Niklas (2000) defined plants as “photosynthetic eukaryotes,” thereby including brown, red, and green macroalgae and microalgae. These groups share several features, including the presence of a complex, dynamic, and polysaccharide-rich cell wall. Cell walls in eukaryotes are thought to have evolved by lateral transfer from cell wall-producing organisms (Niklas, 2004). Green and red algae originate from a primary endosymbiotic event with a cyanobacterium, which is thought to have occurred over 1,500 million years ago (Palmer et al., 2004). Even though extant cyanobacteria have cell walls that are based on a peptidoglycan-polysaccharide-lipopolysaccharide matrix and thus differ markedly from the polysaccharide-rich cell walls of plants, there is preliminary evidence that they may contain some similar polysaccharides (Hoiczyk and Hansel, 2000), and genes already involved in polysaccharide synthesis or those subsequently coopted into wall biosynthesis may have been transferred during endosymbiosis. Independent secondary endosymbiotic events subsequently gave rise to the Euglenozoa (which lack cell walls) and brown algae (which have cell walls; Palmer et al., 2004). Investigations of the diversity of wall composition, structure, and biosynthesis that include algae, therefore, may lend new insights into wall evolution (Niklas, 2004).
Algal cell wall research, in common with that of land plants, has focused on commercially important species and polysaccharides; thus, the most well-described algal wall components include the commercially and ecologically important laminarans, carrageenans, fucans, and alginates (Mabeau and Kloareg, 1997; Campo et al., 2009). However, there are over 35,600 species of seaweed, and their cell wall components exhibit enormous diversity (for review, see Painter, 1983; Kloareg and Quatrano, 1988; De Reviers, 2002). Even though distinct suites of polysaccharides are known to occur in different taxa such that algal cell wall profiles can be used as taxonomic markers (Parker, 1970; Domozych et al., 1980), some wall components have a wider distribution and are also found in other organisms, including land plants.
Renewed interest in plant and algal cell wall composition (Popper and Fry, 2003, 2004; Niklas, 2004; Vissenberg et al., 2005; Van Sandt et al., 2007; Fry et al., 2008a, 2008b; Popper, 2008; Sørensen et al., 2008), perhaps driven by potential industrial applications (Pauly and Keegstra, 2008) and a desire to better understand cell wall functions (Niklas, 2004), has been facilitated by the development of several techniques capable of screening cell wall polymers. Increased information has added detail to the diversity known to exist in cell wall composition, generated as organisms adapted to specific niches (Sarkar et al., 2009). However, it is also becoming apparent that similarities, as well as differences, exist between plant and algal cell walls. Therefore, examination of the patterns of occurrence of wall components suggests that existing diversity is likely to be the result of a variety of different evolutionary scenarios.
A QUESTION OF ORIGIN
Investigation of the occurrence of wall components and the genes involved in their biosynthesis may suggest whether they are innovations within a particular lineage or have a more ancient origin. Mechanisms of cell wall biosynthesis may have evolved several times from diversification of gene families, may have been retained from ancestral organisms, or may have been acquired through horizontal gene transfer. While horizontal gene transfer is a rare event (Becker and Marin, 2009), several endosymbiotic events gave rise to photosynthetic organisms (Fig. 1; Keeling, 2004; Palmer et al., 2004) and could have been accompanied by transfer of wall biosynthesis genes (Niklas, 2004). Therefore, it could be expected that some cell wall genes and their products are common to both algae and plants. However, it is likely that the majority of land plant cell wall components are the products of directly inherited genes that have diversified within a particular lineage (Yin et al., 2009).
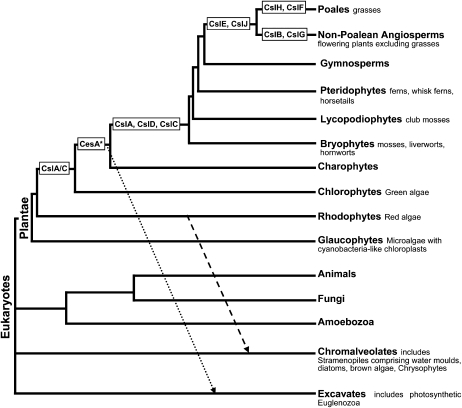
Figure 1.
Emergence of gene families (CesA and Csl) within the cellulose synthase superfamily responsible for cellulose and hemicellulose synthesis (Yin et al., 2009) mapped onto a simplified eukaryote phylogeny (Keeling, 2004; Palmer et al., 2004; Yoon et al., 2004; adapted from Keeling et al., 2009). CesA* represents members of the cellulose synthase family whose proteins assemble into rosette terminal complexes (Yin et al., 2009). CslA/C represents a single gene that is most similar to the land plant CslA and CslC gene families (Yin et al., 2009). The arrows indicate secondary endosymbiosis events involving green algae, which gave rise to the Euglenozoa (dotted arrow), and red algae, which gave rise to the Stramenopiles (dashed arrow; Keeling, 2004).
Convergent Evolution: Several Routes Result in Similar Wall Components
The recent discovery of lignin in the cell walls of a red alga, Calliathron cheilosporioides (Martone et al., 2009), was surprising for a number of reasons. Most significantly, lignin is normally found in vascular plant cell walls (Table I), which probably last shared a common ancestor with red algae over 1 billion years ago (Martone et al., 2009). Furthermore, recorded diversity in wall composition is usually at a more subtle level and tends to mirror known taxonomic groups, such as the presence of acidic sugar residues in bryophyte xyloglucans (Peña et al., 2008). Thus, the existence of a cell wall component in two groups as distantly related as red algae and vascular plants leads us to consider how this may have occurred.
Table I. Occurrence of cell wall components in plant and algal cell walls.
+, Component is likely to be present; ±, component may be present; −, component is likely to be absent; *, component is absent but an unusual sugar residue constituent of the wall component is present.
| Plant Group | Other Wall Components |
||||||||
| Cellulose | Xylan | Mannan | Xyloglucan | RGII | (1→3),(1→4)-β-d-Glucan | AGPs | Lignin | Silica | |
| Brown algae | +a | ±bc | ±d | −e | − | ||||
| Diatoms | ±b | +f | |||||||
| Rhodophytes (red algae) | +a | +g | +h | − | ±i | +j | − | ||
| Chlorophytes (green algae) | +a | +k | +l | *m | +n | +io | −e | − | |
| Charophycean green algae | +a | +p | +q | ±r | − | +s | −e | − | |
| Bryophytes (mosses, liverworts, and hornworts) | +t | +u | +v | +wx | ±y | − | +z | −eaa | +bb |
| Lycopodiophytes (club mosses) | +t | +cc | +dd | +w | +ee | − | +ffgg | − | |
| Equisetophytes (horsetails) | +t | +cc | +dd | +w | +hh | +ii | +jj | +gg | +bbkk |
| Ferns | +t | +ccll | +ddmm | +w | +nn | − | +gg | − | |
| Gymnosperms | +t | +oo | +ddpp | +w | +hhnn | − | +rr | − | |
| Angiosperms, excluding Poales | +t | +oo | +dd | +w | +nn | − | +ss | +ff | − |
| Poalean angiosperms | +t | +tt | +dd | +w | +nn | +uu | +ss | +ff | +bbvv |
Putative occurrence based on labeling with an anti-MLG mAb (Z.A. Popper and E. Demange, unpublished data). Treatment of the brown algal cell walls with 6 m NaOH solubilized polysaccharides that were fragmented by treatment with lichenase (Z.A. Popper and O. Curry, unpublished data).
Bourne et al. (1969) extracted a polymer from Fucus vesiculosus that could be digested by laminarinase and cellulase and appeared to be structurally similar to laminarin.
Putative occurrence of AGPs based on extraction (Schultz et al., 2000) and detection using radial gel permeation (Van Holst and Clarke, 1985; Z.A. Popper, Q. Coster, and T. Slattery, unpublished data).
Lignin-like compounds have been reported from algae and nonvascular plants (Gunnison and Alexander, 1975; Delwiche et al., 1989) but have not been unambiguously confirmed (Ragan, 1984; Lewis, 1999; Peter and Neale, 2004).
Diatoms are reported to contain approximately 5% silica by dry weight (Werner, 1977).
Some red algae, such as B. fuscopurpurea xylans, contain all β-d-(1→3) linkages, whereas others, such as P. palmata, may contain β-d-(1→3) and β-d-(1→4) in the same molecule (Painter, 1983).
mAb labeling and extraction followed by radial gel diffusion (Eder et al., 2008).
