Abstract
One of the drawbacks in improving the aroma properties of tomato (Solanum lycopersicum) fruit is the complexity of this organoleptic trait, with a great variety of volatiles contributing to determine specific quality features. It is well established that the oxylipins hexanal and (Z)-hex-3-enal, synthesized through the lipoxygenase pathway, are among the most important aroma compounds and impart in a correct proportion some of the unique fresh notes in tomato. Here, we confirm that all enzymes responsible for the synthesis of these C6 compounds are present and active in tomato fruit. Moreover, due to the low odor threshold of (Z)-hex-3-enal, small changes in the concentration of this compound could modify the properties of the tomato fruit aroma. To address this possibility, we have overexpressed the ω-3 fatty acid desaturases FAD3 and FAD7 that catalyze the conversion of linoleic acid (18:2) to linolenic acid (18:3), the precursor of hexenals and its derived alcohols. Transgenic OE-FAD tomato plants exhibit altered fatty acid composition, with an increase in the 18:3/18:2 ratio in leaves and fruits. These changes provoke a clear variation in the C6 content that results in a significant alteration of the (Z)-hex-3-enal/hexanal ratio that is particularly important in ripe OE-FAD3FAD7 fruits. In addition to this effect on tomato volatile profile, OE-FAD tomato plants are more tolerant to chilling. However, the different behaviors of OE-FAD plants underscore the existence of separate fatty acid fluxes to ensure plant survival under adverse conditions.
Tomato (Solanum lycopersicum) breeding has often focused on improving yield, fruit size, and disease resistance, while organoleptic properties have largely been neglected. However, consumer demand for higher nutritional and flavor characteristics in tomato fruits is growing. Despite the complexity of this trait, with multiple biosynthetic pathways contributing, quantitative trait loci that affect volatile composition have been recently identified (Tieman et al., 2006; Mathieu et al., 2009). While proper tomato flavor requires low sugar and acid concentrations, tomato aroma is determined by the contribution of over 400 volatile compounds. The importance of each volatile is determined by both its concentration and its odor threshold (Baldwin et al., 2000). A group of approximately 30 compounds participate, either in a positive or a negative manner, in the properties of tomato aroma. Among them, straight-chain C6 aldehydes and alcohols, such as hexanal, (Z)-hex-3-enal, its isomer (E)-hex-2-enal, and (Z)-hex-3-enol, are the most important to tomato flavor, imparting in a correct proportion some of the unique fresh notes to tomato fruit aroma. Indeed, most appreciated tomato varieties have a higher (Z)-hex-3-enal/hexanal ratio than others less demanded by consumers (Carbonell-Barrachina et al., 2006). Therefore, modifying the (Z)-hex-3-enal/hexanal ratio may be important in the aroma perception of tomato fruits, and since the odor threshold for (Z)-hex-3-enal is low, small changes in the concentration of this compound may exert an important variation in the tomato fruit aroma.
These C6 aldehydes and alcohols belong to the complex group of oxylipins, biologically active compounds derived from the oxygenation of unsaturated fatty acids. From the different fatty acids present in plants, hexanal is produced from linoleic acid (18:2), while linolenic acid (18:3) is the precursor of hexenals and derived alcohols. 18:2 and 18:3 are the most abundant fatty acids in plant membrane lipids. In contrast to the biosynthetic pathways of other components of the tomato aroma, the enzymes that participate in the biosynthesis of hexenals and hexanal have been identified and characterized to a large extent (Feussner and Wasternack, 2002). The high specificity of many of the enzymes involved is a feature of this pathway that determines the final products obtained. The first step of this pathway is the production by a specific lipoxygenase (LOX) of the fatty acid hydroperoxide (HPO), derived either from 18:2 or 18:3. According to the position of oxygen insertion, either at the carbon atom 9 or at the carbon atom 13 of the fatty acid backbone, LOXs are classified as 9-LOX or 13-LOX, respectively. In tomato, there are five genes that encode LOXs (TomLoxAto -E) that are differentially expressed during fruit ripening (Chen et al., 2004). TomLoxA, TomLoxB, and TomLoxE are mainly found in fruits and, although their substrate and product specificity is not clear, likely belong to the 9-LOX group based on their sequence similarities and expression (Griffiths et al., 1999; Chen et al., 2004). On the other hand, TomLoxC and TomLoxD are 13-LOX and show differential expression. While TomLoxC is found in fruits, TomLoxD is mainly expressed in leaves and in response to wounding (Heitz et al., 1997; Chen et al., 2004). Interestingly, the major LOX activity in tomato fruit, close to 95%, has 9-LOX specificity (Hatanaka et al., 1992), and no further enzymatic processing of 9-HPOs has been reported. Since the enzymes responsible for HPO modification in fruits have a preference for 13-HPOs, 9-HPOs accumulate in tomato fruits (Matthew et al., 1977). However, minor 13-LOX activity produces a small quantity of 13-HPOs in the fruits that are further cleaved to C6 aldehydes by the action of 13-hydroperoxide lyases (HPLs). From the aldehydes produced by 13-HPL, (Z)-hex-3-enal, derived from 18:3, contributes the most valuable notes to tomato fruit aroma (Boukobza et al., 2001).
Addition of exogenous 18:3 increases the level of (Z)-hex-3-enal produced by tomato fruit homogenates (Boukobza et al., 2001), suggesting that the enzymes required for the synthesis of this aroma compound are fully functional in fruit tissues and that the abundance of 18:3 may be a limiting step in (Z)-hex-3-enal production. Contrary to the situation in leaves, tomato fruit is more abundant in 18:2, precursor of hexanal, which may represent up to 80% of its fatty acid content (Galliard et al., 1977). Conversion of 18:2 to 18:3 is carried out by membrane-bound ω-3 desaturases. In Arabidopsis (Arabidopsis thaliana), three genes, FAD3, FAD7, and FAD8, encode the enzymes that participate in the synthesis of hexadecatrienoic acid (16:3) and 18:3 from dienoic fatty acids. FAD3 catalyzes the desaturation reaction of 18:2 that takes place in the endoplasmic reticulum. It uses phospholipids as acyl substrates and NADH, NADH-cytochrome b5 reductase, and cytochrome b5 as electron donors. In contrast, FAD7 and FAD8 are located at the chloroplast, providing the majority of the trienoic fatty acids present in the chloroplastic membranes (Wallis and Browse, 2002). They use primarily glycolipids as acyl carriers and NAD(P)H, ferredoxin-NAD(P) reductase, and ferredoxin as electron donors.
Metabolic engineering offers an ideal solution to improve the aroma in tomato fruit by increasing the levels of (Z)-hex-3-enal that provides the highly valued fresh notes. To this end, one possible strategy would be to increase the 13-LOX activity specifically involved in the generation of short-chain aldehyde precursors. However, several independent efforts to overexpress the responsible 13-LOX gene led to cosuppression and the consequent depletion of this specific activity (Leon et al., 2002; Chen et al., 2004). A different approach to address this question is to alter the balance between hexenals and hexanal by overexpressing the ω-3 desaturase to increase the content of 18:3, the hexenal precursor. In addition, tomato being a cold-sensitive crop, modifying the unsaturation level of fatty acids present in membrane lipids could contribute to improve the cold tolerance of tomato plants. It is known that modification of the unsaturation degree of the fatty acids is a significant adaptive feature in response to temperature stress (Somerville and Browse, 1991; Iba, 2002). This increase in the trienoic fatty acids present in membrane lipids upon exposure to chilling temperatures is supposed to maintain the required membrane fluidity and to reduce membrane damage, thus ensuring the numerous processes that take place at cell membranes. This capacity of the plants to withstand chilling temperature is not constant but increases noticeably upon exposure to progressively lower temperatures (Guy, 1990). Interestingly, this cold acclimation increases the desaturase activity and the percentage of unsaturated fatty acids (Steponkus et al., 1993). Since most trienoic acids are present in the thylakoid membranes, where the photosynthetic machinery is found, variation of their unsaturation degree at low temperatures could play an important role in maintaining the photosynthetic capacity of the plants.
