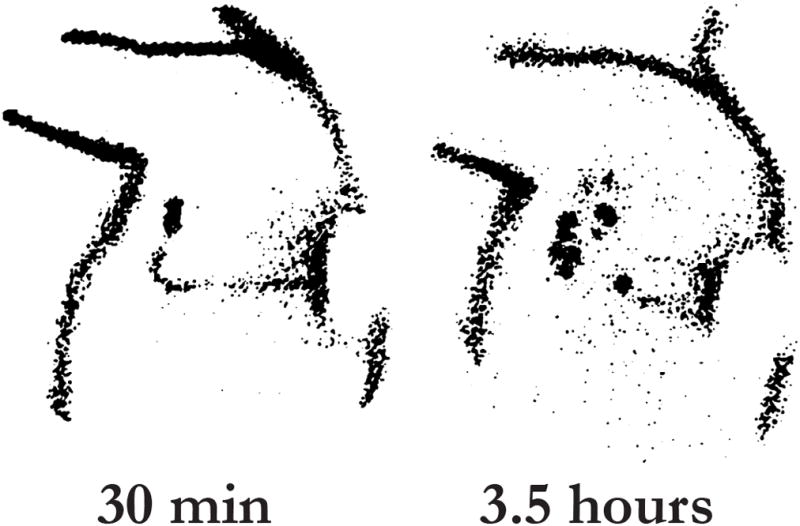
Effect of radiotracer transit time on SN identification. The lymphoscintigram demonstrates the dynamic quality of radiotracers in a patient with melanoma of the left back. The image at 30 minutes depicts the lymphatic channel and SN. Delayed images taken at 3.5 hours show second-tier, non-SNs. Although the SN may not disappear from the lymphoscintigram, its level of radioactivity will decrease with time. Reprinted with permission from Glass EC et al[43].
INTRAOPERATIVE LYMPHATIC MAPPING
Vital Blue Dye
Our initial report of SN biopsy utilized blue dye to direct intraoperative lymphatic mapping [12], and vital blue dye was the gold standard for SN identification in MSLT-I [45]. However, as mentioned above, intraoperative detection of radiotracers using a hand-held gamma probe[23] can identify additional SNs and eliminate the need to raise skin flaps to find the tumor-draining lymphatic channels. In our multicenter validation study based on MSLT-I data, the combination of blue dye and radiotracer appeared to generate the best results [45,46]. Dual-agent mapping with preoperative injection of a radiotracer and intraoperative injection of blue dye has led to a SN identification rate of 99% [32].
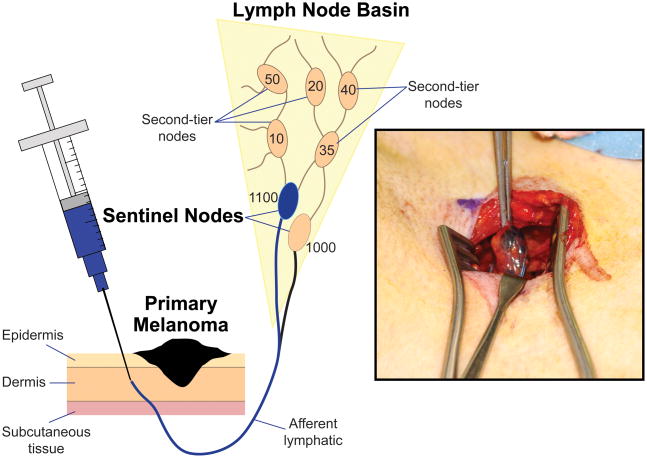
Following preoperative lymphoscintigraphy, the patient is brought to the operating room where vital blue dye is injected intradermally at the primary site 10 to 15 minutes prior to incision. Vital blue dyes complement lymphoscintigraphy by helping visualize the SNs during dissection. Isosulfan blue (Lymphazurin) and Patent Blue V are both effective [22]. Methylene blue has also been used because of its greater availability; however it is less effective at highlighting lymphatic channels and has been associated with soft tissue necrosis [22,47,48]. Vital blue dye is injected intradermally using a tuberculin syringe (27 G) around the primary lesion or biopsy scar (Figure 3). The volume of dye depends on the anatomic site: 1–2 ml for sites on the trunk, extremity and scalp, but only 0.5–0.75 ml on the face because of wider diffusion, particularly around the eye. Injection into the subcutaneous tissue should be avoided because lymphatic density is lower and the dye may not migrate. Also, subcutaneous tissues have separate and distinct lymphatic channels and therefore may lead to incorrect SN identification [22].