The presence of lignin (monolignols H, G, and S) in the rhodophyte C. cheilosporioides was determined by labeling with polyclonal antibodies and gas chromatography-mass spectrometry (Martone et al., 2009).
Presence of (1→4)-β-d-mannans in C. fragile determined by FT-IR, heteronuclear multiple bond correlation NMR, and linkage analysis (Estevez et al., 2009).
RGII has not been detected in green algae, but the prasinophytes contain monosaccharide residues normally present in RGII (York et al., 1985; Becker et al., 1991; Domozych et al., 1991).
Presence in Micrasterias determined by mAb labeling and specific enzyme digestion followed by high-performance anion-exchange chromatography-pulsed-amperometric detection (Eder et al., 2008).
Presence of AGPs in C. fragile determined by mAb labeling, linkage analysis, and NMR (Estevez et al., 2009).
Methylation analysis (Morrison et al., 1993) and CoMPP (Domozych et al., 2009) indicate the presence of 4-linked xylans.
Methylation analysis (Morrison et al., 1993) and CoMPP suggests the presence of (1→4) mannans (Domozych et al, 2009).
Presence of xyloglucan in Chara antheridia suggested by mAb labeling (Domozych et al., 2009).
Presence of AGPs in Chara suggested by mAb labeling (Domozych et al., 2009).
Cellulose has been found in all land plants (Brown, 1985).
Substituted (1→4)-β-d-xylans were detected by mAb labeling in hornwort sporophytes (Carafa et al., 2005).
Hemicelluloses from an aquatic moss, Fontinalis antipyretica, were biochemically analyzed by Geddes and Wilkie (1971, 1972).
Determined by enzyme digestion, followed by identification of the products by HPLC (Popper and Fry, 2003, 2004).
NMR of bryophyte xyloglucans showed them to contain acidic sugars (Peña et al., 2008).
Based on analysis of Driselase-digested material by size-exclusion chromatography/inductively coupled plasma mass spectroscopy, bryophyte walls contain less than 1% (w/w) of the total amount of the RGII present in angiosperms (Matsunaga et al., 2004).
Water-extractable lignans have been detected in bryophytes (Chodat and Cortesi, 1939).
Some plants either specifically accumulate silica or have a high mean relative shoot concentration (MRSC), including a thalloid liverwort (5.452), Equisetum (3.992), and members of the Poales (4.167). The mean value for MRSC in plants is 0.722 (Hodson et al., 2005). The MRSC reduces as follows: liverworts > horsetails > clubmosses > mosses > angiosperms > gymnosperms > ferns (Hodson et al., 2005).
Detected by mAb labeling (Carafa et al., 2005).
Ferns and fern allies contain RGII at levels like that found in angiosperms. The glycosyl sequence is conserved with the exception that an l-rhamnosyl residue is replaced in some members with 3-O-methylrhamnose (Matsunaga et al., 2004). RGII was identified and structurally analyzed by 11B-NMR spectra and glycosyl linkage composition by gas chromatography and gas chromatography/electron impact-mass spectrometry of alditol acetate and trimethylsilyl methyl glucoside derivatives (Matsunaga et al., 2004). 3-O-Methylrhamose is a sugar residue that is found relatively rarely in angiosperms but has been identified in wall preparations from ferns, fern allies, and bryophytes (Popper et al., 2004) and appears to be a component of bryophyte AGPs (Fu et al., 2007).
S-lignin occurs in lycophytes and flowering plants, but biochemical evidence, including analysis of enzyme kinetic properties, suggests that it is derived through separate biosynthetic pathways in each taxa (Weng et al., 2010).
Based on the biochemical analyses of Gómez Ros et al. (2007).
RGII isolated by Driselase digestion followed by size-exclusion chromatography and elucidated by 11B-NMR (Shimokawa et al., 1999).
CoMPP, enzyme digestion followed by matrix-assisted laser-desorption ionization time of flight mass spectrometry, monosaccharide linkage analysis (Sørensen et al., 2008), and specific enzyme digestion followed by thin-layer chromatography and HPLC (Fry et al., 2008b).
Detected by mAb labeling (Verhertbruggen et al., 2009).
Xylans with low levels of substitution detected (Timell, 1962).
A borate-RGII complex is found ubiquitously in higher plant cell walls (Matoh et al., 1996).
Xylans from Cryptomeria japonica were chemically analyzed by Edashige and Ishii (1996).
AGPs detected in loblolly pine (Pinus taeda) using RNA blotting (Loopstra and Sederoff, 1995).
Identified by mAb labeling and biochemical characterization by gas-liquid chromatography-mass spectrometry (Pennell et al., 1989).
Immunolabeling (Trethewey et al., 2005).
There are several evolutionary scenarios that could explain the occurrence of lignin in red algae and vascular plants: (1) lignin could have evolved independently in both lineages; (2) ancient algal genes leading to lignin biosynthesis could have been coopted during the evolution of vascular plants (Niklas and Kutschera, 2009, 2010); (3) the lignin biosynthesis pathway may have existed before the divergence of the embryophytes and subsequently lost from green algae (Xu et al., 2009); or (4) genes for lignin biosynthesis could have been transferred from one organism to another (Niklas, 2004). Lignin is composed of monolignol units. Within vascular plants, gymnosperm lignins are composed almost entirely of guaiacyl units, whereas angiosperm, lycopod (Jin et al., 2005), and Calliathron lignins additionally contain syringyl (S) lignin (Martone et al., 2009). However, S-lignins in lycopods and angiosperms, which diverged approximately 400 million years ago, are derived via distinctly different biosynthetic pathways, implying that S-units evolved in both plant groups via convergent evolution (Weng et al., 2008, 2010). Martone et al. (2009) suggest that S-lignin in Calliathron could represent another example of convergent evolution, and deduction of the lignin biosynthesis pathways in Calliathron could lend support to this theory. Conversely, if lignin was discovered in other algal groups, it could suggest that lignin biosynthesis in land plants has a more ancient origin.
Another potential example of convergent evolution is (1→3),(1→4)-β-d-glucan (MLG), which has been reported from lichens (Honegger and Haisch, 2001; Olafsdottir and Ingolfsdottir, 2001), fungi (Burton and Fincher, 2009; Pettolino et al., 2009), green algae (Eder et al., 2008), horsetails (Equisetum spp.; Fry et al., 2008b; Sørensen et al., 2008), and Poales (Trethewey et al., 2005; Table I).
Within land plants, MLG was only recently discovered in horsetails (Fry et al., 2008b; Sørensen et al., 2008) and was previously thought to have a restricted taxonomic distribution, occurring only in members of the Poales (Trethewey et al., 2005), which last shared a common ancestor with the horsetails over 370 million years ago (Bell and Hemsley, 2000). However, two independent groups, using several methods, concurrently discovered MLG in Equisetum cell walls. Fry et al. (2008b) digested horsetail wall preparations with an MLG-specific enzyme (lichenase; EC 3.2.1.73; Parrish et al., 1960). Quantification of the resulting oligosaccharides by high-pressure liquid chromatography revealed that Equisetum cell walls contain MLG at levels equal to or greater than those found in members of the Poales (Fry et al., 2008b). The presence of MLG in Equisetum cell walls was further supported, and localized within the wall, by monoclonal antibody (mAb) labeling (Sørensen et al., 2008) with a mAb that has a high degree of specificity to MLG (Meikle et al., 1994). The existence of MLG in horsetails was also found to be correlated with the occurrence of a wall-remodeling enzyme capable of grafting MLG to xyloglucan (Fry et al., 2008a).
The cellulose synthase-like gene families CslF, CslH, and CslJ have been shown to be involved in MLG synthesis in grasses (Richmond and Somerville, 2000; Burton et al., 2006, 2008; Doblin et al., 2009). Since these gene families appear to have diverged from within other cellulose synthase-like gene families (Yin et al., 2009) after horsetails had diverged from the lineage that eventually led to the Poales (Fig. 1; Yin et al., 2009), it seems likely that MLG arose independently in horsetails and Poales. The existence of an MLG-like polysaccharide in several groups of only distantly related photosynthetic organisms, putatively including the brown algae (Z.A. Popper, E. Demange, M. Lorenz, and O. Curry, unpublished data; Table I), many of which existed prior to the divergence of the CslF, CslH, and CslJ (Yin et al., 2009), further supports multiple origins of the polymer (Burton and Fincher, 2009).
