Synopsis
Rheumatoid arthritis (RA) likely develops in several phases, beginning with genetic risk, followed by asymptomatic autoimmunity, then finally, clinically-apparent disease. Investigating the phases of disease that exist prior to the onset of symptoms - i.e. the pre-clinical period of RA – will lead to understanding of the important relationships between genetic and environmental factors that may lead to disease, as well as allow for the development of predictive models for disease, and ultimately preventive strategies for RA.
Introduction
The discovery of genetic and environmental factors associated with rheumatoid arthritis (RA), and elevations in autoantibodies and inflammatory markers prior to the onset of symptomatic disease, coupled with similar findings in other autoimmune diseases including type 1 diabetes mellitus (T1DM), has led to the creation of a shared model of autoimmune disease development. In this model, the development of RA follows a natural history divided into phases wherein genetic and environmental interactions initially lead to a period of asymptomatic autoimmunity, evidenced by the presence of RA-related autoantibodies, that later evolves into clinically-apparent disease. It is the initial phases of risk and asymptomatic autoimmunity that encompass ‘pre-clinical’ RA.
In order to understand the genetic and environmental influences that are important to the evolution of RA, as well as develop predictive models and preventive strategies for future symptomatic disease, we must investigate this pre-clinical period. Herein are discussed the following issues related to pre-clinical RA: 1) what is known about the pre-clinical development of autoimmunity and inflammation in RA as well as other autoimmune diseases that may follow a similar model of development as RA, 2) how RA development can be modeled based on studies in pre-clinical RA and other autoimmune diseases, 3) practical issues related to the challenge of defining for research studies ‘pre-clinical’ RA, as compared to clinically-apparent disease, and 4) what aspects of RA evolution including genetic and environmental influences, and predictive and preventive models, could be addressed in studies of the pre-clinical period. Finally, potential methodologies and areas of focus going forward for research into pre-clinical RA will be discussed.
Part 1. Autoantibodies and inflammation in pre-clinical RA as well as other autoimmune diseases
Studies of pre-clinical rheumatoid arthritis
Multiple studies have shown that RA-related autoantibodies are present years prior to the diagnosis of RA (Table 1).(1–11) del Puente et al, who investigated RA in the Pima Indians in the Southwestern United States, showed that rheumatoid factor was present prior to the onset of clinically-apparent RA.(1) Aho et al, who investigated pre-clinical RA in Finland using a biobank of stored pre-diagnosis samples(2, 12–14), and Jonsson et al, who used Icelandic biobank samples(15), also demonstrated that RF (by various methodologies) was present prior to the onset of clinically-apparent RA. Also, in a prospective study of initially healthy family members of patients with RA, Silman et al showed that the presence of RF preceded the onset of clinically-apparent RA.(11)
Table 1.
Summary of selected studies of pre-clinical rheumatoid arthritis (RA)
| Study | Study Design | Biomarkers assessed | Findings | Implications for Prediction of Future RA |
|---|---|---|---|---|
| del Puente et al 1988 (1) |
Prospective; Native Americans, Southwest United States |
RF | RF precedes diagnosis of RA | Increased incidence of RA in RF+ individuals. Rates 2.4–48.3 per 1000 person-years, with highest rates in those with highest RF titers at baseline |
| Silman et al 1992 (11) | Prospective; British; FDRs from families with ≥2 RA cases |
RF | RF precedes diagnosis of RA | Average incidence of RA 8 per 1000 person-years in FDRs; highest rate in FDRs with RF+: 34.8 per 1000 person-years |
| Jonsson et al 1992 (15) | Retrospective; Icelandic; biobank | RF (isotypes) | RF isotype elevations precede diagnosis of RA | Not analyzed |
|
Aho et al 1985–1991 (2, 13, 14) |
Retrospective; Finnish; biobank | RF (isotypes) | RF precedes diagnosis of RA | Not analyzed |
|
Aho et al 1993, 2000 (4, 6) |
Retrospective; Finnish; biobank | AKA, AFA, APF (later studies showed target antigens likely citrullinated) |
AKA, AFA, APF precede diagnosis of RA | RA-related antibodies may be present in subjects who did not develop RA in follow-up period |
|
Rantapaa-Dahlqvist et al 2003 (7) |
Retrospective; Swedish; biobank; N=83 RA cases |
RF, anti-CCP | RF, anti-CCP precede diagnosis of RA; 34% anti-CCP+ ≤1.5 years prior to RA diagnosis; RF-isotypes+ in ~17–34% of cases pre-RA diagnosis |
PPV 22%for future RA if RF-IgA and anti-CCP positive (estimated population prevalence of RA of 1%) |
| Nielen et al 2004 (8) | Retrospective; Dutch; biobank; N=79 RA cases |
RF-IgM, anti-CCP | RF-IgM, anti-CCP precede RA diagnosis; 49% positive for RF-IgM and/or anti-CCP pre-RA. RF-IgM+ or anti-CCP+ median of 2.0 or 4.8 years prior to diagnosis of RA, respectively. |
PPV up to 100% for RA diagnosis within 5 years based on 5-year incidence rates of 0.001 (general population) or 3.9% (estimated from high-risk multicase RA families) |
| Majka et al 2008 (9) | Retrospective; United States’ Military; biobank; N=83 RA cases |
RF, anti-CCP | Pre-RA diagnosis: RF+ 57% of cases, median 6.0 years; anti- CCP+ 61% of cases, median 5.4 years |
Increased age-at-diagnosis of RA associated with longer duration of pre- clinical autoantibody positivity (replicated by Bos et al (17)) |
|
Rantapaa-Dahlqvist et al 2007 (21) |
Retrospective; Swedish; biobank | MCP-1, IL-6, CRP, sPLA2 |
After adjustment, only MCP-1 elevated prior to RA diagnosis | Not analyzed |
|
Nielen et al 2004, 2006 (19, 20) |
Retrospective; Dutch; biobank | RF, anti-CCP, CRP, sPLA2 |
CRP and sPLA2 elevations precede RA diagnosis; CRP ~2 year prior; timing similar to RF/anti-CCP |
Not analyzed |
|
Joregensen et al 2008 (24) |
Retrospective; Norwegian; biobank; N=49 RA cases |
RF-IgM, anti-CCP, multiple cytokines |
RF-IgM and/or anti-CCP precede RA diagnosis by as much as 20 years; anti-TNF elevated <5 years prior to diagnosis of RA. EBV and Parvovirus serologies not different between cases and controls |
Suggests that cytokine elevation may indicate symptomatic disease within 5-years |
| Berglin et al 2004 (113) | Retrospective; Swedish; biobank; N=59 RA cases |
RF, anti-CCP, HLA DRB1 alleles *0404, *0401 |
Anti-CCP+ DRB1*0404 or 0401 high-risk for future RA (OR ~67) | |
|
Johansson et al 2006 (114) |
Retrospective; Swedish; biobank; N=92 RA cases |
RF, anti-CCP; PTPN22 (1858T) |
Anti-CCP+PTPN22: 100% specific for future RA, and high-risk (OR>132) |
Abbreviations: RF=rheumatoid factor; FDR=first-degree relative of proband with RA; AKA=anti-keratin antibodies; AFA=anti-filaggrin antibodies; APF=anti-perinuclear factor antibodies; anti-CCP=anti-cyclic citrullinated peptide antibodies; PPV=positive predictive value; MCP-1=monocyte chemoattractant protein-1; IL-6=interleukin-6; CRP=C-reactive protein; sPLA2=secretory phospholipase A2; HLA=human leukocyte antigen; PTPN22=protein phosphatase 22.
Later studies also showed pre-clinical RA positivity for RF, as well as positivity for the highly RA-specific antibodies to citrullinated protein antigens (ACPAs). Again using a Finnish biobank, Aho et al demonstrated pre-clinical RA positivity of antikeratin (AKA) and antiperinuclear factor (APF) antibodies(3, 4), and antifillagrin antibodies (AFA)(6) – autoantibody targets that based on later findings represented citrullinated antigens.(16) Using stored blood samples available through the Medical Biobank of Northern Sweden, Rantapaa-Dahlqvist et al evaluated for elevations of RF isotypes (immunoglobulins[Ig] M, G and A) and the anti-cyclic citrullinated peptide (anti-CCP) antibody in 98 pre-clinical samples from 83 RA patients, collected a median of 2.5 years prior to symptomatic disease onset.(7) In this study, approximately 34% of patients were positive for anti-CCP within 1.5 years prior to diagnosis of RA, and the prevalence of RF isotype positivity ranged from ~17–34% during this same period. Additionally, in comparison to controls, a combination of both anti-CCP and any RF isotype was highly specific (99%) for the future development of classifiable RA. This report was followed shortly by a similarly-designed retrospective cohort study by Nielen et al that evaluated pre-clinical RA RF and anti-CCP positivity using pre-RA diagnosis samples stored in a Dutch blood donor biobank. (8) In this study, 79 RA patients with stored pre-diagnosis samples were identified, with a median of 13 pre-clinical samples per case. Of these 79 cases, ~28% and ~41% had pre-RA diagnosis elevation of RF (IgM isotype) or anti-CCP, respectively. RF was positive a median of 2.0 years prior to RA diagnosis (range 0.3 to 10.3 years), and anti-CCP was positive a median of 4.5 years prior to RA diagnosis (range 0.1 to 13.8 years).