We report here that overexpression of ω-3 desaturases FAD3 and FAD7 in transgenic tomato plants results in a modification of the fatty acid composition, with a major increase of the unsaturation ratio 18:3/18:2 in leaves and fruits. This altered fatty acid profile leads to changes in the ratio of the aroma compounds (Z)-hex-3-enal/hexanal in both tissues. Moreover, transgenic tomato plants with higher levels of FAD3 and FAD7 desaturases are more tolerant to chilling temperatures.
RESULTS
Expression of the Enzymes Responsible for Oxylipin-Derived Aroma Compounds in Tomato Fruit
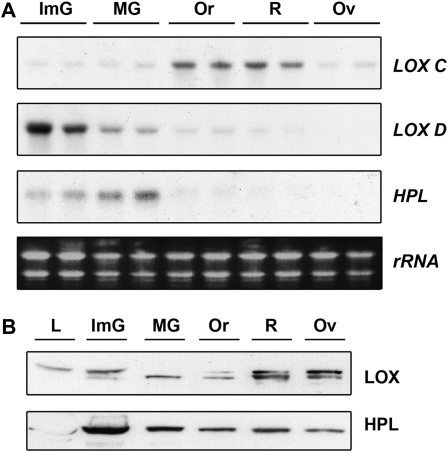
Tomato aroma compounds derived from the oxylipin pathway are synthesized via the subsequent actions of 13-LOX and 13-HPL enzymes on the appropriate fatty acid substrate. Previous reports on the RNA expression pattern of the tomato LOX gene family indicated that these genes are expressed during fruit development and ripening (Heitz et al., 1997; Chen et al., 2004), whereas 13-HPL is expressed predominantly in leaves and flowers (Howe et al., 2000). Yet, 13-HPL activity is also present in tomato fruit homogenates (Boukobza et al., 2001). To clarify these apparently contradictory scenarios, we determined the expression patterns of the 13-LOX genes TomLoxC and TomLoxD and of 13-HPL RNAs during development and ripening of tomato fruits (Fig. 1). TomLoxC RNA was almost undetectable in immature and mature green fruit, but accumulation increased as the fruit developed, reaching a maximum in orange and ripe red fruit and declining in overripe fruit (Fig. 1A). In contrast, TomLoxD mRNA was most abundant in green immature fruit and exhibited a progressive decrease as the fruit developed. At the protein level, immunoblot analysis indicated that 13-LOX enzymes were present at all stages of fruit development and were highest in red and overripe fruit (Fig. 1B). Immunodetection revealed a complex pattern of reacting proteins, suggesting the simultaneous presence of different LOX isoforms.
Figure 1.
Expression of 13-LOX and 13-HPL during tomato fruit ripening. A, Total RNA (10 μg) from immature (ImG), mature green (MG), orange (Or), ripe red (R), and overripe (Ov) fruits was loaded per lane and hybridized with specific probes for 13-LOXs TomLoxC (LOX C) and TomLoxD (LOX D) and HPL. Two biologically independent replicates were loaded for each developmental stage. B, Immunoblot analysis was performed with equal volumes of protein samples from leaves (L) and fruits at different developmental stages and incubated with the polyclonal antibodies indicated on the right. The LOX polyclonal antibody used recognized all tomato LOX isoforms.
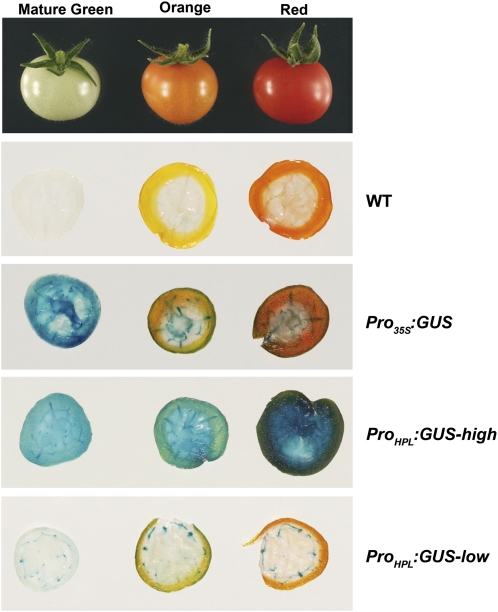
In addition, 13-HPL transcripts were present in green fruit, peaked in mature green fruit, but were absent from ripe fruit (Fig. 1A). At the protein level, 13-HPL levels were highest at the immature green stage and remained essentially constant during subsequent fruit development (Fig. 1B). To confirm this expression pattern, we also generated transgenic tomato plants expressing the uidA gene under the control of the 13-HPL promoter. Histochemical staining of tomato fruits indicated the presence of GUS activity in the different developmental stages studied (Fig. 2). In high-expression lines, GUS staining was present in all fruit tissues, including pericarp, columella, and vascular and placental tissues. Low-expression lines showed a more specific pattern, with GUS staining restricted to vascular bundles.
Figure 2.
Histochemical localization of GUS activity in 13-HPL promoter lines. Tomato fruits at three developmental stages (mature green, orange, and ripe red) were cut transversely and stained for GUS activity as described in “Materials and Methods.” WT, Fruits from nontransformed MicroTom plants; Pro35S, fruits from the constitutive promoter; ProHPL:GUS-high, fruits from a 2-kb promoter transgenic line with high expression level; ProHPL:GUS-low, fruits from a 2-kb promoter transgenic line with low expression level.
Taken together, these data indicate the presence of 13-LOX and 13-HPL proteins in ripe tomato fruits despite low levels of their RNAs in these same tissues and suggest that posttranscriptional processes and/or protein stability may regulate the abundance of oxylipin-synthesizing enzymes.
Molecular Characterization of ω-3 Desaturase Transgenic Plants
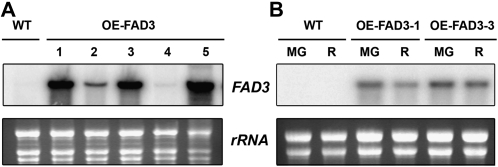
To examine the possibility of modifying the balance between (Z)-hex-3-enal and hexanal by altering the concentration of their precursors 18:3 and 18:2, tomato plants were transformed with the ω-3 desaturase BnFAD3 or StFAD7 cDNAs driven by the constitutive cauliflower mosaic virus (CaMV) 35S promoter. Thirteen transgenic lines carrying the FAD3 construct were obtained, and four of them showed significant accumulation of BnFAD3 mRNA in their leaves (Fig. 3A). Two independent lines, OE-FAD3-1 and OE-FAD3-3, which showed high expression of the transgene, were selected for further characterization. Both lines accumulated BnFAD3 mRNA in mature green and red fruits (Fig. 3B).
Figure 3.
Characterization of OE-FAD3 transgenic tomato lines. A, Total RNA (10 μg) isolated from young fully expanded leaves of representative independent transgenic lines (1, 2, 3, 4, and 5) was fractionated on denaturing agarose gels, transferred to nylon membranes, and hybridized with 32P-radiolabeled BnFAD3. The bottom panel shows the ethidium bromide-stained rRNA as a loading control. B, Total RNA from mature green (MG) and ripe red (R) fruits of the wild type and two representative OE-FAD3 lines hybridized to BnFAD3. WT, Wild type.
To ascertain whether BnFAD3 overexpression resulted in modification of the fatty acid composition, fatty acid analysis was performed using leaves and ripe fruits of OE-FAD3 plants. These transgenic lines showed a marked decrease in the amount of 18:2 accompanied by a slight or no significant increase in 18:3 in leaves and ripe fruits, respectively (Tables I and II). These changes resulted in a modification of the 18:3/18:2 ratio, with leaves from OE-FAD3 plants having a 6-fold higher 18:3/18:2 ratio than wild-type plants (Table I), while in ripe red fruits the ratio was about 1.5-fold higher than that of wild-type plants (Table II).
Table I. Fatty acid composition of total lipids from leaves of wild-type and OE-FAD transgenic tomato plants.
