Abstract
Grass plants develop distinct inflorescences and spikelets that determine grain yields. However, the mechanisms underlying the specification of inflorescences and spikelets in grasses remain largely unknown. Here, we report the biological role of one SEPALLATA (SEP)-like gene, OsMADS34, in controlling the development of inflorescences and spikelets in rice (Oryza sativa). OsMADS34 encodes a MADS box protein containing a short carboxyl terminus without transcriptional activation activity in yeast cells. We demonstrate the ubiquitous expression of OsMADS34 in roots, leaves, and primordia of inflorescence and spikelet organs. Compared with the wild type, osmads34 mutants developed altered inflorescence morphology, with an increased number of primary branches and a decreased number of secondary branches. In addition, osmads34 mutants displayed a decreased spikelet number and altered spikelet morphology, with lemma/leaf-like elongated sterile lemmas. Moreover, analysis of the double mutant osmads34 osmads1 suggests that OsMADS34 specifies the identities of floral organs, including the lemma/palea, lodicules, stamens, and carpel, in combination with another rice SEP-like gene, OsMADS1. Collectively, our study suggests that the origin and diversification of OsMADS34 and OsMADS1 contribute to the origin of distinct grass inflorescences and spikelets.
Morphological innovations are critical for the diversification of animals and plants to adapt to new environments (Linder and Rudall, 2005). Poaceae (grasses) is one of the largest flowering plant families in angiosperms, including many economically important crops such as rice (Oryza sativa), barley (Hordeum vulgare), and maize (Zea mays). Evolutionary changes in the organization and structure of grass inflorescence have resulted in their different morphologies from those of core eudicots and nongrass monocots (Grass Phylogeny Working Group, 2001; Zanis, 2007). The basic inflorescence unit of grasses is the spikelet, which is a short branch with leaf-like organs called glumes enclosing one or more florets. However, the genetic mechanism underlying the specification of grass inflorescence and spikelet morphology is still a challenging question for biologists (Zanis, 2007).
Rice develops a central inflorescence stem that terminates after the formation of several primary and secondary branches. Spikelets are directly produced on primary and secondary branches that are attached on the main axis called the rachis (Itoh et al., 2005). Each rice spikelet consists of a flower with one pistil, six stamens, and two lodicules subtended by an inner bract or prophyll, called the palea, and an outer bract called the lemma. In addition, each spikelet contains two highly reduced leaf-like rudimentary glumes at the base in a distichous pattern and two depressed sterile lemmas at a position opposite to each other above the rudimentary glumes (Itoh et al., 2005). Compared with the lemma and palea, rice sterile lemmas are smaller in size and exhibit characteristic cellular patterns (Li et al., 2009). There has been a hypothesis that sterile lemmas are vestiges derived from lemmas of two sterile florets that were reduced during evolution, but the genetic control of sterile lemma development is largely unknown (Arber, 1934; Takeoka et al., 1993). More recently, Yoshida et al., (2009) and Hong et al. (2010) identified a long sterile lemma1 (g1) mutant (also called elongated empty glume [ele]) with lemma-like sterile lemma structures. G1/ELE is a member belonging to a plant-specific gene family encoding proteins with an unknown function domain, ALOG (for Arabidopsis [Arabidopsis thaliana] LIGHT-DEPENDENT SHORT HYPOCOTYLS1 and Oryza G1). The G1/ELE expression is detectable in sterile lemma primordia throughout their development, suggesting that G1/ELE is key regulator for repressing lemma identity at the sterile lemma positions during rice spikelet development (Yoshida et al., 2009; Hong et al., 2010).
Morphological evolution of plants is likely associated with changes in the number, expression pattern, and interaction of developmental regulatory genes. Angiosperms have more than 250,000 species with flowers varying in the number, organization, and patterning of floral organs (Theissen and Melzer, 2007). Molecular and genetic studies on the model eudicot plants Arabidopsis and Antirrhinum majus have led to the proposal of the classic genetic ABC and revised ABCE models for determining floral organ identity (Coen and Meyerowitz, 1991; Pelaz et al., 2000; Theissen, 2001). Most ABCE genes in Arabidopsis encode MADS box transcription factors (Becker and Theissen, 2003). SEPALLATA (SEP) genes have been shown to act as integrating coregulators with other floral identity genes in floral organ specification (Liu et al., 2009). Extensive studies in eudicot Arabidopsis and petunia (Petunia hybrida) have demonstrated that the SEP genes may redundantly function in specifying the identity of each floral whorl and meristem determinacy (Pelaz et al., 2000; Vandenbussche et al., 2003; Ditta et al., 2004).
Grasses have diverse SEP-like genes, at least five members (OsMADS1, OsMADS5, OsMADS7, OsMADS8, and OsMADS34) in rice and eight in maize (Münster et al., 2002). OsMADS1 (also called LEAFY HULL STERILE1 [LHS1]) is one of the best characterized SEP-like genes in rice, which contributes to determining lemma and palea identity as well as meristem determinacy of inner floral organs (Jeon et al., 2000; Prasad et al., 2001, 2005; Agrawal et al., 2005; Chen et al., 2006b). In grasses, homologs of OsMADS1/LHS1 display distinct expression patterns among different species (Malcomber and Kellogg, 2004), implying that changes in OsMADS1/LHS1 expression patterns may have contributed to the morphological diversification of grass inflorescence architecture (Malcomber and Kellogg, 2004). Transgenic plants with reduced expression of OsMADS7 and OsMADS8 show defects of late flowering, homeotic changes of lodicules, stamens, and carpels into palea/lemma-like organs, and a loss of floral determinacy (Cui et al., 2010). Knockdown of OsMADS1, OsMADS5, OsMADS7, and OsMADS8 causes homeotic transformation of all floral organs except the lemma into leaf-like organs (Cui et al., 2010).
In this study, we showed that the expression of one SEP-like gene, OsMADS34, is detectable in all tested vegetative and reproductive tissues, including inflorescence and spikelet meristems. osmads34 mutants display changed inflorescence morphology and elongated sterile lemmas with lemma/leaf-like cellular patterns. These results suggest that OsMADS34 is involved in controlling rice inflorescence and spikelet morphology by determining the numbers of branches and spikelets as well as the sterile lemma specification. Moreover, analysis of osmads34 osmads1 suggests that OsMADS34 is able to specify rice floral organ identity in combination with OsMADS1.
RESULTS
OsMADS34 Controls Inflorescence Architecture
In order to identify new rice genes controlling rice spikelet/flower development, we generated a rice mutant library using the japonica subspecies 9522 background by treatment with 60Co γ-ray (280 Gy) and screened mutants with defects in spikelet/flower. Two mutant lines displaying obviously altered inflorescence and spikelet morphology were obtained from our rice mutant library (Chen et al., 2006a; Fig. 1). We named them osmads34-1 and osmads34-2 because map-based gene cloning and allelic analyses of the two mutants confirmed that their defects are caused by mutations in OsMADS34 (see below).
Figure 1.
