This study examines secretory and endocytotic trafficking in Arabidopsis by tracking the movement of a brassinosteroid receptor and a boron exporter through the endomembrane system. Both endocytotic and secretory cargo travel through the trans-Golgi network/early endosome (TGN/EE), and the TGN/EE is shown to be an independent organelle that only transiently associates with the Golgi.
Abstract
Plants constantly adjust their repertoire of plasma membrane proteins that mediates transduction of environmental and developmental signals as well as transport of ions, nutrients, and hormones. The importance of regulated secretory and endocytic trafficking is becoming increasingly clear; however, our knowledge of the compartments and molecular machinery involved is still fragmentary. We used immunogold electron microscopy and confocal laser scanning microscopy to trace the route of cargo molecules, including the BRASSINOSTEROID INSENSITIVE1 receptor and the REQUIRES HIGH BORON1 boron exporter, throughout the plant endomembrane system. Our results provide evidence that both endocytic and secretory cargo pass through the trans-Golgi network/early endosome (TGN/EE) and demonstrate that cargo in late endosomes/multivesicular bodies is destined for vacuolar degradation. Moreover, using spinning disc microscopy, we show that TGN/EEs move independently and are only transiently associated with an individual Golgi stack.
INTRODUCTION
As a consequence of their sessile lifestyle, plants have to be able to rapidly adapt their functional responses to environmental cues. In this regard, data have been accumulating that indicate that the repertoire of plasma membrane (PM) proteins is highly dynamic and is constantly being adjusted to suit the plant's needs. These include receptors mediating the transduction of environmental and developmental signals as well as transporters for ions, nutrients, and hormones. By regulating the density of these proteins at the PM through the secretory and endocytic pathways, the plant can effectively adapt to new environmental conditions. Although our knowledge of the compartments through which endocytic cargo passes is still rudimentary (Robinson et al., 2008), one striking example of a regulatory switch between the recycling and degradative pathways of endocytosis is the boron (B) exporter REQUIRES HIGH BORON1 (BOR1; Takano et al., 2002). Under steady state conditions in the presence of low B, BOR1 is found principally at the PM, with a fraction undergoing constitutive cycling. To avoid B toxicity, BOR1 is rapidly internalized and targeted for vacuolar degradation after sensing high external B concentrations (Takano et al., 2005). Unlike BOR1, the steady state distribution of the brassinosteroid receptor BRASSINOSTEROID INSENSITIVE1 (BRI1; Li and Chory, 1997; Friedrichsen et al., 2000) at the PM does not change after application of the ligand (Geldner et al., 2007). However, BRI1 has been shown to cycle between the PM and brefeldin A (BFA)-sensitive endosomal compartments, suggesting that BRI1 is also subject to constitutive endocytic recycling (Geldner et al., 2007). Increasing endosomal concentrations of BRI1 lead to enhanced BR signaling, indicating that plants, like animals, use endosomes as signaling platforms (Russinova et al., 2004; Geldner et al., 2007). Nevertheless, a fraction of endocytosed BRI1 molecules is also targeted to the vacuole (Geldner et al., 2007), so it remains to be determined from which endosomal compartments BRI1 can recycle to the PM and from which point on it becomes destined for degradation. Thus, BRI1 and BOR1 provide evidence that different modes of endocytosis coexist in plant cells, although the respective trafficking pathways have not been precisely defined (Geldner and Jurgens, 2006).
Based on rapid staining with the endocytic tracer FM4-64 and the colocalization of TGN markers and endocytosed PM proteins in the core of BFA compartments, there is now compelling evidence that the trans-Golgi network (TGN), or a subdomain of it, acts as an early endosome (EE) (Dettmer et al., 2006; Lam et al., 2007; Chow et al., 2008). However, evidence for the passage of endocytosed PM proteins through the TGN/EE is limited to the cytokinesis-specific syntaxin KNOLLE, which is removed from the cell plate during M phase (Reichardt et al., 2007).
The TGN was originally defined as a clathrin-coated, tubular network contained within the matrix of a Golgi stack (Staehelin and Moore, 1995). However, TGN-like structures have also been observed more distant from Golgi stacks, and this variation in distance has been ascribed to a maturation process that involves sloughing off at the trans-most Golgi cisterna (Hillmer et al., 1988; Toyooka et al., 2009). More recently, electron tomography has provided evidence for a structural differentiation of the TGN into so-called early and late subcompartments (Staehelin and Kang, 2008), although kinetic studies are needed to confirm this classification. The extent to which the TGN/EE participates in secretory traffic to the PM is also controversial. The study of Toyooka et al. (2009) suggests that part of the TGN separates from the rest to form a cluster of secretory vesicles, which eventually fuse with the PM. On the other hand, it has recently been suggested that cellulose synthase complexes might not pass through the TGN/EE when they move to the PM, thus leading the authors to question the role of the TGN in secretion (Crowell et al., 2009).
Although the ARF GTPase exchange factor GNOM (Geldner et al., 2003) identifies a recycling endosome, a morphological characterization of this compartment at the EM level is lacking. GNOM-bearing membranes and TGN/EE markers accumulate in BFA compartments (Dettmer et al., 2006; Lam et al., 2007; Chow et al., 2008), but it remains to be seen whether the recycling endosome represents an independent compartment, a subdomain of the TGN/EE, or perhaps a compartment that matures out of the latter. Recycling to the PM has also been suggested to occur from a compartment characterized by the presence of SORTING NEXIN1 (SNX1) in Arabidopsis thaliana (Jaillais et al., 2006, 2008). SNX1 is part of the vacuolar sorting receptor-recycling protein complex (retromer) that was previously reported to be localized to the multivesicular body/prevacuolar compartment (MVB/PVC), the equivalent of the late endosome in plants (Oliviusson et al., 2006). However, more recently, SNX1 was shown to localize to the TGN/EE (Niemes et al., 2010a), showing that the plant late endosome has an equivalent function as its counterpart from mammals, from which receptor recycling also does not occur (Braulke and Bonifacino, 2009).
Clearly, the routes of cargo molecules and the compartments of the plant endosomal system need to be better characterized. In particular, it remains to be demonstrated through which domains/compartments receptors and transporters pass on their way to the PM after synthesis and then subsequently during endocytosis: Are they the same or are the synthetic and endocytic populations kept separate? To reconcile the differentiation of the TGN into early and late subcompartments in terms of secretion (Staehelin and Kang, 2008) with its function as an EE, we performed spinning disc confocal microscopy on an Arabidopsis line expressing Golgi and TGN markers. This revealed that TGN/EEs frequently display independent movement and are only transiently associated with individual Golgi stacks. To trace the fate of internalized PM proteins and to distinguish between endocytic and secretory cargo in the TGN, we used immunogold electron microscopy (IEM) and confocal laser scanning microscopy (CLSM) to determine the route(s) of BRI1 and BOR1 throughout the plant endomembrane system. Our results confirm that both endocytic and secretory cargo pass through the TGN/EE and demonstrate that PM proteins found in late endosomes/MVBs are indeed destined for vacuolar degradation. Moreover, CLSM analysis after inducible expression of secretory green fluorescent protein (secGFP) and BRI1-yellow fluorescent protein (YFP) as well as immunogold-EM of a xyloglucan epitope confirmed that at least some secretory cargo molecules pass through the TGN en route to the PM.