Figure 3.
At different times, blue dye and radiotracer are injected intradermally at the site of the primary tumor (radiotracer is injected immediately before preoperative lymphoscintigraphy, and blue dye is injected immediately before the surgical procedure). During intraoperative lymphatic mapping, blue-stained afferent lymphatics are visualized and followed to the first blue-stained node, i.e., the SN (inset). A hand-held gamma probe confirms higher radioactive counts within this SN and can identify additional SNs that are not stained blue.
The time interval from injection of blue dye to surgical dissection depends on the distance from the primary lesion to the regional nodal basin that will be explored. Lymphatic flow is fastest for the distal limbs and slowest for the head/neck region [44,49]. Typically within 5 to 15 minutes, the blue dye has entered the SN and washout from the node is evident after approximately 45 minutes [16]. Since lymphatic function appears to decline in patients older than 50 years [50,51], passive exercise of the affected limb can be undertaken to increase lymphatic flow after blue dye injection. We recommend two to four intradermal injections of blue dye around the primary lesion or biopsy scar after the patient is anesthesized to avoid a painful injection. The injection should produce a small wheal and show the lymphatics closest to the injection site. Care should be taken to not inject too quickly as this may disrupt the lymphatics.
A contraindication to blue dye injection is known allergic reaction to blue dye; anaphylaxis is rare but has been reported [52]. As mentioned above, pregnancy is a relative contraindication because the long-term toxicity to the fetus is unknown. Adverse side effects to blue dye include allergic reactions (“blue hives”), which are seen in up to 2% of cases and may be influenced by blue dye volume [45,52,53]. Other side effects are blue-colored urine for up to 24 hours following administration [52], and a factitious drop in intraoperative oxygen saturation measured by pulse oximetry.
Gamma Probe Detection
The hand-held gamma probe is a radiation detector that provides a count rate from gamma rays. It is an effective tool that facilitates the intraoperative identification of radioactive lymph nodes that may or may not be blue-stained (Figure 3). If SN biopsy is performed shortly after preoperative lymphoscintigraphy, the handheld gamma probe can be used to identify residual radiotracer in the SN. After a few hours, radioactivity decreases in the SN but can be measured in second-tier nodes (Figure 2). Because the radioisotope has a half-life of approximately 5–6 hours, SN biopsy also can be performed the day after lymphoscintigraphy. In this case radioactive counts will be lower and the SN sometimes may be more difficult to identify.
Before the skin is incised, the gamma probe should be moved systematically over the lymphatic basin to confirm the accuracy of the marked skin site; if the patient’s position during surgery differs from that during lymphoscintigraphy, then the skin marks may no longer approximate the location of the SN. Because the preoperative lymphoscintigraphic image given to the surgeon represents only a snapshot of a dynamic process, potential drainage basins (including ectopic locations such as the intermuscular triangle of the back) should be checked with the probe to confirm the absence of radioactivity.
The general function of the probe can be crudely checked by pointing the probe at the injection site to test for a response. The nuclear medicine report and the skin mark act as reference point for the SN search. First, set initial count range to the lowest setting and volume to a clearly audible setting. Correct range is important because an insufficient pitch variation can prevent identification of some radioactive nodes [16]. Record the count activity at the injection site and the background count activity around the basin. Start at the position in the basin closest to the injection site: place the probe perpendicular to the skin surface, and scan in a back and forth pattern moving away from the injection site. When count activity increases, localize the hot spot by scanning perpendicular to the initial scan line. The speed of scanning should be approximately 1 to 5 cm/second. Many probe systems have separate meters for summed counts (over 1–2 seconds) and peak counts; peak counts are useful for quickly scanning a basin.
The distance between the injection site and the drainage basin can affect hot spot identification. The closer the injection site is to the drainage basin, the more likely that background counts will be constant because radioactivity “shines through” from the primary (injection) site. If the injection site is within the probe’s field, counts will be falsely elevated. If the primary tumor is close to the lymphatic basin, shine-through can be minimized by performing WLE of the primary site prior to identifying the SN.
Exploration and Dissection
There are no randomized trials comparing surgical techniques for SN biopsy. At JWCI, we have found that using general anesthesia is more comfortable for the patient and makes it easier for the surgeon to identify deep nodes. However, local anesthesia with sedation can be used in situations where general anesthesia is contraindicated. The skin is prepped in standard fashion. The extremity is free-draped to allow for frequent repositioning during the operation. A hand-held gamma probe covered in a sterile plastic sheath identifies the hot spot on the skin surface. After the injection of local anesthesia, a short skin incision is made. Because the relevant tumor-draining lymphatic channels are well below the skin, this incision does not need to parallel the assumed direction of lymph flow. However, the surgeon must keep in mind that the incision will need to be excised if the SN is positive and CLND is required, and should therefore plan accordingly.
