Abstract
Potassium (K+) is a major plant nutrient required for growth and development. It is generally accepted that plant roots absorb K+ through uptake systems operating at low concentrations (high-affinity transport) and/or high external concentrations (low-affinity transport). To understand the molecular basis of high-affinity K+ uptake in Arabidopsis (Arabidopsis thaliana), we analyzed loss-of-function mutants in AtHAK5 and AKT1, two transmembrane proteins active in roots. Compared with the wild type under NH4+-free growth conditions, athak5 mutant plants exhibited growth defects at 10 μm K+, but at K+ concentrations of 20 μm and above, athak5 mutants were visibly indistinguishable from the wild type. While germination, scored as radicle emergence, was only slightly decreased in athak5 akt1 double mutants on low-K+ medium, double mutants failed to grow on medium containing up to 100 μm K+ and growth was impaired at concentrations up to 450 μm K+. Moreover, transfer of 3-d-old plants from high to low K+ concentrations led to growth defects and leaf chlorosis at 10 μm K+ in athak5 akt1 double mutant plants. Determination of Rb+(K+) uptake kinetics in wild-type and mutant roots using rubidium (86Rb+) as a tracer for K+ revealed that high-affinity Rb+(K+) uptake into roots is almost completely abolished in double mutants and impaired in single mutants. These results strongly indicate that AtHAK5 and AKT1 are the two major, physiologically relevant molecular entities mediating high-affinity K+ uptake into roots during seedling establishment and postgermination growth and that residual Rb+(K+) uptake measured in athak5 akt1 double mutant roots is insufficient to enable plant growth.
Potassium (K+) is essential for plant growth and development, where it has important functions in enzyme activation, osmoregulation, stomatal movements, and maintaining the plasma membrane potential (Glass, 1983; Schroeder et al., 1994; Maathuis and Sanders, 1999; Epstein and Bloom, 2005). As a major plant nutrient, K+ is required in large quantities. The K+ content on a dry weight basis typically ranges from 0.8% to 8% in plants, and the cytosolic K+ concentration is maintained between 80 and 200 mm (Maathuis, 2009). Hence, K+ has to be absorbed by roots from the soil solution efficiently. Since K+ availability in the soil may vary considerably with environmental and soil conditions, plants must be able to adjust to changing K+ concentrations. Classical studies on barley (Hordeum vulgare) roots have described two major uptake components operating primarily at low or high external K+ concentrations, termed mechanism I (high-affinity transport system) and mechanism II (low-affinity transport system; Epstein et al., 1963). Many physiological and molecular genetic studies have since aimed at determining the molecular identity of membrane proteins mediating K+ uptake from the soil solution in Arabidopsis (Arabidopsis thaliana) and other plants (Ashley et al., 2006; Gierth and Mäser, 2007; Ward et al., 2009). The characterization of high-affinity K+ uptake in classical uptake studies was based on K+-starved barley roots (Epstein et al., 1963). The KUP/HAK/KT family transporters were identified as candidate high-affinity K+ uptake transporters (Quintero and Blatt, 1997; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998). Investigations revealed an increase of transcript abundance of HvHAK1, a root-localized K+ transporter of the KUP/HAK/KT family from barley, in K+-deplete conditions (Santa-Maria et al., 1997; Fulgenzi et al., 2008). Similar mechanisms for the uptake of major plant nutrients (Kochian and Lucas, 1982; Siddiqi and Glass, 1983; Siddiqi et al., 1990) and induction of HvHAK1 homologues in response to K+ deprivation have also been described for other plant species like tomato (Solanum lycopersicum; Wang et al., 2002) and pepper (Capsicum annuum; Martinez-Cordero et al., 2004). The KUP/HAK/KT family comprises 13 members in Arabidopsis (Mäser et al., 2001), many of which have been shown to mediate K+ transport or to be involved in K+-dependent growth processes (Fu and Luan, 1998; Kim et al., 1998; Rigas et al., 2001; Elumalai et al., 2002). The K+ transporter AtHAK5 was initially found to be expressed in roots of both K+-replete and K+-deplete plants (Rubio et al., 2000) and was later shown to be induced upon K+ starvation (Ahn et al., 2004; Armengaud et al., 2004; Shin and Schachtman, 2004; Gierth et al., 2005) Analyses of athak5 T-DNA mutants revealed that it constitutes the major component of the inducible high-affinity K+ uptake system (Gierth et al., 2005; Rubio et al., 2008). However, analyses of root uptake kinetics also indicated the presence of residual root K+ uptake at micromolar concentrations, which is consistent with the fact that growth retardation of athak5 mutants could so far only be detected at extremely low K+ concentrations of 1 μm (Qi et al., 2008). Additional K+ uptake at low concentrations is likely mediated by AKT1, a constitutively expressed, Shaker-like K+ channel, and additional root-expressed KUP/HAK/KT transporters and K+ channels.
“Inward-rectifying” K+ channels are a class of K+ channels shown to conduct K+ uptake into many types of plant cells (Schroeder et al., 1984, 1987; Moran and Satter, 1989; Kourie and Goldsmith, 1992; Gassmann and Schroeder, 1994; Maathuis and Sanders, 1995; Kwak et al., 2001). AKT1 encodes a root-expressed member of this plant K+ channel class. Plant “Shaker-type” K+ channels like AKT1 consist of monomers sharing a common protein structure of six transmembrane helices (Anderson et al., 1992; Schachtman et al., 1992; Sentenac et al., 1992; Schwacke et al., 2003; Lebaudy et al., 2007) and in vivo assemble into functional channels as homotetramers or heterotetramers forming a K+-selective pore (MacKinnon, 2003). Expression of AKT1 is mainly confined to roots, and AKT1 has been shown to be involved in mediation of K+ uptake from both micromolar and millimolar external concentrations with growth inhibition of akt1-1 mutants on low-K+ medium depending on the presence of NH4+ (Sentenac et al., 1992; Lagarde et al., 1996; Hirsch et al., 1998; Spalding et al., 1999; Gierth et al., 2005). K+ starvation up-regulates the mRNA level of the wheat (Triticum aestivum) TaAKT1 orthologue in roots (Buschmann et al., 2000).
Using heterologous expression in oocytes, recent studies provided direct evidence that mediation of K+ uptake at low K+ concentrations via AKT1 is possible and strictly requires interaction with the calcium-dependent proteins CBL-Interacting Protein Kinase23 (CIPK23) and Calcineurin B-Like protein1/9 (CBL1/9; Li et al., 2006; Xu et al., 2006). Moreover, only the formation of heterotetramers with AtKC1 subunits can prevent K+ loss through AKT1 at micromolar K+ concentrations by shifting the activation threshold to very negative membrane potentials (Geiger et al., 2009). The interaction requirement provides a means of modulating AKT1 at the protein level (Bregante et al., 2008) and adjusts its activity to external conditions. However, K+-dependent growth of akt1-1 mutants progressed like the wild type in regular low-K+ medium. Only in the presence of high ammonium concentrations of 2 mm was akt1-1 mutant growth reduced on K+-deplete medium (Hirsch et al., 1998), indicating that non-AKT1, ammonium-sensitive K+ uptake is sufficient to sustain growth. From results with Arabidopsis, it can be concluded that several systems, including K+ transporters and K+ channels, may be operating in parallel in roots to mediate adjustable high-affinity K+ uptake. However, the contribution of each system might vary depending on growth conditions or developmental stages.