RESULTS
Independent Movement and Dynamic Association of the TGN/EE with Golgi Stacks
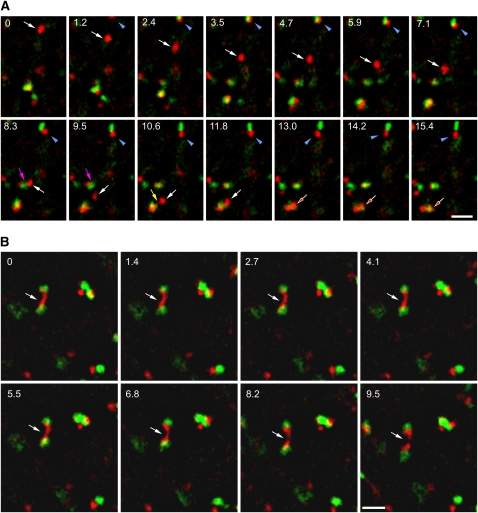
To better characterize the temporal and spatial relationship between Golgi stacks and TGN/EE, we performed spinning disc confocal microscopy on seedlings expressing both the TGN/EE marker VHA-a1-red fluorescent protein (RFP) (Dettmer et al., 2006) and the trans-Golgi marker N-ST-YFP (Grebe et al., 2003). Hypocotyl cells of etiolated seedlings were chosen since their relatively thin peripheral layer of cytoplasm allowed us to follow the dynamics of the organelles in an almost two-dimensional plane. We found that the TGN behaved as a highly mobile and independent organelle (Figure 1A, white arrow; see Supplemental Movie 1 online) that temporarily paused and became closely associated with a Golgi stack (Figure 1A, arrowhead; see Supplemental Movie 1 online). Furthermore, association of individual TGNs was observed (Figure 1A, red arrow; see Supplemental Movie 1 online). Sometimes, a thin protrusion between the partners was visible before the actual merging occurred (Figure 1A, yellow arrow). Evidently, not all TGNs were competent for this interaction (Figure 1A, magenta arrow). Association seemed to be balanced by dissociation events as shown in Figure 1B and Supplemental Movie 2 online for an elongated TGN shared by two Golgi stacks.
Figure 1.
The TGN Is an Independent Organelle That Undergoes Reversible Homotypic Association.
Spinning disc confocal microscopy of hypocotyl cells expressing VHA-a1-RFP and ST-GFP. Numbers = seconds; bars = 5 μM.
(A) The TGN is a highly mobile and independent organelle (white arrows, time point from 0 to 11.8 s) that temporarily pauses and becomes closely associated with a Golgi stack (blue arrowheads, 1.2 to 15.4 s). Homotypic association of individual TGNs was also observed (red arrows, 13.0 to 15.4 s), and sometimes a thin protrusion between the interacting partners was visible (yellow arrows, 10.6 s). Not all TGNs are competent for this interaction (magenta arrow, 8.3 and 9.5 s).
(B) A dissociation event for an elongated TGN shared by two Golgi stacks (white arrows, 0 to 9.5 s).
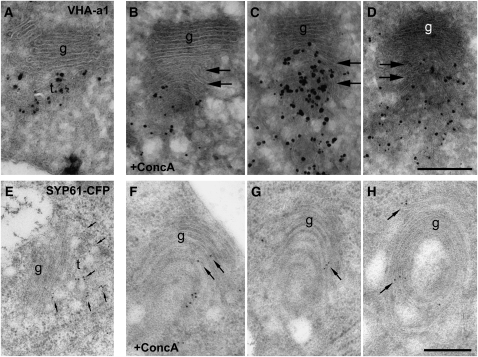
Although the TGN can move as an independent compartment, we previously showed that genetic and pharmacological inhibition of V-ATPase activity not only affects the TGN/EE but also causes a loss of recognizable Golgi morphology (Dettmer et al., 2005,2006; Brux et al., 2008). To test if V-ATPase activity is required for the identity of the TGN/EE, we analyzed the distribution of VHA-a1 and SYP61, two highly specific markers for the plant TGN/EE (Figures 2A and 2E; Dettmer et al., 2006; Robert et al., 2008) in meristematic root cells after a mild treatment with the V-ATPase inhibitor concanamycin (ConcA). In the presence of 0.5 μM ConcA, the number of Golgi cisternae increased from 5 ± 1 to 8 ± 1 (Table 1), and a clear spatial separation between the Golgi stack and the TGN was no longer recognizable (Figures 2B to 2D and 2F to 2H). Furthermore, VHA-a1 and SYP61 were no longer restricted to the TGN but were additionally found on Golgi cisternae, indicating that V-ATPase activity is required for the identity of the TGN/EE (Figures 2B to 2D and 2F to 2H, Table 1).
Figure 2.
The Identity and Independence of the TGN Is Affected by ConcA.
Immunogold labeling of VHA-a1 and SYP61-CFP in ConcA-treated cells. (A) to (D), silver-enhanced cryosections; (E) to (H), HM20 resin sections (see Methods). Bars = 250 nm.
(A) and (E) VHA-a1 and SYP61 are highly specific markers for the plant TGN/EE.
(B) to (D) and (F) to (H) In the presence of ConcA, VHA-a1 and SYP61-CFP were additionally found on Golgi stacks, and a clear spatial separation between the Golgi apparatus and the TGN was no longer recognizable. Furthermore, ConcA induces the proliferation of the Golgi cisternae, accompanied by a loss of identity of the two compartments.
Table 1.
Quantitative Analysis of IEM
| Golgi |
TGN |
MVB |
|||||||
| Protein | Treatment | No. Labeled | No. Gold/μm2 | No. Cisternae | Labeled (%) | No. Gold/μm2 | Labeled (%) | No. Gold/μm2 | Inner Gold |
| BRI1-GFP | control | 9% | 1.1 ± 0.4 | 5 ± 1 | 66% | 22.7 ± 2.1 | 66% | 20.4 ± 2.8 | 93% ± 5% |
| BRI1-GFP | +CHX | 11% | 1.3 ± 0.5 | 5 ± 1 | 59% | 18.6 ± 2.5 | 58% | 18,6 ± 3.5 | 88% ± 7% |
| BOR1-GFP | −B | 11% | 1.1 ± 0.3 | 5 ± 1 | 25% | 5.2 ± 1.0 | 19% | 3.3 ± 0.9* | n.a. |
| BOR1-GFP | +B | 11% | 1.3 ± 0.4 | 5 ± 1 | 66% | 29.1 ± 2.3 | 68% | 21.8 ± 2.2 | 85% ± 4% |
| BOR1-GFP | +CHX, +B | 10% | 1.5 ± 0.4 | 5 ± 1 | 59% | 20.5 ± 1.7 | 60% | 21.3 ± 3.3 | 87% ± 5% |
| BP80 | Control | 12% | 1.3 ± 0.5 | 5 ± 1 | 81% | 42.2 ± 3.5 | 76% | 39.4 ± 3.9 | 30% ± 5% |
| hs:BRI1-YFP | Control | 48% | 8.3 ± 1.0 | 5 ±1 | 50% | 18.1 ± 2.1 | 19% | 3.1 ± 0.8* | n.a. |
| hs:BRI1-YFP | +Wm | 54% | 9.5 ± 1.4 | 5 ± 1 | 50% | 17.2 ± 2.5 | 18% | 3.8 ± 0.8* | n.a. |
| SYP61-CFP | Control | 12% | 1.6 ± 0.6 | 5 ± 1 | 85% | 47.4 ± 3.9 | 10% | 2.4 ± 1.4* | n.a. |
| SYP61-CFP | +ConcA | 63% | 9.2 ± 1.1 | 8 ± 1 | 81% | 31.9 ± 2.7 | 9% | 2.7 ± 1.5* | n.a. |
Labeling density is expressed as the number of gold particles/μm2 ± se, number of cisternae ± sd, and percentage of inner gold particles ± sd. Asterisk indicates that labeling density (≤5 gold/μm2) comparable to background labeling (see Supplemental Table 1 online). n.a., not available; gold particles were too few to allow analysis. For further details (total number of compartments analyzed and total number of gold particles), see Supplemental Table 1 online.