While the mode of MLG synthesis may be different in Poales and Equisetum, it is of interest that the plants share some common morphological and biochemical features. Poales and horsetails exhibit similar body plans (Niklas, 2004). Additionally, they are both known to accumulate silica in their walls (Hodson et al., 2005), which Fry et al. (2008b) suggested may be correlated with the presence of MLG. If this were the case, it could be expected that liverworts, which have the highest relative mean shoot concentration of silica in land plants, could also contain MLG (Hodson et al., 2005). In fact, lichenase digestion of a cell wall preparation from the leafy liverwort Lophocolea bidentata has indicated that at least some liverworts may contain a polysaccharide similar to MLG (Popper and Fry, 2003). A silica transporter and mutants deficient in silica accumulation have been discovered in rice (Oryza sativa; Ma et al., 2006). If these plants exhibited alterations in MLG amount or deposition patterns, this would lend support to an interaction between MLG and silica.
Diversification within a Lineage
Perhaps the best examined cell wall biosynthetic genes are members of the cellulose synthase superfamily, which appears to have diversified within the land plant lineage to give nine cellulose synthase-like families and one cellulose synthase (CesA) family (Yin et al., 2009).
Cellulose is the most abundant naturally occurring polymer (Hess et al., 1928) and has a widespread distribution, being found in plants (Brown, 1985), algae (Naylor and Russell-Wells, 1934), bacteria (Roberts et al., 2002), cyanobacteria (Nobels et al., 2001), and tunicates (Kimura and Itoh, 1995). In land plants, cellulose may account for 20% to 50% (w/w; and in specialized cell walls, such as cotton [Gossypium hirsutum] fibers, up to 98% [w/w]) of the wall, whereas in red algae, it may only account for 1% to 8% (w/w; Kloareg and Quatrano, 1988). CesAs are widespread among eukaryotes and prokaryotes (Tsekos, 1999; Roberts et al., 2002; Roberts and Roberts, 2009), but those that form rosette terminal complexes have only been sequenced from the land plant lineage (Yin et al., 2009) and probably evolved after the divergence of the land plants from the chlorophytes (Yin et al., 2009; Fig. 1). Cellulose in the Chlorophyta and red and brown algae is synthesized by CesA genes whose origin predates that of the plant-specific CesA genes (Yin et al., 2009) and whose products form linear terminal complexes (Tsekos, 1999). Roberts et al. (2002) suggested that the observed differences in cellulose microfibril diameter between different cellulose-containing organisms (Nobels et al., 2001) could be, at least partially, a result of known differences in the arrangement of terminal complexes (Tsekos, 1999).
Mannans and glucomannans are synthesized by CslAs (Dhugga et al., 2004; Liepman et al., 2005; Goubet et al., 2009). While CslAs appear to be absent from green algae (Yin et al., 2009), these algae contain a specific Csl family that is most homologous to land plant CslA and CslC families (Yin et al., 2009; designated CslA/CslC in Fig. 1). Since mannans are known to occur in green algae, including Codium fragile (Estevez et al., 2009) and Acetabularia acetabulum (Dunn et al., 2007), the products of CslA/CslC could be responsible for mannan synthesis. CslA/CslC appears to be absent from brown and red algae (Yin et al., 2009; Fig. 1), suggesting that an absence of reports for mannans in brown algae (Table I) could be due to a lack of the required biosynthetic machinery. However, some red algae have been reported to contain mannans (Percival et al., 2001). The genes responsible for mannan synthesis in red algae may not be CslAs or their sequences may differ significantly from CslAs such that they were not detected in the screen used by Yin et al. (2009). Ostreococcus, an ancient member of the 1,500-million-year-old green lineage and the smallest known eukaryote (Derelle et al., 2006), was the earliest diverging organism found to contain CslA/CslC (Yin et al., 2009). Although Ostreococcus is wall-less, the existence of the products of CslA/CslC may be involved in cell-surface glycosylation, which Palenik et al. (2003, 2007) suggest may help disguise them from grazers. Taken in the context of a lack of all other plant-like Csl genes (Yin et al., 2009), the existence of a gene responsible for mannan synthesis in green algae and the subsequent evolution of a specific family of CslA genes suggest that the presence of mannan in their cell walls could have facilitated the success and diversification of green algae.
Ancient Origins
Xylans represent a case for the possibility that some plant cell wall components are derived from genes that existed before the divergence of green and red algae. Xylans are found ubiquitously in vascular plants and appear to be present in hornworts (Carafa et al., 2005), charophycean green algae (Domozych et al., 2009), chlorophytes, and red algae (Lahaye et al., 2003), suggesting that they have a cosmopolitan distribution among cell walls of photosynthetic organisms (Table I). Xylans can either be (1→3) or (1→4) linked. Painter (1983) hypothesized that red algae were at an evolutionary branch point, as some red algae, including the relatively basal Bangia fuscopurpurea, are composed of (1→3) linkages while others, such as the more recently diverged Palmaria palmata, have been suggested to contain both (1→3) and (1→4) linkages in the same molecule (Turvey and Williams, 1970). Potentially, green algae and land plants derived the genes for (1→4)-β-d-xylan synthesis from red algae. Additionally, while many land plant cell wall polysaccharides appear to be synthesized by Csls that diverged after green algae (Yin et al., 2009; Fig. 1), evidence for the involvement of Csls in xylan biosynthesis appears to be lacking (Zhou et al., 2006). Instead, a large number of glycosyl transferases (GTs), including FRAGILE FIBER8 (GT47; GT numbers refer to those designated by the CAZy database [http://www.cazy.org]; Cantarel et al., 2009), IRREGULAR XYLEM8 (IRX8) and PARVUS (GT8), and IRX9 and IRX14 (GT43), are implicated (Brown et al., 2005, 2007; Lee et al., 2007; Peña et al., 2007; York and O'Neill, 2008). Querying the CAZy database (Cantarel et al., 2009) reveals that Ostreococcus (the smallest known eukaryote and a member of the prasinophycean green algae) appears to lack members of the GT43 family (Hashimoto et al., 2009) thought to be involved in xylan backbone synthesis (Lee et al., 2007). Ostreococcus contains several other GTs (http://www.cazy.org; Cantarel et al., 2009) that could have a role in xylan synthesis, including GT8, which is involved in glucuronoxylan synthesis (Lee et al., 2007), and GT4, which includes a 1,4-β-d-xylan synthase (EC 2.4.2.24; Bailey and Hassid, 1966), thus supporting an origin for xylan biosynthesis that predates the land plant lineage.
Another group of wall components that appear to have ancient origins are arabinogalactan proteins (AGPs), a group of proteoglycans that exhibit considerable structural and functional diversity and are thought to occur ubiquitously in land plants (Basile, 1980; Basile et al., 1989; Pennell et al., 1989; Knox et al., 1991; Lee et al., 2005). However, they may be much more widely distributed. Immunolabeling and chemical analyses have suggested their occurrence in the charophycean green algae (McCann et al., 2007; Domozych et al., 2009) and chlorophytes (Stanley et al., 2005; Eder et al., 2008; Estevez et al., 2009). We have also detected AGPs in extracts from red and brown algae using the radial gel diffusion assay (Z.A. Popper, Q. Coster, and T. Slattery, unpublished data). At least some AGP functions may be conserved between taxa; localization of AGPs in the utricle apical zone in C. fragile (Estevez et al., 2009) may suggest a role in tip growth that correlates with their reported involvement in the tip growth of moss protonemata (Lee et al., 2005).
The model organism Chlamydomonas, which is a flagellated green alga, has walls that are substantially different from those of land plants, not least because they seem to lack cellulose (Roberts, 1974). Instead, the major wall components are layers of crystalline Ara-rich, Hyp-rich glycoproteins (Miller et al., 1974; Roberts, 1974; Bollig et al., 2007). However, closer examination of the Chlamydomonas glycoproteins shows that they appear to share some features with AGPs, including conservation of an inner core of two Ara residues linked to Hyp (Bollig et al., 2007). This lends support to the argument for a degree of conservation between plant and algal wall components. It also highlights the need for extensive sampling, as some green algae share other wall features with land plants (Fig. 1).