Loss-of-function mutants defective simultaneously in AtHAK5 and AKT1 have not been analyzed yet. Since single mutants show only small effects on plant growth on K+-deplete medium in the absence of NH4+ (Hirsch et al., 1998; Gierth et al., 2005; Qi et al., 2008), we generated athak5 akt1 double mutants and investigated whether low-K+ medium has an effect on plant growth in general and seedling establishment in particular. Moreover, we analyzed the kinetics of unidirectional Rb+(K+) influx in roots of double and single mutants.
We show that AtHAK5 and AKT1 are vital for plant growth and development at low K+ concentrations and provide evidence that AtHAK5 and AKT1 are the two essential molecular entities mediating high-affinity Rb+(K+) uptake in roots of Arabidopsis, concluding that the residual Rb+(K+) uptake detected in double mutant roots is insufficient to sustain plant growth.
RESULTS
athak5 Mutants Are Unable to Grow on 10 μm But Not 20 μm K+
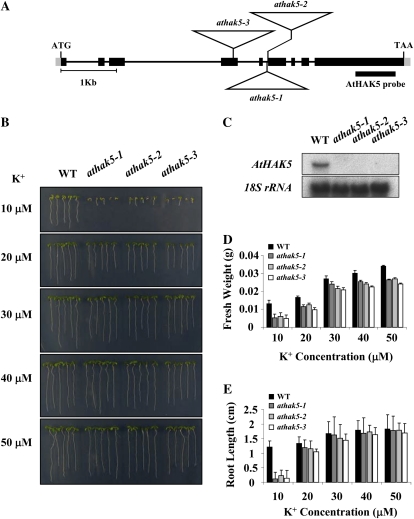
A previous study showed that the plasma membrane protein AtHAK5 constitutes a major component of K+ starvation-induced high-affinity K+ uptake in roots (Gierth et al., 2005). We examined K+-dependent growth of three independent T-DNA insertion lines in the AtHAK5 gene isolated from the SALK collection (Alonso et al., 2003) and named according to previous publications (Gierth et al., 2005; Qi et al., 2008). The positions of T-DNA insertions and the corresponding mutant names are depicted in Figure 1A. No AtHAK5 transcript was detectable in any of the homozygous T-DNA insertion lines when seedlings were grown on low-K+ medium (Fig. 1C).
Figure 1.
Isolation of the athak5 T-DNA insertional mutants, and K+-dependent growth analysis. A, Structure of the AtHAK5 gene. Boxes represent exons, and lines represent introns. The positions of the T-DNA insertions in athak5-1 (SALK_014177), athak5-2 (SALK_005604), and athak5-3 (SALK_130604) are represented by triangles. T-DNA is not drawn to scale. B, The wild type (WT) and athak5 mutant alleles grown for 7 d on medium containing KCl at the concentrations indicated. C, Northern-blot analysis of AtHAK5 transcript levels in the wild type and athak5 mutant alleles. A total of 20 μg of RNA was isolated from 7-d-old seedlings grown on medium containing 50 μm K+. D, Fresh weight of 7-d-old wild-type and athak5 mutant plants. Each bar represents the mean fresh weight (n = 4) of 25 seedlings ± sd. E, Root length of 7-d old wild-type and athak5 mutant plants. Each bar represents the mean root length (n = 4) of 25 seedlings ± sd. [See online article for color version of this figure.]
Because NH4+ specifically inhibits the non-AKT1 pathway of root high-affinity K+ transport (Santa-Maria et al., 1997; Spalding et al., 1999), we used NH4+-free growth medium as described in “Materials and Methods.” To examine the K+-dependent growth phenotype of athak5 mutant plants, we performed comparative growth analyses of the wild type and athak5 mutant alleles (athak5-1, athak5-2, and athak5-3) subjected to low K+ concentrations in the external medium of 10 to 50 μm (Fig. 1B). When grown on medium containing 10 μm K+, athak5 mutant plants exhibited severe growth defects. However, athak5 mutant plants were indistinguishable from wild-type plants when grown on medium containing concentrations of 20 μm K+ or above (Fig. 1B). However, quantification of K+-dependent plant growth revealed that the total fresh weight of athak5 mutant seedlings was strongly reduced compared with that of wild-type plants when grown on 10 μm K+ and was little but significantly reduced on medium containing concentrations of 20 μm K+ or above (Fig. 1D). Conversely, root growth of athak5 mutant plants was significantly reduced compared with the wild type only at 10 μm K+ (Fig. 1E). At concentrations of 20 μm K+ and above, root length of athak5 mutants was similar to the wild type after 7 d of growth. All of the three independent athak5 mutant alleles displayed the same phenotype, indicating that the K+-dependent growth phenotype of athak5 mutant plants results from loss of function of the AtHAK5 gene. These results indicate that AtHAK5, as a major component of root high-affinity K+ uptake, has a significant impact on seedling growth at 10 μm K+ or below.
athak5 akt1 Double Mutant Plants Fail to Establish under Low-K+ Conditions
It has been shown earlier that akt1-1 mutant plants do not grow on medium containing micromolar concentrations (≤100 μm) of K+ in the presence of 2 mm NH4+. However, growth of akt1-1 mutant plants was similar to the wild type in the absence of NH4+. In this study, we found that the athak5 mutant plants could not grow on medium containing 10 μm K+ in the absence of NH4+. Because athak5 mutant plants possess a functional AKT1 channel protein, we hypothesized that a more severe phenotype may occur for athak5 akt1 double mutants than for athak5 single mutants at 10 μm K+. To test this hypothesis, we generated athak5 akt1 double mutants by crossing homozygous lines of these two mutants. Seeds of akt1-1 mutants (CS3762) were obtained from the Arabidopsis Biological Resource Center. We generated double mutants between akt1-1 and three athak5 alleles (athak5-1, athak5-2, and athak5-3). The F1 seeds were grown and allowed to self-fertilize to produce a population of F2 plants. We determined the genotypes of the F2 plants by genomic PCR for AtHAK5 and AKT1. Because the athak5 mutant is in the ecotype Columbia (Col-0) background and akt1-1 is in the ecotype Wassilewskija (Ws-2) background, the resulting progeny represented a mixed Col-0/Ws-2 genetic background. To correct for possible ecotype effects on plant phenotypes, we isolated the wild type, athak5-1, athak5-2, athak5-3, and akt1-1 single mutants, and athak5-1 akt1-1, athak5-2 akt1-1, and athak5-3 akt1-1 double mutants from F2 Col-0 × Ws-2 crosses. Therefore, all lines used in the double mutant experiments represent a mix of Col/Ws ecotypes. Since the athak5-3 cross was the first to yield a mutant homozygous for both T-DNA insertions, all analyses were initially performed with the athak5-3 akt1-1 double mutant and are referred to as athak5 akt1 for simplicity.