Endocytic Cargo Passes through the TGN
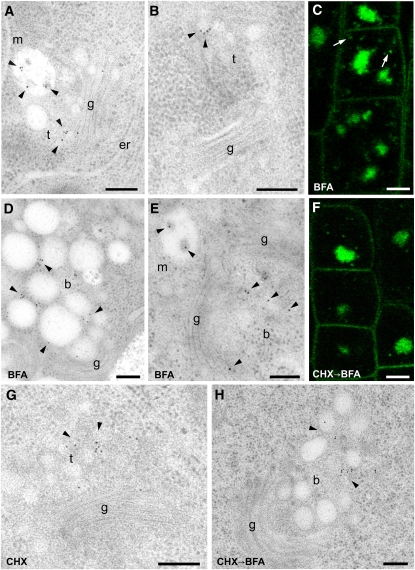
To define the endosomal compartments through which BRI1 and BOR1 pass after their internalization from the PM, we traced these cargoes by IEM. Seedlings expressing BRI1-GFP (Friedrichsen et al., 2000) or BOR1-GFP (Takano et al., 2010) were high-pressure frozen, freeze-substituted, and embedded in Lowicryl HM20 resin. Embedded samples in which the GFP signal, despite the thickness of the resin block, was still detectable were selected (see Supplemental Figure 1 online). Affinity-purified YFP antibodies were used for the IEM. In meristematic root cells, BRI1-GFP was only detectable in the region of the TGN and in MVBs (Figure 3A, Table 1). The tubular-vesicular TGN/EE was positively labeled even when not obviously associated with a Golgi stack (Figure 3B). BFA treatment causes the aggregation of endosomal compartments, including the TGN/EE (Grebe et al., 2003; Dettmer et al., 2006; Robinson et al., 2008). Accordingly, the core of the BFA compartment was positively labeled with BRI1-GFP (Figure 3C). Nevertheless, punctae surrounding the core were also visible (Figure 3C, arrows). IEM analysis confirmed the presence of BRI1-GFP both in the core of the BFA compartment and in MVBs (Figures 3D and 3E). Treatment with the protein synthesis inhibitor cycloheximide (CHX; see Supplemental Figures 2A and 2B online) did not prevent the accumulation of BRI1-GFP in the core of the BFA compartment (Figures 3F and 3H), and BRI1 was still detected at the TGN/EE (Figure 3G), indicating that the signal was not only due to secretory cargo.
Figure 3.
Endocytosed BRI1-GFP Is Found at the TGN and in MVBs.
Immunogold labeling and CLSM analyses of root tips from BRI1-GFP Arabidopsis plants. m, multivesicular body; t, TGN; g, Golgi; er, endoplasmic reticulum; b, BFA compartment. The symbol “→” means that the second inhibitor was added with the first still present. IEM bars = 200 nm; CLSM bars = 5 μm.
(A) BRI1-GFP localizes to both the TGN and MVB (arrowheads).
(B) The TGN is positively labeled (arrowheads) also when it is not closely associated with the Golgi stacks.
(C) The core of the BFA compartment is labeled, and a surrounding punctate pattern is also visible (arrows).
(D) and (E) IEM confirmed the localization of BRI1-GFP in the core of the BFA compartment and on the MVB (arrowheads).
(F) The protein synthesis inhibitor CHX does not prevent the accumulation of BRI1-GFP in the core of the BFA compartment (arrowheads).
(G) In the presence of CHX, BRI1 is still detected at the TGN (arrowheads).
(H) In the presence of CHX and BFA, BRI1 is detected in the BFA compartment (arrowheads).
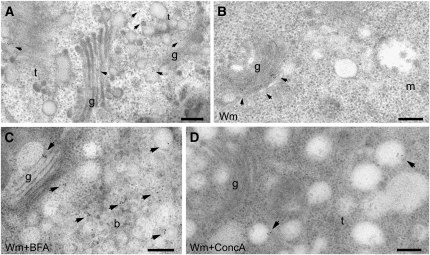
Endocytic Cargo in MVBs Is Destined for Degradation
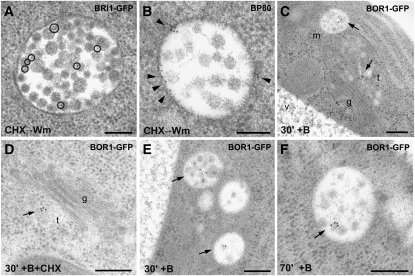
The PI3-kinase inhibitor wortmannin (Wm) (Emans et al., 2002) causes swelling of MVBs, in part due to homotypic fusion events (Wang et al., 2009). As a result, fluorescently tagged PVC markers like the vacuolar sorting receptor BP80 or the Arabidopsis Rab5-like proteins ARA6 and ARA7 show a ring-like appearance after Wm treatment (Tse et al., 2004; Lam et al., 2007; Reichardt et al., 2007). However, in no cases did BRI1-labeled endosomes show a ring-like appearance in response to Wm. We considered this to be a consequence of the fact that BRI1 was always localized on the inner vesicles of the MVBs (Figures 3A, 3E, and 4A, Table 1), rather than preferentially on the boundary membrane, as is the case for BP80 (Figure 4B, Table 1; see Supplemental Figure 3 online). Also, when roots were treated with both CHX and Wm, IEM showed that BRI1-GFP is present exclusively on the inner vesicles (Figure 4A), although MVBs were swollen. A similar distribution pattern was detected for the PM-based boron exporter BOR1. Under low boron conditions, BOR1 was not detectable in any endosomal compartment. However, after 30 min of exposure to boron, BOR1-GFP was localized both to the TGN and to the MVB (Figure 4C). The TGN signal was confirmed also in the presence of CHX (Figure 4D). Moreover, BOR1 also was specifically localized to the inner vesicles of the MVB (Figures 4E and 4F, Table 1), pointing to its subsequent degradation in the vacuole, since after 70 min of high boron treatment the GFP signal had almost disappeared (see Supplemental Figure 4 online). These data indicate that at least the majority of PM proteins found in MVBs will not recycle back to the PM, implying that recycling is more likely to take place at the TGN, through a TGN subdomain or a completely independent recycling endosome derived from it.
Figure 4.
BRI1-GFP and BOR1-GFP, but Not BP80, Are Found on the Inner Vesicles of MVBs.
Immunogold labeling of BP80, BRI1-GFP, or BOR1-GFP in Arabidopsis root tips. m, multivesicular body; t, TGN; v, vacuole; g, Golgi. Bars = 200 nm.
(A) BRI1-GFP is found on the inner vesicles of MVBs (highlighted by circles).
(B) By contrast, BP80 localizes on the boundary membrane of MVBs (arrowheads).
(C) BOR1-GFP localizes both to the TGN and to the MVB after 30 min of exposure to boron (arrowheads).
(D) BOR1-GFP localizes to the TGN (arrow) after 30 min of exposure to boron also in the presence of CHX.
(E) and (F) BOR1-GFP always localizes to the inner vesicles of MVBs, after either 30 or 70 min of exposure to boron.