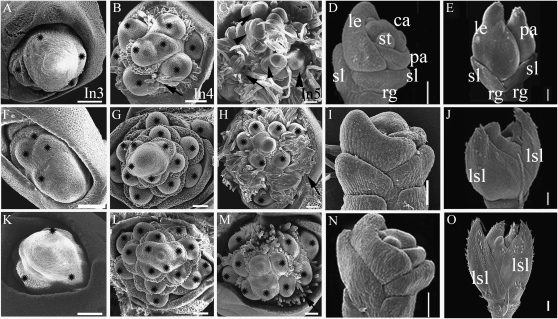
Phenotypes of osmads34, osmads1-z, and osmads1-z osmads34-1 mutants. A to C, Morphologies of the panicle at stage In9 of the wild type (A), osmads34-1 (B), and osmads34-2 (C). D to M, The spikelet of the wild type (D and I), osmads34-1 (E and J), osmads34-2 (F and K), osmads1-z (G and L), and osmads34-1 osmads1-z (H and M) at stage In9. Red and green arrows in D to F, L, and M indicate the place for sections in Figure 4. i1, The epidermis of wild-type lemma with regular bulges. i2, The smooth surface of wild-type sterile lemma. j and k, Epidermal cells of the lemma/leaf-like sterile lemma with smaller bulges (arrows) of osmads34-1 (j) and osmads34-2 (k); the outer surface of the osmads34-2 lemma/leaf-like sterile lemma has smaller bulges (white arrows) and stomata (black arrowhead). l, The abaxial surface of osmads1-z leafy lemma/palea with small bulges (white arrow). m1, The abaxial surface of osmads34-1 osmads1double mutant leafy lemma/palea with smaller bulges (white arrow) and stomata (black arrowhead). m2, The abaxial surface of wild-type leaf with smaller bulges (white arrow) and stomata (black arrowhead). le, Lemma; lo, lodicules; lsl, lemma/leaf-like sterile lemma; pa, palea; pb, primary branch; pi, pistil; ra, rachis; rg, rudimentary glume; sb, secondary branch; sl, sterile lemma; sp, spikelet; st, stamen. Bars = 2 cm in A to C, 2 mm in D to M, and 25 μm in i1, i2, j, k, l, m1, and m2.
Despite a difference in internode length (Supplemental Fig. S1, A–D), no differences in vegetative development and flowering time were detected between wild-type plants and osmads34 mutants. However, osmads34 plants displayed altered inflorescence shape, with reduction of internode length between the primary branches on the main axis (Fig. 1, A–C). The average number of primary branches per panicle increased to about 130% in osmads34-1 (15.2 ± 1.9; n = 10) and 170% in osmads34-2 (18.8 ± 1.6; n = 10) compared with the wild type (11.5 ± 1.3; n = 10). In addition, compared with the wild type (25.0 ± 4.4; n = 10), the number of secondary branches decreased to 85.6% in osmads34-1 (21.4 ± 4.6; n = 10) and 50.0% in osmads34-2 (11.2 ± 3.1; n = 10). Consequently, the average number of spikelets in osmads34 decreased (i.e. 143.7 ± 20 for per wild-type panicle, 111.8 ± 16.9 for osmads34-1, and 59.8 ± 9.5 for osmads34-2; n = 10).
Scanning electron microscopy (SEM) analysis revealed little difference between wild-type and osmads34 inflorescence meristems at stage In3 during the formation of primary branch primordia (Fig. 2, A, F, and K). At stage In4, during the elongation of primary branch primordia, the wild-type inflorescence meristem produced obvious primary branch primordia with elongated hair-like structures (Fig. 2B), whereas osmads34 mutants showed more primary branch primordia with fewer elongated hair-like structures (Fig. 2, G and L). At stage In5, during the formation of secondary branch primordia in the wild type (Fig. 2C), fewer secondary branch primordia were observed in both osmads34-1 and osmads34-2 (Fig. 2, H and M). These observations show that OsMADS34 affects the early development of the rice inflorescence.
Figure 2.
Scanning electron microscopy analysis of wild-type and osmads34 inflorescence and spikelet primordia. A to E, The wild type. F to J, osmads34-1. K to O, osmads34-2. A, F, and K, Early stage (In3 stage) of primary branch formation. Stars mark the primary branch primordia. B, G, and L, Elongation of primary branches (In4 stage). Stars mark the primary branches. C, H, and M, Differentiation of higher order branches in the wild type (In5 stage), but the rachis meristem of the mutant loses its activity. Stars mark the primary branches, arrowheads mark the secondary branches, and black arrows indicate the elongated hair-like structures. D, I, and N, Young spikelet meristems at stage Sp6, when stamen primordia merge. E, J, and O, Young spikelet meristems at stage Sp8. ca, Carpel; le, lemma; lsl, lemma/leaf-like sterile lemma; pa, palea; rg, rudimentary glume; sl, sterile lemma; st, stamen. Bars = 50 μm.
OsMADS34 Specifies the Identity of Sterile Lemmas
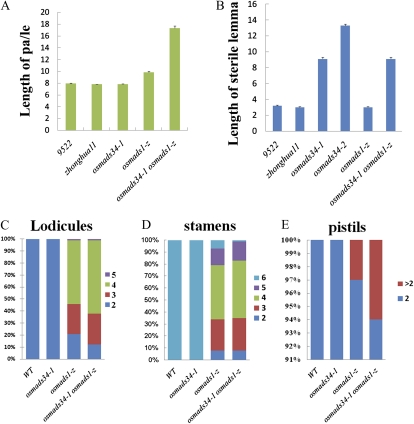
In addition to the altered inflorescence morphology of osmads34 mutants, we observed abnormal spikelet shape of osmads34 mutants with elongated sterile lemmas compared with the wild type. In the wild-type spikelet, sterile lemmas seemed to be bract-like depressed structures subtending the flower (Fig. 1I), and their average length was about 3.21 mm (n = 36; Fig. 3B). By contrast, the sterile lemmas of osmads34-1 displayed elongated lemma/leaf-like structures (Fig. 1J), and osmads34-2 showed longer leaf-like sterile lemmas (Fig. 1K). The average length of the elongated sterile lemmas of osmads34-1 was 9.08 mm (n = 55; Fig. 3B), which was longer than that of the wild-type lemma/palea, 7.8 mm (n = 36; Fig. 3A). In the osmads34-2 mutant, the average length of elongated sterile lemmas was 13.3 mm (n = 36; Fig. 3B). No obvious defects of floral inner organs in osmads34-1 and osmads34-2 mutants were observed (Fig. 1, D–F; Supplemental Fig. S1, E and F).
Figure 3.
Analyses of spikelet organs of osmads34, osmads1-z, and osmads34-1 osmads1-z.A, The length of palea/lemma (pa/le) of the mutants. B, The length of the sterile lemma in the mutants. C to E, The number of floral organs in the wild type (WT), osmads34-1, osmads1-z, and osmads34-1 osmads1-z. For each strain, 100 spikelets were examined. [See online article for color version of this figure.]