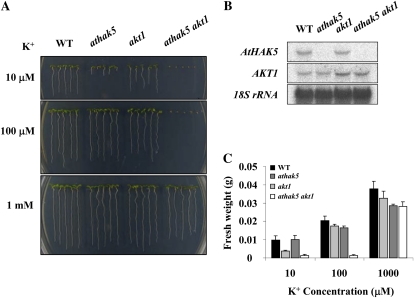
To examine the K+-dependent growth phenotype of athak5 akt1 double mutant plants, we analyzed wild-type, athak5, akt1, and athak5 akt1 plants on plates containing a range of K+ concentrations (Fig. 2A). Interestingly, athak5 akt1 double mutants failed to grow on medium containing up to 100 μm K+. This phenotype was more severe than expected. Quantification of K+-dependent seedling growth revealed significantly reduced fresh weight of athak5 akt1 mutants at 10 and 100 μm K+ and of athak5 at 10 μm K+ (Fig. 2C). athak5 single mutant plants in the Col/Ws background displayed the same growth defect as athak5-3 mutants (Col-0 background; Figs. 1B and 2A). However, when grown on medium containing 1 mm K+, we did not observe significant differences in growth between the wild type and athak5 akt1 double mutant plants. Northern-blot analysis confirmed that full-length transcripts of both AtHAK5 and AKT1 were absent in the athak5 akt1 double mutants (Fig. 2B), but we observed a truncated AKT1 transcript in akt1 mutant plants, consistent with the initial akt1-1 characterization (Hirsch et al., 1998).
Figure 2.
The K+ dependence of the athak5 akt1 double mutant. A, Wild-type (WT), athak5, akt1, and athak5 akt1 plants grown for 7 d on medium containing KCl at the concentrations indicated. B, Northern-blot analysis of AtHAK5 and AKT1 transcript levels in the wild type, athak5, akt1, and athak5 akt1. Seven-day-old seedlings on medium containing 1 mm K+ were transferred to a medium containing 50 μm K+ and allowed to grow for 1 d. C, Fresh weight of 7-d old wild-type, athak5, akt1, and athak5 akt1 plants. Each bar represents the mean fresh weight (n = 4) of 25 seedlings ± sd. [See online article for color version of this figure.]
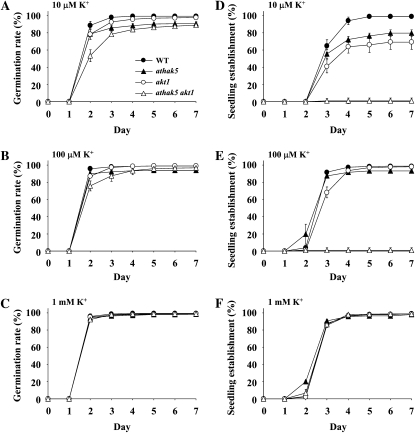
Because the athak5 akt1 double mutant failed to grow on medium containing 100 μm K+ or less, we performed a time-course germination assay with wild-type, single mutant, and double mutant plants. For these experiments, all seeds were harvested at the same time to minimize the effect of seed storage conditions and were evenly spaced on plates to avoid spatial variations. Germination starts with water uptake of the dehydrated seed and finishes with the rupture of the seed coat, where radicle emergence is the visible sign of germination completion (Bewley and Black, 1994; Bentsink and Koornneef, 2008). When seed germination was scored as radicle emergence, the athak5 akt1 double mutant showed a slight delay in germination but approached levels similar to the wild type after 7 d (Fig. 3, A–C). However, when cotyledon emergence was scored, athak5 akt1 double mutants were significantly different from the wild type (Fig. 3, D–F). Under low-K+ conditions (≤100 μm K+), wild-type and athak5 and akt1 single mutant plants showed green cotyledons at day 3 after sowing, whereas none of the athak5 akt1 double mutants showed green cotyledons at this time (Fig. 3, D and E). In contrast, at 1 mm K+, there were no observable differences in germination rate and cotyledon emergence between the wild type and any of the mutants (Fig. 3, C and F).
Figure 3.
Time-course assay of seed germination for the athak5 akt1 double mutant. Seeds were plated on medium containing 0.01 mm (A and D), 0.1 mm (B and E), and 1 mm (C and F) KCl. The number of germinated seeds scored daily after transfer to 22°C is shown. Germination was calculated based on radicle emergence (A–C) and cotyledon appearance (D–F). Error bars show sd (n = 4). WT, Wild type.
athak5 akt1 Double Mutants Displayed K+ Concentration-Dependent Seedling Growth
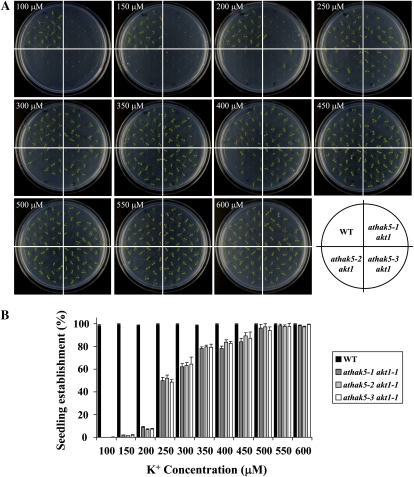
To further characterize the athak5 akt1 double mutant, we compared seedling establishment scored as cotyledon emergence and growth of wild-type and double mutant plants at a range of K+ concentrations (Fig. 4). As expected, 100% of wild-type seeds germinated, and seedlings established in any condition investigated. However, all athak5 akt1 double mutant lines showed a K+ concentration-dependent growth phenotype. At 200 μm K+, only 10% of athak5 akt1 double mutants developed green cotyledons, which increased to about 60% at 300 μm K+, showed an impairment at 400 μm K+, and reached wild-type levels at 500 μm K+ and above (Fig. 4B). Since cotyledon emergence of akt1 single mutants was different from the wild type at K+ concentrations of 100 μm and above (Fig. 3), these results indicate that AtHAK5, which is active and even increased in its expression in akt1-1 mutants (Qi et al., 2008), can be important for plant growth at K+ concentrations up to 450 μm. Since the K+-dependent growth response was similar for all three athak5 akt1 mutant lines, the severe double mutant phenotype can be attributed to the simultaneous functional loss of AtHAK5 and AKT1.
Figure 4.
Effect of K+ on seedling establishment of the athak5 akt1 double mutant. Seeds were plated on medium containing various concentrations of KCl in the absence of NH4+. Seedling establishment was scored based on cotyledon emergence 7 d after transfer to 22°C. Error bars show sd (n = 4). WT, Wild type. [See online article for color version of this figure.]