Data presented are from a single representative experiment that was repeated twice with similar results. Values are means ± sd of triplicate determinations of fatty acid composition.
| Genotype | Fatty Acid |
18:3/18:2 | Lipid Content | |||||||
| 16:0 | 16:1 | 16:2 | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | |||
| mol % | mg g−1 fresh wt | |||||||||
| Wild type | 19.1 ± 0.9 | 6.2 ± 1.7 | 3.8 ± 0.1 | 7.5 ± 0.3 | 2.1 ± 0.2 | 1.9 ± 0.1 | 13.4 ± 0.4 | 46.0 ± 1.1 | 3.43 | 4.29 ± 0.25 |
| OE-FAD3 | 21.5 ± 0.9 | 4.6 ± 0.5 | 2.8 ± 0.2 | 7.1 ± 0.2 | 2.9 ± 0.5 | 7.2 ± 1.2 | 2.8 ± 0.3 | 51.0 ± 1.8 | 18.21 | 4.35 ± 0.12 |
| OE-FAD7 | 20.4 ± 0.7 | 4.9 ± 0.2 | 3.2 ± 0.3 | 7.6 ± 0.2 | 2.2 ± 0.1 | 3.3 ± 1.0 | 9.7 ± 0.4 | 48.7 ± 0.8 | 5.02 | 5.03 ± 0.46 |
| OE-FAD3FAD7 | 26.2 ± 0.2 | 8.6 ± 2.6 | 1.5 ± 0.2 | 6.2 ± 0.1 | 3.9 ± 0.2 | 2.7 ± 0.2 | 2.1 ± 0.1 | 48.8 ± 2.3 | 23.24 | 3.68 ± 0.37 |
Table II. Fatty acid composition of total lipids from ripe red fruits of wild-type and OE-FAD transgenic tomato plants.
Data presented are from a single representative experiment that was repeated twice with similar results. Values are means ± sd of triplicate determinations of fatty acid composition.
| Genotype | Fatty Acid |
18:3/18:2 | Lipid Content | ||||
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | |||
| mol % | mg g−1 fresh wt | ||||||
| Wild type | 26.1 ± 1.3 | 5.2 ± 0.8 | 5.8 ± 1.6 | 43.6 ± 2.2 | 19.2 ± 1.6 | 0.44 | 0.43 ± 0.02 |
| OE-FAD3 | 30.6 ± 0.4 | 13.9 ± 0.4 | 7.0 ± 0.7 | 30.1 ± 0.4 | 18.5 ± 0.4 | 0.61 | 0.30 ± 0.01 |
| OE-FAD7 | 27.8 ± 0.2 | 11.0 ± 0.5 | 9.0 ± 0.4 | 31.8 ± 0.6 | 20.4 ± 0.1 | 0.64 | 0.40 ± 0.01 |
| OE-FAD3FAD7 | 32.4 ± 2.2 | 7.0 ± 0.4 | 9.1 ± 3.1 | 8.6 ± 2.6 | 42.9 ± 3.8 | 4.99 | 0.32 ± 0.03 |
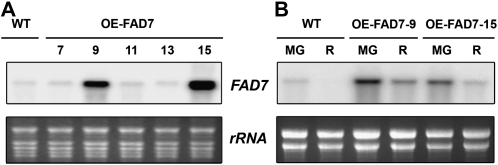
As for the FAD7 construct, out of 23 independent primary transformants, 10 showed a clear accumulation of StFAD7 mRNAs in leaves (Fig. 4A). From these lines, we selected OE-FAD7-9 and OE-FAD7-15 for further characterization, because they expressed the transgene to high levels in leaves. In both OE-FAD7-9 and OE-FAD7-15, StFAD7 was also detected in mature green fruits and, at lower levels, in ripe fruits (Fig. 4B). Fatty acid levels were determined in OE-FAD7 leaves and red ripe fruits (Tables I and II). Compared with wild-type plants, both transgenic lines had reduced 18:2 content and a minimally to insignificant increase in 18:3 content in the tissues studied, which resulted in a 1.5-fold increase in the 18:3/18:2 ratio in both tissues.
Figure 4.
Characterization of OE-FAD7 transgenic tomato lines. A, Total RNA (10 μg) isolated from young fully expanded leaves of representative independent transgenic lines (7, 9, 11, 13, and 15) was fractionated on denaturing agarose gels, transferred to nylon membranes, and hybridized with 32P-radiolabeled StFAD7. The bottom panel shows the ethidium bromide-stained rRNA as a loading control. B, Total RNA from mature green (MG) and ripe red (R) fruits of the wild type and two selected OE-FAD7 lines hybridized to StFAD7. WT, Wild type.
To determine the combined effect of overexpressing both ω-3 desaturases, OE-FAD3 and OE-FAD7 lines were crossed. Selected lines carrying both transgenes were analyzed for simultaneous BnFAD3 and StFAD7 expression in leaves and ripe fruits (Supplemental Fig. S1). These lines clearly showed the accumulation of mRNA derived from both ω-3 desaturases, with similar levels to those observed in the corresponding parental lines (Supplemental Fig. S1). To check how simultaneous overexpression of ω-3 desaturases affected accumulation of the different fatty acids, fatty acid composition was determined in leaves and red ripe fruits of the double transformant (Tables I and II). OE-FAD3FAD7 leaves showed a larger increase in the 18:3/18:2 ratio compared with wild-type or parental OE-FAD3 and OE-FAD7 lines. This modification is mainly caused by a decrease in the 18:2 levels rather than an increment in the amount of 18:3. On the other hand, analysis of fatty acid composition in red ripe fruits showed that OE-FAD3FAD7 lines presented 5-fold lower 18:2 content than wild-type and parental lines. These changes were accompanied by a 2-fold increase in the 18:3 levels, resulting in a significant increase in the 18:3/18:2 ratio in OE-FAD3FAD7 red ripe fruits, being 11-fold higher than in wild-type and parental lines (Tables I and II).
Effect of FAD Overexpression on Oxylipin-Derived Aroma Compounds in Tomato Tissues
The data presented above established that tomato fruits expressed all the enzymes of the LOX pathway for the production of C6 aldehydes. Moreover, OE-FAD transgenic plants present a modification in the fatty acid precursors for hexanal, (Z)-hex-3-enal, and its isomer (E)-hex-2-enal. Therefore, we next explored if the aroma compound profile was modified in ripe fruits of the transgenic tomato plants. In particular, the content in C6 aliphatic compounds and its derived alcohols was determined (Table III).
Table III. Content of main aroma compounds of ripe red fruits and leaves taken from wild-type and OE-FAD transgenic tomato plants.
Data presented are from a single representative experiment that was repeated twice with similar results. Volatile compounds were extracted and analyzed as described in “Materials and Methods.” Values corresponding to hexanal and (Z)-hex-3-enal are shown in boldface for clarity.