Additional studies using stored biobank samples have also demonstrated pre-clinical RA positivity for autoantibodies. Using stored pre-RA samples from 83 United States’ military subjects with RA, Majka et al demonstrated pre-RA diagnosis positivity for RF and anti-CCP.(9) Additionally, they demonstrated that individuals that were older at the time of diagnosis of RA had longer duration of pre-clinical positivity of autoantibodies – a finding that was supported by work by Bos et al.(17) Karlson et al utilized the Nurses’ Health Study (NHS) biobank to demonstrate anti-CCP positivity prior to RA diagnosis, and showed that a lower cut-off for anti-CCP than the kit suggested was also highly specific for future RA.(18)
Multiple studies have also evaluated elevations of inflammatory markers prior to diagnosis of RA, with varying results (Table 1).(19–24) In their Finnish biobank study, Aho et al found no significant elevation of C-reactive protein (CRP) in pre-RA diagnosis samples, although the time of sample collection pre-diagnosis was not reported.(25) Using the same Medical Biobank of Northern Sweden used in the study of pre-RA RF and anti-CCP positivity, Rantapaa-Dahlqvist et al showed a significant elevation of monocyte chemoattractant protein-1 (MCP-1) in 92 pre-RA cases versus controls, but not secretory phospholipase A2 (sPLA2), high-sensitivity CRP (hsCRP) or interleukin(IL)-6.(21) Nielen et al demonstrated, in the Dutch pre-RA sample cohort described above, that CRP was elevated in the pre-RA period, most commonly about 2 years prior to diagnosis, regardless of pre-diagnosis autoantibody positivity. They were unable in this study to demonstrate the chronologic sequence of appearance of CRP versus autoantibodies.(19) They later additionally demonstrated pre-RA elevation of sPLA2 in this cohort, but were unable to determine whether CRP or sPLA2 preceded autoantibodies in the pre-clinical period, and they concluded that the temporal development of autoantibodies and these 2 markers were probably similar.(20) Jorgensen et al utilized samples from a Norwegian biobank to examine in 49 cases with RA pre-diagnosis elevations of autoantibodies (RF and anti-CCP) and multiple cytokines.(24) They found that RF and anti-CCP were elevated in RA cases pre-diagnosis; however, they could not demonstrate any cytokine elevations prior to 5 years before disease onset, and only TNFα was statistically significantly elevated in cases (versus controls) during the 5-year period just prior to diagnosis of RA.(24) Using a single pre-RA diagnosis blood sample from 90 incident RA cases (case status confirmed by chart review) identified in the Women’s Health Study (WHS), Karlson et al were unable to demonstrate significant elevations in CRP levels in cases versus controls (with case samples available a mean of 6.6 years prior to diagnosis), and they were unable to use a single CRP level to predict future RA.(23) However, in a later study using ~170 RA cases from a combination of the NHS and WHS cohorts, Karlson et al were able to demonstrate statistically-significant elevations of soluble tumor necrosis factor receptor II (sTNFRII) prior to RA diagnosis, but not IL-6 or hsCRP.(22)
The prospective studies by del Puente et al(1) and Silman et al(11) suggest that autoantibodies are truly elevated prior to the onset of clinically-apparent disease. However, in the studies of pre-clinical autoantibody and inflammatory marker elevations using stored samples from biobanks, there were not detailed joint-directed questionnaires or examinations performed at baseline or over time to ensure that no clinically-apparent arthritis was present at the time of presumed pre-clinical RA blood collections. As such, it may be that the duration of pre-RA diagnosis autoantibody positivity is overestimated in the studies utilizing stored biobank samples as subjects may have had mild or fluctuating inflammatory symptoms long prior to a confirmed diagnosis of RA.
Also of importance, these pre-RA studies show that not all RA patients have detectable pre-RA diagnosis autoantibody positivity. Methodologic issues may in part explain these findings. For example, patients may not have a stored blood sample available for analysis from the correct time period to demonstrate their pre-diagnosis autoantibody positivity. Also, our current assays for autoantibodies may not detect the earliest pre-clinical autoantibody specificities - perhaps an as-of-yet unknown citrullinated antigen is the earliest autoantibody target in pre-clinical RA? Additionally, not all patients with RA may develop detectable circulating autoantibodies in the pre-clinical period. For example, some patients may develop circulating autoantibodies after clinically-apparent disease develops.
Of note, in the Nielen study, while only ~28% of patients with RA were RF positive pre-diagnosis, ~67% of these same patients were positive for RF by 6 years post-diagnosis, suggesting that circulating RF develops in some cases after symptomatic onset of RA.(8) All of these issues will need to be considered in studies of pre-clinical RA going forward.
The contribution of genetic and environmental factors associated with RA to understanding pre-clinical development
While the exact etiology of RA is unknown, there are multiple genetic and environmental factors that have been associated with disease. Estimates of the genetic contribution to RA have ranged between 30% and 60%.(26, 27) Of the known genetic factors associated with RA, a DRB1 allele containing the ‘shared epitope’ is the most important genetic factor associated with RA.(28, 29) Of the HLA alleles, HLA-DRB1*0401 and HLA-DRB1*0404 (within the HLA-DR4 group) are the most strongly associated with RA, with an approximate relative risk for RA of almost 4 in Caucasians.(30) Additionally, a number of genes outside the HLA-DRB1 gene have recently been associated with RA. These include PTPN22, TRAF1/C5, CTLA4, STAT4, and the list is rapidly growing.(31, 32)
A number of potential environmental factors that could play important roles in modifying either susceptibility to RA or disease severity have been identified. High levels of coffee consumption (33), in particular decaffeinated coffee (34), as well as a positive smoking history (30, 35), exposure to air pollution (36) and environmental exposure to silica-containing dust (37, 38) are also associated with increased risk for RA. Additionally, infections have been associated with RA including pathogens such as the Epstein-Barr Virus (EBV), Mycoplasma or Proteus species.(26, 39–42) Finally, several factors have been identified as possibly protective against development of RA including a history of successful pregnancy (43, 44), oral contraceptive pill use (45–47) and higher vitamin D intake(48).
Several studies have examined the association of environmental factors with asymptomatic RA-related autoantibody positivity. These studies suggest that lack of oral contraceptive use and a history of heavy smoking are associated with an increased prevalence of RF (49–51), while 25,hydroxyvitamin D levels are not associated with RF positivity in individuals without RA (52). Active infection with tuberculosis has also been associated with RF and anti-CCP positivity in individuals without clinically-apparent synovitis.(53, 54) These studies suggest that environmental factors affect RA-related autoimmunity, although prospective evaluation of future risk for RA in these types of autoantibody positive subjects is lacking.
Of recent interest regarding the pathogenesis of RA are interactions between genetic and environmental factors that may lead to RA-related autoimmunity and disease. For example, several studies have reported that smoking in individuals with specific HLA alleles is associated with ACPA-positive RA, suggesting that a gene-environment interaction leads to RA-related autoimmunity.(55–58)
However, while genetic and environmental factors have been associated with RA, the exact role that these factors play in the development of RA-related autoimmunity is not yet clear. Also, it is unclear whether these factors may lead to initial RA related immune dysregulation or transition from asymptomatic autoimmunity to clinically-apparent RA. Importantly, as discussed later, prospective study of the early phases of RA development should help to clarify these issues.
Pre-clinical studies in other autoimmune diseases
In addition to the information about pre-clinical RA available from RA-specific studies, much about the evolution of autoimmunity can be learned by examining the pre-clinical natural history of other autoimmune diseases including type 1 diabetes (T1DM) and systemic lupus erythematosus (SLE). In particular, prospective studies of T1DM have led to the development of a model of disease evolution that may be of importance to RA.
T1DM results from autoimmune-mediated destruction of the pancreatic beta-cells, and it affects approximately 1 in 300 children. Numerous studies of T1DM have established that there are specific high-risk HLA genotypes that predispose to disease.(59, 60) Moreover, the autoimmune attack on the pancreatic beta-cells can be detected years before clinical onset of T1DM via the presence of any of the T1DM-related autoantibodies in the blood including antibodies to the following antigens: islet cell (ICA), insulin (IAA), protein tyrosine phosphatase (IA2), and glutamic acid decarboxylase (GAD65).(61, 62)
Prospective studies of T1DM have established that T1DM exhibits several phases during its development.(62) The first phase is defined as the presence of genetic risk factors that, either alone or in combination, may predispose to the loss of self tolerance. The second phase is reached when transformation from the risk state to a state of immunologic autoreactivity occurs; this second phase of ‘asymptomatic autoimmunity’ is measurable by testing for T1DM-related biomarkers, but this phase is not yet associated with the presence of clinically apparent hyperglycemia. This transition from genetic risk to asymptomatic autoimmunity occurs either because of the introduction of an environmental factor, or perhaps because of the stochastic nature of the immune response. In T1DM, this pre-clinical autoimmune phase is marked by initial immune reactivity to only a small number of autoantigens. The third phase of T1DM is the development of clinically apparent hyperglycemia; this final phase is characterized immunologically by autoreactivity to numerous antigens and extensive immune-mediated tissue destruction of islet cells caused by many pro-inflammatory pathways.
The presence of these three phases of diseases in T1DM is well established, as is the value of prospectively studying children with highly predictive autoantibodies in the pre-clinical phases of disease for epidemiologic and genetic associations.(59, 63–69) Of importance, due to the strong association of T1DM with beta-cell (or islet) autoimmunity (IA), defined as the presence of autoantibodies specific to beta-cell autoantigens, IA has become an alternative endpoint to clinically-apparent hyperglycemia in T1DM research. Advantages of this approach include the opportunity to study the pathological process underlying T1DM in a pre-clinical state, and to verify that a candidate risk factor is not only related to diabetes, but also to the alteration of immune function preceding clinical onset of disease. Studies using as end points both autoimmunity and clinically-apparent diabetes have allowed for differentiation of the risk factors that initiate humoral autoimmunity from those that promote progression from subclinical autoimmunity to diabetes.(59, 69) Of note, prospective studies using asymptomatic IA as an outcome measure have identified important findings regarding dietary and other factors in T1DM including the lack of association of islet-cell autoimmunity with childhood vaccines(67), and the important relationship of timing of cereal exposure during the first year of life to the later development of IA(65). These findings have been of importance in terms of potentially reducing risk for T1DM based on dietary considerations.
The presence of autoantibodies can be also used in the prediction of future T1DM. In first degree relatives (FDRs) of diabetic individuals, the presence of a single autoantibody on one occasion has been found to have a sensitivity and specificity of 95% and 99%, respectively, and a positive predictive value (PPV) of 46% for type 1 diabetes with 5 years of follow-up.(70) Notably, the number of unique T1DM-related autoantibodies that are positive also predicts risk for future T1DM. For example, a person who tests positive for multiple autoantibodies is at a much higher risk of developing T1DM than a person with a single detectable autoantibody.(71) However, T1DM-related autoantibodies may be transiently positive, and there are not yet enough longitudinal data to determine if all people that develop autoantibodies will eventually develop T1DM.(62, 71, 72) Although not 100% predictive of future T1DM development, IA still serves as a very useful intermediate endpoint when studying the autoimmune disease process and potential risk factors for T1DM. In terms of RA, given the high specificity of certain autoantibodies (notably ACPAs) for disease, using autoantibody positivity as a surrogate endpoint for autoimmunity in pre-clinical RA is likely to be of similar great value.