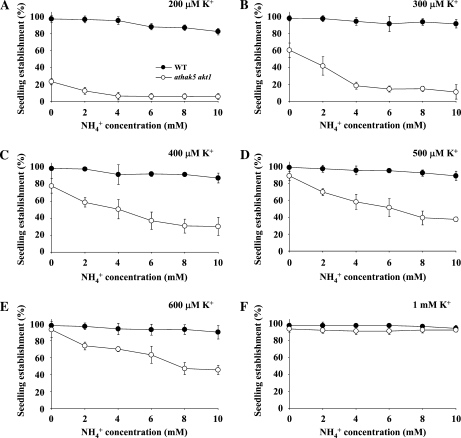
It has been demonstrated previously that high NH4+ concentrations have an inhibitory effect on K+ uptake mediated by KUP/HAK/KT transporters (Santa-Maria et al., 1997; Spalding et al., 1999). To determine whether K+ uptake in the athak5 akt1 double mutant is affected by NH4+ at K+ concentrations between 200 and 600 μm, we measured seedling establishment of the wild type and athak5 akt1 double mutants in the presence of increasing NH4+ concentrations (Fig. 5; Supplemental Fig. S1). Figure 5 shows that seedling establishment of the athak5 akt1 double mutant was strongly inhibited by NH4+, while that of the wild type was only moderately affected. On plates supplemented with 200 μm K+ without NH4+, seedling establishment of the wild type and the athak5 akt1 double mutant was about 97% and 23%, respectively (Fig. 5A). However, in the presence of 4 mm NH4+ or above, seedling establishment of the athak5 akt1 double mutant was further reduced to about 5%.
Figure 5.
Effect of NH4+ on seedling establishment of the athak5 akt1 double mutant. Seeds were plated on medium containing various concentrations of K+ and NH4+. Seedling establishment was calculated based on cotyledon appearance. Error bars show sd (n = 4). WT, Wild type.
Interestingly, the athak5 akt1 double mutant is more sensitive to NH4+ than the wild type at intermediate K+ concentrations (300–600 μm; Fig. 5, B–E); that is, the presence of NH4+ at any K+ concentration leads to reduced seedling establishment in athak5 akt1 double mutants. Even at 600 μm K+, a concentration where no difference could be observed without NH4+ (Fig. 4), seedling establishment is still reduced to 45% in the presence of 10 mm NH4+ (Fig. 5E). In contrast, wild-type seedling establishment remains close to 100% (Fig. 5). Therefore, these results may suggest that an uncharacterized, NH4+-sensitive uptake mechanism is active at intermediate K+ concentrations in roots in addition to AtHAK5 and AKT1. However, increased NH4+ sensitivity of athak5 akt1 double mutants might also point to a role in coping with toxic NH4+ concentrations for AtHAK5 and AKT1.
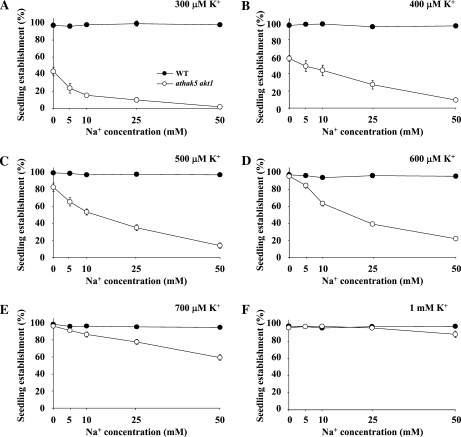
In addition to showing an increased NH4+ sensitivity, athak5 akt1 double mutants also displayed hypersensitivity when exposed to high Na+ concentrations (5–50 mm) in the presence of intermediate K+ concentrations (Fig. 6; Supplemental Fig. S2). Seedling establishment of athak5 akt1 double mutants was gradually reduced with increasing Na+ concentrations in the medium compared with the wild type at K+ concentrations between 300 and 700 μm (Fig. 6, A–E). At 1 mm K+ in the medium, seedling establishment at the different Na+ concentrations was almost indistinguishable between athak5 akt1 double mutants and the wild type (Fig. 6F).
Figure 6.
Effect of Na+ on seedling establishment of the athak5 akt1 double mutant. Seeds were plated on medium containing various concentrations of K+ and Na+. Seedling establishment was calculated based on cotyledon appearance. Error bars show sd (n = 4). WT, Wild type.
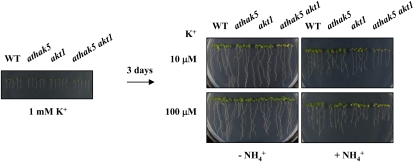
athak5 akt1 Double Mutants Are Hypersensitive to 10 μm But Not to 100 μm K+ after Transfer from K+-Replete Plates
Next, we analyzed the physiological functions of AtHAK5 and AKT1 in postgermination growth. Because growth of athak5 akt1 double mutants would not progress past radicle emergence at low K+ concentrations, we first plated seeds of the wild type, athak5, akt1, and athak5 akt1 on 1 mm K+ medium and then transferred the seedlings to plates containing 10 or 100 μm K+ after 3 d of growth. athak5 akt1 double mutants clearly exhibited leaf chlorosis at 10 μm K+, but plant root length increased similarly although slightly reduced compared with the wild type during the 7-d growth period on 10 μm K+ (Fig. 7). However, no notable difference in growth could be observed between wild-type and athak5 akt1 double mutant seedlings after 7 d of growth at 100 μm K+ in the absence of NH4+. In our postgermination transfer assay, the athak5 akt1 double mutant did not exhibit a significant difference at 10 μm K+ in the absence or presence of NH4+, although general growth inhibition by NH4+ was observed for all plant lines tested (Fig. 7).
Figure 7.
Effect of K+ and NH4+ on postgermination growth of the athak5 akt1 double mutant. Three-day-old seedlings on medium containing 1 mm K+ were transferred to a medium containing 10 or 100 μm K+ in the absence or presence of 2 mm NH4+ and allowed to grow for 7 d. WT, Wild type. [See online article for color version of this figure.]
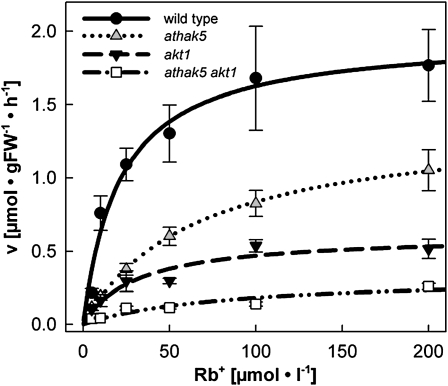
High-Affinity Rb+(K+) Uptake Is Strongly Reduced in Roots of the athak5 akt1 Double Mutant
Analyses of mutant seedling growth on low-K+ medium indicated possible severe impairment in K+ uptake from the external solution in athak5 akt1 double mutants and dependence on at least 10 μm K+ in the growth medium for athak5 single mutants. We directly investigated root K+ uptake in mature mutant and wild-type plants using 86Rb+ as a tracer (Fig. 8; Epstein et al., 1963). The strongest impact on Rb+(K+) uptake kinetics was observed in athak5 akt1 double mutants. Here, high-affinity Rb+(K+) uptake was almost absent, with the Vmax being reduced by 85% (from 1.97 to 0.33 μmol g−1 fresh weight h−1) in double mutant roots (Fig. 8; Table I). Similar results were obtained with athak5-1 akt1-1 double mutants generated from an athak5-1 and akt1-1 cross (Supplemental Fig. S3).
Figure 8.
Kinetics of 86Rb+ uptake in roots of the athak5 akt1 double mutant. 86Rb+ uptake kinetics in roots of intact wild-type, athak5, akt1, and athak5 akt1 plants demonstrate the absence of high-affinity Rb+(K+) uptake in athak5 akt1 double mutants. Hydroponically grown plants were subjected to K+ starvation for 4 d prior to performing uptake experiments. Data represent averages (n = 9–21) ± se from three to six independent experiments. Lines represent results of fitting Michaelis-Menten kinetics to data using the SigmaPlot (SPSS) curve-fitting function (one-term Michaelis-Menten equation). FW, Fresh weight.