Possible Innovations
Rhamnogalacturonan II (RGII) is perhaps the most distinct example of an innovation in wall composition to have occurred in land plants. It has a highly conserved structure and is present in all vascular plants (Matoh et al., 1996), but if present in extant bryophytes it constitutes less than 0.025% (w/w) of the wall (Matsunaga et al., 2004). Since RGII has not been detected in green algae (Domozych et al., 1980; Becker et al., 1994, 1998), it seems likely that its occurrence in land plants could be correlated with specific evolutionary pressures potentially related to terrestrialization. However, the ability to make some of the relatively unusual monosaccharide residues present in RGII, such as 3-deoxy-d-manno-2-octulosonic acid (Kdo; York et al., 1985), may have deeper origins. Most members of the prasinophyceae have scales or a theca (wall) containing Kdo (York et al., 1985; Becker et al., 1991; Domozych et al., 1991). CMP-Kdo synthetase (CKS) is responsible for generating the activated sugar donor CMP-Kdo required for the synthesis of wall polymers containing Kdo, and sequences for the CKS gene are present in every major plant group, including mosses (Royo et al., 2000). Neither Kdo nor CKS has been found in animals or yeasts. However, they are both present in gram-negative eubacteria (Royo et al., 2000), where they probably represent an example of horizontal gene transfer either from the bacteria to the plant or, more unusually, from the plant to the bacteria (Royo et al., 2000). It is of interest that a putative Kdo transferase gene (AtKDTA; Séveno et al., 2010) has recently been characterized. However, AtKDTA appears to represent an example of a gene that was not transferred from a bacterium (in this case, the ancestor of mitochondria) to a plant following an endosymbiotic event. The evidence for this is that AtKDTA synthesizes a protein that localizes to the mitochondria, where Séveno et al. (2010) hypothesize that it may be involved in the synthesis of a lipid A-like molecule. More significantly, in terms of plant cell wall synthesis, AtKDTA null mutants appear to have an unaltered phenotype and conserved RGII structure and amount, implying that AtKDTA is unlikely to be involved in RGII synthesis (Séveno et al., 2010).
Xyloglucan may also represent a relatively recent innovation. It is present in all land plants (Popper and Fry, 2003, 2004), and immunolabeling suggests that it may be present in some members of the charophycean green algae (Ikegaya et al., 2008; Domozych et al., 2009). The occurrence of xyloglucans in other photosynthetic organisms is unknown (Table I). In addition, the enzymes involved in xyloglucan synthesis appear to have continued to diversify within the land plants. This is suggested by the discovery that moss and liverwort xyloglucans contain GalUA and are structurally distinct from xyloglucans synthesized by vascular plants and hornworts (Peña et al., 2008). There are also several xyloglucan side chains that may be restricted to the relatively recently diverged Asteridae (Hoffman et al., 2005). Furthermore, activity of the enzyme xyloglucan endotransglucosylase, involved in xyloglucan modification (Thompson and Fry, 2001) and consequently plant growth and differentiation (Vissenberg et al., 2005), was found in the chlorophyte Ulva linza but appeared to be absent from red and brown algae (Van Sandt et al., 2007). This suggests that xyloglucan or a structurally similar polysaccharide does not occur in the cell walls of either red or brown algae.
Pectins and pectin-like polymers appear to have a relatively cosmopolitan occurrence and are found in red and green algae as well as land plants (Painter, 1983; Domozych et al., 2007; Eder and Lütz-Meindl, 2008). A polysaccharide has even been isolated from the cyanobacterium Microcystis flos-aquae, which contains the monosaccharide residues GalUA, Rha, Man, Xyl, Glc, and Gal in a similar molar ratio to that found in pectin, although the degree of structural similarity has not been determined (Plude et al., 1991). However, arabinans might be expected to be a land plant innovation. Specifically, they could be predicted to occur only in hornworts and vascular plants because they have been implicated in stomatal opening (Jones et al., 2003). However, LM6, a mAb that recognizes short linear stretches of arabinosyl residues, not only labels guard cell walls (Jones et al., 2003) but has also been found to bind to Chara cell walls (Domozych et al., 2009). The recruitment of arabinans in guard cell function, therefore, might be an example of cooption in function of a preexisting wall polymer.
SAMPLING CELL WALL DIVERSITY
Correlating the occurrence of genes and wall components with phylogenies, as given for CesA and Csls (Fig. 1), undoubtedly has the potential to reveal new insights into wall evolution. However, as discussed by Sørensen et al. (2010), it is dependent on adequate sampling. This could be approached by the detailed analysis of representative plants, but screening may help to optimize which plants are selected for further analysis. With conservative estimates of 260,000 vascular plant species alone (Judd et al., 2002; Angiosperm Phylogeny Group, 2003), investigation of the cell wall composition of photosynthetic organisms necessarily demands a high-throughput approach (Sørensen et al., 2010). The total number of samples is further expanded by taking into consideration variation between tissues and between stages in the life cycle (Sørensen et al., 2010). For example, based on analysis of the products released by enzyme digestion of vegetative cells, xyloglucan was thought to be absent from Chara (Popper and Fry, 2003). However, more recent evidence provided by mAb labeling suggests that xyloglucan may occur in the walls of Chara antheridia (Domozych et al., 2009). Additional evidence will be necessary to determine whether xyloglucan actually does occur in Chara cell walls, because although an anti-xyloglucan mAb was capable of recognizing and binding to an epitope present in Chara cell walls, that epitope could be part of a polymer that is not xyloglucan.
Several techniques have been developed that could greatly facilitate the investigation of wall diversity, including Fourier-transform infrared microspectroscopy (FT-IR; Mouille et al., 2003), oligosaccharide mass profiling (OLIMP; Obel et al., 2006), and comprehensive microarray polymer profiling (CoMPP; Willats et al., 2002; Sørensen et al., 2008).
FT-IR is capable of generating a fingerprint that can distinguish between Arabidopsis (Arabidopsis thaliana) mutants with altered cellulose, pectin, and xyloglucan compositions (Mouille et al., 2003). This method could be extended to profile different taxa. However, peaks may shift depending on molecular interactions and the environment within the wall (Kačuráková et al., 2000), a phenomenon that is likely to be even more pronounced between distantly related taxa, making unambiguous peak assignment and attribution difficult.
OLIMP utilizes highly specific hydrolases to digest wall components (Obel et al., 2006). The digestion products are then analyzed by matrix-assisted laser-desorption ionization time of flight mass spectrometry, and structural differences are indicated by changes in observed ions (Obel et al., 2006). This method has been applied to the investigation of Arabidopsis cell wall polysaccharides (Obel et al., 2006; Gille et al., 2009) but could be extremely valuable for screening for the existence of structural differences between polysaccharides within diverse plant taxa. Polysaccharides with unknown or unusual structures could then be subjected to further and more detailed methods of analysis. However, structural analysis of a cell wall component using OLIMP is dependent on its ability to be hydrolyzed, and in some taxa a wall component could be present but resist hydrolysis. We recently found that several species of brown algae can be labeled with a mAb that has a high specificity for (1→3),(1→4)-β-d-glucan (Meikle et al., 1994; Z.A. Popper and E. Demange, unpublished data). In addition, polysaccharides extracted from the wall using strong alkali could be digested with lichenase. However, digestion of brown algal cell walls with lichenase prior to labeling did not prevent the anti-MLG mAb from binding (Z.A. Popper and M. Lorenz, unpublished data). It seems likely that digestion was prevented by the presence of high concentrations of wall-bound phenolic compounds (Schoenwaelder and Clayton, 1998, 1999), which have been shown to inhibit enzyme activity (Barwell et al., 1989; Shibata et al., 2003).
To date, CoMPP is the technique that has been most extensively applied toward screening cell wall diversity (Sørensen et al., 2008) and has already resulted in some interesting discoveries, such as the presence of MLG in horsetail cell walls (Sørensen et al., 2008), as discussed earlier.
Using CoMPP, an indication of the likely presence of specific cell wall components within a particular plant species, tissue, or developmental stage is dependent on the reaction of extracted wall components with mAbs or carbohydrate-binding molecules (Moller et al., 2007). Thus, the availability of mAbs and carbohydrate-binding molecules currently limits the full potential of CoMPP. Increased numbers of mAbs, which are continuing to become available (Pattathil et al., 2010), will increase the power of CoMPP and greatly facilitate the analysis of cell wall diversity. The majority of cell wall-specific mAbs were generated against polysaccharides isolated from flowering plant cell walls, but there are exceptions, including those generated against polysaccharides from brown seaweeds (Vreeland, 1972; Vreeland et al., 1982, 1984). Furthermore, each mAb detects a specific epitope wherever it occurs, although interpretation may be complicated by the fact that the epitope recognized by a mAb can exist in different wall components in different taxa.
CoMPP is frequently followed by more extensive characterization of the cell wall using a variety of techniques to both confirm and add detail to the initial results (Sørensen et al., 2008, 2010). In situ methods enable investigation of the wall components in their native environment, facilitating exploration of their intramural associations.