Systemic lupus erythematosus (SLE) is a multi-system autoimmune disease of unknown etiology. Over 98% of SLE patients are positive for antinuclear antibodies (ANA) and many SLE patients have additional reactivity to specific nuclear antigens including double-stranded DNA (dsDNA), Smith, ribonuclear proteins, and Ro and La.(73–76) Some of these autoantibodies are associated with specific clinical manifestations of disease. For example, autoantibodies to dsDNA are associated with nephritis, and levels of anti-dsDNA antibodies may fluctuate in conjunction with disease activity.(77, 78) Additionally, anti-Ro antibodies have also been shown to be associated with specific manifestations of SLE including skin disease and neutropenia.(74–76) Although these autoantibody systems have been extensively studied for over 50 years, the initiating events for the development of these autoantibodies or the actual roles these antibody specificities play in clinical SLE are not known. However, these SLE-related autoantibodies are present in the serum of lupus patients many years prior to the first clinical evidence of disease.(79, 80) Furthermore, the spectrum/number of autoantibody specificities increases up to the time of diagnosis.(81) Importantly, the discovery of pre-clincial autoantibody positivity and evolution of antibody specificity has led to investigations into potential etiologic agents such as EBV infection in SLE – investigations that could not be performed without pre-clinical studies.(82)
Animal models and pre-clinical rheumatoid arthritis
While animal models of arthritis are not an exact match to human disease, they have provided important insights into the pre-clinical period of arthritis development. In particular, investigations into the relationships between the MHC Class II dependence of disease, a requirement in some instances for the specific exposure to citrullinated autoantigen, and the timing between the evolution of ACPA and clinically apparent arthritis have been helpful in understanding important factors in pre-clinical arthritis. An early example was provided by evidence that citrullinated proteins are found in the synovium after immunization with bovine collagen type II in DBA/1j mice, a strain that is commonly used to study the type II collagen-induced arthritis (CIA) model of RA.(83) However, in this study no ACPAs were detected. In a second study of the same model, ACPAs were found to develop before clinically detectable joint inflammation.(84) In this latter study, two methods were used to show that the ACPAs are pathogenic: first, treatment of mice with a “tolerogenic” form of a citrullinated peptide decreased the arthritis disease severity by ~60% and decreased the levels of ACPAs and epitope spreading to other citrullinated peptides; and second, a mouse monoclonal antibody that recognized citrullinated peptides,and could be used as a representative example of an ACPA, was found to greatly amplify joint inflammation. This occurred only following the administration of low doses of antibodies specific for type II collagen which induced the generation of citrullinated target antigens, presumably through the actions of macrophages and neutrophils containing peptidyl arginine deiminase (PAD) enzymes that participated in the inflammatory response. This second experiment suggested that, in the absence of citrullinated target antigens, there is no target injury; however, when these antigens are present, such as during treatment with monoclonal antibodies specific for type II collagen, ACPAs can greatly amplify inflammation and damage. Recently, additional monoclonal antibodies reactive with citrullinated type II collagen, with or without cross reactivity to other epitopes, were also found to amplify the development of arthritis in mice in vivo.(85) In addition, another study in rats has shown an increased incidence and severity of arthritis when collagen type II is citrullinated before immunization.(86)
Other animal studies have focused on the relationship between cellular and humoral autoimmunity to citrullinated antigens and the shared epitope and class II molecules. For example, immunization with citrullinated vimentin peptide leads to CD4+ T-cell activation and proliferation in transgenic mice expressing human HLA-DRB1*0401.(87) In addition, immunization of human HLA DRB1*0401-transgenic C57BL/6 mice with citrullinated human fibrinogen, but not native human fibrinogen or either form of mouse fibrinogen, resulted in the development of arthritis in a substantial proportion of animals.(88) In this setting, a relatively low level of inflammation was present, but this was accompanied by specific antibody responses to citrullinated human fibrinogen peptide, as well as T-cell responses to citrullinated protein. ACPA-positive arthritis was also found to develop in mice that are immunized with low doses of collagen type II and that have dysregulated MHC expression in the joints owing to transgenic expression of class II transactivator.(89) And finally, a particularly intriguing study has recently shown that immunization of a subset of strains of mice with human fibrinogen alone resulted in destructive arthritis as well as T and B cell reactivity to fibrinogen.(90) In this study, epitope spreading to citrullinated fibrinogen as well as many synovial citrullinated and non-citrullinated autoantigens developed. Remarkably, arthritis could be transferred to naïve mice with either serum or fibrinogen-reactive T cells from immunized mice.
Part 2. The phases of rheumatoid arthritis development: from genetic risk to clinically-apparent disease
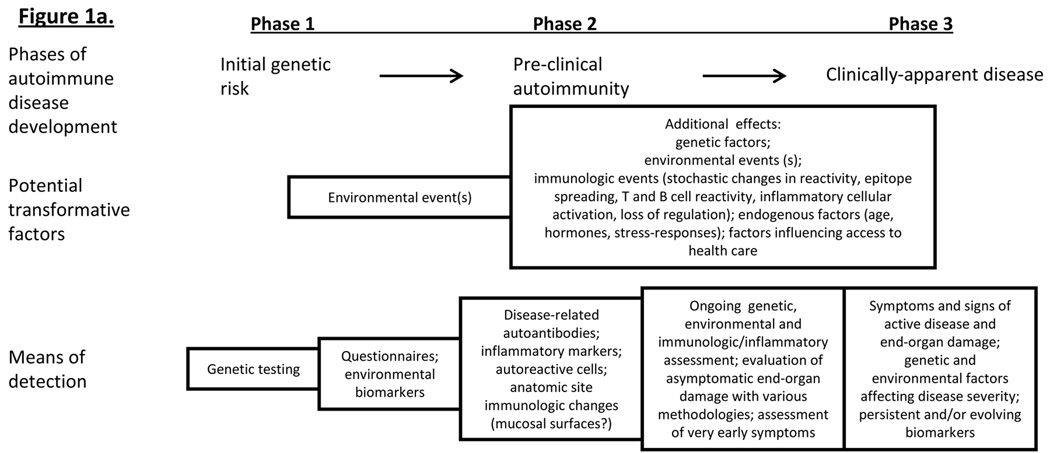
Based on the above discussion, including the presence of pre-clinical RA-related autoantibodies and inflammatory markers, the genetic and environmental factors associated with RA, the models of autoimmune disease development established in prospective studies of T1DM, and the animal models of disease, it is likely that RA develops three phases, outlined as follows and in Figure 1a. The initial phase is characterized by genetic risk for RA, during which no biomarkers of active autoimmunity and inflammation or symptoms are present. Phase 1 may last for years, and is followed by environmental and additional genetic influences that lead to a Phase 2 of disease development - asymptomatic autoimmunity – that is characterized by the presence of RA-related autoantibodies and other immunologic factors such as T and B cell autoreactivity, and perhaps elevated inflammatory markers. Phase 2 may be of variable length, perhaps influenced by genetic, environmental or endogenous factors such as age or gender. As Phases 1 and 2 are asymptomatic, they may collectively be termed ‘pre-clinical’ RA. Finally, there is a transition from asymptomatic autoimmunity to Phase 3, or clinically-apparent disease. During this final phase, patients will have symptoms and signs of active RA, and with numerous autoimmune and inflammatory biomarkers present as well as clear evidence of end-organ damage.
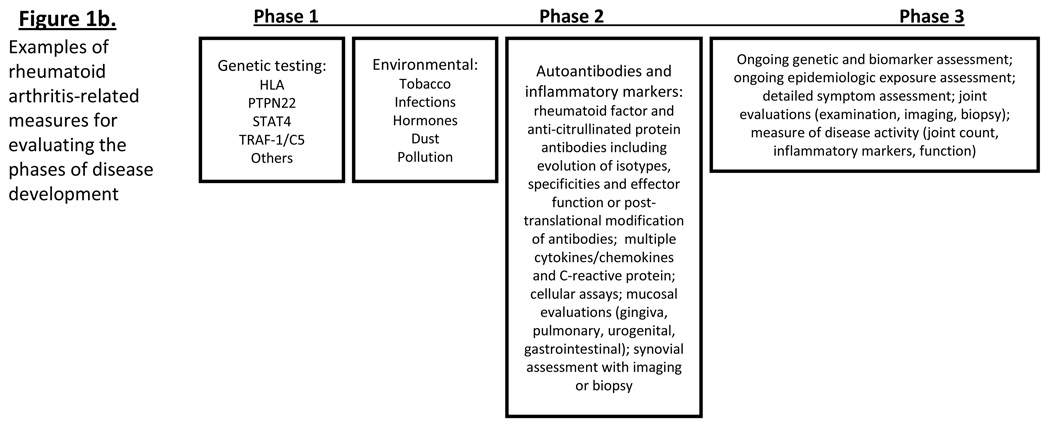
Figure 1*. A model of rheumatoid arthritis (RA) development.
Figure 1a: Based on RA-studies as well as prospective studies in other autoimmune disease (type 1 diabetes mellitus), RA may evolve through 3 phases of disease: Phase 1 = genetic risk, Phase 2 = asymptomatic autoimmunity (identified by presence of autoantibodies) and Phase 3 = clinically-apparent disease. Transition between phases may be caused by interactions between genetic and environmental factors, and/or changes in immune reactivity. Figure 1b: RA-related factors that can be measured during the pre-clinical phases of disease development._ *Figure adapted from Kolfenbach J et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009 Dec 15;61(12):1735-4
Of note, while in comparison to T1DM the natural history of RA is much less well understood, there are several studies that have demonstrated results very consistent this model of disease development for RA. These include numerous studies associating the presence of high risk HLA-DR alleles containing the shared epitope with RA(28, 29), and as described above, the finding of increased levels of autoantibodies in individuals who later develop RA. Additionally, studies, in patients with established RA, demonstrating altered synovial pathology in clinically unaffected joints suggest that there may be a period of time that pre-symptomatic joint inflammation is present during RA development.(91)
Inherent in this model of RA development is that there is a physical location where genetic and environmental factors interact leading to the initial RA-related immune dysregulation and break in tolerance. For example, given the strong association of smoking with RA, and in particular ACPA-positive RA, there is thought that this initial RA-related immune dysregulation occurs at the mucosal surfaces of the mouth (periodontal surfaces) or lung, with later spread of RA-related inflammation to the joints resulting in clinically-apparent synovial disease.(56, 57, 92) This model of mucosal initiation of RA may be true; however, the exact site of initiation of RA is unknown. We will discuss below how the prospective study of pre-clinical RA will allow for detailed exploration of possible sites of initiation of RA-related autoimmunity.
Part 3. Defining pre-clinical RA and transition into clinically-apparent disease
A key aspect of this 3-phase model of RA development is that there is a transition period from the ‘pre-clinical’ state (or Phases 1 and 2), where specific disease markers may be present but there are no symptoms or signs of active inflammatory disease, to a ‘clinical’ period of RA, when symptoms and signs of active inflammatory disease are present. There may be agreement that RA-related autoantibody positivity, in the absence of joint symptoms or other organ injury, in subjects that eventually develop fully-classifiable RA, represents pre-clinical RA; however, defining this transition from pre-clinical to clinical disease is more difficult – at what point does clinically-apparent RA begin?