Table I. Parameters for Michaelis-Menten kinetics of 86Rb+ uptake data from roots of wild-type, athak5, akt1, and athak5 akt1 plants.
Kinetics were determined by curve fitting with the SigmaPlot 8.0 software (SPSS) using a one-term Michaelis-Menten equation (Fig. 8).
| Parameter | Wild Type | athak5 | akt1 | athak5 akt1 |
| Km (μm) | 21.3 | 65.9 | 30.9 | 82.6 |
| Vmax (μmol g−1 fresh weight h−1) | 1.97 | 1.39 | 0.61 | 0.33 |
Uptake kinetics also revealed that in single mutants, high-affinity K+ uptake is impaired. The impact on the apparent affinity (Km) of root Rb+(K+) uptake was stronger in athak5 than in akt1 roots. While the Km shifted from 20.3 μm in the wild type to 65.8 μm in athak5 roots, it changed to only 30.9 μm in akt1 roots under the imposed conditions, indicating that the ability to absorb Rb+(K+) from dilute solutions was more strongly affected in athak5 roots (Fig. 8; Table I). In contrast, the Vmax, representing the maximal uptake velocity, was more strongly reduced in akt1 (0.61 μmol g−1 fresh weight h−1) than in athak5 (1.39 μmol g−1 fresh weight h−1) roots compared with the wild type (1.97 μmol g−1 fresh weight h−1; Fig. 8; Table I). These data strongly indicate that AtHAK5 and AKT1 are the two major molecular components mediating high-affinity K+ uptake in Arabidopsis roots.
DISCUSSION
In this study, we aimed at characterizing the molecular basis of high-affinity K+ uptake into plant roots. Since many plant K+ transporters have been identified, the high-affinity K+ transport system may consist of more than one transporter. In Arabidopsis, two membrane proteins, AtHAK5 and AKT1, have been previously shown to contribute to high-affinity K+ uptake in roots (Hirsch et al., 1998; Spalding et al., 1999; Gierth et al., 2005; Rubio et al., 2008). Here, we provide physiological evidence that AtHAK5 is important for seedling establishment and growth on low-K+ medium and that AtHAK5 and AKT1 are the two essential molecular entities mediating high-affinity K+ uptake into Arabidopsis roots.
AtHAK5 Is a Major Component of the Non-AKT1 Pathway
Among the many K+ transporters encoded in the Arabidopsis genome, members of the KUP/HAK/KT transporter family have commonly been hypothesized to be involved in root high-affinity K+ uptake as a component of the non-AKT1 pathway (Quintero and Blatt, 1997; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998). Because of the high number of KUP/HAK/KT transporters, functional diversity and redundancy among these transporters may be expected and the presence of multiple pathways for high-affinity K+ uptake has long been predicted (Epstein et al., 1963). Of the 13 KUP/HAK/KT transporters, it has been shown that AtHAK5 constitutes the low-K+-inducible component of root high-affinity K+ uptake, because AtHAK5 mRNA abundance is strongly induced by external K+ conditions (Ahn et al., 2004; Shin and Schachtman, 2004; Gierth et al., 2005) and AtHAK5 expression is confined to the epidermis of main and lateral roots (Gierth et al., 2005). In previous reports, direct Rb+ uptake and also K+ depletion studies with athak5 mutants suggested that AtHAK5 is a major contributor to the total high-affinity K+ uptake capacity in roots (Gierth et al., 2005; Rubio et al., 2008). To gain insight into the root high-affinity K+ transport system, we first analyzed the physiological relevance of AtHAK5 for K+ nutrition by investigating the K+-dependent growth of athak5 mutants. We found that athak5 mutants displayed a reduction of root growth at 10 μm K+ when NH4+ was absent (Fig. 1). However, root growth of athak5 mutants was similar to that of the wild type when K+ concentrations were higher than 20 μm. Recently, it has been reported that AtHAK5 is essential for plant growth at very low K+ conditions of 1 μm or below (Qi et al., 2008). The discrepancy between both studies can probably be explained by differences in medium composition and culture or experimental conditions, as has been demonstrated recently for phosphate depletion experiments (Jain et al., 2009). From the K+-dependent growth phenotype of athak5 mutants reported in this study, it can be concluded that AtHAK5 is a major component of the non-AKT1 pathway and is particularly important for plant growth when K+ concentrations are below 10 μm K+.
AtHAK5 and AKT1 Are the Major Transporters of the High-Affinity K+ Transport System
Apart from AtHAK5, AKT1 is considered to contribute to high-affinity K+ uptake in roots. Note, however, that low-affinity transporters also can mediate K+ uptake at micromolar concentrations while saturating at higher K+ concentrations (Epstein et al., 1963) and that inward K+ channel currents show saturation at millimolar K+ concentrations (Schroeder and Fang, 1991; Gierth et al., 2005). AKT1 belongs to the group of Shaker-like K+ channels and is expressed in root hairs, epidermis, cortex, and endodermis cells of mature roots (Lagarde et al., 1996). Previous studies on inward-rectifying K+ channels pointed to the model that these K+ channels could adjust their apparent affinities (Km) for K+ from the low-affinity to the high-affinity range, depending on the presence of other membrane transporters and experimental conditions (Schroeder and Fang, 1991; Schroeder et al., 1994; Duby et al., 2008). For example, it was proposed that blocking or genetically disabling other high-affinity K+ transporters would shift inward-rectifying K+ channels to higher affinities (Schroeder and Fang, 1991; Schroeder et al., 1994). Indeed, the dependence of the apparent K+ affinity for AKT1-mediated root K+ uptake on blocking other transporters with NH4+ (Spalding et al., 1999) or on disruption of AtHAK5 (Gierth et al., 2005; Qi et al., 2008) provides experimental evidence for this early model, showing that disabling of other transporters shifts the apparent uptake affinity of AKT1 to Km values ranging from about 0.8 mm to 20 μm. Recently, the identification of AKT1 regulation through interaction with CBL/CIPK proteins (Li et al., 2006; Xu et al., 2006) and heterotetramer formation with AtKC1 (Geiger et al., 2009) has provided new insights into the molecular nature of this complex K+ channel activity modulation.
Because athak5 mutants have a functional AKT1 protein, we speculated that the athak5 akt1 double mutant would show an aggravated phenotype at low K+ in the absence of NH4+. Analyses of germination and seedling growth of athak5 akt1 double mutants showed that double mutant seeds, when scored as radicle emergence, germinated at a rate slightly reduced but still comparable to the wild type (Fig. 3, A–C). However, athak5 akt1 plants were unable to establish (i.e. to unfold cotyledons and grow further) at K+ concentrations of up to 100 μm (Figs. 3 and 4), and seedling growth was still impaired at K+ concentrations of up to 400 μm (Fig. 4).