| Compound | Content |
|||
| Wild Type | OE-FAD3 | OE-FAD7 | OE-FAD3FAD7 | |
| μg g−1 tissue | ||||
| Fruits | ||||
| Pentanal | 0.024 ± 0.002 | 0.014 ± 0.002 | 0.013 ± 0.003 | 0.011 ± 0.004 |
| Pent-1-en-3-one | 1.649 ± 0.072 | 2.075 ± 0.226 | 2.632 ± 0.486 | 8.065 ± 0.412 |
| Hexanal | 124.696± 13.548 | 52.215± 5.057 | 30.489± 5.163 | 0.896± 0.154 |
| (Z)-Hex-3-enal | 14.550± 2.082 | 23.856± 3.696 | 30.740± 4.751 | 57.195± 2.261 |
| Pent-1-en-3-ol | 0.108 ± 0.037 | 0.397 ± 0.229 | 0.396 ± 0.265 | 0.324 ± 0.229 |
| (E)-Hex-2-enal | 17.638 ± 2.804 | 20.722 ± 2.579 | 23.625 ± 2.730 | 34.986 ± 5.639 |
| (Z)-Pent-2-en-1-ol | 0.008 ± 0.002 | 0.006 ± 0.003 | 0.007 ± 0.004 | 0.032 ± 0.005 |
| (Z)-Hex-3-enol | 0.987 ± 0.251 | 6.001 ± 0.705 | 3.668 ± 0.290 | 5.708 ± 0.556 |
| Leaves | ||||
| Pentanal | 0.061 ± 0.013 | 0.017 ± 0.003 | 0.023 ± 0.004 | 0.019 ± 0.002 |
| Pent-1-en-3-one | 0.773 ± 0.075 | 1.178 ± 0.152 | 1.120 ± 0.107 | 1.130 ± 0.102 |
| Hexanal | 1.668± 0.134 | 0.162± 0.017 | 0.390± 0.021 | 0.047± 0.003 |
| (Z)-Hex-3-enal | 344.116± 58.067 | 356.280± 66.871 | 377.290± 49.092 | 334.068± 26.043 |
| Pent-1-en-3-ol | 0.056 ± 0.011 | 0.060 ± 0.030 | 0.048 ± 0.010 | 0.055 ± 0.003 |
| (E)-Hex-2-enal | 105.707 ± 3.080 | 94.631 ± 10.152 | 87.051 ± 12.664 | 82.786 ± 8.515 |
| (Z)-Pent-2-en-1-ol | 0.017 ± 0.005 | 0.026 ± 0.001 | 0.029 ± 0.003 | 0.031 ± 0.002 |
| (Z)-Hex-3-enol | 0.155 ± 0.021 | 0.339 ± 0.038 | 0.270 ± 0.023 | 0.816 ± 0.039 |
Wild-type tomato fruits have a higher content in hexanal, probably due to the relatively higher abundance of 18:2, especially in ripe fruits (Galliard et al., 1977). In contrast, OE-FAD fruits showed a significant decrease in the amount of hexanal, being almost undetectable in OE-FAD3FAD7 ripe fruits (Table III). Specifically, hexanal content was reduced to more than half in OE-FAD3 fruits, while OE-FAD7 exhibited a 75% decrease in the concentration of hexanal with respect to wild-type fruits. Moreover, the levels of hexanal in OE-FAD3FAD7 were 140-fold lower than in the wild type and approximately 50-fold lower than in both parental lines. This modification is associated with an increase in (Z)-hex-3-enal, its isomer (E)-hex-2-enal, and its derivative (Z)-hex-3-enol (Table III). Both OE-FAD3 and OE-FAD7 showed similar increases in the levels of these compounds, which are on average 60% higher than in wild-type fruits. Interestingly, OE-FAD3FAD7 presented a marked increase in (Z)-hex-3-enal levels that are 4-fold higher than in wild-type fruits and 2-fold higher than in the parental transgenic fruits (Table III). The changes observed in the concentration pattern of these C6 aliphatic aldehydes resulted in a significant alteration in the (Z)-hex-3-enal/hexanal ratio, in particular in OE-FAD3FAD7 ripe fruits. These double transgenic plants showed a 500-fold higher ratio than wild-type fruits. This modification implies a significant variation in the aroma profile in tomato fruits and suggests an improvement of the organoleptic characteristics in these fruits, which may present a richer fresh note.
Other quantitatively minor compounds in tomato fruit aroma changed according to modifications observed in C6 compounds, described above, and the fatty acid of origin [18:2, pentanal; 18:3, pent-1-en-3-one, pent-1-en-3-ol, and (Z)-pent-2-en-1-ol].
As in tomato fruits, OE-FAD leaves displayed a significant decrease in the amount of hexanal, larger in the case of OE-FAD3FAD7 than in the parental lines (Table III). Interestingly, this decrease in the amount of hexanal in the parental lines was more pronounced than in tomato fruits, hexanal contents being reduced by 90% and 77% in OE-FAD3 and OE-FAD7 plant leaves, respectively, when compared with wild-type leaves. However, contents of hexenals [(Z)-hex-3-enal and (E)-hex-2-enal] showed no significant changes in any of the OE-FAD plant leaves.
Cold Response of Transgenic Tomato Plants with Altered Fatty Acid Desaturation
Previous reports demonstrated that overexpression of a chloroplast ω-3 fatty acid desaturase results in a greater tolerance to low temperatures (Kodama et al., 1994; Iba, 2002). To test whether tomato plants overexpressing either a single ω-3 fatty acid desaturase or both FAD3 and FAD7 simultaneously were affected in their tolerance to chilling temperature, experiments were first carried out using plants grown in tissue culture. When plants were held at 0°C for 3 d and then allowed to recover, the wild-type seedlings exhibited a greater bleaching of leaves in comparison with OE-FAD3, OE-FAD7, and OE-FAD3FAD7 (data not shown). To obtain a more quantitative measurement of this phenotypic difference, we determined the relative conductance, which measures cellular electrolytes leached from damaged tissue plotted against total cellular electrolytes and reflects membrane integrity and functionality. It is based on the principle that the greater the damage to cells from freezing injury, the greater the exosmosis of cellular electrolytes into a water solvent (Dexter, 1956). To this end, 12-d-old tomato seedlings grown on Murashige and Skoog (MS) medium at 25°C were chilled at 0°C for 3 d in continuous light (100 μmol m−2 s−1) and returned to the same conditions prior to chilling for recovery. Measurements of the relative conductivity indicated significantly reduced ion leakage in the cold-treated OE-FAD7 and OE-FAD3FAD7 seedlings compared with wild-type seedlings (Supplemental Fig. S2). However, the OE-FAD3 seedlings did not show significant differences from the wild-type seedlings (Supplemental Fig. S2).
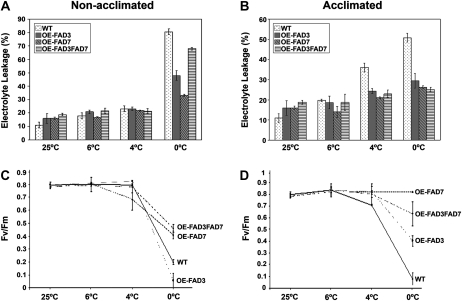
To extend our study on the response of OE-FAD plants to low temperatures, plants were grown in soil and then held at 6°C, 4°C, or 0°C for 3 d. Prior to placing the plants at these temperatures, one group of plants was acclimated by gradually decreasing the temperature from 25°C to 10°C (see “Materials and Methods”). Measuring the relative conductance of the nonacclimated plants indicated that there was a significant difference in electrolyte leakage at 0°C across the lines (Fig. 5A). The OE-FAD7 line had the least electrolyte leakage, followed, in order of increasing electrolyte leakage, by OE-FAD3, the double OE-FAD3FAD7, and the wild type (Fig. 5A). The differences detected at 0°C across the different lines were not apparent at 6°C or 4°C (Fig. 5A) and were marginally detectable at 2°C (data not shown). In addition to electrolyte leakage, the photosynthetic capacity was determined by measuring the ratio of variable to maximal chlorophyll fluorescence (Fv/Fm), which also shows a strong correlation with trienoic fatty acid levels (Routaboul et al., 2000). In the nonacclimated plants Fv/Fm values were not significantly different between the ω-3 FAD overexpressors and the wild type at 6°C or 4°C (Fig. 5C). At 0°C, Fv/Fm values were much lower in the wild-type and OE-FAD3 plants relative to OE-FAD7 and OE-FAD3FAD7 plants (Fig. 5C). In contrast, the photosynthetic capacity of OE-FAD7 and OE-FAD3FAD7 plants was maintained at values close to 50% of those measured at standard temperature conditions (Fig. 5C), and only these plants were able to recover upon exposure to 0°C.
Figure 5.
Chilling tolerance of OE-FAD transgenic tomato plants grown in soil. A and B, Relative conductance measurements of nonacclimated plants (A) and acclimated plants (B) before cold treatment (25°C) and following a 24-h recovery after chilling in the dark for 3 d at 6°C, 4°C, and 0°C. The wild type (WT) is dotted, OE-FAD3 is gray, OE-FAD7 is diagonally hatched, and OE-FAD3FAD7 is horizontally hatched. C and D, Chlorophyll fluorescence (Fv/Fm) from soil-grown wild-type and ω-3 desaturase-overexpressing tomato before cold treatment (25°C) and following a 24-h recovery of nonacclimated (C) and acclimated (D) plants chilled in the dark at 6°C, 4°C, and 0°C. All measurements were taken on the youngest, fully expanded leaves at similar developmental stages for all treatments. Values are means ± sd of three replicates.