The key principle of SN biopsy is to remove all SNs with minimal dissection. Dissection is facilitated by tracing blue-stained lymphatics to the SN. Using the gamma probe to guide the path of dissection will avoid unnecessary tissue trauma. Because tissues will move during dissection, a sudden loss of counts may indicate that the path of dissection has extended past the node; simple retraction of the probe will usually re-establish the count rate. When it appears that a section of tissue contains radioactivity, the surgeon must critically inspect the tissue before continuing dissection. Place the probe on the tissue and point the probe in different directions to ensure that radioactivity is not from the injection site or from tissue located more distally. On occasion, subcutaneous SNs can be identified near primary sites located at the base of the neck, upper back or other sites.
Meticulous, gentle dissection towards the SN is performed to avoid transecting the lymphatics, because disruption of these lymphatics leads to contamination of radiotracer and dye and to an increased risk of seroma. Electrocautery is used to dissect through the subcutaneous tissue and fascia. Blunt dissection towards the hot node is performed using a tonsil clamp. Afferent and efferent lymphatic and blood vessels are ligated with clips and/or suture. Care is taken to neither crush nor cauterize the specimen.
After a node is dissected free, the radioactivity of this node is checked by placing it on a surface away from the patient to avoid interference. The surgeon should check all surfaces of the tissue specimen for radioactivity. The ex vivo count is recorded and a suture is loosely placed to mark the hottest spot without distorting SN architecture; this suture will guide the pathologist during histopathological evaluation. The operative site is then checked for remaining activity. Though the lymphoscintigram may indicate only one hot node, there may actually be multiple hot nodes in a cluster that appear as one due to the limited resolution of the gamma camera. The decision to remove nodes will depend on the clinical definition of a SN (see section below). Lymphatic vessels and lymph nodes may be replaced by tumor and fail to take up mapping agents [36]; therefore the operative site should be inspected and palpated for clinically suspicious lymph nodes. All such lymph nodes should be removed. If nodal metastasis is detected on permanent section, CLND should be planned as a second operation. If no SN can be found, a CLND should be considered for high-risk primary lesions.
Lymphatic mapping in the groin and axilla is associated with high rates of SN identification and low rates of false-negative SNs (1% for the groin and 5% for the axilla) [42,46]. Lymphatic mapping in the head and neck region requires much more experience for comparable success, because this area has a dense and complex pattern of communicating lymphatic channels, often with multiple SNs. Reported false-negative rates for the head-neck region have been as high as 15% [42]. Although our group and other investigators have reported higher success rates [54,55], we find that the head-neck region is the most difficult site to map. SNs of the parotid are usually located superficially and easily removed. There is no consensus on whether to excise external iliac or obturator SNs identified by lymphoscintigraphy; we recommend removing them through a retroperitoneal approach if the lymphoscintigram demonstrates direct drainage to these nodes from the primary site on the skin.
Frozen sections and tumor imprints for melanoma are unreliable and discouraged. Tissue processed for frozen section decreases the amount of tissue available for permanent sections. Since nodal metastases are frequently small and are located near the capsule, the tissue lost during frozen section may be the only site of micrometastasis. Several studies have shown that frozen sections fail to identify micrometastatic (< 2 mm) disease [56,57].
Compared to CLND, SN biopsy has minimal morbidity. Risks of the procedure include wound separation (0.2% to 1.2%), lymphedema (0.6% to 0.7%), seroma/hematoma (2.3% to 5.5%), and surgical site infection (1.1% to 4.6%) [46,58]. Additionally, quality of life assessments indicate that the procedure is well tolerated [59]. To limit complications, we re-approximate the deep and superficial tissues to close dead space, use the gamma probe to guide and therefore minimize dissection, and administer prophylactic antibiotics when appropriate. We do not use drains.
Though the SN concept is simple, the success of SN biopsy is significantly influenced by the surgeon’s experience [12,45]. In MSLT-I, the rate of same-basin nodal recurrence after a negative SN biopsy was 10.3% for the first 25 cases versus 5.2% for subsequent cases [46]. Since all centers were required to satisfactorily perform 30 SN biopsies prior to enrolling patients for the trial, it appears that the learning curve for accurate SN biopsy was approximately 50 cases in MSLT-I. However, MSLT-I was initiated in 1994; since then, widespread use of dual-agent mapping and other technical refinements have undoubtedly improved the learning curve.
