Short-read sequencing technologies support the facile de novo identification of spontaneous and chemically induced mutations in the Arabidopsis (Arabidopsis thaliana) reference genome (Schneeberger et al., 2009b; Ossowski et al., 2010). Here, we show that short-read sequencing is also suitable for the analysis of new mutations in a nonreference inbred accession that differs from the reference genome in about 0.5% of all positions.
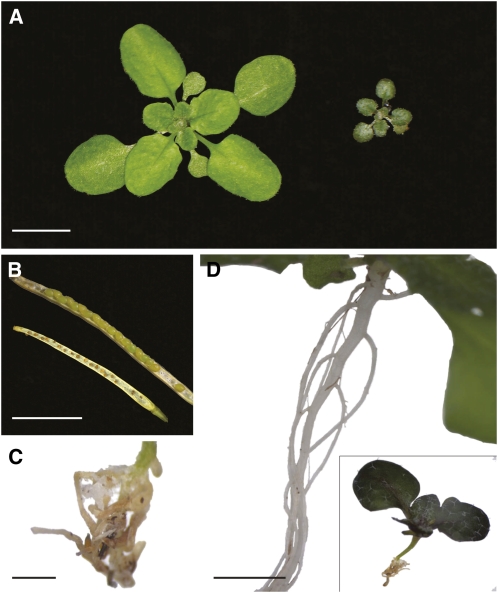
We crossed two normal-appearing, green individuals of Arabidopsis accessions Krotzenburg (Kro-0; CS1301) and Anholt (Anh-1; CS22313) to each other. The F1 plants were all normal, but the F2 population segregated purplish, small, and nonflowering plants (Fig. 1A). Plants could be prompted to flower in high humidity, but the resulting seeds were not viable (Fig. 1B). Leaves were about 10 times smaller than in the wild type, but leaf cell number was reduced only about 3-fold, indicating that both decreased cell expansion and division contributed to the dwarf phenotype. Consistent with the purplish phenotype, several genes involved in biosynthesis of the purple pigment anthocyanin were up-regulated in the dwarf plants. Using a combination of per-gene variance (rank product P value of 0.01; Breitling et al., 2004) and common variance (2-fold change) as criterion, PRODUCTION OF ANTHOCYANIN PIGMENT2 (At1g66390), CHALCONE ISOMERASE (At3g55120), FLAVONOL SYNTHASE1 (At5g08640), and DIHYDROFLAVONOL 4-REDUCTASE (At5g42800) were all differentially expressed. None of these phenotypes, including a disorganized root (Fig. 1, C and D), could be suppressed by treating the plants with cytokinin, gibberellic acid, jasmonic acid, or auxin.
Figure 1.
A, A quarter of F2 plants from a single Anh-1 × Kro-0 cross were purplish dwarfs (right), compared with larger, green siblings. B, Rescue of the flowering defect by spraying plants with water every other day allowed fertilization but did not support normal seed development (bottom, compared with normal silique above). C, Closeup of an abnormal root of a soil-grown plant, with ectopic outgrowths. D, Comparison of an abnormally small root system (inset) of dwarf plants and normal siblings, shown at the same scale. Bars = 1 cm in A, B, and D and 0.1 cm in C.
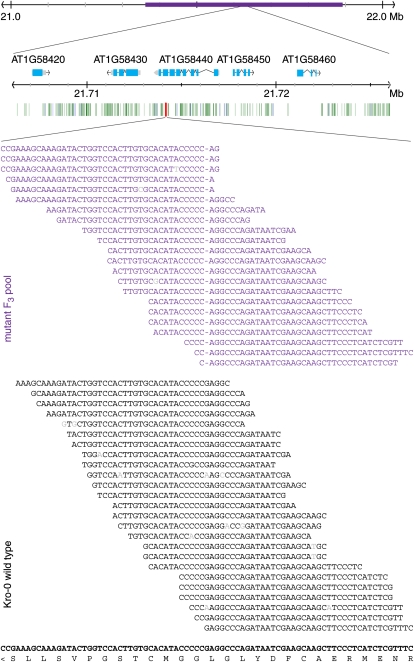
Using conventional mapping with almost 1,900 F2 plants of the Kro-0 × Anh-1 cross, we identified a 530-kb interval, between 21.36 and 21.88 Mb on chromosome 1, that was linked to the dwarf phenotype (Fig. 2). The mapping interval contained 116.5 kb of repetitive DNA, which is often polymorphic and may suppress recombination (Fu et al., 2002), possibly explaining the failure to further reduce the final mapping interval.
Figure 2.
Mapping interval (purple) on chromosome 1, and polymorphisms in the vicinity of the causal mutation (red). Green and blue lines indicate single nucleotide changes and deletions, respectively, shared with the parental Kro-0 strain. The middle section shows alignments of Illumina DNA sequence reads against the reference genome sequence, positions 21,714,424 to 21,714,504 (TAIR9). The amino acid sequence encoded by the reverse strand is given at bottom.