On the other hand, measuring the relative conductance of the acclimated plants indicated that all of the ω-3 FAD overexpressors were reduced in their electrolyte leakage relative to the wild type at 4°C and 0°C (Fig. 5B). This difference was not apparent at 6°C (Fig. 5B). Acclimated OE-FAD plants also exhibited less leaf chlorosis and were better able to recover upon return to normal conditions, independent of the ω-3 desaturase overexpressed (data not shown). However, there were differences in the photosynthetic capacity across the OE-FAD lines at the different chilling temperatures (Fig. 5D). The Fv/Fm values of the OE-FAD7 acclimated plants did not differ across the different tested temperatures, while these values slightly decreased in OE-FAD3FAD7 plants at 0°C but not at the other temperatures (Fig. 5D). The Fv/Fm values of the OE-FAD3 plants were also only decreased at 0°C, and less than the wild-type plants (Fig. 5D). Taken together, these results suggest that unsaturated levels in distinct membranes contribute differently to the response to low temperature.
DISCUSSION
Improving fruit organoleptic characteristics is an overlooked issue in tomato breeding programs. This may in part be due to the complexity of factors that contribute to this trait and the difficulties associated with its evaluation for screening protocols, but it may also in part be due to emphasis by plant breeders on increased yield and disease resistance. However, better aroma of fruits may encourage consumer intake and thus have a beneficial effect in both human nutrition and agricultural business. Increasing the concentration of the oxylipins (Z)-hex-3-enal and (E)-hex-2-enal modifies the aroma profile of tomato fruit by increasing the hexenal/hexanal ratio. This modification may intensify the fresh notes of fruit aroma that constitute a valued trait for consumers. Moreover, perception of the sweetness of tomato fruits can be influenced by acidity and the proportion of volatile compounds present in the fruit (Baldwin et al., 2000).
C6 aldehydes are synthesized via the subsequent action of 13-LOX and 13-HPL enzymes on the appropriate fatty acid substrate. Despite being one of the best characterized pathways, there were some conflicting results on the expression of the genes and enzymes that participate in this pathway. Our results show that 13-LOX are present and active in the ripe fruit. TomLoxC and TomLoxD exhibit an opposite RNA expression pattern during fruit development, as reported previously (Heitz et al., 1997; Chen et al., 2004). TomLoxD mRNA is mainly expressed in green fruits, while TomLoxC transcripts accumulate in ripe fruits (Heitz et al., 1997). Moreover, our data show that the majority of LOX protein is detected in ripe fruits. However, we can speculate that LOX accumulation at this stage is not only attributable to TomLoxC expression but rather to the contribution of other TomLox, including those encoding 9-LOXs, which represent the majority of LOX activity in tomato fruits (Hatanaka et al., 1992; Griffiths et al., 1999). Nonetheless, TomLoxC and TomLoxD expression in fruits may provide the limited amount of 13-HPO required for the production of C6 aldehydes. In this regard, it is important to notice that silencing of the TomLoxC in tomato and of its homolog in potato (Solanum tuberosum; LOXH1) specifically abolishes C6 aldehyde production (Leon et al., 2002; Chen et al., 2004). In addition, our results demonstrate that in spite of the low RNA levels observed, 13-HPL protein is also present in ripe fruit, suggesting a high stability of the protein. Indeed, GUS activity controlled by the 13-HPL promoter followed a similar pattern to 13-HPL expression and HPL accumulation. It is established that GUS protein exhibits a high stability (Taylor, 1997). Moreover, the expression pattern and tissue specificity observed in transgenic plants suggest that the upstream 2-kb sequence used in our study contains all the elements needed for proper expression of the 13-HPL gene.
Apparently, HPL abundance is not a rate-limiting factor for C6 volatile production in tomato fruits. Although TomLoxC levels in tomato fruits may not provide sufficient 13-HPO, overexpression of this 13-LOX has not proved feasible in tomato or potato (Leon et al., 2002; Chen et al., 2004). Linolenic acid (18:3) and linoleic acid (18:2) are the precursors for (Z)-hex-3-enal and hexanal, respectively. Our approach to the modification of the aroma of tomato fruits has been the overexpression of ω-3 desaturases, either FAD3 or FAD7, to alter the biosynthesis and/or ratio of these aldehydes. Analyses of fatty acid composition in leaves and ripe fruits of the tomato transgenic plants showed that, indeed, overexpression of either FAD3 or FAD7, or both, results in a significant modification in the levels of fatty acid unsaturation, with an increase in the 18:3/18:2 ratio in all tissues studied. This was especially true when FAD3 and FAD7 had simultaneously been overexpressed. This additive effect is in agreement with the physiological role of both desaturases in contributing to the biosynthesis of 18:3, as was demonstrated in the Arabidopsis triple mutant fad3 fad7 fad8, which accumulates much less 18:3 than either of the single mutants fad3 or fad7 (Wallis and Browse, 2002). The decrease in the levels of 18:2 of OE-FAD plants is accompanied, in most cases, by an increase in the levels of 18:3, which suggest that it is a direct effect of transgene expression. However, in certain conditions, the reduction of 18:2 is not accompanied by a proportional rise of 18:3, particularly in leaves. These results are in accordance with those observed in the leaves of rice (Oryza sativa) or tobacco (Nicotiana tabacum) FAD3-overexpressing plants (Shimada et al., 2000; Zhang et al., 2005). One possible explanation is the existence of a threshold in the maximal level of 18:3 that cellular membranes may tolerate to permit a proper fluidity degree that ensures cellular processes, the photosynthesis capacity in particular (Falcone et al., 2004). A higher level of 18:3 could thus have a detrimental effect on the normal physiological processes that occur in plant membranes. Moreover, tomato OE-FAD3 leaves showed a larger modification in the 18:3/18:2 ratio than those overexpressing the chloroplastic FAD7, indicating a different contribution of each ω-3 desaturase to the 18:3 content in this tissue. Since the transcriptional abundance of FAD3 and FAD7 in leaves of transgenic plants is similar, it is possible that the specific activity of each enzyme is different or that one of the pathways, eukaryotic or prokaryotic, already prevails and, therefore, the effect of increasing the metabolic flux through that particular pathway may have a limited effect. Similar results were observed in tobacco plants, where FAD3 overexpression caused a larger modification in the 18:3/18:2 ratio than did overexpression of FAD8 (Zhang et al., 2005).
Despite the abundance of 9-LOX activity in tomato fruits, the C9 aldehyde biosynthetic pathway is not functional. One possible reason is that there is no 9-HPL activity in tomato fruit. However, overexpression of 9-HPL in tomato fruit did not result in a qualitative change in the aliphatic aldehyde composition or cause the expected increase in C9 aldehydes (Matsui et al., 2001). On the other hand, the transgenic tomato plants overexpressing ω-3 desaturases show a modification in the amount of hexanal and hexenals and an increase in the (Z)-hex-3-enal/hexanal ratio, which is significantly larger in OE-FAD3FAD7 fruits, in which it is 500 times higher than control plants. There is a good correlation between the 18:3/18:2 ratio and the (Z)-hex-3-enal/hexanal ratio in the transgenic plants. Namely, the decrease in the 18:2 levels leads to a decrease in the levels of hexanal, while the slight increment in the levels of 18:3 leads to an increase in the amount of (Z)-hex-3-enal. Hexanal is related to herbaceous grassy notes in the aroma, while (Z)-hex-3-enal contributes to the more appreciated fresh notes. Thus, the increase in the (Z)-hex-3-enal/hexanal ratio obtained in the OE-FAD tomato plants is assumed to qualitatively improve the aroma and likely the tomato fruit flavor. These data also confirm that the enzymes responsible for the synthesis of these C6 compounds are present and active in tomato fruit and that the limiting factor in the production of (Z)-hex-3-enal is the content of 18:3 derived from the ω-3 desaturases present in the fruits. Indeed, overexpression of yeast Δ-9 desaturase, responsible for the conversion of palmitic acid (16:0) and estearic acid (18:0) to palmitoleic acid (16:1) and oleic acid (18:1), respectively, caused an increase in the (Z)-hex-3-enal and hexanal in the leaves (Wang et al., 2001), possibly because this tissue already contains the rest of the desaturases that further process the fatty acid. In contrast, the endogenous expression of the FAD3 and FAD7 is low in the fruits; therefore, ω-3 desaturase activity in this tissue may not be sufficient to trigger the equivalent increase in C6 aldehydes. Overexpression of these genes would thus be required to produce enough 18:3 to allow a modification in the derived C6 aldehydes.