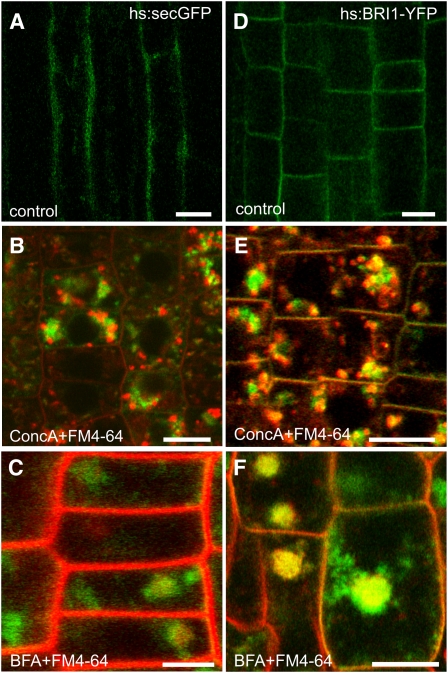
Secretory Traffic Passes through the TGN
To determine if secretory cargo passes the TGN on its way to the PM, we used two transgenic Arabidopsis lines expressing either secGFP, a secreted version of GFP (Zheng et al., 2004), or BRI1-YFP (Geldner et al., 2007), both under the control of a heat shock–inducible promoter. After heat shock, secGFP was weakly detected only in the apoplastic space (Figure 5A), whereas seedlings treated with ConcA showed a GFP signal inside the cells that partially overlapped with the endocytic tracer FM4-64 (Figure 5B). Similarly, BRI-YFP, which localized to the PM after heat shock (Figure 5D), accumulated intracellularly and was colocalized with FM4-64 after treatment with ConcA (Figure 5E). In the presence of BFA, secGFP was hard to detect but present in the core of the BFA compartment (Figure 5C), whereas inducible expression of BRI1-YFP led to much stronger labeling (Figure 5F). All inhibitor treatments were reversible within 2 h after washout and had no adverse effect on cell viability (see Supplemental Figure 5 online). Immunogold labeling on ultrathin HM20 resin sections showed that BRI1-YFP was already detectable in both the Golgi apparatus and the TGN 1 h after the induction (Figure 6A, Table 1), a time point at which, by CLSM, no BRI1-YFP signal was detectable at the PM (see Supplemental Figure 2C online). Upon Wm treatment, Golgi stacks and TGN were specifically labeled, whereas the MVB was not (Figure 6B, Table 1). Finally, in the additional presence of BFA (Figure 6C) and ConcA (Figure 6D), the Golgi stacks and the respective TGN-derived inhibitor-induced compartments were specifically labeled.
Figure 5.
The Effects of ConcA and BFA on hs:secGFP and hs:BRI1-YFP.
CLSM images of cells in the root elongation zone of hs:secGFP ([A] to [C]) or hs:BRI1-YFP ([D] to [F]) seedlings. Bars = 10 μM.
(A) and (D) Untreated cells expressing secGFP (A) or BRI1-YFP (D) 5 h after heat shock.
(B) and (E) FM4-64 was added 5 min before ConcA. Thirty minutes after addition of ConcA, the heat shock started. After 5 h of expression, a strong intracellular signal of both secGFP (B) or BRI1-YFP (E) was detectable.
(C) and (F) BFA was added 30 min before the heat shock. Five hours after the heat shock, root cells were stained with FM4-64 for 30 min. Both secGFP (C) and BRI1-YFP (F) were detected in the core of the BFA compartment.
Figure 6.
Newly Synthesized BRI1-YFP Passes through Both the Golgi and the TGN.
Immunogold labeling of hs:BRI1-YFP in Arabidopsis root tips in the presence of different combinations of inhibitors. g, Golgi; t, TGN; m, MVB; b, BFA compartment. Bars = 200 nm.
(A) After a 1-h heat shock treatment, seedlings were immediately high-pressure frozen and freeze substituted. hs:BRI1-YFP localizes both to the Golgi apparatus and the TGN (arrows).
(B) Treatment with the endocytosis inhibitor Wm started 30 min before the heat shock. hs:BRI1-YFP again localized to both the Golgi and the TGN (arrows), whereas the MVB was not labeled.
(C) Treatment with Wm and BFA started 30 min before the heat shock. hs:BRI1-YFP localized both to the Golgi apparatus and the core of the BFA compartment (arrows).
(D) Treatment with Wm and ConcA started 30 min before the heat shock. hs:BRI1-YFP localized to the TGN (arrows).
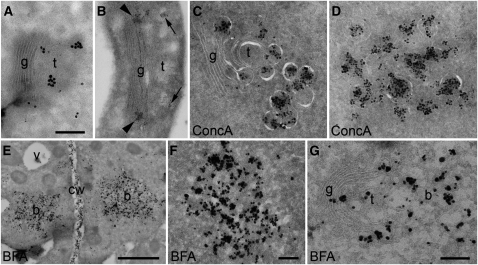
Xyloglucans are processed in the Golgi apparatus (Keegstra and Raikhel, 2001), and CCRC-M1, an antibody directed against fucose-containing xyloglucans (Puhlmann et al., 1994), labels not only the Golgi stack but also a TGN-like/post-Golgi compartment (Moore and Staehelin, 1988; Moore et al., 1991). We used this antibody to detect fucosylated xyloglucans in Arabidopsis root tips via IEM analysis. Immunogold labeling of ultrathin thawed cryosections of high-pressure frozen, freeze-substituted, and rehydrated samples showed that the TGN was labeled by CCRC-M1 (Figures 7A and 7B). Moreover, after treatment with ConcA or BFA, the ConcA-induced aggregates (Figures 7C and 7D) or the BFA compartments (Figures 7E to 7G), respectively, were intensely labeled, confirming that both inhibitors affect secretory trafficking and that the secretory cargo that passes through the TGN also accumulates in the core of BFA compartments.
Figure 7.
Xyloglucans Accumulate after ConcA Treatment and in BFA Compartments.
Immunogold labeling of a fucosylated xyloglucan epitope with CCRC-M1 antibodies. b, BFA compartment; cw, cell wall; g, Golgi stack; t, TGN. Bars = 250 nm in (A) to (D), (F), and (G) and 2 μm in (E).
(A) Labeling of the rims of Golgi stacks and TGN with silver-enhanced Nanogold-F(ab)2.
(B) Labeling of the rims of Golgi stacks (arrowheads) and TGN (arrows) with 12-nm gold particles coupled to IgG. Clustered marker molecules represent secretory vesicles.
(C) and (D) Labeling of aggregated large Golgi-derived secretory vesicles after treatment with ConcA.
(C) Vesicles close to a Golgi stack.
(E) to (G) Labeling of BFA compartments after treatment with BFA.
(E) Overview showing two vesicles aggregates.
(F) Enlarged BFA compartment.
(G) BFA compartment with attached Golgi stack.
DISCUSSION
The TGN/EE: A Golgi-Derived Independent Organelle
The TGN is considered to be part of the plant Golgi apparatus and is localized within a ribosome-excluding Golgi matrix (Moore and Staehelin, 1988). It only leaves the matrix as a consequence of maturation and sloughing off (Mollenhauer, 1971; Mollenhauer and Morre, 1991; Toyooka et al., 2009). A differentiation into early and late subcompartments of the TGN, based upon electron tomography (Staehelin and Kang, 2008), would therefore appear to support this model for Golgi function. However, live-cell imaging using spinning disc confocal microscopy has revealed novel and unexpected information about the TGN/EE, which causes us to reconsider the nature of this compartment. The TGN/EE was seen to leave Golgi stacks and become associated with others, often bypassing the most immediate Golgi. These observations are not in accordance with a subdivision of the TGN based on degrees of maturation of an indivisible Golgi-TGN/EE unit. The TGN/EE should therefore now be recognized as being a dynamic and independent compartment that only temporarily moves together with an individual Golgi stack.
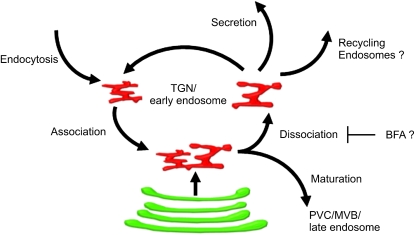
Surprisingly, we observed that distinct TGN/EE units could associate with one another (Figure 8). This homotypic interaction was preceded by the formation of a thin tube-like protrusion that connected the TGN/EEs. It would appear that the association process is reversible, since dissociation events were also detected. In in vitro assays, the yeast Tlg SNARE complex has been shown to be required for homotypic fusion (Brickner et al., 2001), and the early endosomal antigen 1 is involved in homotypic fusion of mammalian early endosomal vesicles (Mills et al., 1998). The dynamic behavior of the plant TGN/EE may also involve membrane fusion and fission, but proof of this awaits the availability of suitable protocols for purification and content mixing of TGN/EE vesicles.
Figure 8.
Model Illustrating the Multiple Functions of the TGN in Plants.