Seed germination, noticeable as radicle emergence, depends on cell expansion, which is driven by passive water uptake during seed imbibition (Bewley and Black, 1994). Dehydrated seeds contain storage reserves of mineral nutrients like K+ that contribute to the osmotic potential in germinating seeds and hence to water uptake. These storage reserves can be used during the initial phase of embryo axis elongation, culminating in seed coat rupture and radicle emergence. Subsequently, the seedling needs to absorb K+ and other mineral nutrients from the external medium in order to sustain growth, since storage reserves are limited. athak5 akt1 mutants did not display an impaired ability to germinate with respect to radicle emergence (Fig. 3, A–C) but appeared to be unable to absorb K+ from low-K+ medium in quantities necessary for progression of seedling growth. Our data thus show that AtHAK5 and AKT1 are essential for postgermination seedling growth and development. Data also show that the functional disruption of these proteins cannot be compensated by other K+ transporters expressed in roots like TRH1/AtKT3 (Rigas et al., 2001; Desbrosses et al., 2003) or AtKC1 (Reintanz et al., 2002; Bregante et al., 2008; Duby et al., 2008; Geiger et al., 2009).
The double mutants analyzed in this study were generated in a mixed Col/Ws background. In general, genetic variation among Arabidopsis ecotypes has been shown to result in quantitative traits of physiological importance (Koornneef et al., 2004). However, all athak5 akt1 crosses showed essentially the same K+-dependent phenotype (Fig. 4), indicating that an impact on phenotype occurrence due to Arabidopsis ecotype differences was unlikely.
In addition to the observed early seedling phenotype, athak5 akt1 double mutants exhibited a K+-dependent phenotype when transferred from high-K+ to low-K+ conditions. Following transfer after 3 d of growth at 1 mm K+, we found that the athak5 akt1 double mutant plants showed K+ deficiency symptoms at 10 μm but grew similar to the wild type at 100 μm K+ in the absence of NH4+ (Fig. 7). During preculture on 1 mm K+, athak5 akt1 double mutants are probably able to accumulate considerable amounts of K+, which can be used during subsequent starvation periods to sustain growth. The obvious, similar-to-wild-type increase in biomass of athak5 akt1 double mutants after transfer to 10 μm K+ (Fig. 7) would support this interpretation. However, while at 10 μm K+ these K+ reserves are rapidly depleted, leading to K+ deficiency symptoms, at 100 μm K+ no deficiency symptoms could be observed. This indicates that under these conditions, residual K+ uptake might occur that is sufficient to prevent the occurrence of deficiency symptoms in plants precultured in K+-replete conditions but is insufficient to supply adequate K+ to plants when grown completely in low-K+ conditions (Figs. 2–4). Rb+(K+) uptake kinetics in roots of mature double mutant plants demonstrated the presence of a minute, residual Rb+(K+) uptake activity (Fig. 8).
Rb+ uptake studies also revealed that high-affinity K+ uptake in roots of athak5 akt1 double mutants was almost completely absent (Fig. 8). The apparent absence of high-affinity uptake in athak5 akt1 double mutants using Rb+ as a tracer for K+ could also be expected if an additional high-affinity uptake system was present that would strongly discriminate between K+ and Rb+. However, the severe growth phenotype of athak5 akt1 double mutants on low-K+ medium (Figs. 3 and 4) excludes this possibility. Furthermore, root inward-rectifying K+ channel currents as well as KUP/HAK/KT transporters have been shown to have clear Rb+ permeabilities (Maathuis and Sanders, 1995; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998), suggesting that a different class of K+ transporter would be needed for this activity. Under low-K+ conditions, the Rb+ uptake activity of athak5 akt1 double mutants compared with the wild type was about 15% (Vmax reduced from 1.97 μmol g−1 fresh weight h−1 in wild-type roots to 0.33 μmol g−1 fresh weight h−1 in athak5 akt1 double mutant roots; Fig. 8; Table I). Although a relatively high apparent affinity could be calculated from the residual uptake kinetics (Km = 82 μm; Table I), this remaining K+ uptake is obviously insufficient to provide adequate amounts of K+ to double mutant plants under low-K+ conditions (Figs. 2 and 7). While this article was being revised, a study investigating athak5 akt1 double mutants became available (Rubio et al., 2010) that, in agreement with our results, reports strongly reduced Rb+ accumulation in double mutant roots. Here, we applied short-term 86Rb+ tracer experiments to determine unidirectional uptake, which allows for detailed analyses of kinetic parameters (Ussing, 1969; Sten-Knudsen and Ussing, 1981) and comparison with classical studies of K+ uptake (Epstein et al., 1963; Kochian and Lucas, 1982; Siddiqi and Glass, 1983).
The uptake kinetics in athak5 and akt1 single mutants indicated impairment in high-affinity K+ uptake components in roots of each mutant line; however, the impact on Km and Vmax was different between athak5 and akt1 roots. The Km increased from 21.3 μm in wild-type roots to 65.9 μm in athak5 roots, a value approaching the apparent affinity in double mutant roots (Fig. 8; Table I). In contrast, in akt1 roots the effect on the apparent affinity was rather low (increasing to 30.9 μm in akt1 roots; Table I). This shows that the ability to absorb K+ from dilute solutions is much more strongly affected in athak5 than in akt1 roots, congruent with previous results showing a higher apparent K+ affinity for AtHAK5 compared with AKT1 (Gierth et al., 2005) and with data obtained from Rb+(K+) depletion experiments using athak5 and akt1 single mutants (Rubio et al., 2008). Moreover, our results using single and double mutants indicate that the potential of the plant to compensate high-affinity uptake mediated by AtHAK5 through posttranslational regulation of AKT1 (Li et al., 2006; Xu et al., 2006; Geiger et al., 2009) is limited.
On the other hand, the maximum capacity of K+ uptake is more strongly affected in akt1 roots (Vmax decreased from 1.97 μmol g−1 fresh weight h−1 in wild-type roots to 0.61 μmol g−1 fresh weight h−1 in akt1 roots) than in athak5 roots (decreased to 1.39 μmol g−1 fresh weight h−1; Fig. 8; Table I). Since AKT1 is active in athak5 roots, these data suggest that under the appropriate conditions (i.e. moderately low or intermediate K+ concentrations and very negative plasma membrane potential), K+ uptake through AKT1 may exceed uptake through AtHAK5 in terms of the absolute amount K+ absorbed per unit of time and fresh weight. However, since growth of akt1 mutants was indistinguishable from wild-type plants in the presence of 100 μm K+ (Fig. 2; Hirsch et al., 1998), AtHAK5-mediated uptake appears to be sufficient for K+ nutrition also under moderately low K+ concentrations.
For neither of the mutants could an impact on plant growth be observed at external K+ concentration of 1 mm (Fig. 2), indicating that at millimolar external concentrations additional K+ uptake is conferred by yet unidentified transporters that could also contribute to K+ acquisition at intermediate (i.e. approximately 400 μm) K+ concentrations but not at 100 μm K+ (Fig. 4). Our results showing that postgermination growth of athak5 akt1 mutants at intermediate K+ concentrations could be further inhibited in the presence of high NH4+ concentrations (Fig. 5; Supplemental Fig. S1) might provide a first indication for the nature of the transporters involved. Concomitantly, the increased Na+ sensitivity of athak5 akt1 double mutants (Fig. 6; Supplemental Fig. S2) may indicate that for this unidentified transporter, high external Na+ is a potent competitor for K+ when K+ is present at intermediate concentrations. Future research investigating the impact in mutants completely lacking the component of K+ uptake operating at low and intermediate concentrations will provide further insight into the overall importance of AtHAK5 for K+ nutrition.