In addition to this improvement in tomato aroma, OE-FAD plants are more tolerant to low temperatures. This is not surprising, since one of the characteristic changes that plants undergo upon exposure to temperature stress is a modification of the unsaturation degree of fatty acid in membrane lipids (Somerville and Browse, 1991). A decrease in temperature generally induces an increase in the unsaturation of these lipids, which helps to compensate the reduction in the fluidity of membranes at low temperatures and thus guarantees plant survival. Tomato is a chilling-sensitive species that normally suffers cold injury, reflected in physiological alterations in the photosynthetic capacity and lesions in leaves and fruits, when exposed to temperatures in the range from 0°C to 12°C. However, MicroTom is more tolerant to low temperatures than other tomato varieties. This difference could be associated with its genetic background, since it was reported that MicroTom carries mutations in genes related to the biosynthesis of gibberellins and brassinosteroids (Marti et al., 2006), and both hormones have been suggested to participate in the cold response.
FAD-overexpressing tomato plants showed clear differences relative to wild-type plants when exposed to low temperatures, especially 0°C. OE-FAD plants showed greater low-temperature tolerance and were better able to maintain PSII activity than wild-type plants. These results suggest that the increase in the fatty acid unsaturation degree of membrane lipids has a greater effect on protecting tomato plants at temperatures close to the freezing point. Moreover, the photosynthetic capacity of OE-FAD7 and OE-FAD3FAD7 plants at 0°C is higher than that of OE-FAD3 or control plants, indicating that fatty acid unsaturation of lipids in thylakoid membranes is crucial to maintain chloroplast functions, similar to what is observed in the fad3 fad7 fad8 mutant in Arabidopsis (Routaboul et al., 2000). This protection of the photosynthetic capacity is also observed in OE-FAD3 plants when these plants are progressively acclimated to low temperatures. These data suggest that an enhanced trafficking of unsaturated lipids from the endoplasmic reticulum toward the thylakoids may occur to protect the chloroplast function and prevent the physiological disorders associated with exposure to freezing temperatures that can endanger plant survival.
In conclusion, modifying the expression of ω-3 desaturases in tomato plants results in an increase in the 18:3/18:2 ratio, which could by itself beneficially affect fruit quality. The increases in the (Z)-hex-3-enal content and in the (Z)-hex-3-enal/hexanal ratio probably lead to an important enhancement in the fresh notes of the tomato aroma. Finally, ω-3 desaturase overexpression enhances the tolerance of tomato plants to chilling temperatures, and the different behaviors of OE-FAD plants underlie the existence of fatty acid fluxes to ensure plant survival under adverse conditions.
MATERIALS AND METHODS
Generation of Constructs for Constitutive Expression of Endoplasmic Reticulum and Chloroplastic ω-3 Desaturases
Endoplasmic reticulum ω-3 desaturase (FAD3) cDNA clone L01418 from Brassica napus was kindly provided by Dr. C. Somerville. A SmaI-SalI fragment containing the complete BnFAD3 open reading frame (ORF) was cloned in the sense orientation into the SmaI-SalI sites of binary plasmid BIN19 (Bevan, 1984) between the CaMV 35S promoter and the ocs terminator (pFAD3). Chloroplastic ω-3 desaturase (FAD7) ORF, including the signal peptide sequence, was amplified from leaf cDNA from potato (Solanum tuberosum ‘Desireé’) using specific primers (5′-CCGTTAACCATGGCAAGTTGGGTTCTATCAG-3′ and 5′-CAGTCGACAGGGTTTTGCTATTTCTCAG-3′) designed from published FAD7 cDNA sequence information (Martin et al., 1999). The PCR product was cloned in pGEM-T Easy (Promega) and sequenced. An HpaI-SalI fragment containing the complete StFAD7 ORF was cloned in the sense orientation into the SmaI-SalI sites of binary plasmid BIN19 between the CaMV 35S promoter and the ocs terminator (pFAD7). Plasmids pFAD3 and pFAD7 were transformed into Agrobacterium tumefaciens strain C58 using the freeze-thaw method (Holsters et al., 1978).
Generation and Analysis of the 13-HPL Promoter-GUS Reporter Fusion
A 2-kb fragment of 13-HPL was isolated from tomato (Solanum lycopersicum) genomic DNA using the Universal Genome Walker kit (Clontech), cloned in pGEM-T Easy, and sequenced. The promoter sequence was fused in frame to the GUS gene coding region present in the plasmid pBI101.2 (Jefferson et al., 1987) and transferred to A. tumefaciens strain C58 using the freeze-thaw method. Tomato transgenic plants carrying the above construct were generated.
For histochemical detection of GUS activity, tomato fruits at three developmental stages (mature green, orange, and ripe red) were cut transversely and incubated overnight at 37°C in 1 mg mL−1 5-bromo-4-chloro-3-indolyl 6-β-glucuronide, 50 mm sodium phosphate buffer (pH 7.0), 0.5% Triton X-100 (v/v), and 25% methanol (v/v); 75% ethanol was then added to stop the reaction.
Tomato Transformation
Transformation of tomato (cv MicroTom) was performed as described (McCormick et al., 1986) with minor modifications. Cotyledons from 8- to 10-d-old tomato plants grown in MS medium were excised and put on a tobacco (Nicotiana tabacum) BY2 cell feeder layer for 24 h. Cotyledons were mechanically wounded and incubated for 2 to 3 min in an overnight Agrobacterium culture diluted to an optical density at 600 nm of 0.6 in MS 2% Suc. Cotyledons were blotted on filter paper and cocultured in the dark for 2 d in MS with a feeder layer. After cocultivation, cotyledons were transferred to MS 2% Suc, 0.7% agarose with 50 mg L−1 kanamycin, 500 mg L−1 claforan, and 2 mg L−1 zeatine (Sigma). After 7 d, explants were transferred to MS 2% Suc, 0.7% agarose with 50 mg L−1 kanamycin, 250 mg L−1 claforan, and 1 mg L−1 zeatine. The following week, zeatine concentration was lowered and kept to 0.5 mg L−1. At the 3rd week, developing shoots were cut from the base and transferred to MS 2% Suc, 0.7% agarose with 50 mg L−1 kanamycin, 250 mg L−1 claforan, and 1 mg L−1 indoleacetic acid. Potential rooted transformants, identified by the ability to grow on kanamycin-containing medium, were acclimated in pots with soil mix and transferred to a greenhouse. Putative transgenic plants were screened by RNA-blot analysis to detect the expression of the transgene. Primary transformants (T0) were allowed to self-fertilize under controlled conditions. Seeds (T1) were harvested and germinated, employing kanamycin selection to determine the segregation patterns of the transgene. Progeny obtained by selfing T1 plants were analyzed, and homozygous plants (T4; FAD3 or FAD7 lines) were retained. T2 FAD3 and FAD7 transgenic lines were crossed by emasculating the flowers and pollinating them with pollen from each line. Progeny was screened by PCR to detect the presence of both transgenes using a CaMV 35S primer (5′-GACGCACAATCCCACTATC-3′) and specific primers for FAD3 and FAD7. Positive plants were verified for expression of the transgenes by RNA-blot analysis, and homozygous plants (T5; OE-FAD3FAD7 lines) were retained.