The TGN can be found either separated or closely associated to the Golgi stack. TGNs can associate homotypically, and the process is reversible. BFA causes the formation of large TGN-derived agglomerates (BFA compartments); we propose that this drug inhibits the process of dissociation between TGNs.
Although BFA-sensitive guanine-nucleotide exchange factors for the ADP-ribosylation factor GTPases (ARF-GEFs) are the known molecular targets of BFA, and BFA treatment of plants lacking the BFA-resistant Golgi-localized ARF-GEF GNOM-LIKE1 prevents COPI vesicle formation (Richter et al., 2007), it is not at all clear how the prevention of coat protein attachment by BFA eventually leads to the formation of BFA compartments. Perhaps a BFA-sensitive ARF-GEF interfering with fission would explain the huge aggregates of TGN/EEs observed in root cells after BFA treatment.
The TGN and Endocytic Recycling
Based on studies with the endocytic tracer FM4-64, two compartments have been identified in Arabidopsis and in general in plants: the tubular-vesicular TGN and the MVB (Dettmer et al., 2006; Lam et al., 2007; Otegui and Spitzer, 2008; Robinson et al., 2008). If the TGN, the main sorting hub for the secretory and vacuolar traffic is also an early endosomal compartment, PM proteins detected in this compartment could therefore be either newly synthesized or internalized. In this investigation, we have been able to differentiate between secretory and endocytic cargo in the TGN, and, as a result, have confirmed the dual function for the TGN/EE.
The PM-localized brassinosteroid receptor BRI1 and the boron exporter BOR1 are both internalized via endocytosis (Russinova et al., 2004; Takano et al., 2005), but while most of BRI1 seems to recycle back to the PM (Geldner et al., 2007), BOR1 is rapidly degraded when high amounts of boron are supplied (Takano et al., 2005). We localized both BRI1 and BOR1 to the TGN and the MVB. Combined with the kinetics of FM4-64 endocytosis (Dettmer et al., 2006), this strongly suggests that the TGN is indeed the first compartment to be reached by endocytosed PM proteins, and it is reasonable to hypothesize that recycling back to the PM (for BRI1) starts from the TGN. In Arabidopsis, a recycling endosome (RE) has been characterized by the presence of the ARF GTPase exchange factor GNOM (Geldner et al., 2003), but its identity at the EM level is lacking. However, the GNOM compartment is BFA sensitive and, like the TGN, is also found in the core of the BFA compartment. Nevertheless, differences in the BFA sensitivities of the two compartments have been reported (Geldner et al., 2009), suggesting that the TGN and RE may be separate entities. Thus, it remains unclear whether the RE is physically separate and independent of the TGN or is merely a subdomain of the TGN. The situation becomes even more complicated when one looks at the endosomal marker GTPases Rab A2 and A3. During interphase, they partially overlap with the TGN marker VHA-a1 but, unlike VHA-a1, become incorporated into the cell plate during cytokinesis (Chow et al., 2008).
In this investigation, we confirmed the presence of rapidly internalized BRI1 and BOR1 in the TGN. By inhibiting protein synthesis with cycloheximide, we distinguished between secretory and endocytic populations of these two PM proteins in the TGN. Under these conditions, positive immunogold signals were still obtained in the TGN. This demonstrates that, at any one time, at least part of the signals for BRI1 and BOR1 found in the TGN are endocytic in origin.
The TGN and Secretory Traffic to the PM
Classically, the TGN is regarded as the final sorting station for secretory cargo to the PM or the vacuole (Staehelin and Moore, 1995). Some secretory cargo molecules, including certain pectins and the cellulose synthase complex, seem to exit the Golgi stack before reaching the TGN (Moore et al., 1991; Zhang and Staehelin, 1992; Crowell et al., 2009). Surprisingly, direct evidence of secretory cargo passing through the TGN is limited, and we have therefore made use of inducible cargo molecules. Both secGFP and BRI1-YFP accumulated intracellularly after ConcA treatment and based on costaining with FM4-64 both molecules were trapped in TGN-derived membranes. In Arabidopsis root cells, BFA does not block secretion (Grebe et al., 2003; Zheng et al., 2004), but we found that, after prolonged incubation with BFA, secGFP was weakly detectable in the core of the BFA compartment, indicating that it does pass through the TGN. Accumulation of BRI1-YFP in BFA compartments could be due to both secretory and endocytic trafficking. Nevertheless, BRI1-YFP was detectable in the TGN 1 h after induction, which argues that it passes through the TGN on its way to the PM. Currently available means to interfere with endocytosis are not sufficient to fully exclude recycling. Improved tools for in vivo imaging are needed to better differentiate between endocytic and secretory cargo.
On the other hand, the presence of fucosylated xyloglucans indicates that secretory cargo is found in the TGN and the BFA compartment, since there is no evidence for endocytic recycling of xyloglucans. This is not the case with pectins, whose presence in BFA compartments has been interpreted to represent endocytic cargo (Baluska et al., 2002, 2005; Samaj et al., 2005). This conclusion is largely based on the specificity of a rhamnogalacturonan antibody used for identifying cross-linked pectins. However, based on immunoblots with purified epitopes, this antibody binds non-cross-linked pectins with the same affinity as cell wall–derived cross-linked pectins (Matoh et al., 1998). Moreover, there is no evidence that BFA treatment prevents continued synthesis of pectins in the Golgi apparatus. Thus, it cannot be claimed with certainty that the presence of pectins in the BFA compartment is due to endocytosis. We therefore conclude that although not all secretory cargo may have to pass through the TGN/EE, it should still be considered as a secretory compartment.
Do MVBs Function in Recycling?
Even though BRI1 is mostly recycled back to the PM, its estimated half-life is 5 h (Geldner et al., 2007), implying that at any time a significant proportion should be found in the pathway leading to vacuolar degradation. We therefore interpret the immunogold labeling of the inner vesicles of the MVB for both BRI1 and BOR1 as indicating that these vesicles are destined for degradation after fusion of the MVB with the vacuole. The presence of BRI1 on the inner vesicles of the MVB is also in accordance with the assumption that signal transduction can only be switched off when the cytosolic C-terminal kinase domain of BRI1 no longer faces the cytosol. Therefore, we consider it unlikely that recycling to the PM takes place from MVBs. Labeling of the inner vesicles also rules out the possibility that recycling might occur through the direct fusion of MVBs with the PM, as the PM proteins would be released into the apoplast in an exosome-like fashion (An et al., 2007).
Based on the presence of vacuolar sorting receptors (VSRs; Tse et al., 2004) and retromer, a protein complex believed to recycle VSRs (Oliviusson et al., 2006), the MVB/PVC has been regarded as a recycling compartment (Foresti and Denecke, 2008; Jaillais et al., 2008; Otegui and Spitzer, 2008). In mammalian cells, which have separate TGN and EE compartments, recycling of the lysosomal acid hydrolase receptor mannosyl 6-phosphate to the TGN occurs mainly from the EE via retromer (Bonifacino and Hurley, 2008). Although some additional recycling from late endosomal compartments cannot be excluded (Ghosh et al., 2003; Braulke and Bonifacino, 2009), the coat protein for facilitating this event is unclear since the previously favored candidate, TIP47, has now been shown to have other functions (Bulankina et al., 2009). Plant retromer interacts with VSRs (Oliviusson et al., 2006), but the recycling of VSRs from the plant MVB/PVC has recently been challenged by Niemes et al. (2010a) who have shown that both the small (sorting nexins) and large subunit of retromer locate to the TGN. Nevertheless, the presence of VSRs at the MVB/PVC raises the question of whether they are functionally relevant in this compartment.