CONTROVERSIES REGARDING THE DEFINITION OF A SENTINEL NODE
The SN is defined as the initial lymph node(s) that directly drain the lymph from the site of the primary lesion [12]. This definition assumes that there is a lymphatic channel connecting the primary lesion to the lymph node. This is somewhat simplistic because there may be more than one lymphatic channel draining the primary site to two or more SNs. In approximately 70% of cases one SN is identified, in 20% two SNs are identified, and in the remaining cases greater than two SNs are identified. After lymph passes through the SN, it drains up the lymphatic channel into second-tier nodes (non-SNs) [44], which often can be identified on delayed lymphoscintigrams (Figure 2) [43] but rarely can be visualized by blue staining because the dye becomes too dilute to see after it leaves the SN.
Despite a good understanding of the theoretical definition of a SN, there is no consensus on the clinical definition of a SN. The SN has been described as the hottest node, the blue node, first node visualized on lymphoscintigraphy, and a node with radioactivity greater than twice or thrice background radioactivity [60,61]. These various definitions confound standardization of SN biopsy. It is important to understand that all clinical definitions of a SN rely on detection techniques (mapping agents, gamma probe), and that these tools have their limitations. If blue dye is the only tracer used, then the SN is defined by its blue stain, because the dye rapidly dilutes and does not progress beyond the first draining node. However, if a radioactive tracer is used in addition to the blue dye, then the definition must be expanded because not all hot nodes are SNs and not all SNs are necessarily blue.
Data from the Sunbelt Melanoma Trial showed that resection of all blue-stained nodes and all nodes with greater than 10% of the hottest node’s radioactivity was associated with a low estimated false-negative rate [32,62]. However, one problem faced in determining a node’s radioactivity is that the ex vivo counts may be two to five times higher than the in vivo counts. Thus, the surgeon does not know a node’s true count until the node is removed. To deal with this conundrum, we measure the background radioactivity of the regional nodal basin prior to incision and excise all blue nodes and all hot nodes that have radioactive counts greater than the background.
FUTURE OF SN BIOPSY
New agents for lymphatic mapping are currently being developed. One agent with exciting potential is 99mTc-diethylenetriamine pentaacetic acid (DPTA)-mannosyldextran, a radiocolloid that binds mannose receptors on antigen-presenting cells of lymph nodes. Studies have shown that this agent has rapid clearance from the primary injection site and accumulates in the SN for at least 24 hours [63,64].
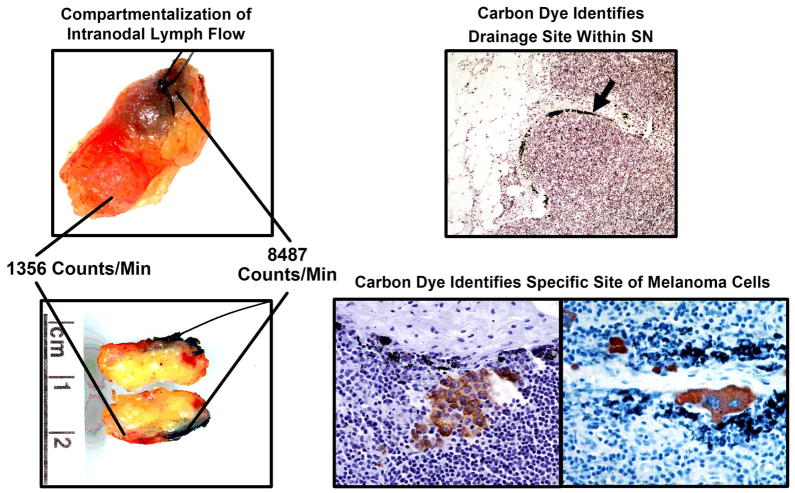
Despite advances in pathologic assessment of the SN, nodal micrometastasis may be missed if the wrong section of the node is examined or if the node is nonsentinel. We developed carbon dye mapping as a means to permanently label a node as the SN and to help the pathologist identify the intranodal site of lymphatic drainage, i.e., the site most likely to harbor tumor cells [13,65,66]. Carbon particles (sterile India ink) are mixed with the blue dye prior to injection. The SN uptakes the blue dye and carbon particles; these carbon particles are seen in the SN and correlate with the intranodal location of tumor cells (Figure 4) [66]. As a result, the location of carbon particles in the SN may assist the pathologist in identifying metastasis. Currently there are no commercial preparations available and use of carbon particles is restricted to research studies.
Figure 4.