To date, given that the main anatomic site affected by RA is the synovial joint, the criteria for determining the presence of ‘clinically-apparent’ RA have largely focused on the joints. In the American College of Rheumatology 1987 Revised Classification Criteria for RA, four of seven criteria need to be fulfilled for RA to be defined.(93) However, it is important to remember that these criteria were developed to help standardize research studies in RA, that all of the disease manifestations that result in criteria fulfillment take time to develop, and that a patient meeting these criteria may have truly transitioned from pre-clinical to clinical RA years prior to fulfilling the ACR criteria for RA.(94, 95)
However, in an attempt to investigate early joint disease that may not meet the 1987 Revised ACR Criteria, there are several investigative groups that have sought to classify inflammatory arthritis (IA) or undifferentiated arthritis (UA) as a distinct clinical entity. Such classification allows for the establishment of prospective clinical studies to determine the long-term outcomes of patients who present with such findings, as well as to identify factors that may predict evolution to classifiable RA. A major focus of these studies is also to identify discriminatory factors that may indicate that early aggressive therapy is warranted. The most commonly utilized criteria for IA/UA are the Norfolk criteria and the Leiden criteria.(96–100) The Norfolk criteria define IA as ≥2 swollen joints of duration greater than 4 weeks.(96) In the Leiden criteria, IA/UA is defined as ≥1 joint with inflammatory findings confirmed by a rheumatologist, with the joint findings not otherwise classifiable (ie gout or pseudogout).(97) Additionally, together the ACR and the European League Against Rheumatism (EuLAR) are currently developing revised RA criteria, in part to address the need to link IA to the same long-term prognostic considerations and need for treatment as are typical for RA as defined by the earlier ACR criteria.(101)
The Norfolk and Leiden criteria for UA/IA, and the new ACR/EuLAR criteria for RA, may be more useful than the current ACR RA criteria in identifying patients earlier in the transition from pre-clinical to clinical RA, and it will be important for studies in pre-clinical RA going forward to have clearly defined criteria for transition from pre-clinical disease, in order to understand the evolution of RA, define when in the spectrum of clinical presentations therapeutic intervention may be necessary, and define a clinical outcome that is a suitable endpoint for prevention trials.
Part 4. What can we learn by investigating pre-clinical rheumatoid arthritis?
Genetic and environmental interactions in pre-clinical RA
As discussed above, the genetic and environmental influences that lead to the pre-clinical phase of disease development are likely key features in the development of RA-related autoimmunity. However, given the observed length of time between the appearance of autoimmunity and diagnosis of RA, standard case-control study approaches using subjects with established RA may not optimally allow for the identification of environmental risk factors for RA or at what point the risk factors influence the development of autoimmunity and disease. For example, as mentioned earlier, there is now considerable data that supports that gene-environmental interactions between smoking and specific HLA alleles are associated with ACPA positivity in established RA.(35, 55–58, 102, 103) However, investigating the relationship between these factors in the pre-clinical period would help determine whether HLA alleles, smoking and ACPA positivity are associated in the absence of synovitis – a finding that would support a role for smoking as an initial step and perhaps causative step in RA-related autoimmunity.
Additionally, hypotheses regarding the specific anatomic site where the initial RA-related immune dysregulation may occur can be evaluated in real-time in a prospective study. For example, there are several studies that hypothesize that RA development is caused by smoking or other inhaled toxins leading to initial immune dysregulation at the mucosal surfaces of the lung.(36, 38, 57) However it is unclear whether the lung is the site of RA-related immune dysregulation or a conduit through which inciting environmental agents enter the body and then lead to immune dysregulation in the joints directly. Real-time prospective study of subjects with RA-related autoimmunity, evidenced by autoantibodies (either prevalent or incident) but no synovitis, would allow for detailed exploration of mucosal surface immune function. Supporting this approach, in preliminary work we have identified evidence of inflammatory lung injury including airway and alveolar disease using high-resolution computed tomography of the lungs of individuals with RA-related autoantibody positivity but without joint symptoms or findings, suggesting that RA-related inflammation may indeed occur initially in the lung.(104)
Evaluation of autoantibody and inflammatory marker evolution in the pre-clinical period of rheumatoid arthritis development
There is a growing body of work that suggests that RA-related autoantibody isotypes, antigen specificity, and post-translational modification and effector function (such as autoantibody glycosylation) may be related to disease severity and perhaps evolution from UA to RA.(105–110) For example, Verpoort et al reported that patients who evolved from UA to RA had a broader spectrum of ACPA-isotypes compared to those with persistent UA at follow-up.(109) Additionally, a study by Ioan-Facsinay et al comparing autoantibodies in North American natives with RA and their healthy FDRs showed ACPA reactivity in both groups (19% in healthy FDRs versus ~91% in RA cases); however, the fine specificities of ACPAs differed between RA cases and healthy controls, with reactivity to citrullinated fibrinogen and vimentin only seen in RA cases.(111) Also in this study, ACPA isotypes were more limited in healthy FDRs compared to RA cases. As a whole, these finding suggest that APCA isotype evolution and specificity may be important for disease development. As such, investigating the evolution of the isotype changes and specificity during the pre-clinical period when the possible transformations from non-pathogenic to pathogenic states may occur, may lead to key insights into RA development, and perhaps identification of the ‘original sin’ in RA – ie the initial autoantigen that leads to RA-related autoimmunity. In regard to this latter point, based on the work of several groups, ACPA reactivity to citrullinated α-enolase CEP-1 epitope may indicate that this citrullinated antigen most strongly links exposure to cigarette smoke and HLA risk alleles in RA.(58) If autoimmunity to this citrullinated antigen could be demonstrated in the pre-clinical period prior to other autoimmune responses to citrullinated antigens, the argument that this antigen is the initial one in RA would be compelling. In addition, prospective evaluation of cytokine/chemokine type (i.e. IL-17, TNFα, or paradigm-specific small molecule profiles) may allow for identification of key inflammatory processes in RA development that may help to identify the inciting factors for this disease as well as allow us to specifically target aspects of the immune system for prevention.
Predictive models for future rheumatoid arthritis
Several prospective and retrospective studies suggest that autoantibodies or combinations of genetic factors and autoantibodies indicate high-risk for future RA. In prospective evaluation (including joint examinations and radiographs of hands and feet approximately every 2 years) of approximately 2700 Pima and/or Papago Native Americans in Arizona, USA (a population with a overall prevalence rate of RA of ~5%), followed up to 19 years, del Puente et al demonstrated increased risk for incident RA in RF-positive (by sheep cell agglutination) individuals, with the highest risk of 48.3 cases per 1000 person-years seen in those with a titer of >1:256 at baseline testing.(1) Also in prospective evaluation of 370 FDRs from multicase RA families followed with periodic joint symptom assessment for a mean of 5 years, Silman et al found the overall rate for incident RA to be approximately 8 per 1000 person-years, with a rate of incident RA in subjects that were positive for RF at baseline of 34.8 cases per 1000 person years (calculated from 4 incident cases of RA out of 24 individuals).(11)
Retrospective biobank studies have also estimated risk for future RA, although these results are more limited due to lack of prospectively collected data. In their Swedish biobank study, Rantaa-Dahlqvist et al estimated PPVs for future RA of 4% if RF-IgM positive alone, 16% if anti-CCP positive alone, and 22% if anti-CCP and RF-IgA positive (these PPV calculations assumed a prevalence of RA in the general population of 1%). Nielen et al estimated a PPV for onset of classifiable RA within 5 years of 5.9% for subjects that were anti-CCP positive alone, assuming an incidence rate of RA over 5-years in the general population of 0.001%. However, this PPV increased to 69.4% if the incidence rate of RA in the population over 5-years used for calculation was 3.9%. Of note, this higher rate of incident RA (3.9% versus 0.001%) used by Nielen et al in their PPV calculations was estimated from Silman et al’s 1992 study of incident RA in individuals from multicase RA families(11), where a strong family history of RA (≥2 member with RA) may serve as a possible surrogate for additional genetic and/or environmental risk for RA.(8) Also, if both RF and anti-CCP were positive, the PPV for RA diagnosis within 5 years was 100%, regardless of which background incidence of RA was used for comparison.
Studies that have evaluated risk for future RA have shown that certain genetic factors, notably HLA alleles containing the ‘shared epitope’, may increase risk for future RA.(112) However, the ability of genetic factors alone to predict future RA may be limited, in part due to the high prevalence of these factors in the population - in North American controls, at least one HLA allele containing the ‘shared epitope’ is present in ~40% of the population, and PTPN22 risk alleles are present in ~16% of the population.(56) As such, prediction of risk for future RA will likely best be done using combinations of genetic, environmental and autoantibody factors.
Several studies have already examined the relationship between genetic factors and autoantibodies in prediction of future RA. Berglin et al, using the Northern Sweden Health and Disease biobank, performed a nested case-control study to show a strong association between pre-RA diagnosis anti-CCP positivity and HLA-DRB1*0404 or *0401 alleles and risk for future RA (Odds ratio 66.8, 95% CI 8.3–539.4).(113) Additionally, using data from the same Swedish biobank and similar study design, Johansson et al showed a strong association of anti-CCP positivity and 2 copies of the 1858T variant allele of PTPN22 with future RA (Odds ratio 132.0, 95% CI ~18-2721).(114) Studies are also underway to utilize gene and environment interactions for prediction of RA, as well as prediction of pre-clinical RA-related autoantibody positivity as a surrogate outcome for evolving RA (Elizabeth Karlson, personal communication).
There may be other factors that act separately or in concert with genetic and environmental factors to influence transition from asymptomatic to symptomatic RA-related autoimmunity. Majka et al (9) and Bos et al (17) reported in their retrospective cohort analyses that subjects with older age at time-of-diagnosis of RA had longer duration of pre-clinical RA-related autoantibody positivity. These findings may be explained by differences in genetic or environmental exposures that vary by age, or by age-related immune senescence. Additionally, these findings suggest that age or other endogenous factors such as gender may have relevance in predicting future onset of RA in at-risk populations.
Also, prospective studies of cohorts of patients initially with early symptomatic IA/UA have allowed for investigation of the effects of genetic and other factors in the persistence and evolution of symptomatic arthritis. Several Dutch studies have shown that factors such as age, sex, number of swollen joints and autoantibody and CRP status may predict persistent IA at 1-year.(97, 99, 115) Additionally, in separate studies, Feitsma et al and van der Helm-van Mil showed that the presence of PTPN22 or HLA risk alleles does not significantly increase risk of transition from UA to RA, but that these genetic risk factors do influence autoantibody presence (HLA) or level (PTPN22).(116, 117) Finally, several pre-clinical studies including those using the Swedish, Dutch and NHS biobanks noted an increase in autoantibody titer and/or inflammatory marker levels in the time period immediately preceding diagnosis.(7, 8, 18, 19, 21, 22) Regarding this latter point, prospective study of biomarker changes may also be of key interest in understanding the kinetics of pre-clinical RA and in predictive models for symptomatic disease.
Although these studies are supportive of the ability of predictive models to determine risk for future RA, it should be noted that estimates of risk for future RA using stored samples from biobanks are limited because such studies were not truly prospective. Importantly, participants were not subjected to detailed baseline or follow-up joint examinations, and pre-RA diagnosis samples were assessed retrospectively in comparison to selected controls after RA had been diagnosed. For these reasons, the relationship between symptom onset and biomarker positivity is inexact.