In addressing this problem, it must first be stated that VSRs (or reporters like GFP-BP80) do not always accumulate in the MVB/PVC. Indeed, the predominant location of VSRs in Arabidopsis roots is the TGN (Reichardt et al., 2007; this article). Second, there is now evidence that VSRs may already interact with their soluble vacuolar cargo ligands in the endoplasmic reticulum (Niemes et al., 2010b). Third, Niemes et al. (2010a) have shown that the steady state distribution of VSRs in tobacco (Nicotiana tabacum) protoplasts can be shifted upstream from the MVB/PVC to the TGN without affecting vacuolar cargo transport. Together, these results cast doubt on the functional relevance of VSRs in the MVB/PVC, suggesting that their presence in this compartment is a consequence of a gradual accumulation of inactive receptors destined for degradation (Niemes et al., 2010a). However, in contrast with BRI1 and BOR1, the VSR BP80 (80 kD binding protein) is mainly localized to the boundary membrane of the MVB. In mammalian cells, ubiquitinylation plays a decisive role in targeting molecules for rapid ESCRT (endosomal sorting complex required for transport)-mediated internalization (Raiborg and Stenmark, 2009), and in Arabidopsis, the ESCRT-related CHMP1A and CHMP1B (CHARGED MULTIVESICULAR BODY PROTEIN 1A/1B) proteins mediate the sorting of auxin carriers into the internal vesicles of the MVB (Spitzer et al., 2009). We might therefore assume that for the purpose of rapid degradation, BOR1, and a portion of BRI1, become ubiquitinylated and gain access to the interior of the MVB with the help of the ESCRT complex (Hurley and Emr, 2006). However, the ubiquitinylation of the cytosolic tail of an intracellular receptor would effectively curtail its role in recycling, and this may be the reason that VSRs are not detectable at high levels inside the MVB. It is also in agreement with the fact that under conditions of high overexpression, BP80 has been detected at the tonoplast (da Silva et al., 2005; Fung et al., 2005), which would indicate that the limiting membrane of the MVB has been incorporated into the tonoplast. Despite the foregoing arguments, we cannot rule out the possibility of a nonretromer-mediated recycling of VSRs from the MVB/PVC. However, in this regard, it should be kept in mind that the cycling of mannosyl 6-phosphate receptors between the TGN and the EE in mammalian cells is reflected at the ultrastructural level: large numbers of budding profiles for CCV and other vesicles are seen at the TGN and the EE shows extensive tubulation (due to the attachment of the sorting nexins). By contrast, although the plant TGN has lots of tubules together with vesicle-budding profiles, the MVB/PVC, in both chemically and high-pressure frozen-fixed specimens reveals neither tubular extensions nor vesicle budding profiles.
As a result of the observations presented here, a number of additional questions concerning the TGN/EE as an independent compartment now arise. We do not know how long a TGN/EE as such survives, nor do we know anything about the mechanisms of TGN-Golgi stack recognition and reattachment. However, the major challenge will be to understand how the dynamic behavior of the TGN/EE, with its complex tasks, is made compatible with its role as the central hub for endocytic and secretory trafficking.
METHODS
Plant Materials and Growth Conditions
Plants expressing BRI1-GFP (Friedrichsen et al., 2000) were a gift from Joanne Chory. Plants expressing hs:BRI1-YFP or BOR1:BOR1-GFP were previously described (Geldner et al., 2007; Takano et al., 2010). All plants used were Arabidopsis thaliana ecotype Columbia-0. Three- to five-day-old BRI1-GFP, hs:secGFP, and hs:BRI1-YFP seedlings were grown on Murashige and Skoog (MS) medium + 1% sucrose. The bor1-1/BOR1:BOR1-GFP plants were grown in MGRL medium (Fujiwara et al., 1992) containing 50 μM Fe-EDTA and 0.3 μM boric acid (−B) or 100 μM boric (+B). All the seedlings were grown at 22°C, with cycles of 16 h of light and 8 h of dark for 3 to 5 d.
Construction of hs:secGFP
SecGFP as an XhoI/HindIII fragment was amplified from the secGFP construct of Batoko et al. (2000) using the following primers: (sense) 5′-CTGATCAACTCGAGGGATCCAAGGAGATATAACAATGAA-3′ and (antisense) 5′-CGTACGGTAAGCTTTTATTTGTATAGTTCATCCATGCCA-3′, and then subcloned into pGII-HS-tNos (Geldner et al., 2007).
Inhibitor Treatments and FM4-64 Staining
Seedlings were incubated in 1 mL of liquid medium (half-strength MS medium þ 0.5% sucrose, pH 5.8) containing 45 or 90 μM BFA, 0.5 μM or 2 μM ConcA, 20 μM Wm, 50 μM CHX, or combinations of these inhibitors. Endocytosis and colocalization studies were performed with 2 μM FM4-64. The seedlings were incubated with inhibitors and dye at room temperature or at 37°C for the indicated times. The following stock solutions were used: 50 mM BFA in DMSO:ethanol (1:1), 10 mM ConcA in DMSO, 20 mM Wm in DMSO, 50 mM CHX in DMSO, and 4 mM FM4-64 in DMSO.
Protein Extraction and Immunoblot Analysis
For total protein extracts, 4-d-old etiolated seedlings were ground on ice and resuspended in extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Triton X-100, and 13 Complete protease-inhibitor mix [Roche]). Protein gel blots and immunodetection were performed as previously described (Pimpl et al., 2006), using affinity-purified rabbit YFP antibodies (0.62 μg/mL).
Affinity-Purified YFP Antibodies
YFP was produced in Escherichia coli BL21(DE3) as a fusion protein with an intein-chitin binding domain (CBD) tag in plasmid vector pTYB11 as described by Visser et al. (2002). The fusion protein was bound to a column of chitin beads, and the tag was removed on-column by intein-mediated splicing induced with 50 mM DTT. Anti-YFP antibodies were raised in rabbits by four subcutaneous injections, each with 100 μg purified YFP (Eurogentec). Anti-YFP IgG was purified from the rabbit serum on a 7-mL column of chitin beads to which 15 mg of the CBD-intein-YFP fusion protein was bound. Briefly, 15 mL of serum was cleared by centrifugation and loaded onto the column in 20 mM NaPi and 100 mM NaCl, pH 7.0. After extensive washing with the same buffer to remove noninteracting proteins, anti-YFP IgG was eluted from the column by decreasing the pH from 3.2 to 3.5 with a steep, 2-mL linear 0 to 20% gradient of 0.1 M Na-citrate, pH 2.7, in 100 to 80% 20 mM NaPi and 100 mM NaCl, pH 7.0, followed by elution with three volumes of 20% 0.1 M Na-citrate, pH 2.7, in 80% 20 mM NaPi and 100 mM NaCl, pH 7.0. One-milliliter fractions of IgG eluting from the column (monitored by absorbance at 280 nm) were collected in tubes containing 0.1 mL 1 M unbuffered KPiPi, pH ∼10, to bring the pH after elution immediately to pH 6.0 to 7.
Transmission Electron Microscopy
For Figures 2E to 2H, 3, 4, and 6, 3- to 5-d-old root tips from Arabidopsis were high-pressure frozen as described by Bubeck et al. (2008). Freeze substitution was performed with a Leica EM AFS2 freeze substitution unit in dry acetone supplemented with 0.2 to 0.4% uranyl acetate at −85°C for 16 h before warming up to −50°C over a 5-h period. Roots were infiltrated and embedded in Lowicryl HM20 (Polysciences) at −50°C and UV polymerized for 3 d. Ultrathin sections were incubated with antibodies against GFP and BP80 at a dilution of 1:1400 and 1:50, respectively. Gold-conjugated secondary antibodies (BioCell GAR10) were used at a dilution of 1:50 in PBS supplemented with 1% (w/v) BSA. Sections were poststained with aqueous uranyl acetate/lead citrate and examined in a JEM 1400 transmission electron microscope (JEOL) operating at 80 kV.