Blue dye and radiotracer identify the first tumor-draining regional lymph nodes (SNs); carbon dye pinpoints the most likely site of tumor cells within these nodes. Unlike blue dye and radioisotopes, carbon is relatively immobile in the node; its intranodal presence therefore can confirm the node as sentinel and help the pathologist identify the most likely intranodal site of any micrometastatic foci. Reprinted with permission from Morton et al. [66]
CONCLUSIONS
SN biopsy is a minimally invasive technique that accurately stages the regional lymph node basin and thereby avoids the potential morbidity of ELND in 80% of patients with clinically localized melanoma. The technique requires a team of dedicated nuclear medicine physicians, surgeons, and pathologists. Surgical technique is paramount to localize and remove all SNs. Experience and familiarity with regional lymphatic drainage patterns to axillary, inguinal and especially head/neck regions can help the surgeon to achieve a high SN identification rate with low morbidity.
Acknowledgments
Supported by grant P01 CA29605 from the National Cancer Institute and by funding from the Samueli Foundation (Corona del Mar, CA; Dr. Bagaria), the Wayne and Gladys Valley Foundation (Oakland, CA), Ruth and Martin H. Weil Fund (Los Angeles, CA), Mrs. Lois Rosen, the Lance Armstrong Foundation (Austin, TX), the John Wayne Cancer Foundation (Newport Beach, CA) and the Wrather Family Foundation (Los Alamos, CA). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
References
- 1.Balch CM, Soong SJ, Atkins MB, Buzaid AC, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–149. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 2.Morton DL, Wanek L, Nizze JA, Elashoff RM, Wong JH. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;214:491–499. doi: 10.1097/00000658-199110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow H. Melanotic cancerous disease. Lancet. 1892;2:872. [Google Scholar]
- 4.Balch CM, Soong SJ, Murad TM, Ingalls AL, Maddox WA. A multifactorial analysis of melanoma. II. Prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979;86:343–351. [PubMed] [Google Scholar]
- 5.Milton GW, Shaw HM, McCarthy WH, Pearson L, Balch CM, Soong SJ. Prophylactic lymph node dissection in clinical stage I cutaneous malignant melanoma: results of surgical treatment in 1319 patients. Br J Surg. 1982;69:108–111. doi: 10.1002/bjs.1800690217. [DOI] [PubMed] [Google Scholar]
- 6.Sim FH, Taylor WF, Ivins JC, Pritchard DJ, Soule EH. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer. 1978;41:948–956. doi: 10.1002/1097-0142(197803)41:3<948::aid-cncr2820410324>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Adamus J, Bandiera DC, Brennhovd IO, Caceres E, Cascinelli N, Claudio F, Ikonopisov RL, Javorskj VV, Kirov S, Kulakowski A, Lacoub J, Lejeune F, Mechl Z, Morabito A, Rode I, Sergeev S, van Slooten E, Szcygiel K, Trapeznikov NN. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977;297:627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi U, Adamus J, Bandiera DC, Brennhovd O, Caceres E, Cascinelli N, Claudio F, Ikonopisov RL, Javorski VV, Kirov S, Kulakowski A, Lacour J, Lejeune F, Mechl Z, Morabito A, Rode I, Sergeev S, van Slooten E, Szczygiel K, Trapeznikov NN, Wagner RI. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982;49:2420–2430. doi: 10.1002/1097-0142(19820601)49:11<2420::aid-cncr2820491133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Soong S, Ross MI, Urist MM, Karakousis CP, Temple WJ, Mihm MC, Barnhill RL, Jewell WR, Wanebo HJ, Harrison R. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Soong SJ, Bartolucci AA, Urist MM, Karakousis CP, Smith TJ, Temple WJ, Ross MI, Jewell WR, Mihm MC, Barnhill RL, Wanebo HJ. Efficacy of an elective regional lymph node dissection of 1 to 4 mm thick melanomas for patients 60 years of age and younger. Ann Surg. 1996;224:255–263. doi: 10.1097/00000658-199609000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton DL, Cagle L, Wong J. Intraoperative lymphatic mapping and selective lymphadenectomy: Technical details of a new procedure for clinical stage I melanoma. Presented at the 42nd Annual Meeting of the Society of Surgical Oncology; Washington, DC. May 20–22, 1990. [Google Scholar]