In conclusion, we have shown that AtHAK5 is essential for plant vitality in very low-K+ conditions, which is supported by the dramatic reduction in Rb+(K+) uptake and mutant phenotype analyses, and that AtHAK5 and AKT1 in conjunction provide the physiologically relevant means of sustaining seedling and plant growth when K+ concentrations in the external medium are limiting.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of the Arabidopsis (Arabidopsis thaliana) AtHAK5 T-DNA insertion lines (athak5-1 [SALK_014177], athak5-2 [SALK_005604; Gierth et al., 2005], and athak5-3 [SALK_130604]) and akt1-1 (Hirsch et al., 1998; CS3762) were obtained from the Arabidopsis Biological Resource Center at Ohio State University. Seeds were surface sterilized, washed, and stored in the dark for 3 d at 4°C to synchronize germination. Plants were grown in K+-free growth medium as described previously (Hirsch et al., 1998). In the NH4+-free growth medium, NH4H2PO4 was substituted by H3PO4. Different concentrations of K+ were supplemented by adding KCl. All media were solidified with 0.7% (w/v) agarose (USB). The average K+ concentration of the agarose was determined as 15 ± 1 mg L−1 by inductively coupled plasma mass spectrometry, leading to a K+ background of 2.5 to 2.8 μm in nominally K+-free 0.7% agarose plates. Plates were placed vertically in a growth chamber set to deliver 16 h of light and 8 h of dark at 22°C. For low-K+ treatment, 7-d-old seedlings grown on high-K+ medium (1 mm K+) were transferred to high-K+ (1 mm) or low-K+ (50 μm) liquid medium for 1 d.
Northern-Blot Analysis
Total RNA was extracted from 7-d-old seedlings using easy-BLUE Total RNA Extraction Kit (iNtRON Biotechnology) following the manufacturer's protocol. RNA (20 μg per lane) was separated on MOPS-formaldehyde agarose gels and transferred to a nitrocellulose membrane. The 32P-labeled probes were prepared using a random priming method. Prehybridization, hybridization, and washes were performed as described by Sambrook and Russell (2001). Signals were developed using the FUJI BAS-2500 system (Fuji Film).
Root Growth Assay
To measure root growth, seeds were plated to a medium containing various concentrations of K+. The plates were placed vertically and photographed after 7 d. Root length was measured using image-analysis software (Scion Image 4.02). Three replicates of 25 seedlings were grown on medium containing various concentration of K+. For postgermination growth, 3-d-old wild-type, athak5, akt1, and athak5 akt1 plants grown vertically on medium containing 1 mm K+ were transferred to medium containing 10 and 100 μm K+ in the presence or absence of 2 mm NH4+ and allowed to grow for 7 d.
Generation of the athak5 akt1 Double Mutants
The athak5 akt1 double mutants were generated by crossing athak5-1, athak5-2, and athak5-3 with akt1-1. Homozygous individuals were isolated in the F2 progeny by PCR genotyping. Subsequently, the double mutant was verified by northern-blot analysis. For physiological analysis, seed pools from three to four homozygous individuals were used. For simplicity, the athak5 akt1 double mutant nomenclature in this report refers specifically to the athak5-3 akt1-1 double mutant.
Germination Assay
Seeds were surface sterilized, washed, and stored in the dark for 3 d at 4°C to synchronize germination. Approximately 100 seeds from the wild type, athak5, akt1, and athak5 akt1 were planted on medium containing different concentrations of K+. Plates were placed horizontally in a growth chamber set to deliver 16 h of light and 8 h of dark at 22°C. Time-course assay of seed germination was scored daily for 7 d. K+ concentration-dependent germination rate was scored at 7 d. For determination of the effects of NH4+ on seedling establishment, plants were grown in NH4+-free medium as described previously (Spalding et al., 1999). Different concentrations of K+ and NH4+ were supplemented by adding KCl and NH4H2PO4, respectively. For determination of the effects of Na+ on seedling establishment, plants were grown in K+-free medium as described previously (Hirsch et al., 1998) with minor modifications. NH4H2PO4 was substituted by H3PO4. K+ was added as KCl, and Na+ was added as NaCl.
86Rb+ Uptake Experiments
For determination of 86Rb+ uptake kinetics, plants were cultivated on a hydroponic system consisting of plastic containers holding 3 L of aerated nutrient solution as described previously (Gierth et al., 2005). Plant culture, composition of the nutrient and the radiolabeled uptake solution, and setup of uptake experiments were as described by Gierth et al. (2005). In brief, intact, K+-starved (4 d) plants were immersed into 10 mL of uptake solution consisting of K+-free nutrient solution supplemented with RbCl. Radioactive 86Rb+ was added at a concentration of 1 μCi μmol−1 Rb+. At the end of the 15-min uptake period, plants were surface dried with tissue paper and immediately washed in a solution consisting of K+-free nutrient solution supplemented with 1.75 mm nonradiolabeled RbCl (4°C, two times for 2 min). Plants were separated into roots and shoots, surface dried, and the root fresh weight was determined. Incorporated radioactivity was measured after addition of water with a scintillation counter (LS 6500; Beckman Coulter). 86Rb+ was purchased from Perkin-Elmer.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of NH4+ on seedling establishment of athak5 akt1 double mutant.
Supplemental Figure S2. Effect of Na+ on seedling establishment of athak5 akt1 double mutant.
Supplemental Figure S3. 86Rb+ uptake in athak5-1 akt1-1 mutants.
Supplementary Material
Acknowledgments
We thank Sonja Hetfeld (University of Cologne) for excellent technical assistance with hydroponics and 86Rb+ uptake experiments, and Marcel Bucher and Aleksandra Polatajko (both University of Cologne) for inductively coupled plasma mass spectrometry analyses.