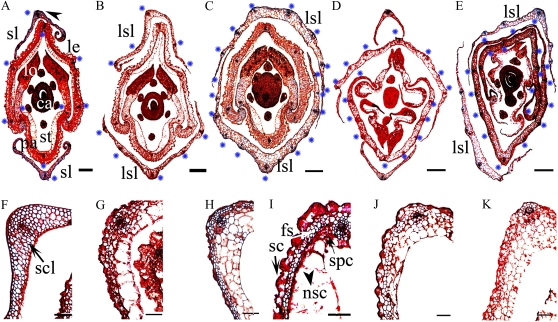
Unlike the cellular pattern of wild-type sterile lemmas containing only one vascular bundle (Fig. 4A), the osmads34-1 lemma/leaf-like sterile lemma had five vascular bundles (Fig. 4B; Supplemental Fig. S2B), and those of osmads34-2 had seven to 11 vascular bundles (Fig. 4C; Supplemental Fig. S2C). Wild-type rice sterile lemmas contained only sclerenchymatous cells between two epidermal layers (Fig. 4F), but osmads34 developed elongated sterile lemmas with similar cellular patterns to those of the wild-type lemma/palea (i.e. silicified cells, fibrous sclerenchyma, spongy parenchymatous cells, and nonsilicified cells from the outer to the inner epidermis; Fig. 4, G–I; Yuan et al., 2009). Consistently, SEM analysis indicated that at early stages, osmads34-1 and osmads34-2 developed abnormally enlarged primordia of sterile lemmas (Fig. 2, D, E, I, J, N, and O). Compared with the smooth outer epidermal surface of wild-type sterile lemmas (Fig. 1i2), epidermal cells of osmads34 lemma/leaf-like sterile lemmas seemed to be transformed from smooth narrow files to cells with compact small bulges (Fig. 1, J and K), which were frequently observed in wild-type lemma/palea (Fig. 1i1). We also found stomata in the elongated sterile lemma of osmads34-2, which are present on the leaf surface (Fig. 1, K and m2). These results suggest the homeotic transformation of the sterile lemma to lemma/leaf like structure in the two osmads34 mutants.
Figure 4.
Histological analysis of mutant spikelets. A and F, The wild type. A, Transverse section of the bottom of a wild-type spikelet at stage In9 (red arrow in Fig. 1D) showing five vascular bundles in the lemma (le; stars), three vascular bundles in the palea (pa), and only one vascular bundle in the sterile lemma (sl). ca, Carpel; lo, lodicules; st, stamen. F, A closeup of the sterile lemma marked by the arrowhead in A. Internal cells in the wild-type sterile lemma are sclerenchymatous cells (scl). I, A closeup of the lemma of the top transverse section (green arrow in Fig. 1D). Note that four types of cell identity could be observed in the wild-type lemma and palea: silicified cells (sc), fibrous sclerenchyma (fs), spongy parenchymatous cells (spc), and nonsilicified cells (nsc). B, Transverse section of the bottom of the osmads34-1 spikelet (red arrow in Fig. 1E) showing five vascular bundles (indicated by blue stars) of the lemma/leaf-like sterile lemma (lsl). G, A closeup of the osmads34-1 sterile lemma of the top transverse section (green arrow in Fig. 1E) showing four cell types: silicified, fibrous sclerenchyma, spongy parenchymatous, and nonsilicified. C, Transverse section of the bottom of an osmads34-2 spikelet (red arrow in Fig. 1F) showing more vascular bundles (indicated by blue stars) in the lemma/leaf-like sterile lemmas. H, A closeup of lemma/leaf-like sterile lemma of osmads34-2 of the top transverse section (green arrow in Fig. 1F) showing four cell types: silicified, fibrous sclerenchyma, spongy parenchymatous, and nonsilicified. D and J, The osmads1-z spikelet. D, Transverse section of the bottom of an osmads1-z spikelet showing leafy lemma/palea, four enlarged lodicules, and reduced stamens (red arrow in Fig. 1L). J, A closeup of leafy lemma of the top transverse section (green arrow in Fig. 1L) showing four cell types (silicified, fibrous sclerenchyma, spongy parenchymatous, and nonsilicified) in osmads1-z. E and K, The osmads34-1 osmads1-z spikelet. E, Transverse section at the bottom of the osmads34-1 osmads1-z spikelet showing leafy-like sterile lemma, leafy lemma/palea, four enlarged lodicules, and reduced stamens (red arrow in Fig. 1M). K, A closeup of the osmads34-1 osmads1double mutant leafy lemma/palea of the top transverse section (green arrow in Fig. 1M). Bars = 100 μm in A to E and 50 μm in F to K.
Map-Based Cloning of OsMADS34
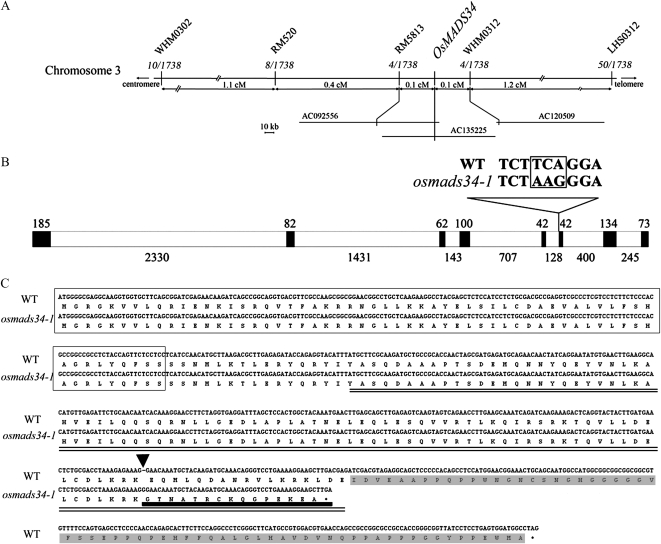
All of the F1 progeny of osmads34 mutants backcrossed with the wild-type rice showed normal inflorescence and spikelet development. In addition, an approximate 3:1 ratio for phenotype segregation was observed in the F2 plants, supporting that the mutation most likely occurs at a single recessive locus. Using a map-based cloning approach and sequencing analysis, we identified a three-nucleotide substitution within an annotated MADS box gene (Os03g54170), OsMADS34 (also called OsMADS19), in osmads34-1 (Fig. 5). The nucleotides at the 3′ exon-intron boundary of the fifth intron and the sixth exon of OsMADS34 in osmads34-1 were changed from TCA to AAG (Fig. 5B). Although no obvious expression change of OsMADS34 was observed in osmads34-1 compared with the wild type (Supplemental Fig. S3B), abnormal splicing at the sixth exon of the OsMADS34 transcript was observed in osmads34-1, causing a frame-shift mutation (Fig. 5, B and C). There was no detectable expression of OsMADS34 in osmads34-2 (Supplemental Fig. S3B), suggesting that osmads34-2 is likely a null mutation of OsMADS34. This is consistent with the phenotypic analysis of osmads34-2. However, in osmads34-2, no sequence mutation of OsMADS34 was observed within a 12,073-bp genomic DNA region consisting of a 3-kb 5′ untranslated region, the OsMADS34 genomic region (introns and exons), and a 3-kb 3′ untranslated region. We assume that osmads34-2 may be caused by epigenetic change(s) of OsMADS34. Notably, the C-terminal motif of OsMADS34 was 65 amino acids, which is much shorter than those of other MADS box proteins (Malcomber and Kellogg, 2005). OsMADS34 was confirmed to be responsible for the defects of osmads34-1 and osmads34-2 by a functional complementation experiment using the full-length OsMADS34 cDNA under the control of a double cauliflower mosaic virus 35S promoter, which was able to rescue the defect of osmads34 inflorescences and spikelets (Supplemental Fig. S3, I, J, and M). Furthermore, ectopic expression of OsMADS34 in the wild type displayed no obvious abnormal phenotypes (Supplemental Fig. S3, H and M).