- 12.Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 13.Chen SL, Iddings DM, Scheri RP, Bilchik AJ. Lymphatic mapping and sentinel node analysis: current concepts and applications. CA Cancer J Clin. 2006;56:292–309. doi: 10.3322/canjclin.56.5.292. [DOI] [PubMed] [Google Scholar]
- 14.Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol. 1988;12:612–618. doi: 10.1097/00000478-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Cochran AJ, Balda BR, Starz H, Bachter D, Krag DN, Cruse CW, Pijpers R, Morton DL. The Augsburg Consensus. Techniques of lymphatic mapping, sentinel lymphadenectomy, and completion lymphadenectomy in cutaneous malignancies. Cancer. 2000;89:236–241. [PubMed] [Google Scholar]
- 16.Chakera AH, Hesse B, Burak Z, Ballinger JR, Britten A, Caraco C, Cochran AJ, Cook MG, Drzewiecki KT, Essner R, Even-Sapir E, Eggermont AM, Stopar TG, Ingvar C, Mihm MC, Jr, McCarthy SW, Mozzillo N, Nieweg OE, Scolyer RA, Starz H, Thompson JF, Trifiro G, Viale G, Vidal-Sicart S, Uren R, Waddington W, Chiti A, Spatz A, Testori A. EANM-EORTC general recommendations for sentinel node diagnostics in melanoma. Eur J Nucl Med Mol Imaging. 2009;36:1713–1742. doi: 10.1007/s00259-009-1228-4. [DOI] [PubMed] [Google Scholar]
- 17.Morton DL, Chan AD. The concept of sentinel node localization: how it started. Semin Nucl Med. 2000;30:4–10. doi: 10.1016/s0001-2998(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 18.Gould EA, Winship T, Philbin PH, Kerr HH. Observations on a “sentinel node” in cancer of the parotid. Cancer. 1960;13:77–78. doi: 10.1002/1097-0142(196001/02)13:1<77::aid-cncr2820130114>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–466. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Holmes EC, Moseley HS, Morton DL, Clark W, Robinson D, Urist MM. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186:481–490. doi: 10.1097/00000658-197710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fee HJ, Robinson DS, Sample WF, Graham LS, Holmes EC, Morton DL. The determination of lymph shed by colloidal gold scanning in patients with malignant melanoma: a preliminary study. Surgery. 1978;84:626–632. [PubMed] [Google Scholar]
- 22.Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214:637–641. doi: 10.1097/00000658-199111000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essner R, Foshag LJ, Morton DL. Intraoperative radiolymphoscintigraphy: A useful adjunct to intraoperative lymphatic mapping and selective lymphadenectomy in patients with clinical stage I melanoma. Society of Surgical Oncology Annual Meeting; Houston, TX. March 17–20, 1994. [Google Scholar]
- 24.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Coventry BJ, Glass EC, Wang HJ. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 25.Estourgie SH, Nieweg OE, Kroon BB. High incidence of in-transit metastases after sentinel node biopsy in patients with melanoma. Br J Surg. 2004;91:1370–1371. doi: 10.1002/bjs.4692. [DOI] [PubMed] [Google Scholar]
- 26.Thomas JM, Clark MA. Selective lymphadenectomy in sentinel node-positive patients may increase the risk of local/in-transit recurrence in malignant melanoma. Eur J Surg Oncol. 2004;30:686–691. doi: 10.1016/j.ejso.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Pawlik TM, Ross MI, Thompson JF, Eggermont AM, Gershenwald JE. The risk of intransit melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol. 2005;23:4588–4590. doi: 10.1200/JCO.2005.12.245. [DOI] [PubMed] [Google Scholar]
- 28.van Poll D, Thompson JF, Colman MH, McKinnon JG, Saw RP, Stretch JR, Scolyer RA, Uren RF. A sentinel node biopsy does not increase the incidence of in-transit metastasis in patients with primary cutaneous melanoma. Ann Surg Oncol. 2005;12:597–608. doi: 10.1245/ASO.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Kang JC, Wanek LA, Essner R, Faries MB, Foshag LJ, Morton DL. Sentinel lymphadenectomy does not increase the incidence of in-transit metastases in primary melanoma. J Clin Oncol. 2005;23:4764–4770. doi: 10.1200/JCO.2005.20.537. [DOI] [PubMed] [Google Scholar]