One of the most frequently used in situ methods is labeling using mAbs or carbohydrate-binding molecules. This method can map the tissue-specific location of cell wall components and, when used in concert with advanced microscopy techniques such as electron tomography, can even enable three-dimensional visualization of a component within the wall (Mastronarde, 1997; Otegui et al., 2001; Otegui and Staehelin, 2004; Segui-Simarro et al., 2004). It has been found that the presence of high concentrations of pectin can mask, or prevent, mAb labeling of xyloglucan (Marcus et al., 2008). While the phenomenon of masking complicates the interpretation of mAb labeling, it also yields details regarding interactions between wall components that can be unveiled by a strategy that combines specific enzyme digestion with mAb labeling (Marcus et al., 2008). Further complications could arise from the presence of cell wall components that render hydrolytic enzymes inactive. However, mAb labeling can also be combined with a variety of chemical pretreatments; incubation of Fucus sections in EDTA prior to mAb labeling was found to improve antibody penetration (Vreeland et al., 1984).
CONCLUSION
Becker and Marin (2009) stated that they were “convinced that many plant ‘innovations’ will actually turn out to be innovations of the streptophyte algae,” whereas Niklas (2004) suggested that some plant cell wall features may have even deeper roots and share origins with more ancient algal ancestors. Both hypotheses may be true for different wall components.
Despite the fact that the chlorophytes and streptophytes (land plants and charophycean green algae) last shared a common ancestor 725 to 1,200 million years ago (Becker and Marin, 2009), it appears that through a combination of shared ancestry and convergent evolution they have some common cell wall characteristics. They also share some wall features with red and brown algae (summarized in Table I), to which they are even more distantly related (Yoon et al., 2004). Therefore, while cell wall differentiation may have been of high adaptive importance (Stebbins, 1992), it appears that a degree of conservation also exists. Further characterization of plant and algal cell wall polysaccharides and the enzymes that synthesize them may reveal the existence of core features common to eukaryotic cell walls, despite the presence of different cooccurring cell wall components and diverse intramural interactions.
Acknowledgments
We thank the editor and reviewers for their helpful comments and suggestions and David Domozych, Iben Sørensen, and William Willats for allowing us to read a preprint edition of “How Did Plant Cell Walls Evolve?”
References
- Andrew IG, Little JWL. (1997) A xyloglucan isolated in etiolated seedlings of Pinus radiata. Phytochemistry 46: 203–207 [Google Scholar]
- Angiosperm Phylogeny Group (2003) An update of the Angiosperm Phylogeny Group for the orders and families of flowering plants: APGII. Bot J Linn Soc 141: 399–436 [Google Scholar]
- Bailey RW, Hassid WZ. (1966) Xylan synthesis from uridine-diphosphate-d-xylose by particulate preparations from immature corncobs. Proc Natl Acad Sci USA 56: 1586–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan B, Andolfo P, Navazio L, Tolomio A, Mariani P. (2001) Cellulose in algal cell wall: an ‘in situ’ localization. Eur J Histochem 45: 51–56 [PubMed] [Google Scholar]
- Barwell CJ, Blunden G, Manandhar PD. (1989) Isolation and characterization of brown algal polyphenols as inhibitors of α-amylase, lipase and trypsin. J Appl Phycol 1: 319–323 [Google Scholar]
- Basile DV. (1980) A possible mode of action for morphoregulatory hydroxyproline-proteins. Bull Torrey Bot Club 107: 325–338 [Google Scholar]
- Basile DV, Kushner BK, Basile MR. (1989) A new method for separating and comparing arabinogalactan proteins in the Hepatica. Bryologist 90: 401–404 [Google Scholar]
- Becker B, Becker D, Kamerling JP, Melkonian M. (1991) 2-Keto-sugar acids in green flagellates: a chemical marker for prasinophyte scales. J Phycol 27: 498–504 [Google Scholar]
- Becker B, Marin B. (2009) Streptophyte algae and the origin of the embryophytes. Ann Bot (Lond) 103: 999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Marin B, Melkonian M. (1994) Structure, composition, and biogenesis of prasinophyte cell coverings. Protoplasma 181: 233–244 [Google Scholar]
- Becker B, Melkonian M, Kamerling JP. (1998) The cell wall (theca) of Tetraselmis striata (Chlorophyta): macromolecular composition and structural elements of the complex polysaccharides. J Phycol 34: 779–787 [Google Scholar]
- Bell PR, Hemsley AR. (2000) Green Plants: Their Origin and Diversity. Cambridge University Press, Cambridge, UK [Google Scholar]
- Bollig K, Lamshöft M, Schweimer K, Marner F-J, Budzikiewicz H, Waffenschmidt S. (2007) Structural analysis of linear hydroxyproline-bound O-glycans of Chlamydomonas reinhardtii: conservation of the inner core in Chlamydomonas and land plants. Carbohydr Res 342: 2557–2566 [DOI] [PubMed] [Google Scholar]
- Bourne EJ, Brush P, Percival E. (1969) The active carbohydrate metabolites of the brown seaweed, Fucus vesiculosus. Carbohydr Res 9: 415–422 [Google Scholar]
- Bremner I, Wilkie KCB. (1971) The hemicelluloses of bracken. II. A galactoglucomannan. Carbohydr Res 20: 193–203 [DOI] [PubMed] [Google Scholar]
- Brown DM, Goubet F, Wong VW, Goodacre R, Stephens E, Dupree P, Turner SR. (2007) Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J 52: 1154–1168 [DOI] [PubMed] [Google Scholar]
- Brown DM, Zeef LAH, Ellis J, Goodacre R, Turner SR. (2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17: 2281–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM., Jr (1985) Cellulose microfibril assembly and orientation: recent developments. J Cell Sci Suppl 2: 13–32 [DOI] [PubMed] [Google Scholar]
- Burton RA, Fincher GB. (2009) (1,3;1,4)-β-d-Glucans in the cell walls of the Poaceae, lower plants, and fungi: a tale of two linkages. Mol Plant 2: 873–882 [DOI] [PubMed] [Google Scholar]
- Burton RA, Jobling SA, Harvey AJ, Shirley NJ, Mather DE, Bacic A, Fincher GB. (2008) The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley (Hordeum vulgare L.). Plant Physiol 146: 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, Stone BA, Newbigin EJ, Bacic A, Fincher GB. (2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-d-glucans. Science 311: 1940–1942 [DOI] [PubMed] [Google Scholar]
- Campo VL, Kawano DF, Da Silva DB, Jr, Carvalho I. (2009) Carrageenans: biological properties, chemical modifications and structural analysis. A review. Carbohydr Polym 77: 167–180 [Google Scholar]
- Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37: D233–D238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafa A, Duckett JG, Knox JP, Ligrone R. (2005) Distribution of cell-wall xylans in bryophytes and tracheophytes: new insights into basal interrelationships of land plants. New Phytol 168: 231–240 [DOI] [PubMed] [Google Scholar]
- Chodat F, Cortesi R. (1939) Sur la coloration des membranes de mousses. Compt Rend Soc Phys Nat Geneve 21: 58–61 [Google Scholar]
- Currie HA, Perry CC. (2007) Silica in plants: biological, biochemical and chemical studies. Ann Bot (Lond) 100: 1383–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche CF, Graham LE, Thomson N. (1989) Lignin-like compounds and sporopollenin in Coleochaete, an algal model for land plant ancestry. Science 245: 399–401 [DOI] [PubMed] [Google Scholar]
- Derelle E, Ferraz C, Rombauts S, Rouzé P, Worden AZ, Robbens S, Partensky F, Degroeve S, Echeynién S, Cooke R, et al. (2006) Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci USA 103: 11647–11652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reviers B. (2002) Biologie et Phylogénie des Algues, Vol 1. Belin, Paris, pp 144–165 [Google Scholar]
- Dhugga KS, Barreiro R, Whitten B, Stecca K, Hazeebrook J, Randhawa GS, Dolan M, Kinney AJ, Tomer D, Nichols S, et al. (2004) Guar seed β-mannan synthase is a member of the cellulase synthase super gene family. Science 303: 363–366 [DOI] [PubMed] [Google Scholar]