Figure 5.
Map-based cloning of OsMADS34. A, Fine mapping of the osmads34-1 on chromosome 3. Names and positions of the molecular markers are indicated. Numbers represent recombinant events. cM, Centimorgan. A total of 1,738 individuals of the F2 mapping population generated by a cross between osmads34-1 and cv Guang-lu-ai 4 (var indica) were analyzed using a set of primers (Supplemental Table S1). Finally, this mutant gene was located within a DNA region of 133 kb. B, A schematic representation of the exon and intron organization of OsMADS34. The mutant sequence has three nucleotide substitutions in the fifth intron. Black boxes indicate exons, and white boxes indicate introns. C, The OsMADS34 cDNA and protein sequences. The mutated protein sequence is underlined by the thick line. The MADS box domain is boxed. The K domain is marked by two lines, and the C domain is shaded. The black triangle marks the extra G in the osmads34-1 mutant cDNA. WT, Wild type.
OsMADS34 Encodes a Grass-Specific SEP-Like Transcription Factor without Activation Activity
Previous studies have shown that OsMADS34 belongs to the AGAMOUS-LIKE1/2/4 (AGL1/2/4) lineage of the SEP subfamily and is closely related to OsMADS1 and OsMADS5, the two relatively well-known SEP-like genes in rice (Zahn et al., 2005). In this study, phylogenetic analysis of AGL1/2/4 lineage members from eudicots and monocots revealed that grass SEP genes from Lolium perenne, rice, Sorghum bicolor, wheat (Triticum aestivum), and maize are divergent from those of eudicots due to their location in three different groups (Supplemental Figs. S4 and S5). In addition, we confirmed that OsMADS34 is a sister of OsMADS1 and OsMADS5 and was likely generated through a gene duplication event that occurred before the diversification of the grass family (Poaceae). We also found that, after the gene duplication, OsMADS34 acquired distinct sequence structure in its C-terminal region, as revealed in the alignment of the protein sequences (Supplemental Fig. S4). Close inspection of the exon-intron structure of OsMADS1, OsMADS5, and OsMADS34 further indicated that the difference in protein sequences is probably caused by changes in exon-intron structure (Supplemental Fig. S4A). More specifically, although exon numbers of these three genes remained unchanged during evolution, the seventh and eighth exons diversified considerably in both length and sequence. In OsMADS5, for example, the eighth exon, which is 34 bp long, has 73.5% identity to the middle part of the eighth exon of OsMADS1. Because genes from nongrass monocots usually have protein and cDNA sequences highly comparable to OsMADS1, it is very likely that the exon-intron structure of the ancestral gene has been retained in OsMADS1. If this is the case, then OsMADS5 may have been generated through pseudoexonization (a process in which exonic sequences become nonexonic sequences) of the first 24 bp and the last 58 bp of the eighth exon of an OsMADS1-like ancestral gene. Similarly, OsMADS34 may have evolved from an OsMADS1-like ancestral gene via the occurrence of a premature stop codon caused by a 1-bp deletion, leading to a shortened C-terminal end of OsMADS34. Therefore, we propose that OsMADS34 has lost the transcription activation function due to the short C terminus. Consistent with a shortened C terminus of OsMADS34, no transcription activation function of OsMADS34 was observed in yeast cells (Supplemental Fig. S6). In addition, we observed that OsMADS34 was able to form homodimers but no heterodimers with OsMADS1 in yeast cells (Supplemental Fig. S6).
Expression Pattern of OsMADS34
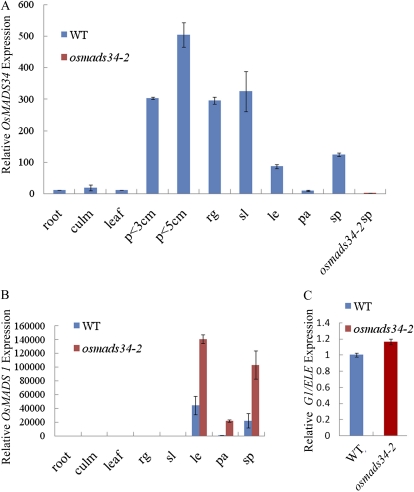
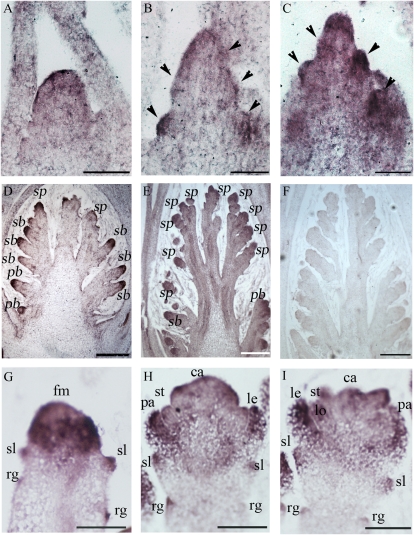
Quantitative reverse transcription (qRT)-PCR analysis revealed the expression of OsMADS34 in roots, culms, leaves, and various stages of the inflorescence (Fig. 6A). RNA in situ hybridization revealed that OsMADS34 was expressed at the apical inflorescence meristem at stage In1 (Fig. 7A) and then highly in the primary branch primordia (Fig. 7, B and C). In particular, the expression of OsMADS34 was strongly detectable in the apical region of secondary branch primordia (Fig. 7, D and E). At stage In7, during the initiation of floral organ primordia, we observed that OsMADS34 was strongly expressed in the developing spikelet primordia, such as the precursors of rudimentary glumes, sterile lemmas, and other floral organs (Fig. 7G). Later, OsMADS34 transcripts were observed in the primordia of carpels, stamens, lemmas/paleas, as well as sterile lemmas and rudimentary glumes (Fig. 7, H and I). Only a background level of signal was observed with the sense probe (Fig. 7F).
Figure 6.
qRT-PCR analysis of OsMADS34, OsMADS1, and G1/ELE mRNA levels. A, Expression levels of the OsMADS34 gene in the wild-type (WT) root, the culm, the leaf, and the spikelet (sp) at stage In9 and in the inflorescence at stages In7 and In8. Dissected rudimentary glume (rg), sterile lemma (sl), lemma (le), and palea (pa) at stage In9 were analyzed. osmads34-2 spikelets at stage In9 were tested. p, Panicle. B, Expression levels of the OsMADS1 gene in wild-type and osmads34-2 lines at stage In9. C, Expression levels of the G1/ELE gene in wild-type and osmads34-2 inflorescences (the panicle length is 0.5 to 1 cm, stages In5 and In6). The error bars indicate sd. Each experiment was biologically repeated three times, each with three technical replicates. [See online article for color version of this figure.]
Figure 7.
In situ analysis of the OsMADS34 gene. A, The inflorescence at stage In1 (establishment of the rachis meristem). B, The inflorescence meristem during the formation of primary rachis branches (arrowheads; stages In2 and In3). C, The inflorescence meristem when primary branches elongate (arrowheads; stage In4). D, The inflorescence meristem when the branch meristem initiates (stage In5). E, The panicle when the top portion of the panicle develops inner floral organ primordia whereas the lower part still remains at the spikelet primordia (stage In6). F, Sense control. G to I, Closeup observation of spikelet meristems at various developmental stages, Sp2 to Sp7. ca, Carpel; fm, floral meristem; le, lemma; lo, lodicules; pa, palea; pb, primary branch; rg, rudimentary glume; sb, secondary branch; sl, sterile lemma; sp, spikelet; st, stamen. Bars = 100 μm in A to F and 25 μm in G to I.