- 30.Rousseau DL, Jr, Ross MI, Johnson MM, Prieto VG, Lee JE, Mansfield PF, Gershenwald JE. Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol. 2003;10:569–574. doi: 10.1245/aso.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faries MB, Morton DL. Surgery and sentinel lymph node biopsy. Semin Oncol. 2007;34:498–508. doi: 10.1053/j.seminoncol.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleicher RJ, Essner R, Foshag LJ, Wanek LA, Morton DL. Role of sentinel lymphadenectomy in thin invasive cutaneous melanomas. J Clin Oncol. 2003;21:1326–1331. doi: 10.1200/JCO.2003.06.123. [DOI] [PubMed] [Google Scholar]
- 34.Wright BE, Scheri RP, Ye X, Faries MB, Turner RR, Essner R, Morton DL. Importance of sentinel lymph node biopsy in patients with thin melanoma. Arch Surg. 2008;143:892–899. doi: 10.1001/archsurg.143.9.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faries MB, Wright BE, Wanek L, Elashoff D, Morton DL. Predictors of occult nodal metastasis in patients with thin melanoma. Arch Surg. doi: 10.1001/archsurg.2009.271. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson JF, Uren RF. Teaching points on lymphatic mapping for melanoma from the Sydney Melanoma Unit. Semin Oncol. 2004;31:349–356. doi: 10.1053/j.seminoncol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Belhocine TZ, Scott AM, Even-Sapir E, Urbain JL, Essner R. Role of nuclear medicine in the management of cutaneous malignant melanoma. J Nucl Med. 2006;47:957–967. [PubMed] [Google Scholar]
- 38.Vidal-Sicart S, Pons F, Fuertes S, Vilalta A, Rull R, Puig S, Palou JM, Ortega M, Castel T. Is the identification of in-transit sentinel lymph nodes in malignant melanoma patients really necessary? Eur J Nucl Med Mol Imaging. 2004;31:945–949. doi: 10.1007/s00259-004-1485-1. [DOI] [PubMed] [Google Scholar]
- 39.Balkissoon J, Rasgon BM, Schweitzer L. Lymphatic mapping for staging of head and neck cancer. Semin Oncol. 2004;31:382–393. doi: 10.1053/j.seminoncol.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Uren RF, Thompson JF, Howman-Giles R, Chung DK. The role of lymphoscintigraphy in the detection of lymph node drainage in melanoma. Surg Oncol Clin N Am. 2006;15:285–300. doi: 10.1016/j.soc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Essner R. The role of lymphoscintigraphy and sentinel node mapping in assessing patient risk in melanoma. Semin Oncol. 1997;24:S8–10. [PubMed] [Google Scholar]
- 42.McMasters KM, Chao C, Wong SL, Wrightson WR, Ross MI, Reintgen DS, Noyes RD, Cerrito PB, Edwards MJ. Interval sentinel lymph nodes in melanoma. Arch Surg. 2002;137:543–547. doi: 10.1001/archsurg.137.5.543. [DOI] [PubMed] [Google Scholar]
- 43.Glass EC, Essner R, Morton DL. Kinetics of three lymphoscintigraphic agents in patients with cutaneous melanoma. J Nucl Med. 1998;39:1185–1190. [PubMed] [Google Scholar]
- 44.Uren RF, Howman-Giles RB, Thompson JF. Demonstration of second-tier lymph nodes during preoperative lymphoscintigraphy for melanoma: incidence varies with primary tumor site. Ann Surg Oncol. 1998;5:517–521. doi: 10.1007/BF02303644. [DOI] [PubMed] [Google Scholar]
- 45.Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE, Roses DF, Karakousis CP, Mozzillo N, Reintgen D, Wang HJ, Glass EC, Cochran AJ. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230:453–463. doi: 10.1097/00000658-199910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morton DL, Cochran AJ, Thompson JF, Elashoff R, Essner R, Glass EC, Mozzillo N, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Coventry BJ, Wang HJ. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242:302–311. doi: 10.1097/01.sla.0000181092.50141.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stradling B, Aranha G, Gabram S. Adverse skin lesions after methylene blue injections for sentinel lymph node localization. Am J Surg. 2002;184:350–352. doi: 10.1016/s0002-9610(02)00945-5. [DOI] [PubMed] [Google Scholar]
- 48.Ruhlen JL. Tissue necrosis. Cutaneous and subcutaneous damage following extravasation of methylene blue. J Kans Med Soc. 1982;83:236–260. [PubMed] [Google Scholar]