Pre-clinical prevention of rheumatoid arthritis
Several clinical trials have demonstrated that treatment with aggressive therapy in early RA (that meets 1987 ACR RA criteria), leads to improved clinical outcomes, and perhaps increased rates of drug-free remission.(118, 119) As such, currently, a major goal for RA treatment is early identification and treatment of disease.(120) In addition, several investigative groups have evaluated the efficacy of disease-modifying therapy in individuals who have UA/IA not yet meeting established ACR criteria for RA. For example, in the Probable Rheumatoid Arthritis Methotrexate versus Placebo Treatment (PROMPT) trial, in patients with UA of ≤2 years’ duration, methotrexate use delayed progression to RA as defined by the 1987 ACR criteria, with the most benefit seen in anti-CCP positive subjects.(98) Another small study investigating abatacept in UA showed a trend towards decreased progression to classifiable RA.(121) Also, several studies utilizing immunomodulation to prevent progression of T1DM have been performed, and while T1DM has not yet been prevented, studies have shown that intervention may lead to decreased insulin usage, and decreased risk for death from ketoacidosis early in disease.(122) Finally, in SLE, a retrospective study of military patients with SLE by James et al found that treatment with hydroxychloroquine in early disease led to decreased progression of 25 SLE-specific autoantibodies as well as clinical findings.(123) Overall, the results from these studies suggest that intervention in an even earlier period of RA-related autoimmunity – ie in the pre-clinical period – may lead to prevention of disease.
Unfortunately, there as of yet are no published results of pre-clinical RA prevention trials. However, interesting data obtained from database studies suggests that certain agents such as HMG-CoA reductase inhibitors (statins) that may have some benefit in treatment of active RA may modify future RA risk.(124–126) Jick et al have shown using a national database in the United Kingdom (N=313 RA cases and 1252 controls) a decreased risk for incident RA in patients using statins for hyperlipidemia (OR 0.59, 95% CI 037–0.96).(127)
Ultimately, these findings and further research may lead to pre-clinical pharmacological immunomodulation to prevent disease. Alternatively, perhaps non-pharmacologic tolerization (based on murine models of disease) or risk-factor modification in the pre-clinical period may be used to abrogate clinically-apparent disease – for example, if a person at high-risk for future RA stopped smoking, this may decrease the risk for developing clinically-apparent arthritis. However, while prevention of RA is of key importance, implementation of prevention trials will only be possible after we have first developed models in prospective studies to identify accurately those at high risk for future RA in a definable and relatively short time period necessary to conduct such studies.
Part 5. Future research in pre-clinical RA
There are multiple approaches to investigate pre-clinical RA, and these are outlined in Table 2. Ideally, the investigation of pre-clinical RA would take an approach similar to the Framingham study, which followed a large population over time to investigate causes of cardiovascular disease using serial measures of biomarkers and clinical outcomes.(128) However, due to the low prevalence of RA, large-scale population studies at single centers may be impractical due to the cost of screening and relatively few outcomes of disease. As such, pre-clinical RA may best be investigated by using research models similar to those used in studies in T1DM – i.e. using a multi-center approach and prospectively following subjects that are likely to be at higher risk for RA-related autoimmunity as well as development of clinically-apparent disease. One example of a population at higher risk for RA is FDRs of RA probands, as these FDRs may have 5 to 7-fold increased risk for incident RA(11)). In a multi-center study in the United States, we are currently following prospectively a large cohort of FDRs of probands with RA (goal N=2100).(129) To date, a substantial proportion (~14%, N~1200) of these FDRs exhibit RA-related autoantibody positivity.(129) Additionally, in Canada, El-Gabalawy et al are currently prospectively following a cohort of North American Natives who are FDRs of probands with RA, and have demonstrated intriguing findings regarding differences in ACPA isotypes and specificities between RA cases and asympatomatic FDRs.(111, 130, 131) These will be valuable cohorts to investigate the effects of genetic and environmental factors on RA-related autoimmunity, as well as to investigate the evolution of RA-related autoimmunity and inflammation over time. While identification of asymptomatic individuals with RA-related autoantibody positivity is of interest, these prospective cohort analyses will also allow for the identification of factors that impact the transition from Phase 1 (genetic risk) to Phase 2 (asymptomatic autoimmunity) – a transition during which the key early interactions leading to RA-related autoimmunity occur. Examples of RA-related measures that may be assessed prospectively in pre-clinical study are provided in Figure 1b.
Table 2.
Potential methodologies for investigating pre-clinical rheumatoid arthritis
| Study Design | Examples | Strengths | Weaknesses |
|---|---|---|---|
|
Retrospective case-control studies using stored pre-diagnosis samples (biobanks): |
Finnish, Swedish and Dutch biobank studies(6–8) United States’ Military/Department of Defense Serum Repository(9) Nurses’ Health Study(18, 22) Women’s Health Study(22) |
Biobanks already established and pre- clinical samples may be obtained from cases with known RA |
Decreased ability to determine future risk for disease; unable to ascertain exact relationship between timing of appearance of RA-related biomarkers and symptoms; limited samples available. |
|
Prospective studies: Populations at high-risk for RA (such as first- degree relatives of probands with RA, twin studies, or populations such as native Americans with high prevalence of disease) |
Ongoing: Holers et al(129): Studies of the Etiologies of Rheumatoid Arthritis (SERA): prospective evaluation of first-degree relatives of probands with RA. El-Gabalawy et al(111, 130, 131): North American Native people in Central Canada (Cree and Ojibway) prospectively followed for evolution of RA-related autoimmunity. Background prevalence of RA in population ~2%. Prior: Native American (Pima and Papago) studies(1) |
Real-time evaluation of evolution of RA- related autoimmunity to determine timing of appearance of biomarkers in relationship to environmental exposures and development of symptomatic disease; possibility for detailed assessment of mucosal biology in pre-clinical RA. |
Costly in terms of money and time; relatively low prevalence of incident RA and prevalence of RA-related autoantibodies limits statistical power. |
|
Other prospective studies: Large-scale population screening to identify individuals with high-risk features for RA including genetic factors and RA-related autoantibody positivity. |
Health-fair screening in Colorado identifying anti- CCP positive asymptomatic individuals who are recruited for follow-up.(132) |
Potentially identifies subjects at risk for ‘sporadic RA’(as are most cases in population); may help to establish prevalence of RA-related autoantibodies in general populations |
Expensive, and relatively low prevalence of RA-related autoantibodies requires large-scale screening to identify at-risk individuals |
|
Coupling of prospective RA studies with other research projects (such as cardiovascular disease studies) |
Multiple studies are likely available | Allows for coupling of research projects perhaps resulting in decreased costs |
Costly in terms of money and effort to establish screening |
|
Other Approaches: Animal models of disease |
Multiple | Allows for detailed evaluation of antigen interactions; may provide for testing of methodologies for tolerization |
Animal models of arthritis have substantial differences from human disease |
| Population screening for multiple autoimmune diseases |
Multiple | Multiple autoimmune diseases may be studied; array technology for multiple autoantibody testing |
Expensive; optimal diagnostic accuracy and reproducibility of testing would need to be established |
Also, based on the prior success of biobank studies of pre-clinical RA, additional samples from yet untapped existing biobanks may be evaluated for RA-related biomarkers. However, as these retrospective cohorts may lack detailed joint evaluations, the ability to investigate the relationship between immune parameters and symptomatic disease may be limited.
Alternatively, to the prospective or biobank studies discussed above, a large general population could be screened for RA-related genetic traits or RA-related autoantibody positivity to identify those at high-risk for disease. From this initial screening, a smaller group of individuals with high-risk genetic and/or autoantibody markers may be followed over time to evaluate the evolution of RA-related autoimmunity. For example, in a health-fair screen of approximately 5000 individuals, we have identified approximately 40 anti-CCP positive subjects without synovitis on examination, and we are now evaluating these subjects prospectively for evolution of RA-related autoimmunity.(132) Such population screening is complicated by cost issues, and the potential for subjects participating in such screening to have existing symptomatic joint disease, thereby missing the ‘pre-clinical’ window of disease. However, with appropriate methodologies, population screening may be a reasonable approach to identify individuals with asymptomatic RA-related autoimmunity.
Of note, given the growing availability of array technologies, it may be possible to screen large populations for risk of developing a wide variety of autoimmune diseases. By testing for multiple autoimmune diseases, this approach might also allow for a more cost-effective way to identify individuals at risk for RA-related autoimmunity as well as those at risk for other autoimmune diseases that also exhibit pre-clinical disease-related autoantibodies including T1DM and SLE mentioned above as well as autoimmune thyroid disease (Graves’ and Hashimoto’s Thyroiditis), primary biliary cirrhosis, and celiac disease.(133, 134) With regard to such a strategy, it is important to understand that in aggregate these diseases affect up to ~7% of the population.(133, 134) Also, there are data to support the possibility that risk for cardiovascular disease (CVD), the leading cause of death in RA patients, may be increased even in the pre-clinical period of RA.(135) As such, pre-clinical evaluation of RA may not only identify RA-specific factors, but also lead to a better understanding of CVD risk in RA, and perhaps CVD prevention.
In addition to the design and implementation of prospective studies to investigate the pre-clinical period of RA development, several other issues regarding research methodologies in pre-clinical RA need to be addressed. First, what constitutes positivity for RA-related autoantibodies? Currently there are multiple methodologies for testing RFs and ACPAs, with each kit having different cut-offs for positivity resulting in a variety of sensitivities and specificities. As such, standardized methodologies for testing RFs and ACPAs as well as for determining what result is considered positive will need to be established in order to homogenize testing results and development of predictive models for future RA. International studies in T1DM have already adopted standardized autoantibody testing, and these tests are performed in a limited number of research laboratories to ensure accuracy and reproducibility of testing. Second, as discussed above, we will need to define what constitutes ‘pre-clinical’ RA as well as what constitutes transition from asymptomatic RA-related autoimmunity to clinically-apparent disease. Likely this will need standardized symptom and joint count assessments, as well as potentially standardized techniques for imaging of the synvovium using MRI and/or ultrasound, and standardized synovial biopsy techniques and tissue processing. Also, it may be useful in pre-clinical studies to establish biomarker end-points that could be employed in prevention trials. Such profiles, which may include autoantibodies and inflammatory markers, may be used as surrogate outcomes for increased risk for future RA. For example, if an asymptomatic individual in a prevention trial has RA-related autoantibodies suggesting high-risk for future RA as well as an elevated CRP suggesting active inflammation, a decrease of CRP in this individual due to a pharmacologic intervention (in absence of joint symptoms) may be considered a clinically-appropriate success in prevention of disease. This approach is aimed at allowing for a more timely assessment of intervention success as joint symptoms may take years to develop. Of note, in terms of symptoms or examination findings, swollen joints may not be the first indication of transition from pre-clinical RA to clinical RA, and perhaps patient-reported measures such as stiffness or pain in absence of detectable swelling are the first indications of symptomatic RA. Detailed studies of the pre-clinical period, closely examining joint symptoms, and perhaps investigating asymptomatic organ injury using synovial biopsy to discover asymptomatic synovitis, or joint or lung imaging to detect asymptomatic RA-related injury may be necessary to understand truly the evolution from pre-clinical to clinical RA. Third, what phenotype of RA will be best to investigate? Will it be best to focus on sero-positive disease as it will be easier to identify pre-clinical sero-positive RA due to the presence of specific autoantibodies? Given that sero-negative RA may be distinct from sero-positive RA in terms of genetic and environmental risk factors, it may be worthwhile to focus efforts going forward on sero-positive disease.(136) Fourth, to understand the predictive power of RA-related biomarkers for future disease, relevant control populations for prospective studies of pre-clinical RA need to be established. This is of particular importance when considering that several pre-clinical studies in RA have already shown that not all subjects with RA-related autoantibody positivity, including ACPAs, may develop symptomatic RA.(4, 15) Finally, standardized approaches for assessing epidemiologic exposures that might impact RA development will need to be established.