Based on the sequences of the flanking markers, we concluded that plants showed the dwarf phenotype if they had inherited both alleles from the Kro-0 grandparent used in the cross to Anh-1. Since the original Kro-0 line did not exhibit the dwarf phenotype and other Kro-0 × Anh-1 crosses did not produce abnormal F2 progeny, we concluded that a spontaneous mutation had occurred in the germline of the particular Kro-0 individual used for the original cross to Anh-1. The F1 plant would have been heterozygous for this mutation. Therefore, we decided to directly compare the mutant genome in this interval with that of the Kro-0 parental genome. Because the size of the final mapping interval made analysis by PCR-based sequencing impractical, we sequenced the entire Kro-0 parental genome at 25-fold coverage, with 36- to 42-bp paired-end reads generated on Illumina's Genome Analyzer. In parallel, we produced 25-fold coverage of the haploid genome from F3 dwarf plants. We pooled genomic DNA from 100 plants to obtain sufficient material for sequencing. Single nucleotide polymorphisms and insertions/deletions were called for both the parent and mutant pool, by independently comparing them with the ecotype Columbia (Col-0) reference genome using SHORE and GenomeMapper (Ossowski et al., 2008; Schneeberger et al., 2009a). For background cleaning, we made use of all variants detected in the Kro-0 parent. To predict mutations specific to the dwarf sample, only those with a SHORE quality value of at least 25 were considered.
Within the 530-kb mapping interval, we identified 5,691 single nucleotide differences in the dwarf pool relative to the Col-0 reference sequence. Of these, 4,023 were predicted with high confidence. This level of polymorphism is similar to that found in other accessions in this region, with 4,036 and 3,511 found in the genomes of Burren (Bur-0; CS22679) and Tsu (Tsu-1; CS22693), respectively (Ossowski et al., 2008). Of the 4,023 high-quality polymorphisms, 531 were predicted to change the coding potential of 63 genes. All but one were shared with the normal Kro-0 parent. The one remaining mutation in the dwarf pool, a 1-bp deletion, resided in the seventh exon of the gene At1g58440, located in the middle of the mapping interval at 21.718 Mb. The deletion disrupted the At1g58440 open reading frame (Fig. 2). Dideoxy sequencing confirmed that the mutation was specific to F3 individuals with the dwarf phenotype. A Col-0 line with a T-DNA insertion in At1g58440 (N522763) showed the same purplish, dwarf, and abnormal root phenotype as these plants. At1g58440 encodes SQUALENE EPOXIDASE1 (SQE1), which catalyzes a key step in sterol metabolism, and the morphological phenotypes of sqe1 mutants are very similar to the ones seen in our dwarfs, including partial rescue by growing plants in 90% humidity (Rasbery et al., 2007; Posé et al., 2009; D. Posé, personal communication).
Our study provides a proof of concept for identifying mutations in a background other than a high-quality reference genome using direct whole genome sequencing. We have recently shown that de novo mutation identification with short-read sequencing in a reference background provides not only very high specificity (i.e. very few false positives) but also very high sensitivity (i.e. very few false negatives; Ossowski et al., 2010). This is in contrast to similar efforts with human genomes (Lupski et al., 2010), reflecting both the more complex nature of human genomes and also the absence of a near-isogenic reference. Moreover, different from other studies aimed at identifying causal mutations for human diseases (Lupski et al., 2010), we took an unbiased approach in this work and did not use any prior information on candidate genes associated with the phenotype in question. In summary, our work indicates that short-read sequencing is a useful and sensitive tool that can be applied to mutation identification, as long as a high-quality reference sequence from close relatives is available. This prospect should be good news for anybody interested in performing mutant screens in nonmodel organisms.
Acknowledgments
We thank Jun Cao and Christa Lanz for help with Illumina sequencing; Kirsten Bomblies for the F1 seeds; Kirsten Bomblies, David Posé, Ignacio Rubio-Somoza, and Marco Todesco for sharing ideas and discussions; and Waldemar Hauf for technical help.
References
- Breitling R, Armengaud P, Amtmann A, Herzyk P. (2004) Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573: 83–92 [DOI] [PubMed] [Google Scholar]
- Fu H, Zheng Z, Dooner HK. (2002) Recombination rates between adjacent genic and retrotransposon regions in maize vary by 2 orders of magnitude. Proc Natl Acad Sci USA 99: 1082–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Reid JG, Gonzaga-Jauregui C, Rio Deiros D, Chen DC, Nazareth L, Bainbridge M, Dinh H, Jing C, Wheeler DA, et al. (2010) Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N Engl J Med 362: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski S, Schneeberger K, Clark RM, Lanz C, Warthmann N, Weigel D. (2008) Sequencing of natural strains of Arabidopsis thaliana with short reads. Genome Res 18: 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski S, Schneeberger K, Lucas-Lledo JI, Warthmann N, Clark RM, Shaw RG, Weigel D, Lynch M. (2010) The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 327: 92–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posé D, Castanedo I, Borsani O, Nieto B, Rosado A, Taconnat L, Ferrer A, Dolan L, Valpuesta V, Botella MA. (2009) Identification of the Arabidopsis dry2/sqe1-5 mutant reveals a central role for sterols in drought tolerance and regulation of reactive oxygen species. Plant J 59: 63–76 [DOI] [PubMed] [Google Scholar]
- Rasbery JM, Shan H, LeClair RJ, Norman M, Matsuda SP, Bartel B. (2007) Arabidopsis thaliana squalene epoxidase 1 is essential for root and seed development. J Biol Chem 282: 17002–17013 [DOI] [PubMed] [Google Scholar]
- Schneeberger K, Hagmann J, Ossowski S, Warthmann N, Gesing S, Kohlbacher O, Weigel D. (2009a) Simultaneous alignment of short reads against multiple genomes. Genome Biol 10: R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Ossowski S, Lanz C, Juul T, Petersen AH, Nielsen KL, Jørgensen JE, Weigel D, Andersen SU. (2009b) SHOREmap: simultaneous mapping and mutation identification by deep sequencing. Nat Methods 6: 550–551 [DOI] [PubMed] [Google Scholar]