Nucleic Acid and Protein Analyses
Total RNA was isolated from tomato fully expanded leaf and fruit at different developmental stages as described previously (Smith et al., 1986; Logemann et al., 1987). For northern-blot analysis, total RNA (10 μg) was fractionated on formaldehyde-denaturing agarose gels, transferred to nylon membranes, and hybridized to specific 32P-radiolabeled probes. RNA-blot hybridization and membrane washing were performed as described elsewhere (Church and Gilbert, 1984). Protein extraction and immunoblotting were as described (Royo et al., 1999).
Plant Growth Conditions
Tomato plants (cv MicroTom) were grown in soil in the greenhouse at 22°C under a 16-h-light/8-h-dark photoperiod. Seeds of transgenic lines were surface sterilized, germinated on MS 2% Suc, 0.7% agarose, and subsequently transferred to soil.
Fatty Acid Analysis
Leaves and red ripe tomato fruits from each line were harvested and immediately frozen in liquid nitrogen, mixed and homogenized to a powder with a mortar and pestle, and stored at −80°C. Fatty acid composition of tomato leaves was determined using the one-step method of Garces and Mancha (1993). Following the addition of 13.2 mL of methanol:toluene:dimethoxypropane:H2SO4 (39:20:5:2, v/v/v/v) and 6.8 mL of heptane to 300 mg of homogenized tomato leaves, the mixture was incubated for 1 h at 80°C, forming a single phase. After cooling, the upper phase containing the fatty acid methyl esters was separated and washed with 5 mL of 6.7% Na2SO4. For tomato ripe red fruit, total lipids from 500 mg of sample were extracted according to Hara and Radin (1978) with 15 mL of hexane:isopropanol (3:2, v/v) and 7.5 mL of 6.7% Na2SO4. The lower phase was reextracted with the same procedure, and both upper phases were pooled together. The mixture was evaporated to dryness with nitrogen, and 3 mL of methanol:toluene:H2SO4 (88:10:2, v/v/v) was added and incubated for 1 h at 80°C. After cooling, the fatty acid methyl esters were extracted with 1 mL of heptane and 5 mL of 6.7% Na2SO4. Fatty acid methyl esters extracted from both tissues were analyzed by gas-liquid chromatography using a HP-5890 (Hewlett-Packard) fitted with a capillary column (30 m length, 0.25 mm i.d., 0.20 μm film thickness) of fused silica (Supelco) and a flame ionization detector. Hydrogen was used as carrier gas with a linear flow rate of 1.34 mL min−1 and a split ratio of 1:50. The injector and detector temperature was 220°C, and the oven temperature was 170°C. Heptadecanoic acid was used as internal standard to calculate the lipid content of the samples.
Determination of Volatiles from Tomato Tissues
Frozen tomato tissues were obtained as described in the previous section and stored at −80°C. Frozen tomato fruit (2 g) or leaf powder (0.6 g) was placed in 2 mL of extraction buffer (150 mm sodium phosphate, pH 6.7, 250 mm sorbitol, 10 mm EGTA, 10 mm MgCl2, and 1% glycerol [v/v]), homogenized with an Ultraturrax homogenizer for 1 min at maximum speed, and incubated at 25°C in a thermostatic water bath. After a 5-min incubation, aliquots of 0.5 mL were placed into vials (12 mL) containing 1.5 mL of saturated CaCl2 solution for enzyme inactivation and sealed immediately.
Volatile compounds were quantified in quadruplicate by high-resolution gas chromatography using the solid-phase microextraction technique (SPME). For this purpose, vials were placed in a heater at 40°C for 10 min and, after this equilibrium time, volatile compounds from head space were adsorbed on a SPME fiber DVB/Carboxen/PDMS 50/30 μm (Supelco). Sampling time was 30 min at 40°C. Desorption of volatile compounds trapped in the SPME fiber was done directly into the gas chromatograph injector. Volatiles were analyzed using a HP-5890 gas chromatograph equipped with a DB-Wax capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm; J&W Scientific). Operating conditions were as follows: N2 as carrier gas, injector and detector at 250°C, column held for 6 min at 40°C and then programmed at 2°C min−1 to 120°C. Quantification was performed using individual calibration curves for each identified compound. Compound identification was carried out on a HRGC-MS Fisons series 8000 equipped with a similar stationary-phase column and two different lengths, 30 and 60 m, matching against the Wiley/NBS Library and by gas chromatography retention time against standards.
Chilling Tolerance Studies
Tissue Culture Seedlings
In separate experiments, tomato seeds were surfaced sterilized with 15% bleach for 15 min and rinsed with four washes of sterile deionized water. Seeds were germinated on plates with MS medium (15 seeds per plate, three plates per genotype) for 12 d at 25°C under 80 μmol m−2 s−1 light intensity and a 16-h-light/8-h-dark photoperiod. Plates were chilled at 0°C in continuous light (100 μmol m−2 s−1) for 3 d and returned to the same conditions prior to chilling for recovery. Samples were collected from nonchilled and chilled plants to determine chilling tolerance by electrolyte leakage (Pennycooke et al., 2003). To this end, seedlings were carefully pulled out of the agar and rinsed with deionized water. One seedling was subsequently placed in a 16-mm glass test tube with 7 mL of deionized water and shaken at 300 rpm for 1 h before measuring the conductivity (C1) with an Accumet Basic AB30 electrical conductivity meter (Fisher Scientific). Tubes were autoclaved for 20 min and then agitated as above before reading the conductivity (C2). Relative injury represents the mean ion leakage as a percentage of the total leakage from killed samples: (C1/C2) × 100. The experiments were repeated twice.
Potted Seedlings
Tomato seedlings were grown for 12 d in Bacto Promix (Michigan Peat Company) under a 12-h photoperiod at 100 μmol m−2 s−1 light intensity provided by cool-white fluorescent lamps using a 25°C/23°C day/night temperature regime. Seedlings were fertilized twice with 0.5 g L−1 of an all-purpose 20:20:20 plant nutrient solution (Schultz). Plants were separated into two groups before chilling treatment: nonacclimated treated and acclimated treated. Nonacclimated treated plants were chilled at 6°C, 4°C, or 0°C for 3 d in the dark. For the acclimation treatment, seedlings of each genotype were transferred to a growth chamber set with a gradual drop in temperature of 15°C for 3 d, 12°C for 3 d, followed by 10°C for 3 d with a 12-h photoperiod under 80 μmol m−2 s−1 light intensity, before chilling at 6°C, 4°C, or 0°C for 3 d, respectively, in the dark. After the chilling treatments, both sets of plants were returned to normal growing conditions (25°C/23°C) and allowed to recover. All experiments were repeated twice. Chilling tolerance was determined on detached leaf discs by electrolyte leakage (Pennycooke et al., 2003). One 5-mm leaf disc was placed in a 16-mm glass test tube with 7 mL of deionized water and shaken at 300 rpm for 1 h before measuring the conductivity (C1) with an Accumet Basic AB30 electrical conductivity meter (Fisher Scientific). Tubes were autoclaved for 20 min and then agitated as above before reading the conductivity (C2). Relative injury represents the mean ion leakage as a percentage of the total leakage from killed samples: (C1/C2) × 100.
To evaluate the effect of freezing on the functionality of the PSII reaction centers, the maximum quantum yield of the PSII photochemistry was measured by the ratio of variable (Fv) to maximal (Fm) chlorophyll fluorescence in a dark-adapted state (Butler and Kitajima, 1975; Venema et al., 1999). Chlorophyll fluorescence was measured with a hand-held portable pulse amplitude-modulated fluorometer (model OS-30p; Opti Sciences) on the youngest, fully expanded leaves of similar developmental stage from all treatments (nonchilled control, nonacclimated chilled, and acclimated chilled). Leaves were dark adapted prior to taking measurements. Dark adaptation was applied with dark leaf clips for 10 to 15 min before each measurement. The Fv/Fm was measured directly by the “mod 2” of the fluorometer.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Abundance of FAD3 and FAD7 mRNA in OE-FAD transgenic tomatoes.