In addition to studies in humans, animal models of autoimmune disease may yield important insights into pre-clinical autoimmunity. In particular, animal models may be utilized to understand initial citrullinated protein-specific autoimmunity, or the role of mucosal surfaces in generation of autoimmunity. Also, animal models may help to identify methods to tolerize individuals to citrullinated or other antigens important to RA in the pre-clinical period of RA development, a time period in which non-pharmaceutical, immunomodulatory strategies may be more effective prior to the extensive epitope spreading that has occurred in RA by the time clinical symptoms and signs of disease appear. Animal studies may also allow the determination of whether novel treatments focused on the citrullination process itself may be beneficial.
Conclusion
Based on multiple studies in RA as well as other autoimmune diseases, RA likely develops in phases exhibited by genetic risk, asymptomatic autoimmunity and finally clinically-apparent disease, with transformations between these phases occurring due to combinations of genetic, environmental and immunologic factors. Investigating the pre-clinical phases of RA development will provide key insights into the factors that lead to disease, as well as allow for development of predictive models for disease, and ultimately prevention strategies for RA. Going forward, studies of pre-clinical RA may utilize bio-repositories, but the most effective approach will likely be the use of prospective cohort studies, with subjects chosen from populations at greater risk for RA such as FDRs or twin studies, or those identified with high-risk markers for RA, either genetic or autoantibody, through community screening. In addition to design and implementation of such prospective studies, the rheumatology community will need to define with greater clarity what constitutes the transition from pre-clinical to clinical RA, as well as standardize autoantibody and inflammatory marker testing. While these latter tasks are daunting, investigation of pre-clinical RA likely gives us the greatest hope for curing or preventing this disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
“When the rheumatic poison is in the system, any disturbing circumstance, even of temporary duration, such as over fatigue, anxiety, grief or anger, by rendering the system more susceptible of its influence, may prove the accidental or exciting cause of the disease”
Henry William Fuller in “On Rheumatism, Rheumatic Gout, and Sciatica” Publishers: Samuel S & William Wood, New York, 1854.
Contributor Information
Kevin D. Deane, Email: Kevin.Deane@UCDenver.edu.
Jill M. Norris, Email: Jill.Norris@UCDenver.edu.
V. Michael Holers, Email: Michael.Holers@UCDenver.edu.
References
- 1.del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum. 1988 Oct;31(10):1239–1244. doi: 10.1002/art.1780311004. [DOI] [PubMed] [Google Scholar]
- 2.Aho K, Heliovaara M, Maatela J, Tuomi T, Palosuo T. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol. 1991 Sep;18(9):1282–1284. [PubMed] [Google Scholar]
- 3.Kurki P, Aho K, Palosuo T, Heliovaara M. Immunopathology of rheumatoid arthritis. Antikeratin antibodies precede the clinical disease. Arthritis Rheum. 1992 Aug;35(8):914–917. doi: 10.1002/art.1780350810. [DOI] [PubMed] [Google Scholar]
- 4.Aho K, von Essen R, Kurki P, Palosuo T, Heliovaara M. Antikeratin antibody and antiperinuclear factor as markers for subclinical rheumatoid disease process. J Rheumatol. 1993 Aug;20(8):1278–1281. [PubMed] [Google Scholar]
- 5.Aho K, Heliovaara M, Knekt P, Reunanen A, Aromaa A, Leino A, et al. Serum immunoglobulins and the risk of rheumatoid arthritis. Ann Rheum Dis. 1997 Jun;56(6):351–356. doi: 10.1136/ard.56.6.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aho K, Palosuo T, Heliovaara M, Knekt P, Alha P, von Essen R. Antifilaggrin antibodies within "normal" range predict rheumatoid arthritis in a linear fashion. J Rheumatol. 2000 Dec;27(12):2743–2746. [PubMed] [Google Scholar]
- 7.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003 Oct;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 8.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004 Feb;50(2):380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 9.Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008 Jun;67(6):801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacGregor AJ, Silman AJ. Rheumatoid factors as predictors of rheumatoid arthritis. J Rheumatol. 1991 Sep;18(9):1280–1281. [PubMed] [Google Scholar]
- 11.Silman AJ, Hennessy E, Ollier B. Incidence of rheumatoid arthritis in a genetically predisposed population. Br J Rheumatol. 1992 Jun;31(6):365–368. doi: 10.1093/rheumatology/31.6.365. [DOI] [PubMed] [Google Scholar]
- 12.Aho K, Palosuo T, Heliovaara M. Predictive significance of rheumatoid factor. J Rheumatol. 1995 Nov;22(11):2186–2187. [PubMed] [Google Scholar]
- 13.Aho K, Palosuo T, Raunio V, Tuomi T. The timing of rheumatoid factor seroconversions. Arthritis Rheum. 1987 Jun;30(6):719–720. doi: 10.1002/art.1780300623. [DOI] [PubMed] [Google Scholar]
- 14.Aho K, Palosuo T, Raunio V, Puska P, Aromaa A, Salonen JT. When does rheumatoid disease start? Arthritis Rheum. 1985 May;28(5):485–489. doi: 10.1002/art.1780280503. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson T, Thorsteinsson J, Kolbeinsson A, Jonasdottir E, Sigfusson N, Valdimarsson H. Population study of the importance of rheumatoid factor isotypes in adults. Ann Rheum Dis. 1992 Jul;51(7):863–868. doi: 10.1136/ard.51.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Venrooij WJ, Pruijn GJ. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis. Arthritis Res. 2000;2(4):249–251. doi: 10.1186/ar95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos WH, Nielen MM, Dijkmans BA, van Schaardenburg D. Duration of pre-rheumatoid arthritis anti-cyclic citrullinated peptide positivity is positively associated with age at seroconversion. Ann Rheum Dis. 2008 Nov;67(11):1642. doi: 10.1136/ard.2007.085456. [DOI] [PubMed] [Google Scholar]
- 18.Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009 Apr;36(4):706–711. doi: 10.3899/jrheum.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielen MM, van Schaardenburg D, Reesink HW, Twisk JW, van de Stadt RJ, van der Horst-Bruinsma IE, et al. Increased levels of C-reactive protein in serum from blood donors before the onset of rheumatoid arthritis. Arthritis Rheum. 2004 Aug;50(8):2423–2427. doi: 10.1002/art.20431. [DOI] [PubMed] [Google Scholar]
- 20.Nielen MM, van Schaardenburg D, Reesink HW, Twisk JW, van de Stadt RJ, van der Horst-Bruinsma IE, et al. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann Rheum Dis. 2006 Apr;65(4):535–537. doi: 10.1136/ard.2005.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rantapaa-Dahlqvist S, Boman K, Tarkowski A, Hallmans G. Up regulation of monocyte chemoattractant protein-1 expression in anti-citrulline antibody and immunoglobulin M rheumatoid factor positive subjects precedes onset of inflammatory response and development of overt rheumatoid arthritis. Ann Rheum Dis. 2007 Jan;66(1):121–123. doi: 10.1136/ard.2006.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlson EW, Chibnik LB, Tworoger SS, Lee IM, Buring JE, Shadick NA, et al. Biomarkers of inflammation and development of rheumatoid arthritis in women from two prospective cohort studies. Arthritis Rheum. 2009 Mar;60(3):641–652. doi: 10.1002/art.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shadick NA, Cook NR, Karlson EW, Ridker PM, Maher NE, Manson JE, et al. C-reactive protein in the prediction of rheumatoid arthritis in women. Arch Intern Med. 2006 Dec 11–25;166(22):2490–2494. doi: 10.1001/archinte.166.22.2490. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen KT, Wiik A, Pedersen M, Hedegaard CJ, Vestergaard BF, Gislefoss RE, et al. Cytokines, autoantibodies and viral antibodies in premorbid and postdiagnostic sera from patients with rheumatoid arthritis: case-control study nested in a cohort of Norwegian blood donors. Ann Rheum Dis. 2008 Jun;67(6):860–866. doi: 10.1136/ard.2007.073825. [DOI] [PubMed] [Google Scholar]
- 25.Aho K, Palosuo T, Knekt P, Alha P, Aromaa A, Heliovaara M. Serum C-reactive protein does not predict rheumatoid arthritis. J Rheumatol. 2000 May;27(5):1136–1138. [PubMed] [Google Scholar]
- 26.Silman AJ. Epidemiology of rheumatoid arthritis. APMIS. 1994 Oct;102(10):721–728. doi: 10.1111/j.1699-0463.1994.tb05226.x. [DOI] [PubMed] [Google Scholar]
- 27.Alarcon GS. Epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 1995 Aug;21(3):589–604. [PubMed] [Google Scholar]
- 28.Reveille JD. The genetic contribution to the pathogenesis of rheumatoid arthritis. Curr Opin Rheumatol. 1998 May;10(3):187–200. doi: 10.1097/00002281-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Nepom GT, Nepom BS. Prediction of susceptibility to rheumatoid arthritis by human leukocyte antigen genotyping. Rheum Dis Clin North Am. 1992 Nov;18(4):785–792. [PubMed] [Google Scholar]
- 30.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010 Jan;69(1):54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plenge RM. Rheumatoid arthritis genetics: 2009 update. Curr Rheumatol Rep. 2009 Oct;11(5):351–356. doi: 10.1007/s11926-009-0050-0. [DOI] [PubMed] [Google Scholar]
- 32.Barton A, Worthington J. Genetic susceptibility to rheumatoid arthritis: an emerging picture. Arthritis Rheum. 2009 Oct 15;61(10):1441–1446. doi: 10.1002/art.24672. [DOI] [PubMed] [Google Scholar]
- 33.Heliovaara M, Aho K, Knekt P, Impivaara O, Reunanen A, Aromaa A. Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann Rheum Dis. 2000 Aug;59(8):631–635. doi: 10.1136/ard.59.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikuls TR, Cerhan JR, Criswell LA, Merlino L, Mudano AS, Burma M, et al. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2002 Jan;46(1):83–91. doi: 10.1002/1529-0131(200201)46:1<83::AID-ART10042>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004 Oct;50(10):3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 36.Hart JE, Laden F, Puett RC, Costenbader KH, Karlson EW. Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ Health Perspect. 2009 Jul;117(7):1065–1069. doi: 10.1289/ehp.0800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999 Oct;107 Suppl 5:793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koeger AC, Lang T, Alcaix D, Milleron B, Rozenberg S, Chaibi P, et al. Silica-associated connective tissue disease. A study of 24 cases. Medicine (Baltimore) 1995 Sep;74(5):221–237. doi: 10.1097/00005792-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Blaschke S, Schwarz G, Moneke D, Binder L, Muller G, Reuss-Borst M. Epstein-Barr virus infection in peripheral blood mononuclear cells, synovial fluid cells, and synovial membranes of patients with rheumatoid arthritis. J Rheumatol. 2000 Apr;27(4):866–873. [PubMed] [Google Scholar]