For Figures 2A to 2D and 7, 3- to 5-d-old seedlings (controls or treated with ConcA for 45 min or 45 μM BFA for 75 min) were high-pressure frozen as described by Ripper et al. (2008). Frozen samples were freeze-substituted in acetone containing 0.075% OsO4, 0.5% glutaraldehyde, and 0.25% uranyl acetate and 2% water (−90°C, 72 h; −60°C, 8 h; −30°C, 8 h; Ripper et al., 2008). Samples were washed and rehydrated with acetone and water containing 0.5% and 0.25% gibberellin, respectively. After additional postfixation in water with 0.35% gibberellin at 0°C for 45 min, samples were washed with water and infiltrated in a mixture of polyvinylpyrrolidone and sucrose (1.8 M sucrose/20% polyvinylpyrrolidone; Tokuyasu, 1989) and mounted in a cryo-ultramicrotome (Leica) for cryosectioning at −115°C. Ultrathin thawed cryosections were labeled with mAb CCRC-M1 (1:5; Zhang and Staehelin, 1992; Carbosource Services, University of Georgia), and goat anti-mouse F(ab′)2 coupled to Nanogold (1:70; Nanoprobes) or goat anti-mouse IgG coupled to 12-nm gold (1:30; Dianova). Nanogold was silver-enhanced with HQSilver (8.5 min, 22°C; Nanoprobes). Labeled and silver-enhanced sections were embedded in methyl cellulose containing 0.3 to 0.45% uranyl acetate.
Quantitative Analysis of IEM
The quantitative analysis was conducted on ultrathin sections previously immunolabeled at the most convenient dilution of the respective antibodies. The sections were analyzed and every endosomal compartment encountered during the screening of cells that did not present folding, scratches, or any source of unspecific labeling was taken into consideration. The area of the single compartments was calculated using the image processing program ImageJ (http://rsbweb.nih.gov/ij/). Standard errors and standard deviations were calculated using Excel (Microsoft).
CLSM
Seedlings were mounted in half-strength MS liquid medium and were observed under a Zeiss Axiovert LSM510 Meta CLSM using a C-Apochromat ×63/1.2 W corr water immersion objective. At the Metadetector, the main beam splitters (HFT) 514 and 488 were used. The following fluorophores (excited and emitted by frame switching in the multitracking mode) were used: GFP (488 nm [40% laser power]/496 to 518 nm), YFP (514 nm [40% laser power]/518 to 539 nm], FM4-64 (488 nm [10% laser power]/625 to 689 nm together with GFP or 514 nm [20% laser power]/636 to 689 nm together with YFP). Pinholes were adjusted to 1 airy unit for each wavelength. Postacquisition image processing was performed using the Zeiss LSM 5 image browser and CorelDrawX4.
Spinning Disc Confocal Microscopy
Seeds were surface sterilized and stratified at 4°C for 2 d before plating on half-strength MS and 0.7% agar at pH 5.8. After 2 h of light exposure, the plates were wrapped in aluminum foil and the seeds were grown for 3 to 4 d at 22°C in the vertical position. Seedlings were mounted in half-strength MS liquid medium. Imaging was performed on a Nikon Ti inverted confocal microscope featuring a Perkin-Elmer Ultra-View spinning disc confocal using a ×60 water immersion objective. YFP was excited at 488 nm, and RFP was excited at 568 nm. Excitation switching and shuttering were performed using the Ultra VIEW discrimination mode, and emission filtering was accomplished using the UltraVIEW emission wheel 527 (W55) for YFP and 455 (W80), 615 (W70) for RFP. Images were acquired with a Hamamatsu EM-CCD high-sensitive (black and white) camera, driven by Volocity software (Improvision, Volocity Version 5.1.0). Typical exposures were 102 or 152 ms for ST-YFP and 717 or 849 ms for VHA-a1-RFP.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: VHA-a1 (At2g28520), BRI1 (At4g38400), BOR1 (At2g47160), SYP61 (At1g28490), and BP-80 (At3g52850).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The BRI1-GFP Signal Is Detectable through the Entire Thickness of the Lowicryl HM20 Resin Block.
Supplemental Figure 2. Efficiency of Cycloheximide Treatment and Time Course of hs:BRI1-YFP Induction.
Supplemental Figure 3. Immunogold Labeling of Endogenous BP80 in Root Tips from Wild-Type Arabidopsis Plants.
Supplemental Figure 4. Upon High Boron Conditions, BOR1-GFP Is Endocytosed and Undergoes Rapid Degradation.
Supplemental Figure 5. Effects of 5 h ConcA and BFA Treatment Are Reversible.
Supplemental Table 1. Further Details on Quantitative Analysis of Immunogold Electron Microscopy.
Supplemental Movie 1. Spinning Disc Confocal Microscopy Showing an Association Event among TGNs.
Supplemental Movie 2. Spinning Disc Confocal Microscopy Showing a Dissociation Event among TGNs.
Supplemental References.
Supplemental Movie Legends.
Supplementary Material
Acknowledgments
We thank Natasha Raikhel for providing the SYP61-CFP line. We acknowledge Stefan Hillmer for expert advice with IEM, Silke Niemes for help with CLSM, and Stephanie Gold, Dagmar Ripper, and Barbara Maier for technical assistance. We thank Ulrike Engel and Christian Ackermann from the Nikon Imaging Center at BioQuant, University of Heidelberg. This work was supported by the Deutsche Forschungsgemeinshaft through SFB 446 (K.S. and Y.-D.S.), RO440/14-1 (D.G.R.), and the ERA-PG project PRECIAR (C.V. and W.v.D.).
References
- An Q., van Bel A.J., Huckelhoven R. (2007). Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal. Behav. 2: 4–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F., Hlavacka A., Samaj J., Palme K., Robinson D.G., Matoh T., McCurdy D.W., Menzel D., Volkmann D. (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130: 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F., Liners F., Hlavacka A., Schlicht M., Van Cutsem P., McCurdy D.W., Menzel D. (2005). Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225: 141–155 [DOI] [PubMed] [Google Scholar]
- Batoko H., Zheng H.Q., Hawes C., Moore I. (2000). A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Hurley J.H. (2008). Retromer. Curr. Opin. Cell Biol. 20: 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke T., Bonifacino J.S. (2009). Sorting of lysosomal proteins. Biochim. Biophys. Acta 1793: 605–614 [DOI] [PubMed] [Google Scholar]
- Brickner J.H., Blanchette J.M., Sipos G., Fuller R.S. (2001). The Tlg SNARE complex is required for TGN homotypic fusion. J. Cell Biol. 155: 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brux A., Liu T.Y., Krebs M., Stierhof Y.D., Lohmann J.U., Miersch O., Wasternack C., Schumacher K. (2008). Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell 20: 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck J., Scheuring D., Hummel E., Langhans M., Viotti C., Foresti O., Denecke J., Banfield D.K., Robinson D.G. (2008). The syntaxins SYP31 and SYP81 control ER-Golgi trafficking in the plant secretory pathway. Traffic 9: 1629–1652 [DOI] [PubMed] [Google Scholar]
- Bulankina A.V., Deggerich A., Wenzel D., Mutenda K., Wittmann J.G., Rudolph M.G., Burger K.N.J., Höning S. (2009). TIP47 functions in the biogenesis of lipid droplets. J. Cell Biol. 185: 641–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.M., Neto H., Foucart C., Moore I. (2008). Rab-A2 and Rab-A3 GTPases define a trans-golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 20: 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell E.F., Bischoff V., Desprez T., Rolland A., Stierhof Y.D., Schumacher K., Gonneau M., Hofte H., Vernhettes S. (2009). Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21: 1141–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva L.L., Taylor J.P., Hadlington J.L., Hanton S.L., Snowden C.J., Fox S.J., Foresti O., Brandizzi F., Denecke J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17: 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Schubert D., Calvo-Weimar O., Stierhof Y.D., Schmidt R., Schumacher K. (2005). Essential role of the V-ATPase in male gametophyte development. Plant J. 41: 117–124 [DOI] [PubMed] [Google Scholar]
- Emans N., Zimmermann S., Fischer R. (2002). Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O., Denecke J. (2008). Intermediate organelles of the plant secretory pathway: Identity and function. Traffic 9: 1599–1612 [DOI] [PubMed] [Google Scholar]