Genetic Interaction between OsMADS34 and OsMADS1
To test whether OsMADS34 has an interaction with OsMADS1 in specifying spikelet development in rice, we identified a new null mutant of OsMADS1 (Supplemental Fig. S7), called osmads1-z, which is allelic with the reported OsMADS1 mutant naked seed rice (Chen et al., 2006b). A 1.312-bp DNA fragment (91–1,403) deletion spanning the first exon and the first intron of OsMADS1 was detected in osmads1-z (Supplemental Fig. S7F), and no obvious OsMADS1 mRNA was observed in this mutant (Supplemental Fig. S7D), suggesting that osmads1-z has a null mutation of OsMADS1. Consistently, the lemma and palea as well as lodicules were transformed into leaf-like structures in osmads1-z (Fig. 4D). In addition, osmads1-z had a changed number of inner floral organs (Fig. 3, C–E).
Compared with osmads1-z (Fig. 1, G and L), double mutant osmads34-1 osmads1-z displayed more severe spikelet organ defects (Fig. 1, H and M). osmads34-1 osmads1-z displayed elongated leaf-like sterile lemmas with more vascular bundles compared with osmads34 mutants (Figs. 1M and 4, E and K; Supplemental Fig. S2, E and J). Much longer leaf-like lemma/palea with flatter appearance was observed in osmads34 osmads1-z (Figs. 1M and 3A). Also, these leafy structures displayed reduced and smaller sclerenchyma cells and spongy parenchymatous cells (Fig. 4, D, E, J, and K). Intriguingly, we also found that stomatas, which are usually present on the leaf surface, were detectable on the surface of leaf-like lemma and palea in osmads34-1 osmads1-z (Fig. 1, m1 and m2), suggesting that the lemma and palea were transformed into leaf-like structures in the double mutant. Moreover, osmads34-1 osmads1-z showed slightly enhanced defects of inner floral organs. Moreover, osmads34-1osmads1-z spikelets showed slightly more severe defects of inner floral organs, such as three to five leafy lodicules (Figs. 1H and 3C), a decreased number of stamens (3.7), and occasionally abnormal carpels (Fig. 3, D and E). Statistically, 21% of spikelets of osmads1-z had two lodicules but only 12% of osmads34-1 osmads1-z spikelets did (Figs. 1H and 3C), and 7% of osmads1 spikelets had six stamens while only 1% of osmads34-1 osmads1-z spikelets did (Fig. 3, D and E).
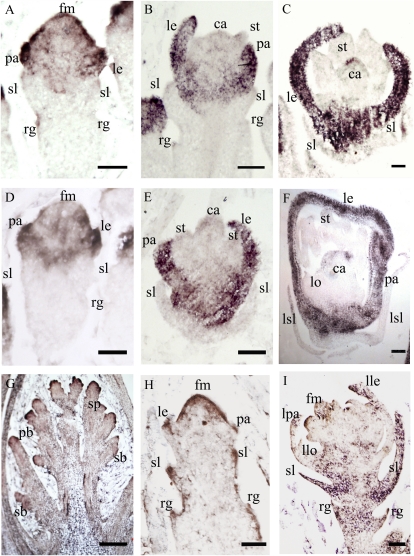
Our observations indicated that osmads34 developed lemma/leaf-like structures at the position of the sterile lemmas. Also, OsMADS1 has been identified as a key regulator of lemma/palea identify, and overexpression of OsMADS1 can transform the glumes into lemma/palea-like organs (Jeon et al., 2000; Prasad et al., 2001, 2005; Agrawal et al., 2005; Chen et al., 2006b). Therefore, we asked whether the defect in osmads34 is caused by ectopic expression of OsMADS1. Similar to the wild type (Fig. 8, A–C), the OsMADS1 expression was observed in the osmads34-1 floral meristem at early stages (Sp4; Fig. 8D) and later in the lemma/palea and carpel (Sp7; Fig. 8, E and F), suggesting that the expression domain of OsMADS1 was not greatly altered in osmads34-1. To precisely quantify the expression of OsMADS1, qRT-PCR was used. At stage In9, the OsMADS1 expression was observed to be increased in the lemma, palea, and whole spikelet in osmads34 (Fig. 6B), while no obvious expression signal of OsMADS1 was observed in the sterile lemmas of osmads34 and wild-type plants (Fig. 6B). Furthermore, we observed that the expression domain of OsMADS34 was quite similar to that in the wild type (Fig. 8, G–I).
Figure 8.
In situ analysis OsMADS1 and OsMADS34 transcripts. A to C, In situ hybridization of OsMADS1 transcripts in the wild type. At an early stage, we observed that OsMADS1 expression is detectable in the spikelet meristem at stage Sp4 (A), then restricted to the primordia of lemma/palea during their initiation at stage Sp6 (B), and disappears from the primordia of the lodicules and stamen where the organ differentiation occurs (B). Subsequently, OsMADS1 expression is strongly detected in lemma and palea and weakly detected in the carpel primordia at stage Sp7 (C). D to F, In situ hybridization of OsMADS1 transcripts in osmads34 at stages Sp4, -6, and -7. G to I, In situ hybridization of OsMADS34 transcripts in osmads1-z (stages In7, Sp4, and Sp6). ca, Carpel; fm, floral meristem; le, lemma; lle, leafy lemma; llo, leafy lodicules; lo, lodicules; lpa, leafy palea; lsl, lemma/leaf-like sterile lemma; pa, palea; pb, primary branch; rg, rudimentary glume; sb, secondary branch; sl, sterile lemma; sp, spikelet; st, stamen. Bars = 100 μm in G and 50 μm in A to F, H, and I.
DISCUSSION
OsMADS34 Is a Key Regulator of Rice Inflorescence Morphology
Grasses including rice, wheat, and maize produce grains from their inflorescences, which are the staple food for human beings. Here, we show that OsMADS34 is a key regulator of rice inflorescence and spikelet architecture. Reduction/loss of OsMADS34 activity causes altered inflorescence morphology, with an increased number of primary branches and a reduced number of spikelets. Also osmads34 mutants display shorter primary branches and fewer secondary branches compared with the wild type (Fig. 1, A–C). This phenotype analysis suggests that OsMADS34 is able to regulate the inflorescence architecture by restricting the primary branch number and preventing the early differentiation of primary branch meristems into secondary branches/spikelets. Consistently, Kobayashi et al. (2010) showed that PANCILE PHYTOMER2 (PAP2)/OsMADS34 plays a positive role in the control of spikelet meristem. The pap2-1 mutant displayed an increased number of primary branches as well as secondary branches (Kobayashi et al., 2010), while osmads34 mutants in this paper have fewer spikelets and reduced secondary branches. This inconsistency may be due to the different genetic backgrounds of these mutants and/or the different mutated sites of these mutants in the gene. pap2-1 was from an insertion of Tos17, an endogenous retrotransposon, in the forth exon of OsMADS34 (Kobayashi et al., 2010).