- 49.Uren RF, Howman-Giles RB, Thompson JF, Roberts J, Bernard E. Variability of cutaneous lymphatic flow rates in melanoma patients. Melanoma Res. 1998;8:279–282. doi: 10.1097/00008390-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Faries MB, Essner R, Foshag LJ, Bilchik AJ, Zogakis T, Morton DL. Low lymph flow decreases with age and correlates with non-sentinel node metastases and poor survival in stage III melanoma (Abstract #4766) Proc Amer Assoc Cancer Res. 2006:47. [Google Scholar]
- 51.Conway WC, Faries MB, Nicholl MB, Terando AM, Glass EC, Sim M, Morton DL. Age-related lymphatic dysfunction in melanoma patients. Ann Surg Oncol. 2009;16:1548–1552. doi: 10.1245/s10434-009-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leong SP, Donegan E, Heffernon W, Dean S, Katz JA. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7:361–366. doi: 10.1007/s10434-000-0361-x. [DOI] [PubMed] [Google Scholar]
- 53.King TA, Fey JV, Van Zee KJ, Heerdt AS, Gemignani ML, Port ER, Sclafani L, Sacchini V, Petrek JA, Cody HS, 3rd, Borgen PI, Montgomery LL. A prospective analysis of the effect of blue-dye volume on sentinel lymph node mapping success and incidence of allergic reaction in patients with breast cancer. Ann Surg Oncol. 2004;11:535–541. doi: 10.1245/ASO.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Carlson GW, Murray DR, Lyles RH, Hestley A, Cohen C. Sentinel lymph node biopsy in the management of cutaneous head and neck melanoma. Plast Reconstr Surg. 2005;115:721–728. doi: 10.1097/01.prs.0000152429.06593.c1. [DOI] [PubMed] [Google Scholar]
- 55.Morton DL, Wen DR, Foshag LJ, Essner R, Cochran A. Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early-stage melanomas of the head and neck. J Clin Oncol. 1993;11:1751–1756. doi: 10.1200/JCO.1993.11.9.1751. [DOI] [PubMed] [Google Scholar]
- 56.Koopal SA, Tiebosch AT, Albertus Piers D, Plukker JT, Schraffordt Koops H, Hoekstra HJ. Frozen section analysis of sentinel lymph nodes in melanoma patients. Cancer. 2000;89:1720–1725. [PubMed] [Google Scholar]
- 57.Gibbs JF, Huang PP, Zhang PJ, Kraybill WG, Cheney R. Accuracy of pathologic techniques for the diagnosis of metastatic melanoma in sentinel lymph nodes. Ann Surg Oncol. 1999;6:699–704. doi: 10.1007/s10434-999-0691-2. [DOI] [PubMed] [Google Scholar]
- 58.Wrightson WR, Wong SL, Edwards MJ, Chao C, Reintgen DS, Ross MI, Noyes RD, Viar V, Cerrito PB, McMasters KM. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10:676–680. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Garreau J, Faries MB, Ye X, Morton DL. Mood state and melanoma outcome in the Multicenter Selective Lymphadenectomy Trial. J Clin Oncol. 2009;27(15S) Abstract 9603. [Google Scholar]
- 60.Nieweg OE, Tanis PJ, Kroon BB. The definition of a sentinel node. Ann Surg Oncol. 2001;8:538–541. doi: 10.1007/s10434-001-0538-y. [DOI] [PubMed] [Google Scholar]
- 61.Morton DL, Bostick PJ. Will the true sentinel node please stand? Ann Surg Oncol. 1999;6:12–14. doi: 10.1007/s10434-999-0012-9. [DOI] [PubMed] [Google Scholar]
- 62.McMasters KM, Reintgen DS, Ross MI, Wong SL, Gershenwald JE, Krag DN, Noyes RD, Viar V, Cerrito PB, Edwards MJ. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann Surg Oncol. 2001;8:192–197. doi: 10.1007/s10434-001-0192-4. [DOI] [PubMed] [Google Scholar]
- 63.Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: a molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14:913–921. doi: 10.1245/s10434-006-9099-4. [DOI] [PubMed] [Google Scholar]
- 64.Wallace AM, Hoh CK, Limmer KK, Darrah DD, Schulteis G, Vera DR. Sentinel lymph node accumulation of Lymphoseek and Tc-99m-sulfur colloid using a “2-day” protocol. Nucl Med Biol. 2009;36:687–692. doi: 10.1016/j.nucmedbio.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haigh PI, Lucci A, Turner RR, Bostick PJ, Krasne DL, Stern SL, Morton DL. Carbon dye histologically confirms the identity of sentinel lymph nodes in cutaneous melanoma. Cancer. 2001;92:535–541. doi: 10.1002/1097-0142(20010801)92:3<535::aid-cncr1352>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 66.Morton DL, Hoon DS, Cochran AJ, Turner RR, Essner R, Takeuchi H, Wanek LA, Glass E, Foshag LJ, Hsueh EC, Bilchik AJ, Elashoff D, Elashoff R. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg. 2003;238:538–549. doi: 10.1097/01.sla.0000086543.45557.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]