- Friedrichsen D.M., Joazeiro C.A., Li J., Hunter T., Chory J. (2000). Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 123: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Hirai M.Y., Chino M., Komeda Y., Naito S. (1992). Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 99: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung K.L., Yim Y.F., Tse Y.C., Miao Y., Sun S.S.M., Jiang L. (2005). Targeting and processing of membrane-anchored YFP fusion proteins to protein storage vacuoles in transgenic tobacco seeds. Seed Sci. Res. 15: 361–364 [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jurgens G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N., Denervaud-Tendon V., Hyman D.L., Mayer U., Stierhof Y.D., Chory J. (2009). Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Hyman D.L., Wang X., Schumacher K., Chory J. (2007). Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 21: 1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Jurgens G. (2006). Endocytosis in signalling and development. Curr. Opin. Plant Biol. 9: 589–594 [DOI] [PubMed] [Google Scholar]
- Ghosh P., Dahms N.M., Kornfeld S. (2003). Mannose 6-phosphate receptors: New twists in the tale. Nat. Rev. Mol. Cell Biol. 4: 202–212 [DOI] [PubMed] [Google Scholar]
- Grebe M., Xu J., Mobius W., Ueda T., Nakano A., Geuze H.J., Rook M.B., Scheres B. (2003). Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13: 1378–1387 [DOI] [PubMed] [Google Scholar]
- Hillmer S., Freundt H., Robinson D.G. (1988). The partially coated reticulum and its relationship to the Golgi-apparatus in higher-plant cells. Eur. J. Cell Biol. 47: 206–212 [Google Scholar]
- Hurley J.H., Emr S.D. (2006). The ESCRT complexes: Structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 35: 277–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miege C., Gaude T. (2008). Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 53: 237–247 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miege C., Rollin C., Gaude T. (2006). AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443: 106–109 [DOI] [PubMed] [Google Scholar]
- Keegstra K., Raikhel N. (2001). Plant glycosyltransferases. Curr. Opin. Plant Biol. 4: 219–224 [DOI] [PubMed] [Google Scholar]
- Lam S.K., Siu C.L., Hillmer S., Jang S., An G., Robinson D.G., Jiang L. (2007). Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19: 296–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chory J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Matoh T., Takasaki M., Takabe K., Kobayashi M. (1998). Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol. 39: 483–491 [Google Scholar]
- Mills I.G., Jones A.T., Clague M.J. (1998). Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr. Biol. 8: 881–884 [DOI] [PubMed] [Google Scholar]
- Mollenhauer H.H. (1971). Fragmentation of mature dictyosome cisternae. J. Cell Biol. 49: 212–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer H.H., Morre D.J. (1991). Perspectives on Golgi apparatus form and function. J. Electron Microsc. Tech. 17: 2–14 [DOI] [PubMed] [Google Scholar]
- Moore P.J., Staehelin L.A. (1988). Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis in Trifolium pratense L.; implication for secretory pathways. Planta 174: 433–445 [DOI] [PubMed] [Google Scholar]
- Moore P.J., Swords K.M., Lynch M.A., Staehelin L.A. (1991). Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J. Cell Biol. 112: 589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemes S., Labs M., Scheuring D., Krueger F., Langhans M., Jesenofsky B., Robinson D.G., Pimpl P. (2010b). Sorting of plant vacuolar proteins is initiated in the ER. Plant J., in press [DOI] [PubMed] [Google Scholar]
- Niemes S., Langhans M., Viotti C., Scheuring D., San Wan Yan M., Jiang L., Hillmer S., Robinson D.G., Pimpl P. (2010a). Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 61: 107–121 [DOI] [PubMed] [Google Scholar]
- Oliviusson P., Heinzerling O., Hillmer S., Hinz G., Tse Y.C., Jiang L., Robinson D.G. (2006). Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui M.S., Spitzer C. (2008). Endosomal functions in plants. Traffic 9: 1589–1598 [DOI] [PubMed] [Google Scholar]
- Pimpl P., Taylor J.P., Snowden C., Hillmer S., Robinson D.G., Denecke J. (2006). Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell 18: 198–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhlmann J., Bucheli E., Swain M.J., Dunning N., Albersheim P., Darvill A.G., Hahn M.G. (1994). Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal alpha-(1→2)-linked fucosyl-containing epitope. Plant Physiol. 104: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Stenmark H. (2009). The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458: 445–452 [DOI] [PubMed] [Google Scholar]
- Reichardt I., Stierhof Y.D., Mayer U., Richter S., Schwarz H., Schumacher K., Jurgens G. (2007). Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr. Biol. 17: 2047–2053 [DOI] [PubMed] [Google Scholar]
- Richter S., Geldner N., Schrader J., Wolters H., Stierhof Y.D., Rios G., Koncz C., Robinson D.G., Jurgens G. (2007). Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature 448: 488–492 [DOI] [PubMed] [Google Scholar]
- Ripper D., Schwarz H., Stierhof Y.D. (2008). Cryo-section immunolabelling of difficult to preserve specimens: Advantages of cryofixation, freeze-substitution and rehydration. Biol. Cell 100: 109–123 [DOI] [PubMed] [Google Scholar]
- Robert S., Chary S.N., Drakakaki G., Li S., Yang Z., Raikhel N.V., Hicks G.R. (2008). Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc. Natl. Acad. Sci. USA 105: 8464–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Jiang L., Schumacher K. (2008). The endosomal system of plants: Charting new and familiar territories. Plant Physiol. 147: 1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russinova E., Borst J.W., Kwaaitaal M., Cano-Delgado A., Yin Y., Chory J., de Vries S.C. (2004). Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16: 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaj J., Read N.D., Volkmann D., Menzel D., Baluska F. (2005). The endocytic network in plants. Trends Cell Biol. 15: 425–433 [DOI] [PubMed] [Google Scholar]
- Spitzer C., Reyes F.C., Buono R., Sliwinski M.K., Haas T.J., Otegui M.S. (2009). The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell 21: 749–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L.A., Kang B.H. (2008). Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 147: 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L.A., Moore I. (1995). The plant Golgi apparatus. Annu. Rev. Plant Biol. 46: 261–288 [Google Scholar]
- Takano J., Miwa K., Yuan L., von Wiren N., Fujiwara T. (2005). Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA 102: 12276–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J., Noguchi K., Yasumori M., Kobayashi M., Gajdos Z., Miwa K., Hayashi H., Yoneyama T., Fujiwara T. (2002). Arabidopsis boron transporter for xylem loading. Nature 420: 337–340 [DOI] [PubMed] [Google Scholar]
- Takano J., Tanaka M., Toyoda A., Miwa K., Kasai K., Fuji K., Onouchi H., Naito S., Fujiwara T. (2010). Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K.T. (1989). Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem. J. 21: 163–171 [DOI] [PubMed] [Google Scholar]
- Toyooka K., Goto Y., Asatsuma S., Koizumi M., Mitsui T., Matsuoka K. (2009). A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 21: 1212–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse Y.C., Mo B., Hillmer S., Zhao M., Lo S.W., Robinson D.G., Jiang L. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser N.V., Hink M.A., Borst J.W., van der Krogt G.N., Visser A.J. (2002). Circular dichroism spectroscopy of fluorescent proteins. FEBS Lett. 521: 31–35 [DOI] [PubMed] [Google Scholar]
- Wang J., Cai Y., Miao Y., Lam S.K., Jiang L. (2009). Wortmannin induces homotypic fusion of plant prevacuolar compartments. J. Exp. Bot. 60: 3075–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.F., Staehelin L.A. (1992). Functional compartmentation of the Golgi apparatus of plant cells: Immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 99: 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Kunst L., Hawes C., Moore I. (2004). A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J. 37: 398–414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.