Most reported MADS box proteins have been shown to form multimeric complexes, which are the prerequisites for their function (Goto et al., 2001; Honma and Goto, 2001; Jack, 2001). In this study, we show that OsMADS34 has no transcriptional activation ability in yeast cells. Therefore, it is possible that other interacting MADS box proteins with transcriptional activation domains are required for OsMADS34 function. Currently, how OsMADS34 regulates the number of primary branches still remains unclear, and future investigation on this aspect will be helpful to understand the molecular basis of grass inflorescence establishment.
OsMADS34 Regulates Sterile Lemma Identity
The rice spikelet has one fertile floret with two rudimentary glumes and two sterile lemmas, which are not observed in the spikelets in other grasses such as maize and wheat. Morphological and genetic studies support the hypothesis that a rice spikelet was originally generated from three florets and the two lateral florets were lost during evolution, only leaving the lemma, called sterile lemmas (Kellogg, 2009; Kobayashi et al., 2010).
In this study, our results show that OsMADS34 is a key regulator for sterile lemma identity and is required to prevent the formation of lemma/leaf-like organs at the position of the sterile lemma primordium within the spikelet meristem, thus permitting the subsequent development of sterile lemmas. Consistently, osmads34 mutants showed the enlarged sterile lemmas with similar cellular structure to those of the wild-type lemma/leaf. We did not observe the expression increase of the lemma/palea marker gene OsMADS1 in the lemma/leaf-like organ at the position of sterile lemmas in osmads34-2. Moreover, no obvious morphological alteration of rudimentary glumes was observed in our osmads34 mutants. By contrast, Kobayashi et al. (2010) reported that 60% of pap2-1 spikelets exhibited elongated rudimentary glumes, and 40% of them developed an ectopic filamentous organ. Sterile lemmas and rudimentary glumes have different morphology, implying that different genes may be involved in their development (e.g. OsMADS34). The fact that sterile lemmas of osmads34 mutants resemble lemma/leaf may indicate a release from their modified state or may be due to a homeotic conversion caused by ectopic expression of a lemma-determining gene. The expression of OsMADS34 in rudimentary glumes and lemmas suggests that there must be other genetic differences between all three organs to make OsMADS34 function context specific. G1/ELE was shown to be a key regulator of sterile lemmas, and g1/ele mutants displayed the transformation of sterile lemmas into lemma-like structures (Yoshida et al., 2009; Hong et al., 2010). We did not observe the detectable signal of G1/ELE in the sterile lemma in the wild-type and osmads34-2 spikelets at stage In9. We observed no great expression change of G1/ELE mRNA levels in wild-type and osmads34-2 inflorescences at stages In5 and In6 (Fig. 6C), suggesting that G1/ELE and OsMADS34 may in parallel regulate the sterile lemma development.
OsMADS34 Specifies Flower Organs Together with OsMADS1
Although OsMADS34 expression was detected in all the tissues, especially in the floral meristem, no defects of floral organs were observed in osmads34 mutants. So we asked whether OsMADS34 has redundant function with other gene(s). The SEP genes in Arabidopsis have been studied extensively. Four SEP genes, designated AtSEP1, AtSEP2, AtSEP3, and AtSEP4, function largely redundantly in regulating flower development (Pelaz et al., 2000; Ditta et al., 2004). Flowers of triple mutants (sep1 sep2 sep3) form sepal-like organs (Pelaz et al., 2000), and floral organs in sep1 sep2 sep3 sep4 quadruple mutants are entirely converted into leaf-like organs (Ditta et al., 2004).
Previous studies have shown that OsMADS1 functions in controlling the differentiation of specific cell types in the lemma and palea (Prasad et al., 2005). In this study, the lemma and palea of osmads1-z/osmads34-1 are transformed into elongated leaf-like organs with smaller bulges, stomata, and more vascular structures, partly resembling the phenotype of the quadruple sep1/2/3/4 mutants in Arabidopsis (Ditta et al., 2004), which further implies that floral organs are modified leaves. The length of sterile lemmas of osmads1-z/osmads34-1 was not obviously increased compared with osmads34 mutants (Fig. 3B). Compared with the osmads1 single mutant, the osmads1 osmads34 double mutant displays enhanced defects of the inner floral organs, suggesting that OsMADS34 is also involved in the identity determination of inner flower organs, redundantly with OsMADS1. Furthermore, we showed that OsMADS34 or OsMADS1 did not greatly alter the expression domain of each other in the spikelet. The expression increase of OsMADS1 in osmads34 may be from a direct or indirect regulatory relationship between OsMADS1 and OsMADS34. Similarly, in the OsMADS7 and OsMADS8 RNA interference plants, the expression of OsMADS1, OsMADS5, and OsMADS34 was increased, suggesting that these genes may be negatively regulated rather than positively modulated, either directly or indirectly (Cui et al., 2010). No protein interaction of OsMADS1 and OsMADS34 was observed by yeast two-hybrid assay, suggesting that OsMADS1 and OsMADS34 may function independently as key regulators for rice spikelet morphological establishment.
OsMADS34 and OsMADS1 Are Diversified SEP-Like Genes Conserved in Grasses
MADS box genes play an essential role in establishing flower morphology in various plants. The SEP genes belonging to a specific MADS box subfamily are critical for determining the “floral state” by specifying floral organs and meristem identity (Zahn et al., 2005). To date, SEP genes have been observed in angiosperms but not in gymnosperms, suggesting that the origin of the SEP lineage contributes greatly to extant angiosperms apart from extant gymnosperms (Becker and Theissen, 2003; Malcomber and Kellogg, 2005; Zahn et al., 2005).
There are five MADS box genes belonging to the SEP subfamily in rice. OsMADS1/LHS1, OsMADS5, and OsMADS34 belong to the LOFSEP subgroup of the SEP family. OsMADS5 might be generated from an OsMADS1-like ancestral gene and may have redundant functions with other genes (Agrawal et al., 2005). The osmads5 mutant displayed no obvious phenotypic defects. OsMADS7/45 and OsMADS8/24 belong to the SEP3 subgroup. Silencing of both OsMADS7 and OsMADS8 reveals that these two genes are associated with the control of flowering time, specification of lodicules, stamens, and carpels, as well as floral determinacy (Pelucchi et al., 2002; Cui et al., 2010). Recently, the AGL6 MADS-box gene OsMADS6 was shown to play a very similar functional role to those of the SEP clade, which specifies floral organs of all four whorls and floral meristem determinacy (Ohmori et al., 2009; Li et al., 2010).
Phylogenetic analysis has shown the uncertain relationship between rice LOFSEP-like genes and eudicot SEP-like genes (Becker and Theissen, 2003; Malcomber and Kellogg, 2005; Zahn et al., 2005; this study). Both functional and evolutionary analysis imply that orthologs of OsMADS34 and OsMADS1 might have been crucial for the origin of the grass spikelet and may have evolved distinct functions from those well-studied Arabidopsis SEP genes. The OsMADS34 transcripts are present in both vegetative and reproductive organs, but Arabidopsis SEP genes are mainly expressed in floral organs (Rounsley et al., 1995; Pelaz et al., 2000). Moreover, osmads34 mutants display altered plant architecture due to the shortened culm length, changed inflorescence, and spikelet morphology. In the ornamental plant Gerbera, a single SEPALLATA-like MADS box gene is involved in various aspects of reproductive transition, inflorescence architecture, meristem patterning, and floral organ identity, suggesting the functional diversity of SEP-like genes in plants (Uimari et al., 2004). In addition, OsMADS34 and OsMADS1 clades include members from grasses, implying that orthologs of OsMADS34 and OsMADS1 may play important functions in specifying the inflorescence and spikelet in grasses (Becker and Theissen, 2003; Zahn et al., 2005). OsMADS1/LHS1 orthologs were shown to have varied expression patterns during spikelet development in grass species (Malcomber and Kellogg, 2004; Reinheimer et al., 2006), suggesting that the OsMADS1 orthologs are involved in the diversification of specifying spikelets in cereals. Therefore, future functional analysis of orthologs of OsMADS34 and OsMADS1 in grasses will help us understand the evolutionary mechanism of inflorescence and spikelet development.