- 40.Oliver JE, Silman AJ. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006 May-Jun;35(3):169–174. doi: 10.1080/03009740600718080. [DOI] [PubMed] [Google Scholar]
- 41.Dixon B. Bacteria and arthritis. BMJ. 1990 Nov 3;301(6759):1043. doi: 10.1136/bmj.301.6759.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebringer A. Rheumatoid arthritis and proteus. Clin Med. 2005 Jul-Aug;5(4):420–421. doi: 10.7861/clinmedicine.5-4-420a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silman AJ, Hochberg MC. Rheumatoid arthritis. In: Silman AJ, Hochberg MC, editors. Epidemiology of the rheumatic diseases. New York: Oxford University Press; 1993. pp. 7–68. [Google Scholar]
- 44.Spector TD, Silman AJ. Is poor pregnancy outcome a risk factor in rheumatoid arthritis? Ann Rheum Dis. 1990 Jan;49(1):12–14. doi: 10.1136/ard.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pladevall-Vila M, Delclos GL, Varas C, Guyer H, Brugues-Tarradellas J, Anglada-Arisa A. Controversy of oral contraceptives and risk of rheumatoid arthritis: meta-analysis of conflicting studies and review of conflicting meta-analyses with special emphasis on analysis of heterogeneity. Am J Epidemiol. 1996 Jul 1;144(1):1–14. doi: 10.1093/oxfordjournals.aje.a008846. [DOI] [PubMed] [Google Scholar]
- 46.Spector TD, Hochberg MC. The protective effect of the oral contraceptive pill on rheumatoid arthritis: an overview of the analytic epidemiological studies using meta-analysis. J Clin Epidemiol. 1990;43(11):1221–1230. doi: 10.1016/0895-4356(90)90023-i. [DOI] [PubMed] [Google Scholar]
- 47.Doran MF, Crowson CS, O'Fallon WM, Gabriel SE. The effect of oral contraceptives and estrogen replacement therapy on the risk of rheumatoid arthritis: a population based study. J Rheumatol. 2004 Feb;31(2):207–213. [PubMed] [Google Scholar]
- 48.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004 Jan;50(1):72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 49.Bhatia SS, Majka DS, Kittelson JM, Parrish LA, Ferucci ED, Deane KD, et al. Rheumatoid factor seropositivity is inversely associated with oral contraceptive use in women without rheumatoid arthritis. Ann Rheum Dis. 2007 Feb;66(2):267–269. doi: 10.1136/ard.2006.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korpilahde T, Heliovaara M, Knekt P, Marniemi J, Aromaa A, Aho K. Smoking history and serum cotinine and thiocyanate concentrations as determinants of rheumatoid factor in non-rheumatoid subjects. Rheumatology (Oxford) 2004 Nov;43(11):1424–1428. doi: 10.1093/rheumatology/keh365. [DOI] [PubMed] [Google Scholar]
- 51.Tuomi T, Heliovaara M, Palosuo T, Aho K. Smoking, lung function, and rheumatoid factors. Ann Rheum Dis. 1990 Oct;49(10):753–756. doi: 10.1136/ard.49.10.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feser M, Derber LA, Deane KD, Lezotte DC, Weisman MH, Buckner JH, et al. Plasma 25,OH vitamin D concentrations are not associated with rheumatoid arthritis (RA)-related autoantibodies in individuals at elevated risk for RA. J Rheumatol. 2009 May;36(5):943–946. doi: 10.3899/jrheum.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elkayam O, Caspi D, Lidgi M, Segal R. Auto-antibody profiles in patients with active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2007 Mar;11(3):306–310. [PubMed] [Google Scholar]
- 54.Elkayam O, Segal R, Lidgi M, Caspi D. Positive anti-cyclic citrullinated proteins and rheumatoid factor during active lung tuberculosis. Ann Rheum Dis. 2006 Aug;65(8):1110–1112. doi: 10.1136/ard.2005.045229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Criswell LA, Saag KG, Mikuls TR, Cerhan JR, Merlino LA, Lum RF, et al. Smoking interacts with genetic risk factors in the development of rheumatoid arthritis among older Caucasian women. Ann Rheum Dis. 2006 Sep;65(9):1163–1167. doi: 10.1136/ard.2005.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kallberg H, Padyukov L, Plenge RM, Ronnelid J, Gregersen PK, van der Helm-van Mil AH, et al. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet. 2007 May;80(5):867–875. doi: 10.1086/516736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006 Jan;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 58.Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009 Dec;41(12):1319–1324. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- 59.Steck AK, Zhang W, Bugawan TL, Barriga KJ, Blair A, Erlich HA, et al. Do non-HLA genes influence development of persistent islet autoimmunity and type 1 diabetes in children with high-risk HLA-DR,DQ genotypes? Diabetes. 2009 Apr;58(4):1028–1033. doi: 10.2337/db08-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheehy MJ. HLA and insulin-dependent diabetes. A protective perspective. Diabetes. 1992 Feb;41(2):123–129. doi: 10.2337/diab.41.2.123. [DOI] [PubMed] [Google Scholar]
- 61.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008 Feb;41(1):11–18. doi: 10.1080/08916930701619169. [DOI] [PubMed] [Google Scholar]
- 62.Achenbach P, Bonifacio E, Koczwara K, Ziegler AG. Natural history of type 1 diabetes. Diabetes. 2005 Dec;54 Suppl 2:S25–S31. doi: 10.2337/diabetes.54.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 63.Norris JM, Beaty B, Klingensmith G, Yu L, Hoffman M, Chase HP, et al. Lack of association between early exposure to cow's milk protein and beta-cell autoimmunity. Diabetes Autoimmunity Study in the Young (DAISY) JAMA. 1996 Aug 28;276(8):609–614. doi: 10.1001/jama.1996.03540080031025. [DOI] [PubMed] [Google Scholar]
- 64.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003 Oct 1;290(13):1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 65.Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003 Oct 1;290(13):1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 66.Graves PM, Rotbart HA, Nix WA, Pallansch MA, Erlich HA, Norris JM, et al. Prospective study of enteroviral infections and development of beta-cell autoimmunity. Diabetes autoimmunity study in the young (DAISY) Diabetes Res Clin Pract. 2003 Jan;59(1):51–61. doi: 10.1016/s0168-8227(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 67.Graves PM, Barriga KJ, Norris JM, Hoffman MR, Yu L, Eisenbarth GS, et al. Lack of association between early childhood immunizations and beta-cell autoimmunity. Diabetes Care. 1999 Oct;22(10):1694–1697. doi: 10.2337/diacare.22.10.1694. [DOI] [PubMed] [Google Scholar]
- 68.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007 Sep 26;298(12):1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 69.Lamb MM, Yin X, Barriga K, Hoffman MR, Baron AE, Eisenbarth GS, et al. Dietary glycemic index, development of islet autoimmunity, and subsequent progression to type 1 diabetes in young children. J Clin Endocrinol Metab. 2008 Oct;93(10):3936–3942. doi: 10.1210/jc.2008-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Chase HP, et al. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J Autoimmun. 1996 Jun;9(3):379–383. doi: 10.1006/jaut.1996.0051. [DOI] [PubMed] [Google Scholar]
- 71.Taplin CE, Barker JM. Natural evolution, prediction, and prevention of type 1 diabetes in youth. Endocr Res. 2008;33(1–2):17–33. doi: 10.1080/07435800802080328. [DOI] [PubMed] [Google Scholar]
- 72.Kishiyama CM, Chase HP, Barker JM. Prevention strategies for type 1 diabetes. Rev Endocr Metab Disord. 2006 Sep;7(3):215–224. doi: 10.1007/s11154-006-9015-z. [DOI] [PubMed] [Google Scholar]
- 73.Maddison PJ, Reichlin M. Quantitation of precipitating antibodies to certain soluble nuclear antigens in SLE. Arthritis Rheum. 1977 Apr;20(3):819–824. doi: 10.1002/art.1780200310. [DOI] [PubMed] [Google Scholar]
- 74.Lee LA, Gaither KK, Coulter SN, Norris DA, Harley JB. Pattern of cutaneous immunoglobulin G deposition in subacute cutaneous lupus erythematosus is reproduced by infusing purified anti-Ro (SSA) autoantibodies into human skin-grafted mice. J Clin Invest. 1989 May;83(5):1556–1562. doi: 10.1172/JCI114052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miranda-Carus ME, Askanase AD, Clancy RM, Di Donato F, Chou TM, Libera MR, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J Immunol. 2000 Nov 1;165(9):5345–5351. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 76.Kurien BT, Newland J, Paczkowski C, Moore KL, Scofield RH. Association of neutropenia in systemic lupus erythematosus (SLE) with anti-Ro and binding of an immunologically cross-reactive neutrophil membrane antigen. Clin Exp Immunol. 2000 Apr;120(1):209–217. doi: 10.1046/j.1365-2249.2000.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hahn BH. Antibodies to DNA. N Engl J Med. 1998 May 7;338(19):1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 78.Davis P, Percy JS, Russell AS. Correlation between levels of DNA antibodies and clinical disease activity in SLE. Ann Rheum Dis. 1977 Apr;36(2):157–159. doi: 10.1136/ard.36.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003 Oct 16;349(16):1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 80.McClain MT, Arbuckle MR, Heinlen LD, Dennis GJ, Roebuck J, Rubertone MV, et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004 Apr;50(4):1226–1232. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 81.Arbuckle MR, James JA, Dennis GJ, Rubertone MV, McClain MT, Kim XR, et al. Rapid clinical progression to diagnosis among African-American men with systemic lupus erythematosus. Lupus. 2003;12(2):99–106. doi: 10.1191/0961203303lu334oa. [DOI] [PubMed] [Google Scholar]
- 82.Gross AJ, Hochberg D, Rand WM, Thorley-Lawson DA. EBV and systemic lupus erythematosus: a new perspective. J Immunol. 2005 Jun 1;174(11):6599–6607. doi: 10.4049/jimmunol.174.11.6599. [DOI] [PubMed] [Google Scholar]
- 83.Vossenaar ER, Nijenhuis S, Helsen MM, van der Heijden A, Senshu T, van den Berg WB, et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003 Sep;48(9):2489–2500. doi: 10.1002/art.11229. [DOI] [PubMed] [Google Scholar]