- Doblin MS, Pettolino FA, Wilson SM, Campbell R, Burton RA, Fincher GB, Newbigin E, Bacic A. (2009) A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-d-glucan synthesis in transgenic Arabidopsis. Proc Natl Acad Sci USA 14: 5996–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Serfis A, Kiemle SN, Gretz MR. (2007) The structure and biochemistry of charophycean cell walls. I. Pectins of Penium margaritaceum. Protoplasma 230: 99–115 [DOI] [PubMed] [Google Scholar]
- Domozych DS, Sørensen I, Willats WGT. (2009) The distribution of cell wall polymers during antheridium development and spermatogenesis in the charophycean green alga, Chara corallina. Ann Bot (Lond) 104: 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domozych DS, Stewart KD, Mattox KR. (1980) The comparative aspects of cell wall chemistry in the green algae (Chlorophyta). J Mol Evol 15: 1–12 [DOI] [PubMed] [Google Scholar]
- Domozych DS, Wells B, Shaw PJ. (1991) The basket scales of the green alga Mesostigma viridae: chemistry, immunology and ultrastructure. J Cell Sci 100: 397–407 [Google Scholar]
- Dunn EK, Shoue DA, Huang X, Kline RE, Mackay AL, Carpita NC, Taylor IEP, Mandoli DF. (2007) Spectroscopic and biochemical analysis of regions of the cell wall of the unicellular ‘mannan weed,’ Acetabularia acetabulum. Plant Cell Physiol 48: 122–133 [DOI] [PubMed] [Google Scholar]
- Edashige Y, Ishii T. (1996) Structures of cell-wall polysaccharides from suspension-cultured cells of Cryptomeria japonica. Mokuzai Gakkaishi 42: 895–900 [Google Scholar]
- Eder M, Lütz-Meindl U. (2008) Pectin-like carbohydrates in the green alga Micrasterias characterized by cytochemical analysis and energy filtering TEM. J Microsc 231: 201–214 [DOI] [PubMed] [Google Scholar]
- Eder M, Tenhaken R, Driouich A, Lütz-Meindl U. (2008) Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (Streptophyta). J Phycol 44: 1221–1234 [DOI] [PubMed] [Google Scholar]
- Estevez JM, Fernández PV, Kasulin L, Dupree P, Ciancia M. (2009) Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 19: 212–228 [DOI] [PubMed] [Google Scholar]
- Fry SC, Mohler KE, Nesselrode BHWA, Franková L. (2008a) Mixed-linkage β-glucan:xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant J 55: 240–252 [DOI] [PubMed] [Google Scholar]
- Fry SC, Nesselrode BH, Miller JG, Mewburn BR. (2008b) Mixed-linkage (1→3,1→4)-β-d-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytol 179: 104–115 [DOI] [PubMed] [Google Scholar]
- Fu H, Yadav MP, Nothnagel EA. (2007) Physcomitrella patens arabinogalactan proteins contain abundant terminal 3-O-methyl-l-rhamosyl residues not found in angiosperms. Planta 226: 1511–1524 [DOI] [PubMed] [Google Scholar]
- Geddes DS, Wilkie KCB. (1971) Hemicelluloses from the stem tissues of the aquatic moss Fontanalis antipyretica. Carbohydr Res 18: 333–335 [Google Scholar]
- Geddes DS, Wilkie KCB. (1972) A galactoglucomannan from the stem tissues of the aquatic moss Fontanalis antipyretica. Carbohydr Res 23: 349–357 [Google Scholar]
- Gille S, Hānsel U, Ziemann M, Pauly M. (2009) Identification of plant cell wall mutants by means of a forward chemical genetic approach using hydrolases. Proc Natl Acad Sci USA 106: 14699–14704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez Ros LV, Gabaldón C, Pomar F, Merino F, Pedreño MA, Barceló AR. (2007) Structural motifs of syringyl peroxidases predate not only the gymnosperm-angiosperm divergence but also the radiation of tracheophytes. New Phytol 173: 63–78 [DOI] [PubMed] [Google Scholar]
- Goubet F, Barton CJ, Mortimer JC, Yu X, Zhang Z, Miles GP, Richens J, Liepman AH, Seffen K, Dupree P. (2009) Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J 60: 527–538 [DOI] [PubMed] [Google Scholar]
- Gunnison D, Alexander M. (1975) Basis for the resistance of several algae to microbial decomposition. Appl Microbiol 29: 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Higuchi T, Ishikawa H. (1960) Formation of lignin in tissue culture of Pinus strobus. Plant Cell Physiol 1: 173–182 [Google Scholar]
- Hashimoto K, Tokimatsu T, Kaeano S, Yoshizawa AC, Okuda S, Goto S, Kanehisa M. (2009) Comprehensive analysis of glycosyltransfereases in eukaryotic genomes for structural and functional characterization of glycans. Carbohydr Res 344: 881–887 [DOI] [PubMed] [Google Scholar]
- Hess K, Haller R, Katz JR. (1928) Die Chemie der Zellulose und Ihrer Begleiter. Akademische Verlagsgesellschaft, Leipzig, Germany [Google Scholar]
- Hodson MJ, White PJ, Mead A, Broadley MR. (2005) Phylogenetic variation in the silicon composition of plants. Ann Bot (Lond) 96: 1027–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M, Jia Z, Peña MJ, Cash M, Harper A, Blackburn AR, II, Darvill A, York WS. (2005) Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydr Res 115: 1826–1840 [DOI] [PubMed] [Google Scholar]
- Hoiczyk E, Hansel A. (2000) Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J Bacteriol 182: 1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger R, Haisch A. (2001) Immunocytochemical location of the (1,3)(1,4)-beta-glucan lichenin in the lichen-forming ascomycete Cetraria islandica (Icelandic moss). New Phytol 150: 739–746 [Google Scholar]
- Ikegaya H, Hayashi T, Kaku T, Iwata K, Sonobe S, Shimmen T. (2008) Presence of xyloglucan-like polysaccharide in Spirogyra and possible involvement in cell-wall attachment. Phycol Res 56: 216–222 [Google Scholar]
- Jin ZF, Matsumoto Y, Tange T, Akiyama T, Higuchi M, Ishii T, Iiyama K. (2005) Proof of the presence of guaiacyl-syringyl lignin in Selaginella tamariscina. J Wood Sci 51: 424–426 [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ. (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci USA 100: 11783–11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ. (2002) Plant Systematics: A Phylogenetic Approach. Sinauer Associates, Sunderland, MA [Google Scholar]
- Kačuráková M, Capek P, Sasinkova V, Weller N, Ebringerova A. (2000) FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym 43: 195–203 [Google Scholar]
- Keeling P, Leander BS, Simpson A. (2009) Eukaryotes: Eukaryota, organisms with nucleated cells. http://tolweb/org/Eukaryotes/3/2009.10.28 (January 29, 2009)
- Keeling PJ. (2004) Diversity and evolutionary history of plastids and their hosts. Am J Bot 91: 1481–1493 [DOI] [PubMed] [Google Scholar]
- Kimura S, Itoh T. (1995) Evidence for the role of the glomerulocyte in cellulose synthesis in the tunicate Metandrocarpa uedai. Protoplasma 186: 24–33 [Google Scholar]
- Kloareg B, Quatrano RS. (1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Annu Rev 26: 259–315 [Google Scholar]
- Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K. (1991) Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root pattern formation. Plant J 1: 317–326 [DOI] [PubMed] [Google Scholar]
- Lahaye M, Rondeau-Mouro C, Deniaud E, Buléon A. (2003) Solid-state 13C NMR spectroscopy studies of xylans in the cell wall of Palmaria palmata (L. Kuntze, Rhodophyta). Carbohydr Res 338: 1559–1569 [DOI] [PubMed] [Google Scholar]
- Lee C, O'Neill MA, Tsumuraya Y, Darvill AG, Ye ZH. (2007) The irregular xylem9 mutant is deficient in xylan xylosyltransferase activity. Plant Cell Physiol 48: 1624–1634 [DOI] [PubMed] [Google Scholar]
- Lee KJD, Sakata Y, Mau SL, Pettolino F, Bacic A, Quatrano RS, Knight CD, Knox JP. (2005) Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell 17: 3051–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NG. (1999) A 20th century roller coaster ride: a short account of lignification. Curr Opin Plant Biol 2: 153–162 [DOI] [PubMed] [Google Scholar]
- Liepman AH, Wilkerson CG, Keegstra K. (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loopstra CA, Sederoff RR. (1995) Xylem-specific gene expression in loblolly pine. Plant Mol Biol 27: 277–291 [DOI] [PubMed] [Google Scholar]