References
- Ahn SJ, Shin R, Schachtman DP. (2004) Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol 134: 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 89: 3736–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengaud P, Breitling R, Amtmann A. (2004) The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol 136: 2556–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley MK, Grant M, Grabov A. (2006) Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot 57: 425–436 [DOI] [PubMed] [Google Scholar]
- Bentsink L, Koornneef M. (2008) Seed dormancy and germination. The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, pp 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. (1994) Seeds: Physiology of Development and Germination. Plenum Press, New York [Google Scholar]
- Bregante M, Yang Y, Formentin E, Carpaneto A, Schroeder JI, Gambale F, Lo SF, Costa A. (2008) KDC1, a carrot Shaker-like potassium channel, reveals its role as a silent regulatory subunit when expressed in plant cells. Plant Mol Biol 66: 61–72 [DOI] [PubMed] [Google Scholar]
- Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI. (2000) Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiol 122: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbrosses G, Josefsson C, Rigas S, Hatzopoulos P, Dolan L. (2003) AKT1 and TRH1 are required during root hair elongation in Arabidopsis. J Exp Bot 54: 781–788 [DOI] [PubMed] [Google Scholar]
- Duby G, Hosy E, Fizames C, Alcon C, Costa A, Sentenac H, Thibaud JB. (2008) AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J 53: 115–123 [DOI] [PubMed] [Google Scholar]
- Elumalai RP, Nagpal P, Reed JW. (2002) A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell 14: 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Bloom AJ. (2005) Mineral Nutrition of Plants: Principles and Perspectives. Sinauer Associates, Sunderland, MA [Google Scholar]
- Epstein E, Rains DW, Elzam OE. (1963) Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA 49: 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HH, Luan S. (1998) AtKuP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell 10: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgenzi FR, Peralta ML, Mangano S, Danna CH, Vallejo AJ, Puigdomenech P, Santa-Maria GE. (2008) The ionic environment controls the contribution of the barley HvHAK1 transporter to potassium acquisition. Plant Physiol 147: 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W, Schroeder JI. (1994) Inward-rectifying K+ channels in root hairs of wheat: a mechanism for aluminum-sensitive low-affinity K+ uptake and membrane potential control. Plant Physiol 105: 1399–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Becker D, Vosloh D, Gambale F, Palme K, Rehers M, Anschuetz U, Dreyer I, Kudla J, Hedrich R. (2009) Heteromeric AtKC1/AKT1 channels in Arabidopsis roots facilitate growth under K+ limiting conditions. J Biol Chem 284: 21288–21295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M, Mäser P. (2007) Potassium transporters in plants: involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 581: 2348–2356 [DOI] [PubMed] [Google Scholar]
- Gierth M, Mäser P, Schroeder JI. (2005) The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137: 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM. (1983) Regulation of ion-transport. Annu Rev Plant Physiol Plant Mol Biol 34: 311–326 [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280: 918–921 [DOI] [PubMed] [Google Scholar]
- Jain A, Poling MD, Smith AP, Nagarajan VK, Lahner B, Meagher RB, Raghothama KG. (2009) Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol 150: 1033–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI. (1998) AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 10: 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. (1982) Potassium transport in corn roots. 1. Resolution of kinetics into a saturable and linear component. Plant Physiol 70: 1723–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D. (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55: 141–172 [DOI] [PubMed] [Google Scholar]
- Kourie J, Goldsmith MHM. (1992) K+ channels are responsible for an inwardly-rectifying current in the plasma membrane of mesophyll protoplasts of Avena sativa. Plant Physiol 98: 1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI. (2001) Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol 127: 473–485 [PMC free article] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C. (1996) Tissue specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9: 195–203 [DOI] [PubMed] [Google Scholar]
- Lebaudy A, Very AA, Sentenac H. (2007) K+ channel activity in plants: genes, regulations and functions. FEBS Lett 581: 2357–2366 [DOI] [PubMed] [Google Scholar]
- Li L, Kim BG, Cheong YH, Pandey GK, Luan S. (2006) A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJ. (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12: 250–258 [DOI] [PubMed] [Google Scholar]
- Maathuis FJ, Sanders D. (1999) Plasma membrane transport in context: making sense out of complexity. Curr Opin Plant Biol 2: 236–243 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. (1995) Contrasting roles in ion transport of two K+-channel types in root cells of Arabidopsis thaliana. Planta 197: 456–464 [DOI] [PubMed] [Google Scholar]
- MacKinnon R. (2003) Potassium channels. FEBS Lett 555: 62–65 [DOI] [PubMed] [Google Scholar]
- Martinez-Cordero MA, Vicente M, Francisco R. (2004) Cloning and functional characterization of the high-affinity K+ transporter HAK1 of pepper. Plant Mol Biol 56: 413–421 [DOI] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D, et al. (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N, Satter RL. (1989) K+ channels in plasmalemma of motor cells of Samanea saman. Dainty J, Michelis MI, Marré E, Rasi-Coldogno F, , Plant Membrane Transport. Elsevier, Amsterdam, pp 529–530 [Google Scholar]
- Qi Z, Hampton CR, Shin R, Barkla BJ, White PJ, Schachtman DP. (2008) The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J Exp Bot 59: 595–607 [DOI] [PubMed] [Google Scholar]
- Quintero FJ, Blatt MR. (1997) A new family of K+ transporters from Arabidopsis that are conserved across phyla. FEBS Lett 415: 206–211 [DOI] [PubMed] [Google Scholar]
- Reintanz B, Szyroki A, Ivashikina N, Ache P, Godde M, Becker D, Palme K, Hedrich R. (2002) AtKC1, a silent Arabidopsis potassium channel alpha-subunit modulates root hair K+ influx. Proc Natl Acad Sci USA 99: 4079–4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann K, Grabov A, Dolan L, Hatzopoulos P. (2001) Trh1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 13: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Aleman F, Nieves-Cordones M, Martinez V. (2010) Studies on Arabidopsis athak5, atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K uptake. Physiol Plant (in press) [DOI] [PubMed] [Google Scholar]
- Rubio F, Nieves-Cordones M, Aleman F, Martinez V. (2008) Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol Plant 134: 598–608 [DOI] [PubMed] [Google Scholar]
- Rubio F, Santa-Maria GE, Rodriguez-Navarro A. (2000) Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol Plant 109: 34–43 [Google Scholar]
- Sambrook J, Russell DW. (2001) Molecular Cloning: A Laboratory Manual, Ed 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Santa-Maria GE, Rubio F, Dubcovsky J, Rodriguez-Navarro A. (1997) The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 9: 2281–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. (1992) Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 258: 1654–1658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Fang HH. (1991) Inward-rectifying K+ channels in guard cells provide a mechanism for low-affinity K+ uptake. Proc Natl Acad Sci USA 88: 11583–11587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hedrich R, Fernandez JM. (1984) Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature 312: 361–362 [Google Scholar]
- Schroeder JI, Raschke K, Neher E. (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W. (1994) Perspectives in the physiology and structure of inward rectifying K+ channels in higher-plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct 23: 441–471 [DOI] [PubMed] [Google Scholar]
- Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flügge UI, Kunze R. (2003) ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol 131: 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C. (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science 256: 663–665 [DOI] [PubMed] [Google Scholar]
- Shin R, Schachtman DP. (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA 101: 8827–8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MY, Glass ADM. (1983) Studies of the growth and mineral nutrition of barley varieties. 2. Potassium uptake and its regulation. Can J Bot 61: 1551–1558 [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ, Rufty TW. (1990) Studies of the uptake of nitrate in barley. I. Kinetics of nitrogen-13-labeled nitrate influx. Plant Physiol 93: 1426–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD. (1999) Potassium uptake supporting plant growth in the absence of AKT1 channel activity: inhibition by ammonium and stimulation by sodium. J Gen Physiol 113: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sten-Knudsen O, Ussing HH. (1981) The flux ratio equation under nonstationary conditions. J Membr Biol 63: 233–242 [DOI] [PubMed] [Google Scholar]
- Ussing HH. (1969) The interpretation of tracer fluxes in terms of membrane structure. Q Rev Biophys 1: 365–376 [DOI] [PubMed] [Google Scholar]
- Wang YH, Garvin DF, Kochian LV. (2002) Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots: evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiol 130: 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Mäser P, Schroeder JI. (2009) Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 71: 59–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.