MATERIALS AND METHODS
Plant Materials
Three rice (Oryza sativa) mutants, osmads34-1, osmads34-2, and osmads1-z, were used in this study. The 9522 cultivar was used as a wild-type strain for phenotype observation and for RNA analysis. Plants for phenotype analysis and RT-PCR were grown in the paddy field of the Shanghai Jiaotong University. RNA for qRT-PCR on dissected organs was extracted from spikelets at heading time, and the plants were grown in the paddy field of Sanya, China (north latitude 18°09′34″–18°37′27″, east longitude 108°56′30″–109°48′28″).
Microscopy and Image Processing
The sample was fixed in 50% ethanol, 10% formalin, and 5% acetic acid, materials were sectioned and viewed with a Leica light microscope (DFC402C), and SEM was performed as described previously (Li et al., 2006). Fresh tissues of wild-type and mutant plants were photographed using a Nikon E995 digital camera. Images were processed using Photoshop 7.0 software (Adobe).
Map-Based Cloning of OsMADS34
The OsMADS34 gene was first mapped to the long arm of chromosome 3, between two simple sequence repeat markers, RM468 and RM3525, by using 96 F2 plants of osmads34-1 and cv Guang-lu-ai 4 (subspecies indica). Then, using 1,738 F2 plants, the OsMADS34 locus was narrowed between the simple sequence repeat marker RM5813 and the developed insertion/deletion marker WHM0312. Mutations in osmads34-1 were determined by PCR amplification and sequence analysis.
In Situ Hybridization
OsMADS34-specific probe was generated by inserting the cDNA fragment into pMD18-T (TaKaRa; gene-specific primers 34IF and 34IR), and this DNA fragment was subcloned into pBluescript SK+ and sequenced for confirmation. The OsMADS1-specific probe was made according to the method reported previously (Agrawal et al., 2005). RNA hybridization and immunological detection of the hybridized probes were performed according to the protocol described previously (Chu et al., 2006).
RT-PCR, qRT-PCR, and Complementation Tests
RNA from root, culm, leaf, inflorescence, and young flowers was isolated as described previously (Li et al., 2006). The cDNA was synthesized using 5 μg of total RNA in a 20-μL reaction with oligo(dT) primers (SuperScript III reverse transcriptase; Invitrogen). qRT-PCR analyses were performed using the CFX96 Real-Time PCR System (Bio-Rad) using the primers reported by Cui et al. (2010). Reactions contained the Brilliant SYBR Green QPCR Master Mix (Fermentas) in a final volume of 25 μL with 2 pmol of the appropriate primers (Supplemental Table S1) and 5 μL of cDNA. PCR cycling conditions for amplification were 95°C for 5 min followed by 40 cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 20 s. Each experiment had three biological repeats, each with three technical replicates. Data acquisition and analyses were performed using the Bio-Rad CFX Manager software. Samples were normalized using ACTIN1 expression; relative expression levels were measured using the comparative 2(−ΔCt) analysis method (Schmittgen and Livak, 2008).
For complementation tests, OsMADS34 cDNA was cloned into a binary vector expressed under the control of a double cauliflower mosaic virus 35S promoter and then transformed into osmads34-1 and osmads34-2 by Agrobacterium tumefaciens-mediated transformation (Hiei et al., 1994).
Yeast Two-Hybrid Assay
The insert DNA for clones pGAK/OsMADS34 and pGBD/OsMADS34 was prepared by PCR with the full-length OsMADS34 cDNA as the template and OsMADS34-YF and OsMADS34-YR as primers. The plasmids pGAK and pGBD and the PCR products were digested with EcoRI and BamHI and then ligated to generate plasmids. pGAK/OsMADS1ΔC was constructed according to the method reported previously (Lim et al., 2000) with primers OsMADS1-YF and OsMADS1-YR. Other processes were performed in accordance with the manufacturer's instructions (Clontech).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AK070981 (OsMADS1), AK100227 (OsMADS34), and AB512480 (G1/ELE).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phenotypes of osmads34 mutants.
Supplemental Figure S2. Histological analysis of the mutant spikelets.
Supplemental Figure S3. Allelic and complementary analyses of osmads34 mutants and ectopic expression of OsMADS34 in the wild type.
Supplemental Figure S4. Sequence analysis of the OsMADS34 gene and its homologs.
Supplemental Figure S5. Sequence alignment of the OsMADS34 gene and its homologs.
Supplemental Figure S6. OsMADS34 has no transactivational activity, forms homodimers, and has no interaction with OsMADS1 in yeast cells.
Supplemental Figure S7. Analysis and identification of the osmads1-z mutant.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank the anonymous reviewers for very helpful comments and H.Q. Yang and C.H. Shi for providing pHB vector and the naked seed rice mutant, respectively. We thank Z.J. Luo and M.J. Chen for mutant screening and generation of F2 populations, M. Long for sections, X.Y. Gao for SEM, and H. Ma and H. Yu for valuable discussion.