- Love J, Percival E. (1964) The polysaccharides of the green seaweed Codium fragile. III. A β-1→4-linked mannan. J Chem Soc 3345–3350 [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. (2006) A silicon transporter in rice. Nature 440: 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11: 1360–1385 [DOI] [PubMed] [Google Scholar]
- Mabeau S, Kloareg B. (1997) Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J Exp Bot 38: 1573–1580 [Google Scholar]
- Mackie IM, Percival E. (1959) The constitution of xylan from the green seaweed Caulerpa filiformis J Chem Soc 1151–1156 [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, Willats WG, Knox JP. (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martone PT, Estevez JM, Lu F, Ruel K, Denny MW, Somerville C, Ralph J. (2009) Discovery of lignin in seaweed reveals convergent evolution of cell wall architecture. Curr Biol 19: 169–175 [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. (1997) Dual-axis tomograph: an approach with alignment methods that preserve resolution. J Struct Biol 120: 343–352 [DOI] [PubMed] [Google Scholar]
- Matoh T, Kawaguchi S, Kobayashi M. (1996) Ubiquity of a borate-rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol 37: 636–640 [Google Scholar]
- Matsunaga T, Ishii T, Matsumoto S, Higuchi M, Darvill A, Albersheim P, O'Neill MA. (2004) Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes: implications for the evolution of vascular plants. Plant Physiol 134: 339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann A, Benanti G, Knox JP, Popper ZA. (2007) The occurrence of arabinogalactan proteins in charophytes (stoneworts). Physiol Plant 130: abstract 125 [Google Scholar]
- Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, Stone BA. (1994) A (1,3;1,4)-beta-glucan-specific monoclonal antibody and its use in the quantification and immunocytochemical location of (1,3;1,4)-beta-glucans. Plant J 5: 1–9 [DOI] [PubMed] [Google Scholar]
- Miller DH, Mellman IS, Lamport DTA, Miller M. (1974) The chemical composition of the cell wall of Chlamydomonas gymnogama and the concept of a plant cell wall protein. J Cell Biol 63: 420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller I, Sørensen I, Bernal AJ, Blaukopf C, Lee K, Øbro J, Pettolino F, Roberts A, Mikkelsen J, Knox JP, et al. (2007) High-throughput mapping of cell wall polymers within and between plants using novel microarrays. Plant J 50: 1118–1128 [DOI] [PubMed] [Google Scholar]
- Morrison JC, Greve LC, Richmond PA. (1993) Cell wall synthesis during growth and maturation of Nitella internodal cells. Planta 189: 321–328 [DOI] [PubMed] [Google Scholar]
- Mouille G, Robin S, Lecomte M, Pagent S, Höfte H. (2003) Classification and identification of Arabidopsis cell wall mutants using Fourier-transform infrared (FT-IR) microspectroscopy. Plant J 35: 393–404 [DOI] [PubMed] [Google Scholar]
- Naylor G, Russell-Wells B. (1934) On the presence of cellulose and its distribution in the cell walls of brown and red algae. Ann Bot (Lond) 48: 635–641 [Google Scholar]
- Niklas KJ. (2000) The evolution of plant body plans: a biomechanical perspective. Ann Bot (Lond) 85: 411–438 [Google Scholar]
- Niklas KJ. (2004) The cell walls that bind the tree of life. Bioscience 54: 831–841 [Google Scholar]
- Niklas KJ, Kutschera U. (2009) The evolutionary development of plant body plans. Funct Plant Biol 36: 682–695 [DOI] [PubMed] [Google Scholar]
- Niklas KJ, Kutschera U. (2010) The evolution of the land plant life cycle. New Phytol 185: 27–41 [DOI] [PubMed] [Google Scholar]
- Nobels DR, Romanovicz DK, Brown RM., Jr (2001) Cellulose in cyanobacteria: origin of vascular plant cellulose synthase? Plant Physiol 127: 529–542 [PMC free article] [PubMed] [Google Scholar]
- Obel N, Erben V, Pauly M. (2006) Functional wall glycomics through oligosaccharide mass profiling. Hayashi T, , The Science and Lore of the Plant Cell Wall: Biosynthesis, Structure and Function. BrownWalker Press, Boca Raton, FL, pp 258–266 [Google Scholar]
- Olafsdottir ES, Ingolfsdottir K. (2001) Polysaccharides from lichens: structural characteristics and biological activity. Planta Med 67: 199–208 [DOI] [PubMed] [Google Scholar]
- Otegui MS, Mastronarde DN, Kang BH, Bednarek KY, Staehelin LA. (2001) Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualised by high resolution electron tomography. Plant Cell 13: 2033–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Staehelin LA. (2004) Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta 218: 501–515 [DOI] [PubMed] [Google Scholar]
- Painter TJ. (1983) Algal polysaccharides. Aspinall GO, ed, The Polysaccharides, Vol 2. Academic Press, New York, pp 195–285 [Google Scholar]
- Palenik B, Brahansha B, Larimer FW, Land M, Hauser L, Chain P, Lamerdin J, Regala W, Allen EE, McCarren J, et al. (2003) The genome of a motile marine Synechococcus. Nature 424: 1037–1042 [DOI] [PubMed] [Google Scholar]
- Palenik B, Grimwood J, Aerts A, Rouzé P, Salamov A, Putman N, Dupont C, Jorgensen R, Derelle E, Rombouts S, et al. (2007) The tiny eukaryote Ostreococcus provides genomic insight into the paradox of plankton speciation. Proc Natl Acad Sci USA 104: 7705–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JD, Soltis JE, Chase MW. (2004) The plant tree of life: an overview and some points of view. Am J Bot 91: 1437–1445 [DOI] [PubMed] [Google Scholar]
- Parker BC. (1970) Significance of cell wall chemistry to phylogeny in the algae. Ann N Y Acad Sci 175: 417–427 [Google Scholar]
- Parrish FW, Perlin AS, Reese ET. (1960) Selective enzymolysis of poly-β-d-glucans and the structure of the polymers. Can J Chem 38: 2094–2104 [Google Scholar]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, et al. (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M, Keegstra K. (2008) Physiology and metabolism ‘tear down this wall.’ Curr Opin Plant Biol 11: 233–235 [DOI] [PubMed] [Google Scholar]
- Peña MJ, Darvill AG, Eberhard S, York WS, O'Neill MA. (2008) Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from xyloglucans synthesised by hornworts and vascular plants. Glycobiology 18: 891–904 [DOI] [PubMed] [Google Scholar]
- Peña MJ, Zhong R, Zhou GK, Richardson EA, O'Neill MA, Darvill AG, York WS, Ye ZH. (2007) Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19: 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Knox PJ, Scofield GN, Selvendran RR, Roberts K. (1989) A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol 108: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival E, Rupérez P, Fulgencio SC. (2001) Dietary fibre and physicochemical properties of Spanish seaweeds. Eur Food Res Technol 212: 349–354 [Google Scholar]
- Peter G, Neale D. (2004) Molecular basis for the evolution of xylem lignification. Curr Opin Plant Biol 7: 737–742 [DOI] [PubMed] [Google Scholar]
- Pettolino F, Sasaki I, Turbic A, Wilson SM, Bacic A, Hrmova M, Fincher GB. (2009) Hyphal cell walls from the plant pathogen Rhynchosporium secalis contain (1,3;1,6)-β-d-glucans, galacto- and rhamnomannans, (1,3;1,4)-β-d-glucans and chitin. FEBS J 276: 4122–4133 [DOI] [PubMed] [Google Scholar]
- Plude JL, Parker DL, Schommer OJ, Timmerman RJ, Hagstrom SA, Joers JM, Hnasko R. (1991) Chemical characterization of polysaccharide from the slime layer of the cyanobacterium Microcystis flos-aquae C3-40. Appl Environ Microbiol 57: 1696–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA. (2008) Evolution and diversity of green plant cell walls. Curr Opin Plant Biol 11: 286–292 [DOI] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. (2003) Primary cell wall composition of bryophytes and charophytes. Ann Bot (Lond) 91: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. (2004) Primary cell wall composition of pteridophytes and spermatophytes. New Phytol 164: 165–174 [DOI] [PubMed] [Google Scholar]
- Popper ZA, Sadler IH, Fry SC. (2004) 3-O-Methyl rhamnose in lower land plant primary cell walls. Biochem Syst Ecol 32: 279–289 [Google Scholar]