Supplemental Figure S2. Low-temperature treatment of wild-type and OE-FAD transgenic plants grown in tissue culture.
Supplementary Material
Acknowledgments
We gratefully acknowledge Pilar Paredes for excellent technical assistance. We thank Professor C. Somerville for providing the pBnFAD3 vector.
References
- Baldwin EA, Scott JW, Shewmaker CK, Schuch W. (2000) Flavor trivia and tomato aroma: biochemistry and possible mechanism for control of important aroma components. HortScience 35: 1013–1022 [Google Scholar]
- Bevan M. (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12: 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukobza F, Dunphy PJ, Taylor AJ. (2001) Measurement of lipid oxidation-derived volatiles in fresh tomatoes. Postharvest Biol Technol 23: 117–131 [Google Scholar]
- Butler WL, Kitajima M. (1975) Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta 376: 116–125 [DOI] [PubMed] [Google Scholar]
- Carbonell-Barrachina A, Agustí A, Ruiz J. (2006) Analysis of flavor volatile compounds by dynamic headspace in traditional and hybrid cultivars of Spanish tomatoes. European Food Research and Technology A 222: 536–542 [Google Scholar]
- Chen G, Hackett R, Walker D, Taylor A, Lin Z, Grierson D. (2004) Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol 136: 2641–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. (1984) Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter ST. (1956) The evaluation of crop plants for winter hardiness. Adv Agron 8: 203–239 [Google Scholar]
- Falcone DL, Ogas JP, Somerville CR. (2004) Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feussner I, Wasternack C. (2002) The lipoxygenase pathway. Annu Rev Plant Biol 53: 275–297 [DOI] [PubMed] [Google Scholar]
- Galliard T, Matthew JA, Wright AJ, Fishwick MJ. (1977) The enzymic breakdown of lipids to volatile and non-volatile carbonyl fragments in disrupted tomato fruits. J Sci Food Agric 28: 863–868 [Google Scholar]
- Garces R, Mancha M. (1993) One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem 211: 139–143 [DOI] [PubMed] [Google Scholar]
- Griffiths A, Prestage S, Linforth R, Zhang J, Taylor A, Grierson D. (1999) Fruit-specific lipoxygenase suppression in antisense-transgenic tomatoes. Postharvest Biol Technol 17: 163–173 [Google Scholar]
- Guy CL. (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 41: 187–223 [Google Scholar]
- Hara A, Radin NS. (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90: 420–426 [DOI] [PubMed] [Google Scholar]
- Hatanaka A, Kajiwara T, Horino H, Inokuchi K. (1992) Odor-structure relationships in n-hexenols and n-hexenals. Z Naturforsch C 47: 183–189 [DOI] [PubMed] [Google Scholar]
- Heitz T, Bergey DR, Ryan CA. (1997) A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol 114: 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M, de Waele D, Depicker A, Messens E, van Montagu M, Schell J. (1978) Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet 163: 181–187 [DOI] [PubMed] [Google Scholar]
- Howe GA, Lee GI, Itoh A, Li L, DeRocher AE. (2000) Cytochrome P450-dependent metabolism of oxylipins in tomato: cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiol 123: 711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba K. (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53: 225–245 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H, Hamada T, Horiguchi G, Nishimura M, Iba K. (1994) Genetic enhancement of cold tolerance by expression of a gene for chloroplast ω-3 fatty acid desaturase in transgenic tobacco. Plant Physiol 105: 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Royo J, Vancanneyt G, Sanz C, Silkowski H, Griffiths G, Sanchez-Serrano JJ. (2002) Lipoxygenase H1 gene silencing reveals a specific role in supplying fatty acid hydroperoxides for aliphatic aldehyde production. J Biol Chem 277: 416–423 [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 [DOI] [PubMed] [Google Scholar]
- Marti E, Gisbert C, Bishop GJ, Dixon MS, Garcia-Martinez JL. (2006) Genetic and physiological characterization of tomato cv. Micro-Tom. J Exp Bot 57: 2037–2047 [DOI] [PubMed] [Google Scholar]
- Martin M, Leon J, Dammann C, Albar JP, Griffiths G, Sanchez-Serrano JJ. (1999) Antisense-mediated depletion of potato leaf omega3 fatty acid desaturase lowers linolenic acid content and reduces gene activation in response to wounding. Eur J Biochem 262: 283–290 [DOI] [PubMed] [Google Scholar]
- Mathieu S, Cin VD, Fei Z, Li H, Bliss P, Taylor MG, Klee HJ, Tieman DM. (2009) Flavour compounds in tomato fruits: identification of loci and potential pathways affecting volatile composition. J Exp Bot 60: 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Fukutomi S, Wilkinson J, Hiatt B, Knauf V, Kajwara T. (2001) Effect of overexpression of fatty acid 9-hydroperoxide lyase in tomatoes (Lycopersicon esculentum Mill.). J Agric Food Chem 49: 5418–5424 [DOI] [PubMed] [Google Scholar]
- Matthew JA, Chan HW, Galliard T. (1977) A simple method for the preparation of pure 9-D-hydroperoxide of linoleic acid and methyl linoleate based on the positional specificity of lipoxygenase in tomato fruit. Lipids 12: 324–326 [DOI] [PubMed] [Google Scholar]
- McCormick S, Neidermeyer J, Fry J, Barnason A, Horsch R, Fraley R. (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep 5: 81–84 [DOI] [PubMed] [Google Scholar]
- Pennycooke JC, Jones ML, Stushnoff C. (2003) Down-regulating α-galactosidase enhances freezing tolerance in transgenic petunia. Plant Physiol 133: 901–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul JM, Fischer SF, Browse J. (2000) Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol 124: 1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo J, Leon J, Vancanneyt G, Albar JP, Rosahl S, Ortego F, Castanera P, Sanchez-Serrano JJ. (1999) Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests. Proc Natl Acad Sci USA 96: 1146–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Wakita Y, Otani M, Iba K. (2000) Modification of fatty acid composition in rice plants by transformation with a tobacco microsomal ω-3 fatty acid desaturase gene (NtFAD3). Plant Biotechnol 17: 43–48 [Google Scholar]
- Smith CJS, Slater A, Grierson D. (1986) Rapid appearance of an mRNA correlated with ethylene synthesis encoding a protein of molecular weight 35000. Planta 168: 94–100 [DOI] [PubMed] [Google Scholar]
- Somerville C, Browse J. (1991) Plant lipids: metabolism, mutants, and membranes. Science 252: 80–87 [DOI] [PubMed] [Google Scholar]
- Steponkus PL, Uemura M, Webb MS. (1993) A contrast of the cryostability of the plasma membrane of winter rye and spring oat: two species that widely differ in their freezing tolerance and plasma membrane lipid composition. Steponkus PL, , Advances in Low-Temperature Biology, Vol 2 JAI Press, London, 211–312 [Google Scholar]
- Taylor CB. (1997) Promoter fusion analysis: an insufficient measure of gene expression. Plant Cell 9: 273–275 [Google Scholar]
- Tieman DM, Zeigler M, Schmelz EA, Taylor MG, Bliss P, Kirst M, Klee HJ. (2006) Identification of loci affecting flavour volatile emissions in tomato fruits. J Exp Bot 57: 887–896 [DOI] [PubMed] [Google Scholar]
- Venema JH, Posthumus F, van Hasselt PR. (1999) Impact of sub-optimal temperature on growth, photosynthesis, leaf pigments and carbohydrates of domestic and high-altitude wild Lycopersicon species. J Plant Physiol 155: 711–718 [Google Scholar]
- Wallis JG, Browse J. (2002) Mutants of Arabidopsis reveal many roles for membrane lipids. Prog Lipid Res 41: 254–278 [DOI] [PubMed] [Google Scholar]
- Wang C, Xing J, Chin CK, Ho CT, Martin CE. (2001) Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry 58: 227–232 [DOI] [PubMed] [Google Scholar]
- Zhang M, Barg R, Yin M, Gueta-Dahan Y, Leikin-Frenkel A, Salts Y, Shabtai S, Ben-Hayyim G. (2005) Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J 44: 361–371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.