References
- Agrawal GK, Abe K, Yamazaki M, Miyao A, Hirochika H. (2005) Conservation of the E-function for floral organ identity in rice revealed by the analysis of tissue culture-induced loss-of-function mutants of the OsMADS1 gene. Plant Mol Biol 59: 125–135 [DOI] [PubMed] [Google Scholar]
- Arber A. (1934) The Gramineae: A Study of Cereal, Bamboo, and Grass. Cambridge University Press, Cambridge, UK [Google Scholar]
- Becker A, Theissen G. (2003) The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol 29: 464–489 [DOI] [PubMed] [Google Scholar]
- Chen L, Chu HW, Yuan Z, Pan AH, Liang WQ, Huang H, Shen MS, Zhang DB. (2006a) Isolation and genetic analysis for rice mutants treated with 60 Co γ-ray. Journal of Xiamen University 45: 82–85 [Google Scholar]
- Chen ZX, Wu JG, Ding WN, Chen HM, Wu P, Shi CH. (2006b) Morphogenesis and molecular basis on naked seed rice, a novel homeotic mutation of OsMADS1 regulating transcript level of AP3 homologue in rice. Planta 223: 882–890 [DOI] [PubMed] [Google Scholar]
- Chu H, Qian Q, Liang W, Yin C, Tan H, Yao X, Yuan Z, Yang J, Huang H, Luo D, et al. (2006) The FLORAL ORGAN NUMBER4 gene encoding a putative ortholog of Arabidopsis CLAVATA3 regulates apical meristem size in rice. Plant Physiol 142: 1039–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz EM. (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353: 31–37 [DOI] [PubMed] [Google Scholar]
- Cui R, Han J, Zhao S, Su K, Wu F, Du X, Xu Q, Chong K, Theißen G, Meng Z. (2010) Functional conservation and diversification of class E floral homeotic genes in rice (Oryza sativa). Plant J 6: 767–781 [DOI] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. (2004) The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol 14: 1935–1940 [DOI] [PubMed] [Google Scholar]
- Goto K, Kyozuka J, Bowman JL. (2001) Turning floral organs into leaves, leaves into floral organs. Curr Opin Genet Dev 11: 449–456 [DOI] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group (2001) Phylogeny and subfamilial classification of the grasses (Poaceae). Ann Mo Bot Gard 88: 373–457 [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282 [DOI] [PubMed] [Google Scholar]
- Hong L, Qian Q, Zhu K, Tang D, Huang Z, Gao L, Li M, Gu M, Cheng Z. (2010) ELE restrains empty glumes from developing into lemmas. J Genet Genomics 37: 101–115 [DOI] [PubMed] [Google Scholar]
- Honma T, Goto K. (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409: 525–529 [DOI] [PubMed] [Google Scholar]
- Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y. (2005) Rice plant development: from zygote to spikelet. Plant Cell Physiol 46: 23–47 [DOI] [PubMed] [Google Scholar]
- Jack T. (2001) Relearning our ABCs: new twists on an old model. Trends Plant Sci 6: 310–316 [DOI] [PubMed] [Google Scholar]
- Jeon JS, Jang S, Lee S, Nam J, Kim C, Lee SH, Chung YY, Kim SR, Lee YH, Cho YG, et al. (2000) leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell 12: 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA. (2009) The evolutionary history of Ehrhartoideae, Oryzeae, and Oryza. Rice 2: 1–4 [Google Scholar]
- Kobayashi K, Maekawa M, Miyao A, Hirochika H, Kyozuka J. (2010) PANICLE PHYTOMER2(PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice. Plant Cell Physiol 51: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liang W, Jia R, Yin C, Zong J, Kong H, Zhang D. (2010) The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice. Cell Res 20: 299–313 [DOI] [PubMed] [Google Scholar]
- Li H, Xue D, Gao Z, Yan M, Xu W, Xing Z, Huang D, Qian Q, Xue Y. (2009) A putative lipase gene EXTRA GLUME1 regulates both empty-glume fate and spikelet development in rice. Plant J 57: 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, Yuan Z, Xu B, Chu HW, Wang J, et al. (2006) The rice Tapetum Degeneration Retardation gene is required for tapetum degradation and anther development. Plant Cell 18: 2999–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Moon YH, An G, Jang SK. (2000) Two rice MADS domain proteins interact with OsMADS1. Plant Mol Biol 44: 513–527 [DOI] [PubMed] [Google Scholar]
- Linder H, Rudall P. (2005) Evolutionary history of Poales. Annu Rev Ecol Evol Syst 36: 36107–36124 [Google Scholar]
- Liu C, Xi W, Shen L, Tan C, Yu H. (2009) Regulation of floral patterning by flowering time genes. Dev Cell 16: 711–722 [DOI] [PubMed] [Google Scholar]
- Malcomber ST, Kellogg EA. (2004) Heterogeneous expression patterns and separate roles of the SEPALLATA gene LEAFY HULL STERILE1 in grasses. Plant Cell 16: 1692–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcomber ST, Kellogg EA. (2005) SEPALLATA gene diversification: brave new whorls. Trends Plant Sci 10: 427–435 [DOI] [PubMed] [Google Scholar]
- Münster T, Deleu W, Wingen LU, Cacharrón J, Ouzunova M, Faigl W, Werth S, Kim JTT, Saedler H, Theissen G. (2002) Maize MADS-box genes galore. Maydica 47: 287–301 [Google Scholar]
- Ohmori S, Kimizu M, Sugita M, Miyao A, Hirochika H, Uchida E, Nagato Y, Yoshida H. (2009) MOSAIC FLORAL ORGANS1, an AGL6-like MADS box gene, regulates floral organ identity and meristem fate in rice. Plant Cell 21: 3008–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405: 200–203 [DOI] [PubMed] [Google Scholar]
- Pelucchi N, Fornara F, Favalli C, Masiero S, Lago C, Pè ME, Colombo L, Kater MM. (2002) Comparative analysis of rice MADS-box genes expressed during flower development. Sex Plant Reprod 15: 113–122 [Google Scholar]
- Prasad K, Parameswaran S, Vijayraghavan U. (2005) OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J 43: 915–928 [DOI] [PubMed] [Google Scholar]
- Prasad K, Sriram P, Kumar CS, Kushalappa K, Vijayraghavan U. (2001) Ectopic expression of rice OsMADS1 reveals a role in specifying the lemma and palea, grass floral organs analogous to sepals. Dev Genes Evol 211: 281–290 [DOI] [PubMed] [Google Scholar]
- Reinheimer R, Malcomber ST, Kellogg EA. (2006) Evidence for distinct roles of the SEPALLATA gene LEAFY HULL STERILE1 in Eleusine indica and Megathyrsus maximus(Poaceae). Evol Dev 8: 293–303 [DOI] [PubMed] [Google Scholar]
- Rounsley SD, Ditta GS, Yanofsky MF. (1995) Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7: 1259–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108 [DOI] [PubMed] [Google Scholar]
- Takeoka Y, Shimizu M, Wada T. (1993) Panicles. Matsuo T, Hoshikawa K, , Science of the Rice Plant, Vol 1 Food and Agriculture Policy Research Center, Tokyo, pp 295–338 [Google Scholar]
- Theissen G. (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol 4: 75–85 [DOI] [PubMed] [Google Scholar]
- Theissen G, Melzer R. (2007) Molecular mechanisms underlying origin and diversification of the angiosperm flower. Ann Bot (Lond) 100: 603–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uimari A, Kotilainen M, Elomaa P, Yu D, Albert VA, Teeri TH. (2004) Integration of reproductive meristem fates by a SEPALLATA-like MADS-box gene. Proc Natl Acad Sci USA 101: 15817–15822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche M, Zethof J, Souer E, Koes R, Tornielli GB, Pezzotti M, Ferrario S, Angenent GC, Gerats T. (2003) Toward the analysis of the petunia MADS box gene family by reverse and forward transposon insertion mutagenesis approaches: B, C, and D floral organ identity functions require SEPALLATA-like MADS box genes in petunia. Plant Cell 15: 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Suzaki T, Tanaka W, Hirano HY. (2009) The homeotic gene long sterile lemma(G1) specifies sterile lemma identity in the rice spikelet. Proc Natl Acad Sci USA 106: 20103–20108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Gao S, Xue DW, Luo D, Li LT, Ding SY, Yao X, Wilson ZA, Qian Q, Zhang DB. (2009) RETARDED PALEA1 controls palea development and floral zygomorphy in rice. Plant Physiol 149: 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn LM, Kong H, Leebens-Mack JH, Kim S, Soltis PS, Landherr LL, Soltis DE, Depamphilis CW, Ma H. (2005) The evolution of the SEPALLATA subfamily of MADS-box genes: a preangiosperm origin with multiple duplications throughout angiosperm history. Genetics 169: 2209–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanis MJ. (2007) Grass spikelet genetics and duplicate gene comparisons. Int J Plant Sci 168: 93–110 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.