- Ragan M. (1984) Fucus ‘lignin’: a reassessment. Phytochemistry 23: 2029–2032 [Google Scholar]
- Richmond T, Somerville C. (2000) The cellulose synthase superfamily. Plant Physiol 124: 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Roberts E, Delmer DP. (2002) Cellulose synthase (CesA) genes in the green alga Mesotaenium caldariorum. Eukaryot Cell 1: 847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Roberts AW. (2009) Cellulose synthase (CesA) genes in the red alga Porphyra yezoensis Ueda. J Phycol 43: 42. [DOI] [PubMed] [Google Scholar]
- Roberts K. (1974) Crystalline glycoprotein cell walls of algae: their structure, composition and assembly. Philos Trans R Soc Lond B 268: 129–146 [DOI] [PubMed] [Google Scholar]
- Royo J, Gómez E, Hueros G. (2000) CMP-KDO synthetase: a plant gene borrowed from gram-negative eubacteria. Trends Genet 16: 432–433 [DOI] [PubMed] [Google Scholar]
- Sapei L, Gierlinger N, Hartmann J, Nöske R, Strauch P, Paris O. (2007) Structural and analytical studies of silica accumulation in Equisetum hymale. Anal Bioanal Chem 389: 1249–1257 [DOI] [PubMed] [Google Scholar]
- Sarkar P, Bosneaga E, Auer M. (2009) Plant cell walls throughout evolution: towards a molecular understanding of their design principals. J Exp Bot 60: 3615–3635 [DOI] [PubMed] [Google Scholar]
- Schoenwaelder MAE, Clayton MN. (1998) Secretion of phenolic substances into the zygote wall and cell plate in embryos of Hormosira and Acrocarpia (Fucales, Phaeophyceae). J Phycol 34: 969–980 [Google Scholar]
- Schoenwaelder MAE, Clayton MN. (1999) The presence of phenolic compounds in isolated cell walls of brown algae. Phycologia 38: 161–166 [Google Scholar]
- Schultz CJ, Johnson KL, Currie G, Bacic A. (2000) The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell 12: 1751–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segui-Simarro JM, Austin JR, II, White EA, Staehelin LA. (2004) Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16: 836–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séveno M, Séveno-Carpentier E, Voxeur A, Menu-Bouaouiche L, Rihouey C, Delmas F, Chevalier C, Driouich A, Lerouge P. (2010) Characterization of a putative 3-deoxy-d-manno-2-octulosonic acid (Kdo) transferase gene from Arabidopsis thaliana. Glycobiology 20: 617–628 [DOI] [PubMed] [Google Scholar]
- Shibata T, Nagayama K, Tanaka R, Yamaguchi K, Nakamura T. (2003) Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2s, lipoxygenases and cyclooxygenases. J Appl Phycol 15: 61–66 [Google Scholar]
- Shibuya N, Misaki A. (1978) Structure of hemicellulose isolated from rice endosperm cell wall: mode of linkages and sequences in xyloglucan, β-glucan and arabinoxylan. Agric Biol Chem 42: 2267–2274 [Google Scholar]
- Shimokawa T, Ishii T, Matsunaga T. (1999) Isolation and structural characterization of rhamnogalacturonan II-borate complex from Pinus densiflora. J Wood Sci 45: 435–439 [Google Scholar]
- Sørensen I, Domozych D, Willats WGT. (2010) How have plant cell walls evolved? Plant Physiol 153: 366–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen I, Pettolino FA, Wilson SM, Doblin MS, Johansen B, Bacic A, Willats WGT. (2008) Mixed-linkage (1→3),(1→4)-β-d-glucan is not unique to the Poales and is an abundant component of Equisetum arvense cell walls. Plant J 54: 510–521 [DOI] [PubMed] [Google Scholar]
- Stanley MS, Perry RM, Callow JA. (2005) Analysis of expressed sequence tags from the green alga Ulva linza (Chlorophyta). J Phycol 41: 1219–1226 [Google Scholar]
- Stebbins GL. (1992) Comparative aspects of plant morphogenesis: a cellular, molecular and evolutionary approach. Am J Bot 79: 589–598 [Google Scholar]
- Timell TE. (1962) Studies on ferns (Filicineae). 1. The constitution of a xylan from cinnamon fern (Osmunda cinnamomea). Svensk Papperstidning 65: 122–125 [Google Scholar]
- Thompson JE, Fry SC. (2001) Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. Plant J 26: 23–34 [DOI] [PubMed] [Google Scholar]
- Trethewey JAK, Campbell LM, Harris PJ. (2005) (1→3),(1→4)-β-d-Glucans in the cell walls of the Poales (sensu lato): an immunogold labelling study using a monoclonal antibody. Am J Bot 92: 1669–1683 [DOI] [PubMed] [Google Scholar]
- Tsekos I. (1999) The sites of cellulose synthesis in algae: diversity and evolution of cellulose-synthesising enzyme complexes. J Phycol 35: 635–655 [Google Scholar]
- Turvey JR, Williams EC. (1970) The structure of some xylans from red algae. Phytochemistry 9: 2383–2388 [Google Scholar]
- Van Holst GJ, Clarke AE. (1985) Quantification of arabinogalactans-protein in plant extracts by single radial gel diffusion. Anal Biochem 148: 446–450 [DOI] [PubMed] [Google Scholar]
- Van Sandt VST, Stieperaere H, Guisez Y, Verbelen JP, Vissenberg K. (2007) XET activity is found near sites of growth and cell elongation in bryophytes and some green algae: new insights into the evolution of primary cell wall elongation. Ann Bot (Lond) 99: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Verhof R, Schols HA, McCleary BV, McKee L, Gilbert HJ, Knox JP. (2009) Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J 59: 413–425 [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Oyama M, Osata V, Yokoyama R, Verbelen JP, Nishitani K. (2005) Differential expression of AtXTH17, AtXTH18, AtXTH19 and AtXTH20 genes in Arabidopsis roots: physiological roles in specific cell wall construction. Plant Cell Physiol 46: 192–200 [DOI] [PubMed] [Google Scholar]
- Vreeland V. (1972) Immunocytochemical localization of the extracellular polysaccharide alginic acid in the brown seaweed, Fucus disticus. J Histochem Cytochem 20: 358–367 [DOI] [PubMed] [Google Scholar]
- Vreeland V, Larsen B, Laetsch WM. (1982) Monoclonal antibodies to Fucus cell wall antigens: localization and specificity patterns (abstract). J Cell Biol 95: 127a6890553 [Google Scholar]
- Vreeland V, Slomich M, Laetsch WM. (1984) Monoclonal antibodies as molecular probes for cell wall antigens of the brown alga, Fucus. Planta 162: 506–517 [DOI] [PubMed] [Google Scholar]
- Weng JK, Akiyama T, Bonawitz ND, Li X, Ralph J, Chapple C. (2010) Convergent evolution of syringyl lignin biosynthesis via distinct pathways in the lycophyte Selaginella and flowering plants. Plant Cell 22: 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JK, Li X, Stout J, Chapple C. (2008) Independent origins of syringyl lignin in vascular plants. Proc Natl Acad Sci USA 105: 7887–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D. (1977) Silicate metabolism. The Biology of Diatoms. University of California Press, Berkeley, CA, pp 110–149 [Google Scholar]
- Willats WGT, Rasmussen SE, Kristensen T, Mikkelsen JD, Knox JP. (2002) Sugar-coated microarrays: a novel slide surface for the high-throughput analysis of glycans. Proteomics 2: 1666–1671 [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhang D, Hu J, Zhou X, Ye X, Reichel KL, Stewart NR, Syrenne RD, Yang X, Gao P, et al. (2009) Comparative gene analysis of lignin biosynthesis gene families across the plant kingdom. BMC Bioinformatics (Suppl 11) 10: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Huang J, Xu Y. (2009) The cellulose synthase superfamily in fully sequenced plants and algae. BMC Plant Biol 9: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya D. (2004) A molecular timeline for the origin of photosynthetic eukaryotes. Mol Biol Evol 21: 809–818 [DOI] [PubMed] [Google Scholar]
- York WS, Darvill AG, McNeil M, Albersheim P. (1985) 3-Deoxy-d-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr Res 138: 109–126 [Google Scholar]
- York WS, O'Neill MA. (2008) Biochemical control of xylan biosynthesis: which end is up? Curr Opin Plant Biol 11: 258–265 [DOI] [PubMed] [Google Scholar]
- Zhou GK, Zhong R, Richardson EA, Morrison WH, II, Nairn CJ, Wood-Jones A, Ye ZH. (2006) The poplar glycosyltransferase GT47C is functionally conserved with Arabidopsis Fragile Fiber8. Plant Cell Physiol 47: 1229–1240 [DOI] [PubMed] [Google Scholar]