- 84.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006 Apr;116(4):961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009 Feb 16;206(2):449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lundberg K, Nijenhuis S, Vossenaar ER, Palmblad K, van Venrooij WJ, Klareskog L, et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther. 2005;7(3):R458–R467. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003 Jul 15;171(2):538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 88.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008 Apr 14;205(4):967–979. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanazawa S, Ota S, Sekine C, Tada T, Otsuka T, Okamoto T, et al. Aberrant MHC class II expression in mouse joints leads to arthritis with extraarticular manifestations similar to rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006 Sep 26;103(39):14465–14470. doi: 10.1073/pnas.0606450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ho PP, Lee LY, Zhao X, Tomooka BH, Paniagua RT, Sharpe O, et al. Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J Immunol. 2010 Jan 1;184(1):379–390. doi: 10.4049/jimmunol.0901639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kraan MC, Versendaal H, Jonker M, Bresnihan B, Post WJ, t Hart BA, et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998 Aug;41(8):1481–1488. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 92.Smolik I, Robinson D, El-Gabalawy HS. Periodontitis and rheumatoid arthritis: epidemiologic, clinical, and immunologic associations. Compend Contin Educ Dent. 2009 May;30(4):188–190. 92, 94 passim; quiz 98, 210. [PubMed] [Google Scholar]
- 93.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 94.Aletaha D, Breedveld FC, Smolen JS. The need for new classification criteria for rheumatoid arthritis. Arthritis Rheum. 2005 Nov;52(11):3333–3336. doi: 10.1002/art.21410. [DOI] [PubMed] [Google Scholar]
- 95.Silman AJ, Symmons DP. Selection of study population in the development of rheumatic disease criteria: comment on the article by the American College of Rheumatology Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1995 May;38(5):722–723. doi: 10.1002/art.1780380527. [DOI] [PubMed] [Google Scholar]
- 96.Symmons DP, Silman AJ. The Norfolk Arthritis Register (NOAR) Clin Exp Rheumatol. 2003 Sep-Oct;21(5 Suppl 31):S94–S99. [PubMed] [Google Scholar]
- 97.van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007 Feb;56(2):433–440. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 98.van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007 May;56(5):1424–1432. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 99.Verpoort KN, van Dongen H, Allaart CF, Toes RE, Breedveld FC, Huizinga TW. Undifferentiated arthritis--disease course assessed in several inception cohorts. Clin Exp Rheumatol. 2004 Sep-Oct;22(5 Suppl 35):S12–S17. [PubMed] [Google Scholar]
- 100.van Aken J, van Bilsen JH, Allaart CF, Huizinga TW, Breedveld FC. The Leiden Early Arthritis Clinic. Clin Exp Rheumatol. 2003 Sep-Oct;21(5 Suppl 31):S100–S105. [PubMed] [Google Scholar]
- 101.Panel ACoRAaELARE., editor. New Rheumatoid Arthritis Criteria; Philadelphia, Pennsylvania, U.S.A. American College of Rheumatology Annual Meeting.2009. [Google Scholar]
- 102.Pedersen M, Jacobsen S, Garred P, Madsen HO, Klarlund M, Svejgaard A, et al. Strong combined gene-environment effects in anti-cyclic citrullinated peptide-positive rheumatoid arthritis: a nationwide case-control study in Denmark. Arthritis Rheum. 2007 May;56(5):1446–1453. doi: 10.1002/art.22597. [DOI] [PubMed] [Google Scholar]
- 103.Verpoort KN, Papendrecht-van der Voort EA, van der Helm-van Mil AH, Jol-van der Zijde CM, van Tol MJ, Drijfhout JW, et al. Association of smoking with the constitution of the anti-cyclic citrullinated peptide response in the absence of HLA-DRB1 shared epitope alleles. Arthritis Rheum. 2007 Sep;56(9):2913–2918. doi: 10.1002/art.22845. [DOI] [PubMed] [Google Scholar]
- 104.Deane KD, Simonian PL, Lynch DA, Julien PJ, Weisman MH, Norris JM, et al. Rheumatoid arthritis (RA)-related autoantibodies are associated with pulmonary airway abnormalities in individuals without RA (Abstract 340) Arthritis Rheum. 2008;58(9):S292. [Abstract] [Google Scholar]
- 105.Aho K, Tuomi T, Heliovaara M, Palosuo T. Rheumatoid factors and rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;74:41–44. doi: 10.3109/03009748809102938. [DOI] [PubMed] [Google Scholar]
- 106.Dorner T, Egerer K, Feist E, Burmester GR. Rheumatoid factor revisited. Curr Opin Rheumatol. 2004 May;16(3):246–253. doi: 10.1097/00002281-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 107.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7(5):R949–R958. doi: 10.1186/ar1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Verpoort KN, Cheung K, Ioan-Facsinay A, van der Helm-van Mil AH, de Vries-Bouwstra JK, Allaart CF, et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 2007 Dec;56(12):3949–3952. doi: 10.1002/art.23127. [DOI] [PubMed] [Google Scholar]
- 109.Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA, Ioan-Facsinay A, Drijfhout JW, van Tol MJ, et al. Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 2006 Dec;54(12):3799–3808. doi: 10.1002/art.22279. [DOI] [PubMed] [Google Scholar]
- 110.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995 Mar;1(3):237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 111.Ioan-Facsinay A, Willemze A, Robinson DB, Peschken CA, Markland J, van der Woude D, et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008 Oct;58(10):3000–3008. doi: 10.1002/art.23763. [DOI] [PubMed] [Google Scholar]
- 112.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 113.Berglin E, Padyukov L, Sundin U, Hallmans G, Stenlund H, Van Venrooij WJ, et al. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res Ther. 2004;6(4):R303–R308. doi: 10.1186/ar1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johansson M, Arlestig L, Hallmans G, Rantapaa-Dahlqvist S. PTPN22 polymorphism and anti-cyclic citrullinated peptide antibodies in combination strongly predicts future onset of rheumatoid arthritis and has a specificity of 100% for the disease. Arthritis Res Ther. 2006;8(1):R19. doi: 10.1186/ar1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van der Helm-van Mil AH, Detert J, le Cessie S, Filer A, Bastian H, Burmester GR, et al. Validation of a prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: moving toward individualized treatment decision-making. Arthritis Rheum. 2008 Aug;58(8):2241–2247. doi: 10.1002/art.23681. [DOI] [PubMed] [Google Scholar]
- 116.Feitsma AL, Toes RE, Begovich AB, Chokkalingam AP, de Vries RR, Huizinga TW, et al. Risk of progression from undifferentiated arthritis to rheumatoid arthritis: the effect of the PTPN22 1858T-allele in anti-citrullinated peptide antibody positive patients. Rheumatology (Oxford) 2007 Jul;46(7):1092–1095. doi: 10.1093/rheumatology/kem006. [DOI] [PubMed] [Google Scholar]
- 117.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006 Apr;54(4):1117–1121. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 118.Allaart CF, Breedveld FC, Dijkmans BA. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J Rheumatol Suppl. 2007 Nov;80:25–33. [PubMed] [Google Scholar]
- 119.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum. 2008 Feb;58(2 Suppl):S126–S135. doi: 10.1002/art.23364. [DOI] [PubMed] [Google Scholar]
- 120.Cush JJ. Early rheumatoid arthritis -- is there a window of opportunity? J Rheumatol Suppl. 2007 Nov;80:1–7. [PubMed] [Google Scholar]
- 121.Emery P, Durez P, Dougados M, Legerton CW, Becker JC, Vratsanos G, et al. The impact of T-cell co-stimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept. Ann Rheum Dis. 2009 Nov 23; doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rewers M, Gottlieb P. Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future. Diabetes Care. 2009 Oct;32(10):1769–1782. doi: 10.2337/dc09-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain MT, Arbuckle MR, et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16(6):401–409. doi: 10.1177/0961203307078579. [DOI] [PubMed] [Google Scholar]
- 124.Endres M. Statins: potential new indications in inflammatory conditions. Atheroscler Suppl. 2006 Apr;7(1):31–35. doi: 10.1016/j.atherosclerosissup.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 125.McInnes IB, McCarey DW, Sattar N. Do statins offer therapeutic potential in inflammatory arthritis? Ann Rheum Dis. 2004 Dec;63(12):1535–1537. doi: 10.1136/ard.2004.022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klareskog L, Hamsten A. Statins in rheumatoid arthritis--two birds with one stone? Lancet. 2004 Jun 19;363(9426):2011–2012. doi: 10.1016/S0140-6736(04)16485-4. [DOI] [PubMed] [Google Scholar]
- 127.Jick SS, Choi H, Li L, McInnes IB, Sattar N. Hyperlipidaemia, statin use and the risk of developing rheumatoid arthritis. Ann Rheum Dis. 2009 Apr;68(4):546–551. doi: 10.1136/ard.2008.091967. [DOI] [PubMed] [Google Scholar]
- 128.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951 Mar;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kolfenbach JR, Deane KD, Derber LA, O'Donnell C, Weisman MH, Buckner JH, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009 Dec 15;61(12):1735–1742. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oen K, Robinson DB, Nickerson P, Katz SJ, Cheang M, Peschken CA, et al. Familial seropositive rheumatoid arthritis in North American Native families: effects of shared epitope and cytokine genotypes. J Rheumatol. 2005 Jun;32(6):983–991. [PubMed] [Google Scholar]
- 131.El-Gabalawy HS, Robinson DB, Hart D, Elias B, Markland J, Peschken CA, et al. Immunogenetic risks of anti-cyclical citrullinated peptide antibodies in a North American Native population with rheumatoid arthritis and their first-degree relatives. J Rheumatol. 2009 Jun;36(6):1130–1135. doi: 10.3899/jrheum.080855. [DOI] [PubMed] [Google Scholar]
- 132.Deane KD, Striebich CC, Goldstein BL, Derber LA, Parish MC, Feser ML, et al. Identification of undiagnosed inflammatory arthritis in a community health fair screen. Arthritis Rheum. 2009 Nov 30;61(12):1642–1649. doi: 10.1002/art.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bizzaro N. The predictive significance of autoantibodies in organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2008 Jun;34(3):326–331. doi: 10.1007/s12016-007-8059-5. [DOI] [PubMed] [Google Scholar]
- 134.Rose NR. Predictors of autoimmune disease: autoantibodies and beyond. Autoimmunity. 2008 Sep;41(6):419–428. doi: 10.1080/08916930802031686. [DOI] [PubMed] [Google Scholar]
- 135.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005 Feb;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 136.Alarcon GS, Koopman WJ, Acton RT, Barger BO. Seronegative rheumatoid arthritis. A distinct immunogenetic disease? Arthritis Rheum. 1982 May;25(5):502–507. doi: 10.1002/art.1780250503. [DOI] [PubMed] [Google Scholar]